The role of complement in the pathogenesis of autoimmune hemolytic anemia (AIHA) has been controversial and may depend on a number of factors, including the affinity and isotype of the pathogenic antibodies involved. We have recently shown that mouse erythrocytes deficient in the membrane C3 regulatory protein, complement receptor 1–related gene/protein y (Crry), but not decay-accelerating factor (DAF), were spontaneously eliminated in vivo by complement. Here, by generating a mouse deficient in both DAF and Crry, we further delineated the roles of Crry and DAF in regulating alternative and classical pathway C3 activation. By using immunoglobulin-, Fcγ receptor (FcγR)–, C3-, C4-, and C5-deficient mice, we also determined the mechanism by which membrane C3 regulator-deficient erythrocytes are cleared from the circulation. Finally, we evaluated the relative importance of the Fc receptor versus the complement pathway in disposing antibody-opsonized DAF/Crry–deficient erythrocytes. We conclude that (1) Crry plays a more dominant role than DAF in regulating the alternative pathway of complement, whereas DAF and Crry are equally effective in preventing antibody-induced runaway complement activation on mouse erythrocytes; (2) DAF/Crry–deficient erythrocytes are eliminated by the alternative pathway of complement via complement receptor–mediated erythrophagocytosis in the spleen; and (3) when opsonized with an immunoglobulin G2a (IgG2a) autoantibody, Crry/DAF–deficient erythrocytes are eliminated more rapidly by complement than by the Fc receptor pathway. These results shed new light on the relative activities of Crry and DAF and underscore the critical roles of membrane C3 regulators in preventing spontaneous and antibody-induced erythrocyte damage in vivo.
Introduction
In autoimmune hemolytic anemia (AIHA), binding of autoantibodies to erythrocyte surface antigens results in abnormally accelerated clearance of erythrocytes through either extravascular or intravascular hemolysis.1 Although both the Fc receptor (FcR) pathway and the complement system are thought to be involved in the hemolytic process,1-3 several recent studies in the mouse have indicated a primary role for the FcR pathway and questioned the relevance of complement in this process.4-7 Thus, injection of rabbit polyclonal antimouse erythrocyte immunoglobulin G (IgG) or a murine IgG2a antierythrocyte autoantibody caused hemolytic anemia in wild-type and C3 knockout mice but not in FcR γ-chain knockout mice.4-7 More recently, the affinity and IgG isotypes of antimouse erythrocyte antibodies have been demonstrated to be critical in determining the activation of the FcR and/or the complement pathway in experimental murine AIHA.8
We have recently shown that mouse erythrocytes deficient in one of the membrane-bound complement-regulatory proteins, complement receptor 1–related gene/protein y (Crry), were spontaneously eliminated in vivo by the complement system.9 This finding suggested that membrane complement-regulatory proteins play a critical role in determining erythrocyte sensitivity to complement damage under normal conditions, and probably in AIHA. Crry is a rodent (mouse and rat)–specific membrane complement regulator that can inhibit both antibody-induced classical pathway and alternative pathway complement activation.10,11 It overlaps in function with decay-accelerating factor (DAF) and membrane cofactor protein,12 2 membrane C3 inhibitors that are universally expressed in mammalian species including the mouse and human. Although the expression of membrane cofactor protein in the mouse is restricted to the testis,13,14 both DAF and Crry are expressed on mouse erythrocytes.9,10,15 The observation that Crry-deficient, but not DAF-deficient, mouse erythrocytes were spontaneously cleared by complement9 raised the question of whether DAF is redundant on mouse erythrocytes. Here, by creating a mutant mouse that is deficient in both DAF and Crry, we have delineated the relative roles of Crry and DAF on mouse erythrocytes in regulating alternative and antibody-induced complement activation. By using a number of mutant mouse lines as transfusion recipients, we also addressed the mechanism by which Crry/DAF–deficient erythrocytes are spontaneously cleared from the circulation. We describe here that although Crry played a more critical role than DAF in regulating the alternative pathway of complement, DAF and Crry were equally active in preventing antibody-induced runaway complement activation on mouse erythrocytes. We also determined that Crry/DAF–deficient mouse erythrocytes were cleared spontaneously by the alternative pathway of complement, via complement receptor–mediated extravascular hemolysis without involving the terminal lytic complex (C5b-9). Finally, we demonstrated that Crry/DAF–deficient erythrocytes opsonized with a murine antierythrocyte IgG2a autoantibody were eliminated more rapidly by the complement system than by the FcR pathway. These findings shed new light on the relative roles of Crry and DAF in vivo, and suggested that membrane complement regulators prevent extravascular hemolysis under normal physiologic conditions and influence the hemolytic pathway in AIHA.
Materials and methods
Mice
Because Crry mutation was lethal,16 to generate viable Crry- or Crry/DAF–deficient mice, a C3 mutation had to be introduced. Therefore, all Crry- or Crry/DAF–deficient mice used in this study also carried the C3 mutation. The generation of DAF and C3 double-deficient (DAF/C3−/−) mice, and of Crry and C3 double-deficient (Crry/C3−/−) mice, was described previously.9,16 To generate mice deficient in Crry as well as DAF, Crry/C3−/− and DAF/C3−/− mice were crossed. Crry/DAF/C3 triple-deficient (Crry/DAF/C3−/−) mice were identified from the F2 generation by fluorescence-activated cell sorter (FACS) analysis of erythrocytes for the absence of DAF and Crry expression, and confirmed by Southern blot analysis of tail DNA.15,16 These mice had a mixed C57Bl/6 and 129J background. C3 knockout mice backcrossed to C57Bl/6 background were generated as previously described17 and were kindly provided by Dr Rick Wetsel (University of Texas Health Sciences Center at Houston). C4 and IgM μ-chain18,19 knockout (C3−/−, C4−/−, Ig−/−) mice on a C57Bl/6 background, and C5-deficient (C5−/−) mice, strain B10.D2-H2, which has a C57Bl/10SnJ background, were obtained from the Jackson Laboratories (Bar Harbor, ME). FcR γ-chain knockout (FcRγ−/−) mice (deficient in both FcγRI and FcγRIII)20 on a mixed 129J/C57Bl background were from a breeding colony maintained at the University of Pennsylvania animal facility. Normal C57Bl/6 and 129J mice were obtained from the Jackson Laboratories. Unless specified, all recipient mice used in transfusion experiments were males.
FACS analysis
A monoclonal hamster antimouse DAF21 was provided by Dr Noriko Okada (Nagoya City University, Japan). Phycoerythrin (PE)–conjugated goat antihamster IgG was obtained from Pharmingen (San Diego, CA). A polyclonal rabbit antimouse Crry was provided by Dr Michael Holers (University of Colorado Health Sciences Center, Denver). Fluorescein isothiocyanate (FITC)–conjugated goat antirabbit IgG was obtained from Sigma (St Louis, MO). Total blood cells were obtained by tail vein bleeding. Cells were washed 3 times in phosphate-buffered saline (PBS) and resuspended in PBS at 2 × 107 cells per milliliter. After staining with primary and secondary antibodies, cells were analyzed by FACScan (Becton Dickinson, San Jose, CA) by gating erythrocytes in forward and side scattering.
C3 deposition assays
Antibody-induced C3 deposition on mouse erythrocytes was performed as previously described.9 Erythrocytes (1 × 106 cells in 200 μL PBS) were opsonized with a monoclonal mouse IgG2a erythrocyte autoantibody 34-3C (50 μg/mL). The hybridoma for 34-3C was originally derived from the autoimmune NZB mice8 22 and was kindly provided by Dr Raphael Clynes (The Rockefeller University, New York, NY). The 34-3C antibody was purified from ammonium sulfate–precipitated concentrated tissue culture supernatant followed by protein A-G affinity chromatography. Antibody-opsonized cells were incubated with mouse complement (prepared in-house from 129J/C57Bl/6 mice, used at 1:40 dilution) in gelatin-veronal buffered saline (GVBS++) (Sigma) at 37°C for 30 minutes. Cells were washed 3 times in PBS and then stained with a FITC-conjugated goat antimouse C3 (ICN, Aurora, OH) and analyzed by FACS for C3 deposition.
Assessment of erythrocyte survival in vivo
To determine the clearance of complement regulator–deficient erythrocytes in vivo, cells (from 150 μL blood) from Crry/C3−/−, DAF/C3−/−, or DAF/Crry/C3−/− mice were labeled ex vivo with biotin as previously described9 and were introduced into various host mice via the tail vein. In some experiments, the biotinylated cells were also opsonized with monoclonal antibody (mAb) 34-3C, an antimouse erythrocyte IgG2a autoantibody (1 × 107 cells in 200 μL containing 50 μg/mL 34-3C). Blood samples were collected at 5 minutes after erythrocyte infusion and at various indicated time points thereafter. Collected erythrocytes were stained with R-(PE)–conjugated streptavidin (Molecular Probes, Eugene, OR), and the percentage of biotinylated cells was determined at each time point.
To study erythrophagocytosis, DAF/Crry/C3−/− erythrocytes (3 × 108) were labeled ex vivo with carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes) in 1 mL PBS containing 5 μM CFSE. The labeling reaction was carried out at room temperature for 5 minutes, after which 200 μL fetal bovine serum was added to quench the labeling reaction. The cells were then washed several times in PBS and used in transfusion experiments. CFSE-labeled DAF/Crry/C3−/− erythrocytes were transfused into wild-type or C3−/− recipient mice, and after 24 hours, the spleen and liver were collected and embedded in OCT (Sakura, Torrance, CA) medium. Cryosections of spleen and liver (5 μm thickness) were made, and were examined under a fluorescence microscope for the presence of CFSE+ cells.
Results
Relative activities of Crry and DAF in regulating spontaneous and antibody-induced complement activation on mouse erythrocytes
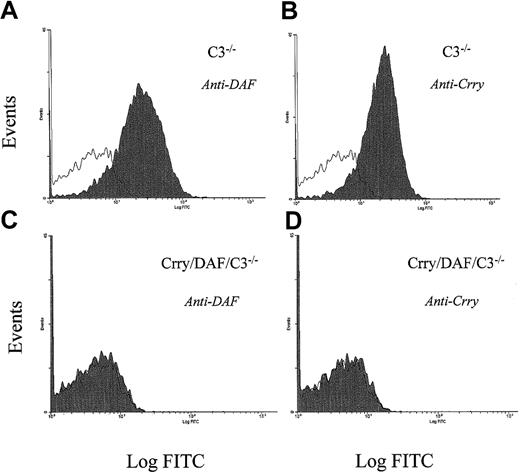
In a previous study, we observed that when transfused into wild-type (C3-sufficient) mice, Crry/C3−/− but not DAF/C3−/− mouse erythrocytes were spontaneously eliminated by complement.9 Experiments using male and female C57Bl/6, 129J, or mixed 129J/C57Bl/6 mice as recipients showed that this phenomenon was not strain- or sex-dependent (data not shown). Because both Crry and DAF function as regulators of C3 activation of the classical and the alternative pathways,12,23,24 the finding that Crry/C3−/− but not DAF/C3−/− erythrocytes were susceptible to complement clearance was rather unexpected and implied differential activities of Crry and DAF on mouse erythrocytes. To further delineate the relative roles of Crry and DAF on mouse erythrocytes, we generated a Crry/DAF/C3−/− mouse by crossbreeding Crry/C3−/− and DAF/C3−/−mice. The successful generation of Crry/DAF/C3−/− mice was indicated by the complete absence of Crry and DAF on their erythrocytes, as shown by FACS analysis (Figure1), and confirmed by Southern blot analysis of tail DNA (data not shown). We then carried out transfusion experiments to compare the sensitivities of Crry/DAF/C3−/−, Crry/C3−/−, and DAF/C3−/− mouse erythrocytes to complement damage in vivo. Figure 2A demonstrates that Crry/DAF/C3−/− cells appeared to be more vulnerable than Crry/C3−/− cells to spontaneous elimination in vivo, as lower numbers of Crry/DAF/C3−/− cells were consistently recovered at 5 minutes after transfusion into wild-type (C3-sufficient) mice. Nevertheless, the overall clearance kinetics of Crry/DAF/C3−/− cells, when followed between 5 minutes and 3 days, was very similar to that of Crry/C3−/− cells (Figure 2B). This experiment confirmed that Crry plays a more critical role than DAF in preventing spontaneous complement damage of mouse erythrocytes.9 It also suggested, however, that although DAF deficiency alone was inconsequential (Figure 2A),9 15 the absence of DAF in the context of Crry deficiency may have a detectable, although by no means dramatic, influence on the susceptibility of these cells to spontaneous complement attack.
FACS analysis for the presence of DAF and Crry in the erythrocytes of Crry/DAF/C3−/− mice.
FACS analysis showed that both DAF (panels A, C) and Crry (panels B, D) are absent from the erythrocytes of Crry/DAF/C3−/− mice (panels C, D). Erythrocytes from C3−/− mice (panels A, B) were used as a control. Open areas represent background signals in the absence of antibodies.
FACS analysis for the presence of DAF and Crry in the erythrocytes of Crry/DAF/C3−/− mice.
FACS analysis showed that both DAF (panels A, C) and Crry (panels B, D) are absent from the erythrocytes of Crry/DAF/C3−/− mice (panels C, D). Erythrocytes from C3−/− mice (panels A, B) were used as a control. Open areas represent background signals in the absence of antibodies.
Relative activities of Crry and DAF on mouse erythrocytes and demonstration of the requirement of C3 in the clearance of Crry/DAF/C3−/− erythrocytes.
(A) Percentage of transfused DAF/C3−/− (●; n = 4), Crry/C3−/− (○; n = 4), and Crry/DAF/C3−/− (▴; n = 4) erythrocytes recovered from age-matched wild-type (C57Bl/6) recipient mice. Erythrocytes were pooled from 6 to 8 donor mice and labeled with biotin, and 3 × 108 cells were transfused into each recipient mouse. (B) Transformation of data from panel A to show the clearance kinetics from 5 minutes (taken as 100%) to 3 days after transfusion. (C) Sensitivity of C3−/−, DAF/C3−/−, Crry/C3−/−, and Crry/DAF/C3−/− erythrocytes to antibody-induced C3 deposition in vitro (n = 8 mice in each genotype). Cells were opsonized with 34-3C (50 μg/mL) and then treated with mouse serum (1:40). MFI indicates mean fluorescent intensity. Both DAF/C3−/− and Crry/C3−/−cells incurred higher C3 deposition than C3−/− cells (P < .01 and P < .001, respectively, Student t test. MFIs were as follows: for C3−/−, 1.92 ± 0.28; for DAF/C3−/−, 3.25 ± 0.29; for Crry/C3−/−, 4.52 ± 0.26). The difference between Crry/C3−/− and DAF/C3−/−cells is also significant (P < .01, Student ttest). However, Crry/DAF/C3−/− cells incurred much more C3 deposition than either DAF/C3−/− or Crry/C3−/− erythrocytes (MFI, 44.60 ± 4.82). Error bars indicate the means ± SE. (D) Elimination of Crry/DAF/C3−/− erythrocytes in vivo was prevented by C3 deficiency (filled circles; n = 3). Open circles represent results from wild-type recipient mice (n = 4).
Relative activities of Crry and DAF on mouse erythrocytes and demonstration of the requirement of C3 in the clearance of Crry/DAF/C3−/− erythrocytes.
(A) Percentage of transfused DAF/C3−/− (●; n = 4), Crry/C3−/− (○; n = 4), and Crry/DAF/C3−/− (▴; n = 4) erythrocytes recovered from age-matched wild-type (C57Bl/6) recipient mice. Erythrocytes were pooled from 6 to 8 donor mice and labeled with biotin, and 3 × 108 cells were transfused into each recipient mouse. (B) Transformation of data from panel A to show the clearance kinetics from 5 minutes (taken as 100%) to 3 days after transfusion. (C) Sensitivity of C3−/−, DAF/C3−/−, Crry/C3−/−, and Crry/DAF/C3−/− erythrocytes to antibody-induced C3 deposition in vitro (n = 8 mice in each genotype). Cells were opsonized with 34-3C (50 μg/mL) and then treated with mouse serum (1:40). MFI indicates mean fluorescent intensity. Both DAF/C3−/− and Crry/C3−/−cells incurred higher C3 deposition than C3−/− cells (P < .01 and P < .001, respectively, Student t test. MFIs were as follows: for C3−/−, 1.92 ± 0.28; for DAF/C3−/−, 3.25 ± 0.29; for Crry/C3−/−, 4.52 ± 0.26). The difference between Crry/C3−/− and DAF/C3−/−cells is also significant (P < .01, Student ttest). However, Crry/DAF/C3−/− cells incurred much more C3 deposition than either DAF/C3−/− or Crry/C3−/− erythrocytes (MFI, 44.60 ± 4.82). Error bars indicate the means ± SE. (D) Elimination of Crry/DAF/C3−/− erythrocytes in vivo was prevented by C3 deficiency (filled circles; n = 3). Open circles represent results from wild-type recipient mice (n = 4).
We next evaluated the relative activities of Crry and DAF in regulating antibody-induced (classical pathway) complement activation on mouse erythrocytes. For this assay, C3−/−, Crry/C3−/−, DAF/C3−/−, and Crry/DAF/C3−/− cells were opsonized with mAb 34-3C, an IgG2a antimouse erythrocyte autoantibody,8 22 and C3 deposition was measured by FACS analysis after the opsonized cells were put into a reaction with normal mouse serum in vitro. Figure2C shows that both Crry/C3−/− and DAF/C3−/−erythrocytes were significantly more sensitive than C3−/−cells to 34-3C–induced C3 deposition. Remarkably, Crry/DAF/C3−/− cells incurred C3 deposition that was at least an order of magnitude higher than either Crry/C3−/−or DAF/C3−/− cells (Figure 2C). This result indicated that there was significant compensation between DAF and Crry in regulating the classical pathway of complement. Thus, although lack of either DAF or Crry caused an increment in erythrocyte sensitivity to classical pathway of complement attack, antibody-induced runaway complement activation on mouse erythrocytes occurred only when both DAF and Crry were missing.
Crry/DAF/C3−/− mouse erythrocytes were eliminated by the alternative pathway of complement
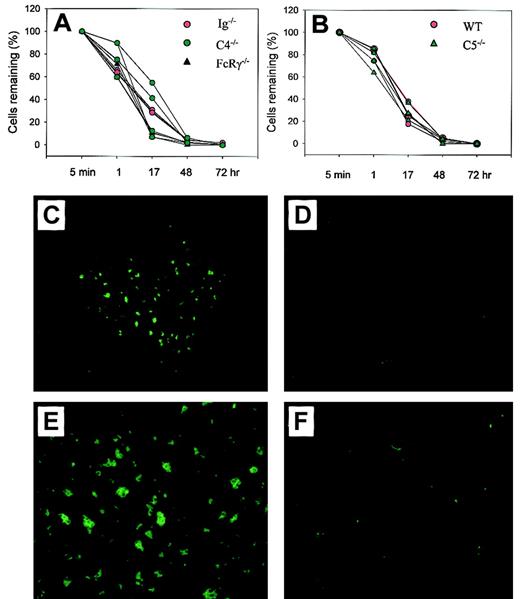
As previously shown for Crry/C3−/−erythrocytes,9 the elimination of transfused Crry/DAF/C3−/− erythrocytes in wild-type recipient mice was C3-dependent (Figure 2D). To determine if this process was related to the phenomenon of natural antibody-mediated transfusion reaction, which involved the activation of the classical pathway of complement, we first transfused Crry/DAF/C3−/− erythrocytes into Ig−/− mice. These mice lacked the production of IgM and class-switched antibodies19 and should not be able to mount a classical pathway of complement-mediated transfusion reaction. Figure 3 shows that Crry/DAF/C3−/− erythrocytes were cleared normally in Ig−/− mice, as they were in wild-type recipient mice. Thus, elimination of Crry/DAF/C3−/− erythrocytes was independent of the antibody repertoire of the recipient. To exclude the possibility that Crry/DAF/C3−/− erythrocytes might have autoantibodies that were prebound to their surface and that could have activated the classical pathway of complement upon transfer into a C3-sufficient host, we studied the survival of Crry/DAF/C3−/− cells in C4−/− mice. These mice had targeted deletion of the C4 gene and thus lacked the classical pathway of complement.18 Figure 3A shows that C4 deficiency similarly did not protect Crry/DAF/C3−/− cells from spontaneous elimination. This result suggested that elimination of Crry/DAF/C3−/− cells was mediated by the alternative rather than the classical pathway of complement. Finally, transfusion of Crry/DAF/C3−/− erythrocytes into FcRγ−/− mice demonstrated normal kinetics of clearance (Figure 3A), confirming that the FcR pathway was also not involved in the spontaneous destruction of Crry/DAF/C3−/−cells.
The elimination of Crry/DAF/C3−/−erythrocytes by the alternative pathway of complement via complement receptor (CR)–mediated erythrophagocytosis.
(A) Crry/DAF/C3−/− erythrocytes were cleared normally in Ig−/− (red circles; n = 4), C4−/− (green circles; n = 4), and FcRγ−/− mice (black triangles; n = 3). (B) Crry/DAF/C3−/− erythrocytes had similar clearance kinetics in C5-deficient mice (green triangles; n = 4) as in wild-type mice (red circles; n = 3). (C-F) Sequestration of CFSE-labeled Crry/DAF/C3−/− erythrocytes in the spleen of wild-type recipients (panel C, × 100 ; panel E, × 200), but not that of C3−/− mice (panel D, × 100; panel F, × 200), at 24 hours after transfusion.
The elimination of Crry/DAF/C3−/−erythrocytes by the alternative pathway of complement via complement receptor (CR)–mediated erythrophagocytosis.
(A) Crry/DAF/C3−/− erythrocytes were cleared normally in Ig−/− (red circles; n = 4), C4−/− (green circles; n = 4), and FcRγ−/− mice (black triangles; n = 3). (B) Crry/DAF/C3−/− erythrocytes had similar clearance kinetics in C5-deficient mice (green triangles; n = 4) as in wild-type mice (red circles; n = 3). (C-F) Sequestration of CFSE-labeled Crry/DAF/C3−/− erythrocytes in the spleen of wild-type recipients (panel C, × 100 ; panel E, × 200), but not that of C3−/− mice (panel D, × 100; panel F, × 200), at 24 hours after transfusion.
Crry/DAF/C3−/− erythrocytes were cleared by extravascular hemolysis without involving the terminal lytic pathway of complement
We next examined whether Crry/DAF/C3−/− erythrocytes were destroyed via intravascular hemolysis by the lytic pathway of complement (C5b-9) or via extravascular hemolysis through complement receptor (CR)–mediated erythrophagocytosis. Figure 3B shows that when Crry/DAF/C3−/− erythrocytes were transfused into C5−/− mice, they were eliminated as usual, with kinetics not dissimilar to that observed in wild-type recipient mice. Thus, an intact lytic pathway was not required, implying that Crry/DAF/C3−/− erythrocytes were cleared primarily, if not exclusively, through CR-mediated extravascular hemolysis. To confirm this implication, Crry/DAF/C3−/− cells were labeled with CFSE, and their possible sequestration in the liver and spleen of wild-type recipient mice was examined. The use of CFSE allowed the direct examination of frozen liver and spleen sections under a fluorescence microscope, and pilot experiments had established that CFSE-labeled Crry/DAF/C3−/− cells had clearance kinetics similar to that of biotin-labeled cells when transfused into wild-type mice (data not shown). Figure 3C,E shows that, 24 hours after transfusion, CFSE+ cell clusters were observed in the spleens of wild-type recipient mice. No trapping of CFSE-labeled cells was observed in the spleens of C3−/− recipient mice (Figure 3D,F), which failed to clear Crry/DAF/C3−/− cells from their circulation (Figure 2B). No fluorescence above background level was observed in the liver of either wild-type or C3−/− recipient mice (data not shown), suggesting that complement-opsonized Crry/DAF/C3−/− erythrocytes were removed by splenic rather than liver phagocytes.
Membrane regulators of complement can significantly influence the hemolytic pathway and kinetics in AIHA
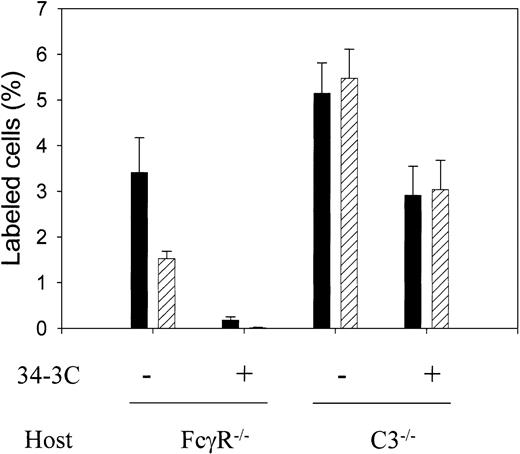
The marked sensitivity displayed by Crry/DAF/C3−/−erythrocytes to antibody-induced complement activation in vitro (Figure2C) suggested that membrane regulators of complement may significantly influence the hemolytic pathways in AIHA, that is, the relative contribution of the FcR versus the complement pathway in the pathogenesis of AIHA. To test this hypothesis, we compared the relative efficiency of the FcR pathway with that of the complement pathway in clearing antibody-opsonized Crry/DAF/C3−/−erythrocytes in vivo. To isolate the function of the FcR from the complement pathway and vice versa, we used C3−/− or FcRγ−/− mice, respectively, as recipients for transfusing antibody-opsonized Crry/DAF/C3−/−erythrocytes. Figure 4 shows that when Crry/DAF/C3−/− erythrocytes opsonized in vitro with 50 μg/mL 34-3G (IgG2a autoantibody) were transfused into FcRγ−/− mice (complement-dependent clearance), they were rapidly eliminated within the first 5 minutes. In contrast, when such cells were transfused into C3−/− mice (FcR-dependent clearance), the kinetics of clearance was much slower. A high percentage of the transfused cells remained at 5 minutes, and little change was detected between 5 minutes and 3 hours (Figure 4). Thus, in the absence of Crry and DAF from the cell surface, complement was shown to be a more efficient pathway for disposing of erythrocytes opsonized with a complement-fixing autoantibody.
More rapid clearance of membrane complement regulator–deficient erythrocytes by the complement than by the FcR pathway.
Biotin-labeled Crry/DAF/C3−/− erythrocytes (3 × 108 per recipient) were either opsonized (+) or not (−) with 50 μg/mL mAb 34-3C, and transfused into FcγR−/− mice (complement-mediated pathway) or C3−/− mice (FcR-dependent pathway). The number of biotin-positive cells (expressed as percentage of total cells) in the recipient mice (n = 4 in FcγR−/− mice experiment; n = 6 and n = 7 for antibody-sensitized and nonsensitized, respectively, in C3−/− mice experiment) at 5 minutes (filled bars) and 3 hours (hatched bars) was determined. Very few of the antibody-opsonized cells were recovered from FcγR−/− mice at 5 minutes and almost none at 3 hours. In contrast, a high percentage of the antibody-opsonized cells remained in the circulation of C3−/− mice at 5 minutes and 3 hours. Error bars indicate means ± SE.
More rapid clearance of membrane complement regulator–deficient erythrocytes by the complement than by the FcR pathway.
Biotin-labeled Crry/DAF/C3−/− erythrocytes (3 × 108 per recipient) were either opsonized (+) or not (−) with 50 μg/mL mAb 34-3C, and transfused into FcγR−/− mice (complement-mediated pathway) or C3−/− mice (FcR-dependent pathway). The number of biotin-positive cells (expressed as percentage of total cells) in the recipient mice (n = 4 in FcγR−/− mice experiment; n = 6 and n = 7 for antibody-sensitized and nonsensitized, respectively, in C3−/− mice experiment) at 5 minutes (filled bars) and 3 hours (hatched bars) was determined. Very few of the antibody-opsonized cells were recovered from FcγR−/− mice at 5 minutes and almost none at 3 hours. In contrast, a high percentage of the antibody-opsonized cells remained in the circulation of C3−/− mice at 5 minutes and 3 hours. Error bars indicate means ± SE.
Discussion
Murine erythrocytes express 2 functionally overlapping membrane regulators of C3 activation, DAF and Crry, as well as a membrane inhibitor of the terminal complement attack complex, CD59. In a previous study, we found that targeted deletion of the DAF and CD59 genes in the mouse significantly increased the sensitivity of its erythrocytes to antibody-induced complement lysis but, surprisingly, had minimal effect on its erythrocyte sensitivity to spontaneous complement attack.9 In contrast, deficiency of Crry on the mouse erythrocytes rendered them susceptible to spontaneous elimination by a C3-dependent mechanism.9 These findings questioned the relative roles of Crry and DAF on murine erythrocytes and made us wonder about the mechanism by which Crry-deficient mouse erythrocytes were spontaneously cleared from the circulation.
In the present study, we have addressed these questions, as well as the concept that membrane regulators of complement may also significantly influence the hemolytic pathways in AIHA. To define the relative roles of Crry and DAF, we generated a Crry/DAF/C3−/− mouse and compared its erythrocyte sensitivity to complement attack with that of DAF/C3−/− and Crry/C3−/− mice. We found that although Crry/DAF/C3−/− erythrocytes appeared to be mildly more sensitive than Crry/C3−/− cells to spontaneous complement elimination (Figure 2A), the overall clearance kinetics of Crry/DAF/C3−/− cells was nevertheless very similar to that of Crry/C3−/− cells (Figure 2B). In contrast, combined deficiency of Crry and DAF dramatically increased the sensitivity of murine erythrocytes to the classical pathway of complement attack (Figure 2C). Thus, in the absence of either Crry or DAF, there was an increment in the sensitivity of murine erythrocytes to antibody-induced C3 deposition. However, when both Crry and DAF were removed, erythrocytes became exceedingly sensitive to the classical pathway of complement activation (Figure 2C). This observation implied that there was significant compensation between Crry and DAF in the regulation of the classical pathway of complement, with the result that either Crry or DAF was sufficient to prevent antibody-induced runaway complement activation on murine erythrocytes. While we cannot rule out the possibility that a lower-level expression was responsible for DAF's playing a lesser role than Crry in regulating the alternative pathway of complement on mouse erythrocytes, our results of the classical pathway assay would suggest that DAF is relatively more active as a regulator of the classical than of the alternative pathway of complement. In this regard, it is of relevance to note a previous in vitro study comparing the efficacies of recombinant soluble murine Crry-immunoglobulin and DAF-immunmoglobulin fusion proteins. In that study, DAF was shown to be more active than Crry as a regulator of the classical pathway of complement, whereas Crry was more active than DAF as a regulator of the alternative pathway of complement.25
Using a number of knockout mouse strains as recipients in the transfusion experiments, we also defined the pathways by which Crry/DAF–deficient mouse erythrocytes were spontaneously cleared from the circulation. We demonstrated that the clearance of such cells was dependent on C3 but not on C4 or C5, suggesting that the classical pathway or the terminal lytic cascade of complement was not involved. That the genetic background of the recipient mice (129J, C57Bl/6) had no effect on the fate of transfused Crry/DAF/C3−/− cells, and the finding that complete deficiency of immunoglobulins in the recipient mice failed to protect the transfused cells from elimination, lent further support to the conclusion that the process did not represent a natural antibody-mediated transfusion reaction. Furthermore, the use of FcRγ−/− mice as transfusion recipients demonstrated a lack of involvement of the FcR pathway. Taken together, these findings suggested that Crry/DAF/C3−/−cells were removed by erythrophagocytosis after incurring surface C3 deposition via the alternative pathway of complement. This conclusion was corroborated by the localization of CFSE-labeled Crry/DAF/C3−/− cell aggregates in the spleens of wild-type but not C3−/− recipient mice 24 hours after the transfusion experiment (Figure 3). Although the identity of the C3 ligand or its receptor responsible for this process remains to be determined, it was apparent that erythrophagocytosis occurred in the spleen rather than in the liver.
Our study has demonstrated that membrane complement regulators are absolutely required for protecting erythrocytes from spontaneous complement damage. Whether these proteins are also required for homeostatic protection of other types of adult mouse tissues remains to be examined although, as alluded to earlier, Crry-deficient mouse embryos, like erythrocytes, were also susceptible to spontaneous complement attack.16 The revelation of an essential role of Crry and DAF in protecting erythrocytes under normal physiologic conditions raised the issue of relevance of membrane complement regulators in autoimmune hemolytic anemia. Although complement has long been considered to be a major effector pathway in the pathogenesis of AIHA,1-3 its importance in AIHA, relative to that of the FcR pathway, has been questioned by a number of recent studies in a murine AIHA model.5-7 It now appears that the affinity and IgG isotype of the pathogenic antibodies may determine, to a large extent, the relative contribution of the 2 pathways.8Thus, the murine mAb 34-3C, an IgG2a antierythrocyte autoantibody, caused a predominantly FcR-mediated hemolysis in which the complement system played a secondary role.8 In comparison, an IgG1-class switch variant of the same antibody invoked only the FcγR pathway, whereas an IgG3 variant activated only the complement system.8 By using C3−/− or FcγR−/− mice as transfusion recipients to isolate the FcR pathway or the complement pathway, we demonstrated here that Crry/DAF/C3−/− erythrocytes opsonized with the same IgG2a autoantibody were eliminated much more quickly by the complement pathway than by the FcR pathway. Thus, in addition to the nature of the pathogenic antibodies involved, the status of membrane complement-regulatory proteins on erythrocytes can also significantly influence the hemolytic pathways in AIHA.
We thank Dr Noriko Okada for antimouse DAF antibodies, Dr Michael Holers for the antimouse Crry antibody, Dr Rick Wetsel for C3 knockout breeder mice, and Dr Alan Schreiber for FcγR knockout breeder mice.
Prepublished online as Blood First Edition Paper, August 1, 2002; DOI 10.1182/blood-2002-06-1875.
Supported by an Established Investigator Award from the American Heart Association and by National Institutes of Health grants AI 44970, AI 49344 (W.C.S.), and AI 40576 and AI 44912 (H.M.).
H.M. and T.M. contributed equally.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Wen-Chao Song, Center for Experimental Therapeutics, University of Pennsylvania School of Medicine, 1351 BRBII/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail:song@spirit.gcrc.upenn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal