Antibodies against human leukocyte antigen (HLA) class II, such as 1D10 or Lym-1, are currently being evaluated for the treatment of B-cell lymphomas. Previous studies have demonstrated that, in addition to IgG Fc receptors, the human myeloid IgA receptor (FcαRI, CD89) also effectively triggered tumor cell killing. Therefore, we used the variable light and heavy chain sequences from another murine anti–HLA class II hybridoma, F3.3, to generate a panel of chimeric human/mouse antibodies, including human immunoglobulin A1 (IgA1), IgA2, IgG1, IgG2, IgG3, and IgG4. Antibody production was accomplished by stable transfection of baby hamster kidney cells, and binding activity and specificity were confirmed by enzyme-linked immunosorbent assay (ELISA) and Western blotting. All constructs demonstrated similar binding to HLA class II. Functional studies revealed that chimeric IgG1, IgA1, and IgA2 triggered similar levels of tumor cell lysis. Analyses of effector populations, however, demonstrated that killing by chimeric IgG1 constructs was triggered mainly by human mononuclear cells and complement, while IgA1 and IgA2 mediated effective lysis by polymorphonuclear neutrophils. Importantly, IgG1 and both IgA isotypes were equally effective at killing freshly isolated human chronic lymphocytic leukemia cells. Chimeric IgA antibodies against HLA class II may constitute attractive reagents for lymphoma therapy.
Introduction
Over the last 5 years, monoclonal antibodies have broadened the therapeutic armentarium in oncology.1,2Hematologic malignancies are recognized as particularly promising targets,3 which is also reflected by today's list of approved antibodies.4 Among those investigated in early clinical trials against B-cell lymphomas are antibodies against human leukocyte antigen (HLA) class II, such as Lym-15and Hu1D10.6 Preclinical studies demonstrated that HLA class II antibodies were particularly effective in killing B-lymphoma cells. For example, they inhibited B-cell proliferation, triggered apoptosis and complement-dependent cytotoxicity (CDC), and mediated antibody-dependent cellular cytotoxicity (ADCC) by mononuclear and polymorphonuclear neutrophil (PMN) effector cells.7-10 Importantly, studies against xenogenic tumors in severe combined immunodeficient (SCID) mice confirmed the potential of HLA class II antibodies in vivo,11,12although HLA class II antibodies were less efficient than anti-idiotype antibodies in syngenic lymphoma models.13 However, HLA class II is constitutively expressed on normal B cells, monocytes/macrophages, and dendritic cells, and exposure to interferon (IFN)–γ may induce HLA class II expression on many other cell types, including activated T cells and endothelial cells. Correspondingly, HLA class II antibodies demonstrated considerable toxicity in primate models,14 and side effects in phase 1 clinical trials were more pronounced than those usually observed with rituximab. Importantly, however, some durable responses were documented in this phase 1 trial, even at low antibody doses.15
Our understanding of what effector mechanisms operate with monoclonal antibodies in vivo is comparatively limited.16 Novel studies demonstrated that some antibodies act by directly stimulating immune effector cells17-20 or by blocking inhibitory immune interactions.21 Traditionally, however, effector mechanisms were divided into direct mechanisms, which are mediated by the antibody's variable region—including growth inhibition, induction of apoptosis, and blockade of growth factors—and indirect mechanisms, which are triggered by the constant regions of antibodies. The latter include complement-dependent tumor cell lysis and effector cell–mediated killing by ADCC or phagocytosis. Effector cell–mediated mechanisms typically require interactions between antibodies' constant Fc region and their cellular receptors. For IgG, 3 classes of leukocyte receptors are distinguished: FcγRI (CD64), FcγRII (CD32), and FcγRIII (CD16), which associate with the common FcRγ chain for signaling.22 Recent studies in mice, in which expression and function of activating Fc receptors were disrupted by “knockout” of this FcRγ chain, have demonstrated that Fcγ receptors are critical for the therapeutic efficacy of rituximab and herceptin,23 currently the most widely used antibodies in oncology. Like rituximab and herceptin, most of the tumor-directed unconjugated antibodies employed in clinical trials today are of human IgG1 isotype, because this was demonstrated to be highly effective in activating human complement and in triggering ADCC by human mononuclear cells.24 Additionally, human IgG1 effectively binds to the major histocompatibility complex (MHC) class I–related Fc receptor (FcRn), which protects immunoglobulins from lysosmal degradation and, thereby, prolongs the plasma half-life of therapeutic antibodies.25
In addition to human IgG1, antibodies of human IgA isotype were also demonstrated to effectively trigger effector cell–mediated lysis of solid and lymphoma tumor cells.26,27 IgA represents the most abundantly produced antibody isotype in humans and is critically involved in the host defense at mucosal surfaces.28 Two isoforms—IgA1 and IgA2—are distinguished, with IgA2 having a shorter hinge region and an increased resistance to enzymatic degradation by bacterial proteases.29 Both IgA1 and IgA2 bind with approximately 5 × 107 M−1 affinity to the myeloid IgA receptor FcαRI (CD89).30 Like other immunoglobulin receptors, FcαRI requires association with the immunoreceptor tyrosine-based activation motif (ITAM)–containing FcRγ chain for in vivo expression and function.31FcαRI is constitutively expressed on monocytes/macrophages, PMNs, and some types of dendritic cells, but—importantly for tumor immunotherapy—not on noncytotoxic cells. Functionally, FcαRI was demonstrated to mediate phagocytosis, oxidative burst, cytokine release, antigen presentation, and ADCC.32 As FcαRI-directed bispecific antibodies proved more effective than the respective Fcγ-directed constructs in triggering lymphoma cell lysis,33 we wondered how the natural ligands of FcαRI—IgA1 or IgA2 antibodies—would compare with their respective IgG counterparts. To address this issue, we generated chimeric mouse/human HLA class II–directed antibodies of IgG or IgA isotypes and analyzed their potential to trigger lysis of lymphoma cells. Interestingly, IgA antibodies against HLA class II were highly effective in triggering tumor cell killing by PMNs, which are increasingly recognized as an important effector cell population for immunosurveillance against malignant tumors.34
Materials and methods
Experiments reported here were approved by the Ethical Committee of the University of Erlangen-Nürnberg (Germany), in accordance with the Declaration of Helsinki.
Monoclonal antibodies and antibody constructs
HLA class II hybridomas F3.3 (mIgG1) and Lym-1 (mIgG2a) were from the Tenovus Research Laboratory (Southampton, United Kingdom) and the American Type Culture Collection (ATCC, Manassas, VA), respectively. Fc receptor antibodies A77 (mIgG1) and My43 (mIgM) against FcαRI (CD89), 197 (mIgG2a) and 22 (mIgG1) against FcγRI (CD64), and F(ab′)2 fragments of 3G8 (mIgG1) against FcγRIII (CD16) were from Medarex (Annandale, NJ). F(ab′) fragments of AT10 (mIgG1) to FcγRII were obtained from Tenovus Research Laboratory.
Cloning of chimeric antibodies
Genes for the antibody variable regions (VL, variable light chain region, and VH, variable heavy chain region) were cloned as described in Boel et al.35 Briefly, mRNA was isolated from the original F3.3 hybridoma, and cDNA was generated by reverse transcriptase (RT)–polymerase chain reaction (PCR) using oligo(dT) primers and Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). Variable regions were amplified by PCR using Pwo polymerase (Peqlab, Erlangen, Germany) and a set of V gene–specific primers as described in Vidarsson et al.36 Variable regions were inserted into pUC-HAVT20 vector to incorporate the HAVT20 leader sequence. After sequencing (GenBank accession numbers AY058910 [VL] and AY058911[VH]) using M13 primers and ABI-Big-Dye-terminator mix (Applied Biosystems, Foster City, CA), HAVT20-V inserts were subcloned into pNUT vectors encoding human constant regions for κ–light chain, and γ1, γ2, γ3, γ4 (GenBank accession numbersAF237583 [γ1], AF237584 [γ2], AF237585 [γ3)], AF237586[γ4]), α1 or α2 heavy chains, respectively. The resulting vectors contained methotrexate and ampicillin resistance genes and a Zn-responsive metallothionein promoter. PCR reactions were performed in a DNA Thermal Cycler 480 (Perkin-Elmer, Shelton, CT), sequencing reactions were performed in an Eppendorf Mastercycler Gradient (Eppendorf, Hamburg, Germany), and sequencing reactions were run and analyzed with an ABI PRISM 310 automated sequencer (Applied Biosystems). cDNA for variable light and heavy chains of Lym-1 were similarly isolated from the Lym-1 hybridoma and cloned into IgG1 and IgA2 vectors. Sequences of VL and VH were identical to GenBank accession numbers X53483 and X53484.
Production of chimeric antibodies
Heavy and light chain pNUT expression vectors were cotransfected into BHK-21 cells by calcium phosphate precipitation. Transfected cells were selected in 10 μM methotrexate (Sigma, St Louis, MO). For antibody production, single cell clones were generated by limiting dilution, and cells were grown in Iscove Modified Dulbecco Medium (IMDM) supplemented with 10% heat-inactivated fetal calf serum (FCS; both Invitrogen) and 100 μM ZnCl2. For functional experiments, transfectoma supernatants were adjusted to give the indicated chimeric antibody concentrations.
Immunoblotting
Chimeric antibodies were run on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) with gradient gels (4%-15%), using the Phast system (Amersham Pharmacia, Buckinghamshire, United Kingdom) under reducing and nonreducing conditions. After transfer onto nitrocellulose membranes (Amersham Pharmacia), membranes were washed with distilled water and blocked for 1 hour with 5% fat-free milk in phosphate-buffered saline (PBS) containing 0.1% Tween. Immunoblots were incubated at 4°C overnight with horseradish peroxidase–conjugated polyclonal goat antihuman κ–light chain antibody (diluted at 1:3000) and antihuman IgG (diluted at 1:2000; both from Serotec, Oxford, United Kingdom) or rabbit antihuman IgA (diluted at 1:2500; Dako, Glostrup, Denmark). After extensive washing with PBS containing 0.1% Tween, blots were detected with the enhanced chemoluminescent reaction reagent ECL Plus (Amersham Pharmacia).
ELISA
Concentrations of chimeric antibodies were determined by sandwich ELISA, using IDEC-C2B8 (mouse/human chimeric IgG1κ; Hoffmann-La Roche, Basel, Switzerland) or human polyclonal serum IgA (Sigma) as standards. Briefly, Maxisorp ELISA plates (Nunc, Roskilde, Denmark) were coated with 2 μg/mL of polyclonal rabbit antihuman IgA or 1 μg/mL of rabbit antihuman IgG (both from Dako). Subsequently, serial dilutions of standards and samples were added. For detection, horseradish peroxidase–conjugated goat antihuman κ–light chain antibody (Serotec) was used. Reactions were developed with O-phenylenediamine dihydrochloride with urea hydrogen peroxide in phosphate-citrate buffer (Sigma), measuring optical density (OD) values at 450 nm. Antibody concentrations were calculated from the standard curves.
Immunofluoresence analyses
For indirect immunofluorescence, 50 μL of transfectoma supernatants were added to target cells. After washing 3 times in phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin (Sigma), 1 μg of mouse antihuman κ–light chain, IgA or IgG (all from Biotest, Denville, NJ), isotype-specific mouse antihuman IgG1, IgG2, IgG3, IgG4 (all from CLB, Amsterdam, NL), IgA1 or IgA2 (both from Southern Biotechnology, Birmingham, AL) was added. Cells were washed again and stained with fluorescein isothiocyanate (FITC)–labeled F(ab′)2 fragments of polyclonal goat antimouse antibodies (Jackson Immunoresearch, West Grove, PA). After washing again, cells were analyzed on a flow cytometer (Coulter EPICS Profile, Brea, CA). For each cell population, relative fluorescence intensity was calculated as the ratio of mean linear fluorescence intensity of relevant to irrelevant isotype-matched antibodies.
Tumor cell targets
Malignant B-cell lines RAJI (Burkitt lymphoma), and ARH-77 (mature B cells) were from the ATCC. Cells were kept in RF10+ medium consisting of RPMI 1640 supplemented with 10% heat-inactivated fetal calf serum (FCS), 100 U/mL penicillin, 100 U/mL streptomycin, and 4 mM l-glutamine (all from Invitrogen). For analysis of freshly isolated tumor cells, citrate-anticoagulated blood from patients with CD5/CD19-positive B–chronic lymphocytic leukemia (B-CLL) were layered over a Ficoll (Biotech, Dreieich, Germany) gradient. After centrifugation, mononuclear cells were isolated from the interface. Remaining erythrocytes were removed by hypotonic lysis. Viability of cells was tested by trypan blue exclusion. Generation of human HLA class II–transfected L66 mouse fibroblasts was described in Würflein et al.37
Isolation of mononuclear and neutrophil effector cells
After informed consent was obtained, 10 to 20 mL of peripheral blood was drawn from healthy volunteers or from patients receiving recombinant human granulocyte colony–stimulating factor (rhG-CSF; Neupogen, 3 to 5 μg/kg of body weight, Hoffmann La-Roche) based on clinical indications. For analysis during growth factor treatment, patients had at least 3 days of cytokine therapy. Neutrophils were isolated by a method slightly modified from that described in Elsässer et al.9 Briefly, citrate-anticoagulated blood was layered over a discontinuous Percoll (Biochrom, Berlin, Germany) gradient consisting of 70% and 62% Percoll. After centrifugation, neutrophils were collected at the interface between the 2 percoll layers and mononuclear cells were collected from the serum/Percoll interface. Remaining erythrocytes were removed by hypotonic lysis. Purity of neutrophils was determined by cytospin preparations and exceeded 95%. Mononuclear cells (MNCs) typically contained approximately 60% CD3+ T cells, 20% CD56+ natural killer (NK) cells, and only 10% CD14-expressing monocytes, as determined by immunofluorescence. Viability of cells tested by trypan blue exclusion was higher than 95%.
Antibody-dependent cellular cytotoxicity assays
ADCC assays were performed as described in Elsässer et al.9 Briefly, target cells were labeled with 200 μCi (7.4 MBq) 51Cr for 2 hours. After extensive washing with RF10+, cells were adjusted to 105/mL. Whole blood, plasma, or isolated effector cells (50 μL), sensitizing antibodies at a concentration of 1 μg/mL (unless otherwise indicated), and RF10+ were added to round-bottomed microtiter plates (Nunc). Assays were started by adding target cells (50 μL), resulting in a final volume of 200 μL and an effector-to-target (E/T) cell ratio of 40:1 with isolated effector cells, unless otherwise indicated. After 3 hours at 37°C, assays were stopped by centrifugation, and 51Cr release from triplicates was measured in counts per minute (cpm). Percentage of cellular cytotoxicity was calculated with the following formula: % specific lysis = (experimental cpm − basal cpm )/ (maximal cpm − basal cpm) × 100, with maximal 51Cr release determined by adding perchloric acid (3% final concentration) to target cells and basal release measured in the absence of sensitizing antibodies and effector cells. Antibody-independent cytotoxicity (effectors without target antibodies) was observed in whole blood assays and with mononuclear effector cells, but not with PMNs.
For analysis of effects induced by Fc-receptor blockade, antibodies 197 (blocking FcγRI, CD64), AT10 F(ab′) (FcγRII, CD32), or 3G8 F(ab′)2 (FcγRIII, CD16) were added at 10 μg/mL. For blockade of FcαRI (CD89), 50 μL of My43 supernatant was used. Percentage inhibition was calculated as follows: % inhibition = (% lysis without BA − % lysis with BA)/ (% lysis without BA) × 100, where BA indicates blocking antibody.
C5-deficient human serum (Sigma) was used to address the role of complement in human IgG1–mediated lymphoma cell killing.
Statistical analyses
Group data are reported as mean ± SEM. Differences between groups were analyzed by unpaired (or, when appropriate, paired) Student t tests. Significance was accepted when theP value was less than .05.
Results
Generation of chimeric antibodies
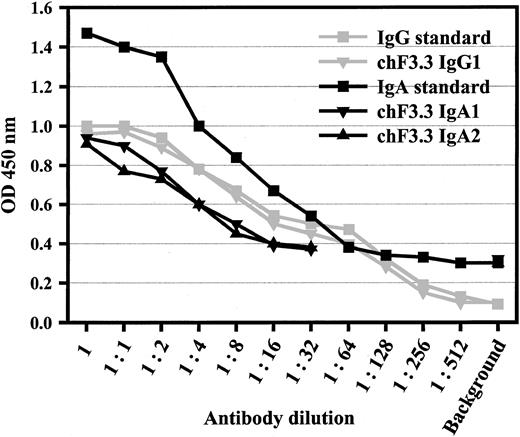
Variable regions of murine pan–HLA class II antibody F3.3 were cloned from the hybridoma9 and sequenced (GenBank accession numbers AY058910 [VL] and AY058911[VH]). cDNA for the light chain variable region was joined with a human κ constant region, and the heavy chain region with human α1, α2, γ1, γ2, γ3, or γ4 heavy chain constant regions, respectively. VL-κ and VHheavy chain vectors were cotransfected into baby hamster kidney (BHK) cells, and stably transfected single cell clones were selected by limiting dilution. Sandwich ELISAs served to measure antibody concentrations in supernatants, which were in the range of 5 μg/mL (Figure 1). The design of the ELISA—additionally—confirmed pairing of heavy and light chains.
Sandwich ELISA for antibody quantification.
ELISA plates were coated with polyclonal antibodies against human IgA or human IgG. After chimeric antibodies were added in serial dilutions, peroxidase-labeled antihuman κ antibody was used for detection. Polyclonal human IgA or rituxan served as standards for IgAs or IgGs, starting from 50 or 5 μg/mL, respectively. This assay also demonstrated the assembly of light and heavy chains of the chimeric antibodies. Data from 1 of 4 experiments with similar results are shown for IgG1, IgA1, and IgA2.
Sandwich ELISA for antibody quantification.
ELISA plates were coated with polyclonal antibodies against human IgA or human IgG. After chimeric antibodies were added in serial dilutions, peroxidase-labeled antihuman κ antibody was used for detection. Polyclonal human IgA or rituxan served as standards for IgAs or IgGs, starting from 50 or 5 μg/mL, respectively. This assay also demonstrated the assembly of light and heavy chains of the chimeric antibodies. Data from 1 of 4 experiments with similar results are shown for IgG1, IgA1, and IgA2.
Characterization of antibodies
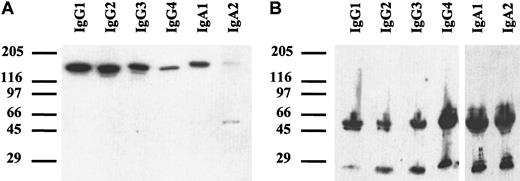
Chimeric antibodies of all isotypes were analyzed by SDS-PAGE. Under nonreducing conditions (Figure 2A), a single band of approximately 150 to 160 kDa was detected, corresponding to the expected size of monomeric IgG or IgA. For IgA2, an additional band of approximately 50 kDa was seen, which probably represented light chain homodimers. In the IgA2m1 isoform, which was used in our experiments, light chains form disulfide bonds with each other but not with their respective heavy chains.38 Under reducing conditions, all isotypes demonstrated single bands of approximately 50 to 60 kDa for the heavy chains and 25 kDa for the light chains (Figure 2B).
Western blots of chimeric antibodies.
Antibodies were separated by SDS-PAGE with gradient gels (4%-15%) under nonreducing (A) or reducing (B) conditions. After transfer onto nitrocellulose, immunoblots were incubated with peroxidase-labeled polyclonal goat antihuman κ–light chain antibody and goat antihuman IgG or rabbit antihuman IgA, respectively. Blots were developed with enhanced chemoluminescent reaction reagent (ECL). Thus, the correct size of all isotypes of chimeric HLA class II antibody F3.3 was demonstrated.
Western blots of chimeric antibodies.
Antibodies were separated by SDS-PAGE with gradient gels (4%-15%) under nonreducing (A) or reducing (B) conditions. After transfer onto nitrocellulose, immunoblots were incubated with peroxidase-labeled polyclonal goat antihuman κ–light chain antibody and goat antihuman IgG or rabbit antihuman IgA, respectively. Blots were developed with enhanced chemoluminescent reaction reagent (ECL). Thus, the correct size of all isotypes of chimeric HLA class II antibody F3.3 was demonstrated.
Binding characteristics of chimeric antibodies
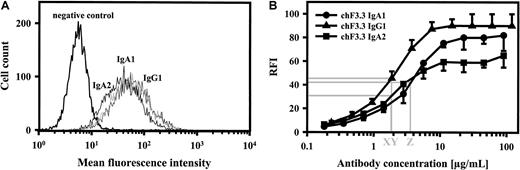
Binding of chimeric antibodies to HLA class II–transfected L66 cells was investigated by indirect immunofluorescence using FITC-labeled antihuman κ–light chain antibody. As shown for chimeric IgG1, IgA1, and IgA2, all isotypes demonstrated virtually identical binding at saturating concentrations (Figure3A). Dilution experiments, furthermore, confirmed that half-maximal binding was achieved at similar antibody concentrations (Figure 3B). These data suggested that binding affinities of our HLA class II antibodies were similar,39but we cannot exclude differences in Kon or Koff, which may be affected by antibody constant regions.40 IgG1-4 and IgA1 or IgA2 isotype identity was confirmed with isotype-specific secondary antibodies (Table1). Thus, chimeric antibodies of all isotypes demonstrated correct heavy and light chain assembly and similar binding characteristics, which was important for subsequent functional studies.
Antigen binding of chimeric antibodies.
Saturating concentrations of chimeric IgA1, IgA2, and IgG1 isotypes of HLA class II antibody F3.3 demonstrated similar binding to HLA class II–transfected L66 cells, as measured by indirect immunofluorescence (panel A; 1 of 2 experiments with similar results is shown). In order to estimate antibody affinity, dilutions of chimeric antibodies were investigated for antigen binding, and antibody concentrations at half-maximal binding were determined (panel B; mean ± SEM for each of 3 experiments is displayed). Thus, IgG1, IgA2, and IgA1 demonstrated half-maximal binding at 1.8 μg/mL (X, Y) and 3.5 μg/mL (Z), respectively. FITC-labeled antihuman kappa–light chain antibody was used for staining.
Antigen binding of chimeric antibodies.
Saturating concentrations of chimeric IgA1, IgA2, and IgG1 isotypes of HLA class II antibody F3.3 demonstrated similar binding to HLA class II–transfected L66 cells, as measured by indirect immunofluorescence (panel A; 1 of 2 experiments with similar results is shown). In order to estimate antibody affinity, dilutions of chimeric antibodies were investigated for antigen binding, and antibody concentrations at half-maximal binding were determined (panel B; mean ± SEM for each of 3 experiments is displayed). Thus, IgG1, IgA2, and IgA1 demonstrated half-maximal binding at 1.8 μg/mL (X, Y) and 3.5 μg/mL (Z), respectively. FITC-labeled antihuman kappa–light chain antibody was used for staining.
Confirmation of chimeric antibodies' isotypes
| Chimeric antibodies . | Human Ig–specific antibodies . | |||||
|---|---|---|---|---|---|---|
| IgA1 . | IgA2 . | IgG1 . | IgG2 . | IgG3 . | IgG4 . | |
| chF3.3 IgA1 | 7.2 | 1.3 | 1.2 | 1.0 | 1.0 | 1.0 |
| chF3.3 IgA2 | 1.0 | 4.5 | 1.2 | 1.0 | 1.0 | 1.0 |
| chF3.3 IgG1 | 1.0 | 1.1 | 11.4 | 1.0 | 1.2 | 1.0 |
| chF3.3 IgG2 | 1.0 | 1.2 | 1.3 | 3.6 | 1.0 | 1.0 |
| chF3.3 IgG3 | 1.0 | 1.1 | 1.2 | 1.0 | 13.3 | 1.0 |
| chF3.3 IgG4 | 1.0 | 1.2 | 1.3 | 1.0 | 1.0 | 7.1 |
| Chimeric antibodies . | Human Ig–specific antibodies . | |||||
|---|---|---|---|---|---|---|
| IgA1 . | IgA2 . | IgG1 . | IgG2 . | IgG3 . | IgG4 . | |
| chF3.3 IgA1 | 7.2 | 1.3 | 1.2 | 1.0 | 1.0 | 1.0 |
| chF3.3 IgA2 | 1.0 | 4.5 | 1.2 | 1.0 | 1.0 | 1.0 |
| chF3.3 IgG1 | 1.0 | 1.1 | 11.4 | 1.0 | 1.2 | 1.0 |
| chF3.3 IgG2 | 1.0 | 1.2 | 1.3 | 3.6 | 1.0 | 1.0 |
| chF3.3 IgG3 | 1.0 | 1.1 | 1.2 | 1.0 | 13.3 | 1.0 |
| chF3.3 IgG4 | 1.0 | 1.2 | 1.3 | 1.0 | 1.0 | 7.1 |
Isotypes of chimeric F3.3 antibodies were confirmed by indirect immunofluorescence of HLA class II–transfected L66 cells using human IgA1-, IgA2-, IgG1-, IgG2-, IgG3-, or IgG4-specific antibodies. FITC-labeled antimouse IgG antibody was used for staining. Data from 1 of 2 experiments are presented as RFIs, calculated as the ratio of mean linear fluorescence intensity of relevant to irrelevant, isotype-controlled antibodies.
Comparison of chimeric antibodies in ADCC
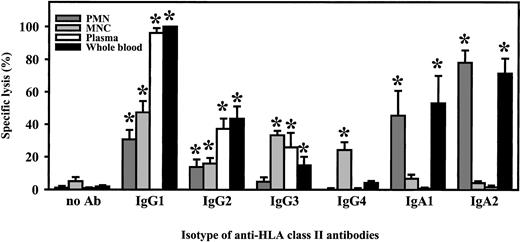
For functional studies, HLA class II–positive mature B-cell line ARH-77 was used as target cell line in 3-hour 51Cr release assays. Relative fluorescence intensity for HLA class II was 191.5 ± 28.4. All isotypes of chimeric HLA class II antibodies (IgA1, IgA2, IgG1-4) were compared in their capacity to mediate ADCC. Unseparated blood served as effector source, which was then fractionated into polymorphonuclear or mononuclear effector cells or into complement-containing plasma to identify effector mechanisms of chimeric antibodies. In unseparated blood, highest killing levels were observed with the chimeric IgG1 antibody, which was predominantly mediated by plasma but to which mononuclear and PMN effector cells also contributed (Figure 4). Plasma-mediated killing was completely abrogated when plasma was heat-inactivated (30 minutes at 56°C) or when C5-deficient human serum was used, indicating that lysis was complement dependent (data not shown). Considerably lower tumor cell killing was observed with other IgG isotypes. Interestingly, human IgA1 and IgA2 also effectively triggered lymphoma cell lysis. Analyses of effector mechanisms demonstrated that IgA-mediated killing was exclusively triggered by PMNs. When RAJI Burkitt lymphoma cells were used as targets (relative fluorescence intensity [RFI] for HLA class II expression, 134.8 ± 21.6) in ADCC by isolated PMNs, IgG1 antibodies proved to be even less effective. Significant killing was observed only at high effector-to-target ratios. In contrast, IgA-mediated lysis of RAJI cells by isolated PMNs was comparable to results with ARH-77. Notably, significant ADCC was observed over a broad range of effector-to-target ratios (Figure 5A) and antibody concentrations (Figure 5B).
Killing of lymphoma cells by chimeric antibodies.
Lysis of ARH-77 lymphoma cells was measured in 3-hour 51Cr release assays, using 1 μg/mL of the indicated chimeric isotypes of HLA class II antibody F3.3. Unseparated blood (whole blood) served as effector source, which was then fractionated into polymorphonuclear (PMN) or mononuclear (MNC) effector cells or into complement-containing plasma to identify effector mechanisms of chimeric antibodies. Isolated PMNs or MNCs were used at an effector-to-target-cell ratio of 40:1. Data are presented as mean percentage specific lysis ± SEM from at least 4 independent experiments. Significant (P < .05) antibody-mediated lysis is marked by an asterisk (*).
Killing of lymphoma cells by chimeric antibodies.
Lysis of ARH-77 lymphoma cells was measured in 3-hour 51Cr release assays, using 1 μg/mL of the indicated chimeric isotypes of HLA class II antibody F3.3. Unseparated blood (whole blood) served as effector source, which was then fractionated into polymorphonuclear (PMN) or mononuclear (MNC) effector cells or into complement-containing plasma to identify effector mechanisms of chimeric antibodies. Isolated PMNs or MNCs were used at an effector-to-target-cell ratio of 40:1. Data are presented as mean percentage specific lysis ± SEM from at least 4 independent experiments. Significant (P < .05) antibody-mediated lysis is marked by an asterisk (*).
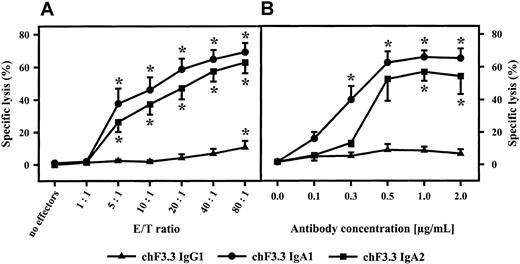
Influence of E/T ratio and antibody concentration on IgA-mediated ADCC.
IgA1-, IgA2-, or IgG1-mediated killing of Raji Burkitt lymphoma cells was investigated using different E/T ratios (A) or antibody concentrations (B). Antibody concentration in panel A was 1 μg/mL; E/T ratio in panel B was 40:1. Data are presented as mean percentage specific lysis ± SEM observed with isolated PMNs from at least 3 different donors. Significant killing (P < .05) is indicated by an asterisk (*).
Influence of E/T ratio and antibody concentration on IgA-mediated ADCC.
IgA1-, IgA2-, or IgG1-mediated killing of Raji Burkitt lymphoma cells was investigated using different E/T ratios (A) or antibody concentrations (B). Antibody concentration in panel A was 1 μg/mL; E/T ratio in panel B was 40:1. Data are presented as mean percentage specific lysis ± SEM observed with isolated PMNs from at least 3 different donors. Significant killing (P < .05) is indicated by an asterisk (*).
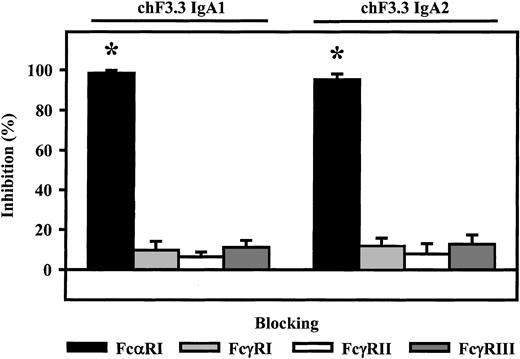
Fc receptor involvement in ADCC by chimeric IgA antibodies
Involvement of Fc receptors in ADCC mediated by chimeric IgA1 and IgA2 was investigated using blocking antibodies to FcαRI (CD89), FcγRI (CD64), FcγRII (CD32), or FcγRIII (CD16). With PMN effector cells, blocking FcαRI resulted in complete inhibition of lysis (98.3% ± 1.4% or 95.2% ± 2.9%, n = 4), while blockade of Fcγ receptors did not significantly affect IgA-induced ADCC (Figure6).
Role of Fc receptors in IgA-mediated killing.
Fc receptor–blocking antibodies My43 (FcαRI), 197 (FcγRI), AT10 F(ab′) (FcγRII), or 3G8 F(ab′)2 (FcγRIII) were used to identify which Fc receptor triggered ARH-77 killing by chimeric IgA1 or IgA2 versions of HLA class II antibody F3.3. PMNs served as effector cells. All Fc receptor antibodies were used at 10 μg/mL. As demonstrated, IgA-mediated lysis was completely inhibited by FcαRI antibody, but not by antibodies directed against Fcγ receptors. Data are presented as mean percentage specific lysis ± SEM from experiments with 4 different donors. Significant inhibition (P < .05) is indicated by an asterisk (*).
Role of Fc receptors in IgA-mediated killing.
Fc receptor–blocking antibodies My43 (FcαRI), 197 (FcγRI), AT10 F(ab′) (FcγRII), or 3G8 F(ab′)2 (FcγRIII) were used to identify which Fc receptor triggered ARH-77 killing by chimeric IgA1 or IgA2 versions of HLA class II antibody F3.3. PMNs served as effector cells. All Fc receptor antibodies were used at 10 μg/mL. As demonstrated, IgA-mediated lysis was completely inhibited by FcαRI antibody, but not by antibodies directed against Fcγ receptors. Data are presented as mean percentage specific lysis ± SEM from experiments with 4 different donors. Significant inhibition (P < .05) is indicated by an asterisk (*).
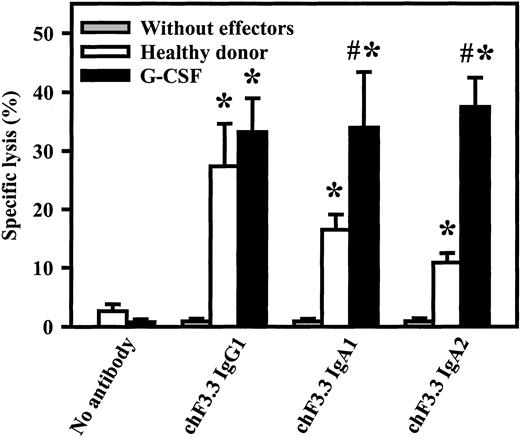
Killing of primary B-CLL cells by chimeric HLA class II antibodies
Primary tumor cells are generally more difficult to kill than lymphoma cell lines. Therefore, chimeric IgG1, IgA1, or IgA2 versions of HLA class II antibody F3.3 were compared in ADCC against freshly isolated B-CLL cells. As effector source, unseparated blood from healthy volunteers or from G-CSF–primed patients was used (Figure7). G-CSF–primed blood contained significantly higher PMN numbers than healthy donor blood (29 000 ± 7000/μL vs 2900 ± 500/μL, n = 4), while FcαRI expression was not affected by G-CSF application.41 Both donor groups mediated significant killing with all 3 antibody isotypes. Analyses of effector mechanisms confirmed results obtained with cell lines: IgG1 killed via complement and MNCs, while IgA acted exclusively via PMNs (data not shown). Notably, both IgA1- and IgA2-mediated lysis were significantly (P < .05) enhanced in blood from donors undergoing G-CSF therapy compared with healthy donors.
Killing of primary B-CLL cells by chimeric HLA class II antibodies.
Chimeric IgG1, IgA1, or IgA2 versions of HLA class II antibody F3.3 were compared in ADCC against 51Cr-labeled, freshly isolated B-CLL cells. As effector source, we used unseparated blood from healthy donors or from G-CSF–primed patients (G-CSF). Both donor groups mediated significant killing (indicated by *) with all 3 antibody isotypes. Notably, only IgA1- and IgA2-mediated lysis, not IgG1-mediated lysis, was significantly enhanced during G-CSF therapy. Killing without antibody or without effectors was consistently low. Data are presented as mean percentage specific lysis ± SEM from experiments with at least 4 different donors from each group. Significant (P < .05) differences between healthy donors and G-CSF–primed patients are indicated by #.
Killing of primary B-CLL cells by chimeric HLA class II antibodies.
Chimeric IgG1, IgA1, or IgA2 versions of HLA class II antibody F3.3 were compared in ADCC against 51Cr-labeled, freshly isolated B-CLL cells. As effector source, we used unseparated blood from healthy donors or from G-CSF–primed patients (G-CSF). Both donor groups mediated significant killing (indicated by *) with all 3 antibody isotypes. Notably, only IgA1- and IgA2-mediated lysis, not IgG1-mediated lysis, was significantly enhanced during G-CSF therapy. Killing without antibody or without effectors was consistently low. Data are presented as mean percentage specific lysis ± SEM from experiments with at least 4 different donors from each group. Significant (P < .05) differences between healthy donors and G-CSF–primed patients are indicated by #.
Discussion
As demonstrated in this study, recombinant chimeric IgA isoforms of pan–HLA class II antibody F3.3 proved highly effective in killing human lymphoma cell lines and, more important, primary tumor cell isolates from CLL patients. Notably, the killing mechanisms of IgA and IgG1 antibodies differed significantly. While IgG1 effectively triggered complement-dependent lysis and ADCC by mononuclear effector cells, IgA antibodies acted exclusively via ADCC by PMNs. Although monocytes also express CD89 and were reported to kill via FcαRI-directed bispecific antibodies,42 monocyte numbers were probably too small to trigger ADCC under our assay conditions. With PMN effector cells, however, IgA antibodies triggered effective killing at low antibody concentrations and at low E/T ratios. To address whether these results were F3.3 specific, we generated chimeric IgG1 and IgA2 variants of HLA class II antibody Lym-1. Preliminary results with these constructs confirmed IgA's killing potential, although binding and killing levels were lower compared with F3.3 (data not shown)—as expected on the basis of data on the respective murine antibodies.37 Furthermore, IgA-mediated tumor cell killing was significantly enhanced when blood from patients during G-CSF therapy was investigated. Previous studies with FcαRI-directed bispecific antibodies demonstrated that this enhanced killing was related to increased PMN counts in G-CSF–primed blood compared with control blood.33
In our experiments, IgA-mediated killing by PMNs was completely blocked by FcαRI (CD89) antibodies, indicating that CD89 was the responsible IgA receptor on PMNs. Recently, a novel leukocyte IgA/IgM receptor was identified on B cells and monocytes,43 and the transferrin receptor (CD71) was demonstrated to specifically bind human IgA1.44 Both receptors were reported to be not expressed on PMNs, while they should be present on lymphoma target cells. The biological functions of both receptors with respect to IgA biology and potential immunotherapeutic applications of IgA antibodies have yet to be defined.
Today, human IgG1 is the most widely used antibody isotype in oncology, because it effectively triggers NK cell–mediated ADCC and CDC and—by binding to FcRn—often has an extended plasma half-life. However, human IgG1 also has potential disadvantages for immunotherapy. For example, antibody-triggered complement activation significantly correlated with more severe side effects in lymphoma patients,45 while the contribution of CDC for clinical efficacy of therapeutic antibodies is controversial.46,47 Furthermore, human IgG1 binds to all 3 classes of Fcγ receptors—FcγRI (CD64), FcγRII (CD32), and FcγRIII (CD16)—some of which are expressed on cells without cytotoxic potential. Additionally, these 3 Fcγ receptor classes also contain isoforms that do not trigger ADCC, such as FcγRIIIb on PMNs, or that trigger strong inhibitory signals on otherwise cytotoxic cells, such as FcγRIIb on monocytes/macrophages.48 The in vivo relevance of inhibitory Fc receptors was elegantly demonstrated in mice, in which the inhibitory FcγRII was deleted by homologous recombination. In animals lacking this isoform, herceptin was significantly more effective than in wild-type mice.23Further support for the role of activating Fc receptors for antibody efficacy in vivo came from clinical studies addressing the function of the cytotoxic FcγRIII isoform. In these studies, a genetic polymorphism of aminoacid 158 in FcγRIIIa—which was documented to affect binding of IgG1—was demonstrated to be associated with response to rituximab therapy.49 Consequently, IgG1 variants were generated with preferential binding to FcγRIIIa and reduced affinity for the inhibitory FcγRIIb.50 However, neither these IgG variants nor molecules with altered complement-activating capacity51 have yet progressed to the clinic.
Application of IgA antibodies may be another, or a complementary approach, to improve effector cell recruitment for antibody therapy. As demonstrated here, IgA antibodies would be expected to effectively recruit PMNs, the most populous effector cell in vivo, which can be further expanded and activated by application of G-CSF or GM-CSF. However, PMNs' capacity to trigger tumor cell lysis is strongly dependent on the selection of appropriate tumor target antigens.9,37,52 Experiments with chimeric HER-2/neu and CD19 antigens, in which intra- and extracellular domains were exchanged, demonstrated that intracellular domains of target antigens were critical for PMN-mediated killing.53 Further studies are required to analyze whether IgA antibodies may overcome PMNs' “antigen restriction,” as was partially observed with FcαRI-directed bispecific antibodies.33 Data reported here were obtained with monomeric IgA1 or IgA2. After covalently binding to J chain, both IgA isoforms form natural dimers. These dimers may improve antibody-mediated signaling in tumor cells and thereby increase apoptosis induction, as demonstrated for chemically dimerized CD19 or CD20 antibodies.54,55 J chain–containing IgA dimers may, furthermore, bind to the polymeric Ig receptor, which facilitates IgA secretion into mucosal surfaces.56 By this route, IgA antibodies may reach certain tumors from the luminal surface, a possibility that appears particularly interesting for epithelial cancers. Further in vivo studies are required to analyze whether these potential benefits of IgA antibodies translate into therapeutic efficacy. However, such studies are complicated by the low homologies between man and mouse IgA and their respective receptors.32,57 Human FcαRI-transgenic mice may represent the first step toward relevant animal models for the evaluation of human IgA antibodies.58
We thank S. Gehr, B. Bock, and H. Vile for their expert technical assistance.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-03-0687.
Supported by the ELAN Fonds from the University Erlangen-Nürnberg, by the Wilhelm Sander Stiftung, and by a Research Training Grant from the European Community Biotechnology program (BIO4-CT97-5084 to G.V.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thomas Valerius, Division of Hematology/Oncology, Department of Medicine III, University of Erlangen-Nürnberg, Krankenhausstr 12, 91054 Erlangen, Germany; e-mail: thomas.valerius@med3.imed.uni-erlangen.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal