Rheumatoid arthritis (RA) is a chronic, inflammatory disease of the synovium of uncertain pathogenesis. A number of phenotypic and functional T-cell defects have been described in RA, including abnormal clonal expansions and suppressed proliferative responses, which suggest a defect in T-cell differentiation. Here, we show that RA patients possess fewer naive CD4+ T cells than healthy controls. Furthermore, a smaller proportion of these cells contains a T-cell receptor excision circle (TREC). Patients with RA also have unusual populations of T cells. These include immature cells characterized as CD45RBbrightCD45RA+CD62L− by flow cytometry and a large population that coexpresses CD45RA and CD45RO. These cells are hyperresponsive to mitogen and TCR stimulation when compared to naive cells. Additionally, an unusual putative central memory subset expressing CD62L, but not CD45RA, appears in RA patients at the expense of more typical cells. Levels of C-reactive protein correlate inversely with the TREC content of naive T cells and positively with the sizes of naive and immature atypical T-cell subsets. These data suggest that inflammation drives proliferation of naive T cells in RA and encourages their differentiation into atypical, hyperresponsive progeny. TREC content of individual naive and atypical T-cell subsets suggests an ontogeny consistent with this hypothesis. These studies provide further evidence of a T-cell differentiation defect in RA, which could explain some of the well-characterized immunologic features of the disease.
Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory disease of the synovium whose pathogenesis is uncertain. A number of features, such as major histocompatibility complex (MHC) association,1 presence of circulating autoantibodies,2 and infiltration of synovium with activated lymphocytes,3 suggest an autoimmune diathesis, though there are competing theories.4 Furthermore, RA T cells are hyporesponsive to stimulation and share a number of features with anergic T cells,5-7 and RA patients are clinically immunosuppressed. They have an excess of infections and malignancies of the immune system.8,9 Additionally, unusual T-cell expansions have been documented in RA patients10,11 along with distortion of the naive T-cell repertoire.12 13 These defects suggest abnormal T-cell development or differentiation in RA that could underlie autoimmunity because inappropriate development and maturation of lymphocytes may bypass tolerogenic mechanisms.
Evidence exists for defective T-cell reconstitution in RA patients with therapy-induced lymphopenia. Patients undergoing autologous stem cell transplantation (ASCT) retained CD4+ lymphopenia for more than 12 months of follow-up.14,15 Furthermore, patients who underwent lymphocytotoxic monoclonal antibody therapy did not experience replenishment of their CD4+ and CD8+T-cell compartments, even when studied 7 years after a brief course of therapy.16 This is in contrast to what happens in patients with hematologic malignancies or solid tumors, most of whom have reconstituted T-cell compartments within 1 year of lymphoablative therapy.17-19 Age-related thymic involution may partly explain this disparity, but, until recently, the best available surrogate measures of thymic function were numbers of circulating naive T cells and radiographic estimation of thymic size.20 The development of techniques for measuring T-cell receptor excision circles (TRECs) has now provided a novel marker. When T cells rearrange their antigen-specific receptors, certain DNA recombination events must take place.21 These involve the excision of small circles of DNA that remain within the cells as episomes. The formation of the TCR-α chain, for example, gives rise to 2 discrete TRECs.22 These do not replicate with the cell but are shared among the progeny; hence, the proportion of peripheral blood T cells containing a TREC provides an estimate of recent thymic function. This discovery has been used to study T-cell reconstitution following chemotherapy for HIV,23 ASCT for myeloma,24and bone marrow transplantation for severe combined immunodeficiency.25 In each situation, peripheral blood TREC content predicted subsequent lymphocyte recovery. Furthermore, the presence of TRECs in the blood of adults suggests residual thymic function beyond adolescence.26
Numerous dynamic processes also influence TREC measurements. For example, the TREC content of naive T cells is influenced by thymic activity on the one hand, proliferation of cells within that subset and differentiation of TREC-containing cells into a more mature phenotype on the other. Thus, in a recent analysis of HIV patients, the proliferation and differentiation of T cells, but not thymic function, were reported to be the major determinants of the TREC content of naive T cells.27 Similarly, when TRECs were measured in patients with RA compared with age-matched control subjects, a significant deficit was demonstrated.28 This might have reflected diminished thymic output, but there was also evidence of enhanced lymphocyte proliferation that might have diluted TRECs.
Defects of thymic function and T-cell differentiation could clearly underlie some of the characteristic immunologic phenomena seen in RA patients. Thus, impaired thymic activity could contribute to the reconstitution defect after lymphodepleting therapies. Similarly, differentiation defects may result in unusual clonal expansions. A better understanding of T-cell dynamics in RA should help to address these possibilities. In this paper we confirm that lymphocytes proliferate abnormally in RA patients. This contributes to reduced TRECs in peripheral blood and gives rise to T cells of atypical phenotype. Although inflammation provides a proliferative stimulus, similar abnormalities appear to be present at disease onset. We suggest that abnormal T-cell dynamics may contribute to autoreactivity in persons in whom RA eventually develops.
Patients, materials, and methods
Patients and healthy controls
Patients with RA, defined according to the American College of Rheumatology criteria,29 were recruited from a number of rheumatology clinics (Table 1). These included the Early Arthritis Clinic, which recruits patients within 12 months of initial onset of symptoms. These patients had not received disease-modifying antirheumatic drugs (DMARDs), but they might have been taking nonsteroidal anti-inflammatory drugs (NSAIDs) or analgesics at the time of recruitment. Diagnoses were subsequently verified after additional follow-up. Other patients attended the Resistant RA Clinic, which manages patients with established disease that has proved refractory to conventional DMARDs. Healthy controls were recruited from among local blood donors. Informed consent was obtained from each participant.
Patient and control data
| . | Control subjects . | Patients with early RA . | Patients with resistant RA . |
|---|---|---|---|
| n | 28 | 26 | 32 |
| Male/female | 14/14 | 10/16 | 8/24 |
| Median disease duration, month (range) | — | 5.6 (2-11) | 126 (22-444) |
| Median CRP, mg/L (range) | — | 24 (5-131) | 81 (5-192) |
| Rheumatoid factor positive, % patients | — | 70 | 66 |
| MTX, % patients | — | None | 50 |
| SSZ, % patients | — | None | 17 |
| Steroids, % patients | — | None | 45 |
| . | Control subjects . | Patients with early RA . | Patients with resistant RA . |
|---|---|---|---|
| n | 28 | 26 | 32 |
| Male/female | 14/14 | 10/16 | 8/24 |
| Median disease duration, month (range) | — | 5.6 (2-11) | 126 (22-444) |
| Median CRP, mg/L (range) | — | 24 (5-131) | 81 (5-192) |
| Rheumatoid factor positive, % patients | — | 70 | 66 |
| MTX, % patients | — | None | 50 |
| SSZ, % patients | — | None | 17 |
| Steroids, % patients | — | None | 45 |
MTX, current methotrexate therapy; SSZ, current sulfasalazine therapy.
T-cell subset separation
Blood samples (15 mL) were collected into heparin. Peripheral blood mononuclear cells (PBMCs) were recovered using a Ficoll density gradient centrifugation, and CD4+ T cells were separated by negative selection (Metachem, Meylan, France). Purity was checked using 3-color flow cytometry (CD3/CD4/CD8; Serotec, Oxford, United Kingdom) and ranged from 92% to 98%. Purified CD4+T cells were stained for CD45RB (fluorescein isothiocyanate [FITC]; DAKO, Ely, United Kingdom), CD45RA (phycoerythrin [PE]; Serotec), CD45RO (PE-CY5; Serotec), and CD62L (energy-coupled dye [ECD]; Coulter, Wycombe, United Kingdom) using conventional methods. Naive T cells were sorted according to CD45RBbright, CD45RA+, and CD62L+phenotypes using a FACS-Vantage cell sorter (Becton Dickinson, Oxford, United Kingdom). Memory cells were identified as CD45RBdull, CD45RA−, and CD45RObright.
Real-time polymerase chain reaction quantification of TRECs
DNA was extracted from lymphocytes using standard proteinase K digestion followed by phenol/chloroform extraction from either total CD4+ populations after magnetic separation or from naive cells after cell sorting. We performed SYBR-green real-time polymerase chain reaction (PCR) based on the coding TREC sequence using an ABI 7700 Sequence Detection System (PE Applied Biosystems, Warrington, United Kingdom). We designed primers to amplify a DNA fragment of 83 bp across the remaining recombination sequence δrec/ψalpha (F-CAC CTC TGG GCT ACG TGC TAG and R-GAA CAC ATG CTG AGG TTT AAA GAG AAT). Optimization was performed according to the manufacturer's instructions using DNA extracted from umbilical cord blood mononuclear cells and a cloned target inserted into a TA-cloning plasmid (Invitrogen, Leek, The Netherlands). This method quantitated TREC molecules per microgram DNA. We subsequently developed a method for the quantification of TRECs based on TREC copy number relative to the GAPDH gene copy number. The latter was proportionate to the number of cells analyzed per sample (F-AAC AGC GAC ACC CAT CCT C and R-CAT ACC AGG AAA TGA GCT TGA CAA). This analysis provides a final value that represents TREC DNA as a proportion ofGAPDH DNA, equivalent to the percentage of cells containing a TREC (F.P. et al, manuscript submitted).
Proliferation assay
CD4+ T cells were separated as above from 50 mL blood. Naive and atypical subsets were sorted under sterile conditions according to the expression of CD45RB, CD45RA, and CD62L. Cells were cultured in RPMI 1640 supplemented with penicillin/streptomycin, glutamine, and 10% human AB Rh-positive serum (Sigma-Aldrich, Poole, United Kingdom), and proliferation was assessed in response to phytohemagglutinin (PHA; 0.01-10 μg/mL; Sigma) or to anti-CD3 antibody (OKT3 0.01-1 μg/mL) plus or minus anti-CD28 (YTH913.12, 5 μg/mL) co-coated on plastic. Irradiated autologous CD4+T-cell–depleted PBMCs were used as feeder cells in a 1:1 ratio to CD4+ T cells. Proliferation was quantified by the incorporation of 3H-thymidine (1 μCi/well [0.037 MBq/well]) after 5 days of culture.
Statistical analysis
Nonparametric tests were used throughout. The Mann-WhitneyU test for 2 independent samples was used to compare healthy controls with RA patients. Spearman rank correlation coefficient was used to correlate 2 variables. Linear regression was used to seek dependency between clinical parameters and laboratory outcomes. The following variables were tested as predictors in linear regression analysis: age, sex, disease duration, CRP, current methotrexate therapy, current sulfasalazine therapy, and current corticosteroid use.
Results
T-cell differentiation is distorted in patients with RA
We initially compared the proportion of circulating CD4+ T cells expressing naive and memory surface markers in RA patients and in healthy controls. Controversy remains over the most reliable surface phenotype of naive T cells. Novel markers have recently been identified, such as the presence of CD103 or CCR7 and the lack of CD27,30-32 but no single marker may be specific. CCR7, for example, has also recently been identified on certain memory T-cell subsets.33 Traditionally, isoforms of the tyrosine phosphatase CD45 (RB, RA, and RO), along with expression of the lymph node homing receptor CD62L, have been used to distinguish naive from memory T cells. CD45RB is highly expressed by naive T cells and is gradually lost after antigenic exposure (bright → dull), in parallel with an increase in CD45RO expression (negative → dull → bright).34 CD45RA and CD62L are also expressed primarily by naive T cells, though they can be reexpressed at a later stage of differentiation.30,33 35 We therefore used these markers to examine T-cell maturity in our patient and control populations (Figure 1).
T-cell differentiation subsets in RA patients and healthy controls.
(A) Atypical differentiation in RA patients. Representative flow cytometry plots of a healthy control (57-year-old man) and a patient with RA (42-year-old woman) are shown. CD4+ T cells were isolated by negative selection using magnetic beads. Cell surface staining was performed with antibodies against CD45RB, CD45RA, CD45RO, and CD62L. CD45RBbright cells are plotted as green throughout and CD45RBdull cells as pink. Naive cells are defined by the expression of CD45RBbright, CD45RA+, and CD62L+, as indicated by the ellipses. Memory cells are defined as CD45RBdull, CD45RA−, and CD45ROhigh, as indicated by the square. Compared with the healthy control, the RA patient has a smaller naive population, CD45RBdull CD45RA+CD62L+ cells (dotted ellipse in control) are absent, and various atypical subsets have appeared (asterisk, hexagon, and arrows; see text for details). In RA patients, a significant proportion of CD45RA+ cells are also CD45RObright. (B) Naive and memory CD4+ T cells in RA patients. Total lymphocytes were recovered from a panel of healthy controls and RA patients. CD4+ T cells were separated and analyzed as described, and the proportion of naive and memory cells were plotted against age. ♦ indicate healthy controls; ⋄, patients with early RA; ▵, patients with resistant RA. Statistical analysis: controls, age versus naive T-cell proportion, r = −0.930,P < .0001; age versus memory T-cell proportion,r = +0.865, P < .0001. RA patients (both groups combined): age versus naive T-cell proportion,r = −0.480, P = .002. RA patients: age versus memory T-cell proportion, r = +0.295,P = .016.
T-cell differentiation subsets in RA patients and healthy controls.
(A) Atypical differentiation in RA patients. Representative flow cytometry plots of a healthy control (57-year-old man) and a patient with RA (42-year-old woman) are shown. CD4+ T cells were isolated by negative selection using magnetic beads. Cell surface staining was performed with antibodies against CD45RB, CD45RA, CD45RO, and CD62L. CD45RBbright cells are plotted as green throughout and CD45RBdull cells as pink. Naive cells are defined by the expression of CD45RBbright, CD45RA+, and CD62L+, as indicated by the ellipses. Memory cells are defined as CD45RBdull, CD45RA−, and CD45ROhigh, as indicated by the square. Compared with the healthy control, the RA patient has a smaller naive population, CD45RBdull CD45RA+CD62L+ cells (dotted ellipse in control) are absent, and various atypical subsets have appeared (asterisk, hexagon, and arrows; see text for details). In RA patients, a significant proportion of CD45RA+ cells are also CD45RObright. (B) Naive and memory CD4+ T cells in RA patients. Total lymphocytes were recovered from a panel of healthy controls and RA patients. CD4+ T cells were separated and analyzed as described, and the proportion of naive and memory cells were plotted against age. ♦ indicate healthy controls; ⋄, patients with early RA; ▵, patients with resistant RA. Statistical analysis: controls, age versus naive T-cell proportion, r = −0.930,P < .0001; age versus memory T-cell proportion,r = +0.865, P < .0001. RA patients (both groups combined): age versus naive T-cell proportion,r = −0.480, P = .002. RA patients: age versus memory T-cell proportion, r = +0.295,P = .016.
Figure 1A illustrates flow cytometry data for a representative healthy control (57 years old) and a patient with early RA (42 years old). CD45RBbright populations are plotted in green, and CD45RBdull populations are plotted in pink. CD45RO expression is characterized as bright, intermediate, or negative, whereas CD45RA and CD62L expression is positive or negative. In healthy controls (upper panels), CD45RBbright CD45RA+cells are also CD62L+ and CD45RO−, and the CD45RBdull population is relatively homogenous (CD45RA− CD45RObright CD62L−). For the current analysis, we designated naive T cells as CD45RBbright CD45RA+ CD62L+ (Figure1A, ellipse) and memory cells as CD45RBdullCD45RA− CD45RObright (Figure 1A, square). In patients with RA (lower panels), we observed a reduction in CD45RBbright CD45RA+ CD62L+ naive T-cell frequency (ellipse). This was compensated, however, by the appearance of 2 atypical CD45RBbright populations: CD45RBbright CD45RA+ CD62L− cells (asterisk; also known as CD45RO− and subsequently referred to as atypical RO− cells) and CD45RBbrightCD45RA+ CD45RObright cells (black arrow; also known as CD62L− and subsequently referred to as atypical RObright cells). The retention of CD45RB and CD45RA expression by these cells suggested that they were relatively immature. Within the CD45RBdull population, CD45RO and CD62L were again heterogeneous in the RA patients. In particular, 2 prominent atypical CD45RBdull subsets appeared—CD45RBdull CD45RA+CD45RObright (white arrow; also known as CD62L− and subsequently referred to as atypical RBdull cells) and CD45RBdullCD45RA− CD45RO− (hexagon; also CD62L+ and referred to subsequently as atypical CD62L+ cells). There were also reduced numbers of CD45RBdull CD62L+ cells with positive CD45RA expression in RA patients compared with healthy controls (dotted ellipse). These data suggest a significant perturbation of T-cell differentiation in RA. In particular, the coexpression of CD45RA and CD45RO by a significant proportion of peripheral blood T cells considerably overestimates the size of naive and memory T-cell subsets based purely on these markers.
In view of these data, we analyzed the proportion of naive and memory peripheral blood CD4+ T cells in a cross-section of healthy controls and RA patients. In controls (Figure 1B, Table2), the percentage of naive and memory cells decreased and increased, respectively, with age. In RA patients fewer naive T cells were present at all ages, though the proportion of memory cells remained similar to that in healthy controls.
Cell subset frequency and TREC content of the patient and control populations
| . | Control (n = 28) . | Early RA (n = 26) . | Resistant RA (n = 32) . |
|---|---|---|---|
| Naive CD4+ T cells* | 11 (3.85-25.6) | 1.50 (0.15-12.8) | 3.7 (0.28-10.3) |
| Memory CD4+ T cells* | 44 (15-61) | 36 (15-53) | 35 (12-68) |
| Atypical RBbright CD4+ T cells* | 2.5 (0.1-7.5) | 10.2 (1.0-33) | 14 (5-40) |
| Total TREC (% cells)† | 4.77 (1.5-10.85) | 0.82 (0.01-6.40) | 1.85 (0.33-4.7) |
| Naive TREC (% cells)† | 8.0 (2.68-17.4) | 4.70 (0.47-8.96) | 1.77 (0.09-7.24) |
| . | Control (n = 28) . | Early RA (n = 26) . | Resistant RA (n = 32) . |
|---|---|---|---|
| Naive CD4+ T cells* | 11 (3.85-25.6) | 1.50 (0.15-12.8) | 3.7 (0.28-10.3) |
| Memory CD4+ T cells* | 44 (15-61) | 36 (15-53) | 35 (12-68) |
| Atypical RBbright CD4+ T cells* | 2.5 (0.1-7.5) | 10.2 (1.0-33) | 14 (5-40) |
| Total TREC (% cells)† | 4.77 (1.5-10.85) | 0.82 (0.01-6.40) | 1.85 (0.33-4.7) |
| Naive TREC (% cells)† | 8.0 (2.68-17.4) | 4.70 (0.47-8.96) | 1.77 (0.09-7.24) |
Median (range) values represent percentages of the total CD4+ T-cell population.
Median (range) values represent the TREC content of CD4+T-cell populations.
Frequency of TREC-containing T cells is reduced in RA patients
The reduced number of naive T cells in patients with RA could have resulted from reduced thymic production or from accelerated differentiation or death. Recent thymic emigrants can be identified by the presence of a TREC,21,23 and the proportion of T cells containing a TREC provides an estimate of recent thymic activity. We developed an assay based on real-time PCR to provide an accurate measure of TRECs in peripheral blood. In healthy controls, there was a strong inverse correlation between age and TREC content of the peripheral blood CD4+ T-cell pool, reflecting the acknowledged decrease in thymic function with age. In RA patients a weak inverse relationship with age was still present, but TREC levels were lower than those in controls (Figure2A, Table 2), irrespective of disease duration. These data confirm observations previously reported in RA patients.28
TREC content of CD4+ T cells is decreased in RA patients.
(A) TREC content of total CD4+ T cells is decreased in RA patients. CD4+ T cells were negatively selected using magnetic beads, and DNA was extracted from the enriched population. TREC content was measured by real-time PCR. Controls (♦) are compared with RA patients (⋄, early RA; ▵, resistant RA). CD4+T-cell TREC content in RA patients was lower than in healthy controls (P < .0001). TREC content decreased with age in both groups (controls, r = −0.816, P < .0001; RA, r = −0.229, P = .132). (B) TREC content of naive CD4+ T cells is lower in RA patients. Naive CD4+ T cells were sorted by flow cytometry (on the basis of their CD45RBbright, CD45RA+, CD62L+ phenotype) from total CD4+ T cells. TREC content was measured as above. Controls (♦) are compared with RA patients (⋄, early RA; ▵, resistant RA). In contrast to total TREC values, there was no significant relationship between the TREC content of naive T cells and age. TREC content of naive T cells in RA patients remained significantly different from that in controls (P < .0001). TREC content was lower in early and resistant RA groups than in controls (controls vs early patients,P = .014; controls vs resistant patients,P < .0001). There was also a significant difference in TREC content between patients with early and resistant RA (P = .005).
TREC content of CD4+ T cells is decreased in RA patients.
(A) TREC content of total CD4+ T cells is decreased in RA patients. CD4+ T cells were negatively selected using magnetic beads, and DNA was extracted from the enriched population. TREC content was measured by real-time PCR. Controls (♦) are compared with RA patients (⋄, early RA; ▵, resistant RA). CD4+T-cell TREC content in RA patients was lower than in healthy controls (P < .0001). TREC content decreased with age in both groups (controls, r = −0.816, P < .0001; RA, r = −0.229, P = .132). (B) TREC content of naive CD4+ T cells is lower in RA patients. Naive CD4+ T cells were sorted by flow cytometry (on the basis of their CD45RBbright, CD45RA+, CD62L+ phenotype) from total CD4+ T cells. TREC content was measured as above. Controls (♦) are compared with RA patients (⋄, early RA; ▵, resistant RA). In contrast to total TREC values, there was no significant relationship between the TREC content of naive T cells and age. TREC content of naive T cells in RA patients remained significantly different from that in controls (P < .0001). TREC content was lower in early and resistant RA groups than in controls (controls vs early patients,P = .014; controls vs resistant patients,P < .0001). There was also a significant difference in TREC content between patients with early and resistant RA (P = .005).
When we examined naive T-cell TREC content specifically, the inverse relationship between TREC content and age was lost (Figure 2B, Table2). Fewer TRECs remained in RA patients, but there was greater variability and the distinction from healthy controls was less marked. There were fewer TRECs in the naive T cells of patients with established resistant RA than there were in patients with early disease (P = .005).
Inflammation predicts the TREC content of naive T cells
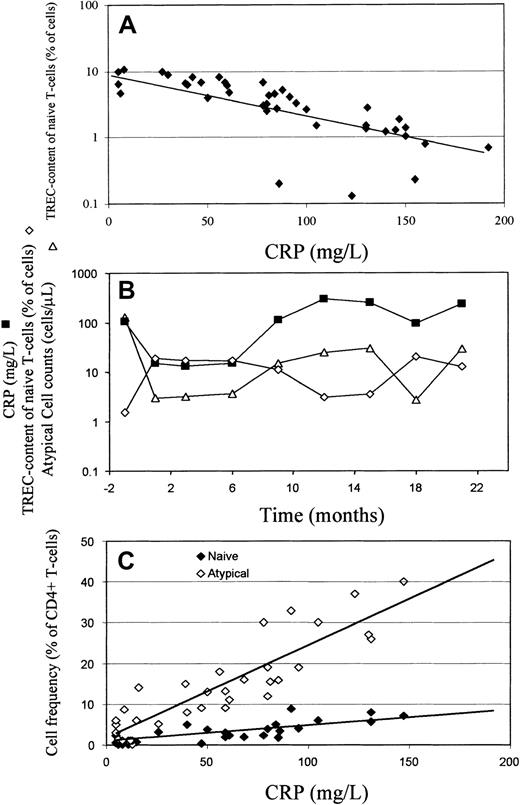
We applied linear regression to seek a clinical correlate of naive CD4+ T-cell TREC content. This analysis suggested a model in which the TREC content of naive CD4+ T cells could be predominantly explained by an inverse relationship with CRP. Figure3A illustrates this relationship in a cross-section of RA patients, and Figure 3B illustrates it in an RA patient with relapsing and remitting disease who was followed up longitudinally for more than 2 years.
TREC content of naive CD4+ T cells correlates negatively with CRP and CRP correlates positively with circulating naive and atypical CD4 ± T cells.
(A) Cross-sectional study. TREC content of naive CD4+ T cells was plotted against CRP for RA patients, illustrating a negative correlation (r = −0.750,P < .0001). (B) Longitudinal study. Longitudinal data from a patient with RA (24-year-old woman) with fluctuating disease severity. CRP (▪) and TREC content of naive (CD45RBbright CD45RA+ CD62L+) CD4+ T cells (⋄) are negatively correlated, whereas CRP and atypical (CD45RBbright CD45RA+CD62L−) cell counts (▵) are positively correlated. (C) Cross-sectional study. The proportion of circulating CD4+ T-cells of naive (♦) and atypical (⋄) phenotype was determined as described in Figure 1 and plotted against CRP. There was a positive correlation between CRP and frequency of atypical cells (r = +0.537, P < .0001) and a weaker correlation with the frequency of naive cells (r = +0.366,P = .017).
TREC content of naive CD4+ T cells correlates negatively with CRP and CRP correlates positively with circulating naive and atypical CD4 ± T cells.
(A) Cross-sectional study. TREC content of naive CD4+ T cells was plotted against CRP for RA patients, illustrating a negative correlation (r = −0.750,P < .0001). (B) Longitudinal study. Longitudinal data from a patient with RA (24-year-old woman) with fluctuating disease severity. CRP (▪) and TREC content of naive (CD45RBbright CD45RA+ CD62L+) CD4+ T cells (⋄) are negatively correlated, whereas CRP and atypical (CD45RBbright CD45RA+CD62L−) cell counts (▵) are positively correlated. (C) Cross-sectional study. The proportion of circulating CD4+ T-cells of naive (♦) and atypical (⋄) phenotype was determined as described in Figure 1 and plotted against CRP. There was a positive correlation between CRP and frequency of atypical cells (r = +0.537, P < .0001) and a weaker correlation with the frequency of naive cells (r = +0.366,P = .017).
Atypical CD4+ T cells in RA patients are the progeny of naive T cells
Assuming peripheral blood T cells mature and differentiate at a relatively constant rate during adult life, a gradual fall in thymic output will be reflected by a parallel reduction of total T-cell TREC content (Figure 2A).20,22,23 26 In contrast, provided intrathymic T-cell differentiation is invariant, the TREC content of naive T cells leaving the thymus should remain relatively constant with age. If this is the case (Figure 2B, healthy controls), the simplest explanation for reduced TRECs in naive T cells of RA patients is a dilutional effect of cell proliferation. If these cells also alter their surface phenotypes, this may account for the appearance of T cells of unusual phenotype in RA patients (Figure 1A).
To seek evidence that atypical T cells derive from naive T cells, we measured the TREC content of various CD4+ T-cell subsets in 3 healthy controls and 3 patients with early RA. Cells were sorted according to their expression of CD45RB, RA, and CD62L following a primary sort based on CD45RO expression. Five populations were isolated from the healthy controls and 8 from the RA patients. The proportion of T cells containing a TREC within each subset was then quantified. T cells can only acquire TRECs within the thymus; they subsequently lose them as they differentiate and expand. It follows that a TREC-poor T-cell subset must be more mature than a TREC-rich T-cell subset and that TREC content can be used to provide a surrogate marker of T-cell ontogeny. In Figure 4, T-cell subset TREC content is related to that of naive cells. In healthy controls, TRECs are most abundant in naive T cells. They are next most frequent in CD45RBbright CD45RA−CD45ROdull CD62L+ and then in CD45RBbright CD45RA− CD45ROdullCD62L− cells. They are progressively less abundant in the CD45RBdull subsets, though these may not all form part of a single differentiation pathway (see “Discussion”). In RA patients the atypical RO− and RObright subsets appear closely related to naive T cells in that their TREC content is similar. The lower TREC content in the atypical RBdullcells suggests a more pronounced proliferation of these cells. As illustrated in Figure 1, we were unable to isolate CD45RBdull CD45RA+ CD62L+ cells from RA patients, but atypical 62L+ cells have a similarly low TREC content.
Atypical T cells are the progeny of naive T cells.
CD4+ T cells were isolated by negative selection, and cell surface staining was performed for CD45RB, CD45RA, CD45RO, and CD62L. Cells were sorted according to their expression of CD45RO and then of CD45RB, CD45RA, and CD62L. DNA was extracted from the subsets illustrated, and TREC content was measured by real-time PCR. Results from 3 healthy controls (22, 33, and 57 years old) and from 3 patients with early RA (45, 61, and 62 years old) were pooled. Values are normalized to the naive cell TREC content of each person. Subsets dominant in healthy controls are shown by solid bars, and atypical subsets dominant in RA patients are shown by hatched bars. Results represent the mean and SD of 3 independent experiments.
Atypical T cells are the progeny of naive T cells.
CD4+ T cells were isolated by negative selection, and cell surface staining was performed for CD45RB, CD45RA, CD45RO, and CD62L. Cells were sorted according to their expression of CD45RO and then of CD45RB, CD45RA, and CD62L. DNA was extracted from the subsets illustrated, and TREC content was measured by real-time PCR. Results from 3 healthy controls (22, 33, and 57 years old) and from 3 patients with early RA (45, 61, and 62 years old) were pooled. Values are normalized to the naive cell TREC content of each person. Subsets dominant in healthy controls are shown by solid bars, and atypical subsets dominant in RA patients are shown by hatched bars. Results represent the mean and SD of 3 independent experiments.
These results support the hypothesis that there is abnormal, inflammation-driven, T-cell proliferation and differentiation in RA patients that results in the appearance of T-cell subsets of atypical phenotype. Further support for the hypothesis is shown in Figure 3C, which illustrates a positive correlation between the frequency of atypical RO−/bright T cells and CRP and a weaker correlation between naive T-cell frequency and CRP. A similar relationship between CRP and atypical cell counts is illustrated longitudinally in Figure 3B.
Atypical cells have a lower activation threshold than naive cells
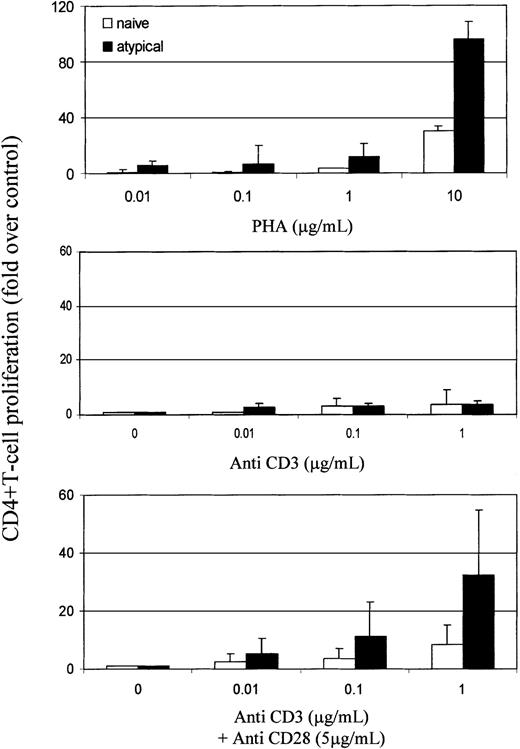
The role of CD45RO versus RA in T-cell activation is uncertain, but memory cells require less stimulation than naive cells to proliferate.36-38 The threshold necessary to activate naive T cells through their TCR is also lowered once they have been induced to proliferate by proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6).39 40 We therefore analyzed the response of naive and atypical RBbright CD4+ T cells to mitogen and TCR stimulation in 5 RA patients (Figure5). CD4+ T cells were sorted according to their expression of CD45RBbrightCD45RA+ CD62L+ CD45RO− (naive) and CD45RBbright CD45RA+ CD62L−CD45RO−/bright (atypical) and were stimulated with PHA and with anti-CD3 and CD28 antibodies. Atypical cells were more responsive than naive cells to mitogen and TCR stimulation. Notably, they proliferated strongly to CD3 plus CD28 but not to CD3 alone.
Activation threshold is lower in atypical cells than in naive cells.
CD4+ T cells were separated, and naive and atypical subsets were sorted from 50 mL blood. Proliferation was assessed in response to PHA (0.01-10 μg/mL; Sigma) or anti-CD3 (OKT3, 0.01-1 μg/mL) with or without anti-CD28 (YTH913.12, 5 μg/mL) antibodies. Proliferation was quantified by the incorporation of 3H-thymidine (1 μCi/well) after 5 days of culture and was normalized to cells in medium only. Results represent the means and SD of independent experiments using 5 RA patients.
Activation threshold is lower in atypical cells than in naive cells.
CD4+ T cells were separated, and naive and atypical subsets were sorted from 50 mL blood. Proliferation was assessed in response to PHA (0.01-10 μg/mL; Sigma) or anti-CD3 (OKT3, 0.01-1 μg/mL) with or without anti-CD28 (YTH913.12, 5 μg/mL) antibodies. Proliferation was quantified by the incorporation of 3H-thymidine (1 μCi/well) after 5 days of culture and was normalized to cells in medium only. Results represent the means and SD of independent experiments using 5 RA patients.
Discussion
We have demonstrated a significant perturbation of T-cell dynamics in RA. Patients had fewer naive T cells than controls, but they also had T-cells of various atypical phenotypes (Figure 1). Additionally, the TREC content of all T-cell subsets was lower in RA patients than in controls (Figure 2 and Table 2). These data are readily explained by abnormal naive T-cell proliferation and phenotypic differentiation, though we cannot exclude an additional defect of thymic T-cell production (see below). Furthermore, the predictive value of CRP (Figures 3, 5) implicates inflammation as the driving force behind the observed abnormalities. Together these results are consistent with a model in which inflammatory stimuli promote the proliferation of naive T cells in RA and their differentiation into subsets of atypical phenotype. Recently, Koetz et al28 also demonstrated reduced total TRECs in RA patients compared with healthy controls. Interestingly, they showed a reduction in telomere length of patients' naive (CD45RO−) T cells, suggesting an increased replicative history, even in patients with recent onset of disease. Combined with our data showing reduced TRECs in various T-cell subsets, these observations provide strong evidence of the increased turnover of naive T cells in RA.
With the aid of current knowledge of T-cell differentiation, we have used TREC content to construct hypothetical maturation pathways for healthy controls and RA patients (Figure6). In healthy controls, a predominant pathway leads to conventional memory cells (CD45RBdullCD45RA− CD45RObright CD62L−). Recently, a second memory subset has been defined, the so-called central memory subset, though its precise lineage is not defined.35 41 We postulate that the CD45RBdullCD45RA+ CD45RO+ CD62L+ cells found in healthy controls equate to this subset.
Model for T-cell differentiation in healthy controls and patients with RA.
The models are based on flow cytometry data (Figure 1) and TREC content of individual subsets (Figure 4). Each subset is represented by a box. White boxes represent subsets found in healthy controls, and gray boxes represent subsets found predominantly in RA patients. Subsets are characterized as TREC rich, TREC high, TREC intermediate (int), and TREC poor, in relation to other subsets from the same pathway (ie, health or RA). In healthy controls we have proposed 2 pathways, culminating in conventional and central memory T cells. In RA, there is proliferation, differentiation, or both within the naive subset, indicated by a circular arrow, resulting in the appearance of atypical subsets that are TREC high. The conventional memory pathway can still be identified, but the central memory pathway cannot. Atypical CD45RBdull CD45RA− CD45RO−CD62L+ cells are also present.
Model for T-cell differentiation in healthy controls and patients with RA.
The models are based on flow cytometry data (Figure 1) and TREC content of individual subsets (Figure 4). Each subset is represented by a box. White boxes represent subsets found in healthy controls, and gray boxes represent subsets found predominantly in RA patients. Subsets are characterized as TREC rich, TREC high, TREC intermediate (int), and TREC poor, in relation to other subsets from the same pathway (ie, health or RA). In healthy controls we have proposed 2 pathways, culminating in conventional and central memory T cells. In RA, there is proliferation, differentiation, or both within the naive subset, indicated by a circular arrow, resulting in the appearance of atypical subsets that are TREC high. The conventional memory pathway can still be identified, but the central memory pathway cannot. Atypical CD45RBdull CD45RA− CD45RO−CD62L+ cells are also present.
In RA patients, the pathway leading to conventional memory cells appears intact. Atypical subsets appear to develop directly from naive T cells. CD45RA expression is never terminated along this pathway, despite the acquisition of CD45RO, giving rise to the large population of CD45RA CD45RO double-positive cells in RA patients. Additionally, central memory cells are not evident in RA patients but a further atypical subset appears (CD45RBdull CD45RA−CD45RO− CD62L+). Of note, the coexpression of CD45RA and CD45RO by a significant proportion of peripheral blood T cells in RA patients considerably overestimates the size of naive and memory T-cell subsets based solely on the expression of these markers.
Our data have implications for the pathogenesis of RA. Atypical cells have a reduced threshold for activation (Figure 5) and premature loss of surface CD62L (Figure 1), which may result in disordered migration with the bypass of lymph nodes and the arrival at peripheral sites. Under specific circumstances, such as tissue trauma or infection, this could result in inappropriate autoreactivity. Consistent with this hypothesis, synovial T cells in RA patients have recently been likened to cytokine-activated T cells,42 and the latter have been shown to elicit a proinflammatory response from monocytes.43 Additionally, clonal expansion of unusual CD4+ T-cell subsets lacking CD28 and CD7 expression or expressing CD57 have been reported in RA.10,11,44,45 In particular autoreactive CD4+CD7−CD28−lymphocytes11 resemble CD4+CD57+ T cells45 46 and have been associated with RA pathogenesis. Our atypical cells express CD28 on their surface (data not shown) and, more important, proliferate strongly through CD3 and CD28 but not to CD3 alone (Figure 5), distinguishing them from those cells.
Proliferation and ultimate exhaustion of the naive repertoire could also contribute to the immunosuppression and T-cell hyporesponsiveness characteristic of RA, as previously suggested.47 We also observed an apparent loss of central memory cells in RA patients, which may be critical for efficient secondary immune responses.33 Additionally, inappropriate trafficking of antigen-inexperienced T-cells, with bypass of lymph nodes caused by the loss of CD62L expression, may, under some circumstances, promote anergy induction through nonprofessional antigen presentation. Through these mechanisms, the increased incidence of infections and immune malignancies in RA could be a further consequence of the abnormalities that we have documented. Because these abnormalities are inflammation driven, it could be argued that they are secondary and of doubtful pathogenic significance. Their presence in patients with symptoms of recent onset, however, suggests otherwise, though this could reflect a significant preclinical phase of RA.48 On the other hand, oligoclonal T-cell expansions have been described in the first-degree relatives of RA patients, suggestive of a primary T-cell proliferative defect.49 Dysregulated T-cell proliferation may, therefore, represent a critical pathogenetic event in RA.
Our results also reinforce the concept that TREC measurements reflect dynamic processes. Thus, though originally defined as a surrogate measure of thymic activity, T-cell proliferation also has a significant influence on peripheral blood TREC concentrations. Indeed, using mathematical modeling, a cogent argument has been made that TREC variability in HIV patients is better explained by changes in peripheral T-cell division rates than by an effect on thymic function.27 Similarly, the proliferative abnormalities we have documented prevent us from making a definitive statement about thymic activity in RA patients. Notwithstanding this caveat, RA may well have a suppressive effect on thymic function. For example, several of the cytokines associated with active RA—including IL-1, IL-6, TNF-α, transforming growth factor-β, and oncostatin M—induce thymic atrophy in laboratory models.50-53 Drugs used in RA, particularly corticosteroids, also adversely affect thymic activity.
In summary, we have confirmed and illuminated some of the T-cell differentiation defects in RA. In particular we have demonstrated that RA patients have unusual, hyperresponsive peripheral blood T-cell subsets. Inflammation appears to drive the appearance of these cells, though they are already present in patients with recent-onset disease. Thus, a primary defect may exist, and we have argued how the inappropriate proliferation of T cells could contribute to RA-associated immunosuppression and disease pathogenesis. Last, our data reinforce the importance of early and aggressive therapy for RA. Although T-cell abnormalities may predate clinical signs and symptoms, they appear to be perpetuated by inflammation. Therefore, the control of inflammation, particularly through the use of cytokine blockade, should minimize dysregulation of proliferation.
We thank Professor Alan Tennant for his assistance with statistical analysis and Professor Herman Waldmann for the supply of YHT913-12 antibody.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-03-0671.
Supported by the Arthritis Research Campaign (P0566), The United Kingdom Medical Research Council, the Dutch Arthritis Association (NR99-1-301), and the Candlelighters Trust.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John D. Isaacs, Molecular Medicine Unit, Clinical Sciences Bldg, St James's University Hospital, Leeds, LS9 7TF, United Kingdom; e-mail: rrrjdi@leeds.ac.uk.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal