In allogeneic hematopoietic stem cell transplant recipients, restoration of humoral immunity is delayed and can remain impaired for years. In many severe combined immune deficiency (SCID) patients given haploidentical bone marrow (BM), lesions in humoral immunity are exacerbated by poor engraftment of donor B cells. The nature of these defects is important to understand as they render patients susceptible to infection. Previous work in mice suggested that in utero transplantation (IUT) of allogeneic BM might offer several advantages for the correction of primary immune deficiencies. In SCID mice given fully allogeneic BM in utero, the lymphoid compartment was restored with minimal evidence of graft-versus-host disease (GVHD). The present report examines B-cell reconstitution and function in mice that have received allogeneic IUT. Results are compared with those of adult mice given total body irradiation (TBI) followed by transplantation with allogeneic BM. In addition to enumerating the various B-cell subsets present in BM, spleen, and peritoneal cavity (PC), B-cell competence was assessed by challenging mice with T cell–independent (TI) and T cell–dependent (TD) antigens. The results demonstrated that all B-cell subsets in the BM and periphery were restored in allogeneic IUT and TBI mice, as were antibody responses after TI challenge. Upon immunization with TD antigens, however, IUT and TBI mice exhibited suboptimal responses as measured by the capacity to isotype switch and generate germinal center (GC) B cells. Thus, although allogeneic BM transplantation results in complete recovery of the B-cell compartment, certain elements of the humoral response remain defective.
Introduction
Stem cell transplantation is a proven therapy for the treatment of certain malignancies and blood disorders. Although successful reconstitution of the hematopoietic system is best achieved with autologous or human leukocyte antigen (HLA)–identical grafts, many patients receive partially matched bone marrow (BM) or peripheral stem cells. In recipients of allogeneic grafts, restoration of humoral competence is delayed, with B-cell counts and antibody (Ab) production taking up to 2 years to normalize.1 The slow rate of humoral reconstitution is correlated with high infection rates2,3 and is thought to reflect the normal pattern of B-cell ontogeny4-7 as well as the effects of graft-versus-host disease (GVHD).4,6,8,9 Even with the return of normal B-cell and immunoglobulin (Ig) levels, defects in humoral immunity can persist. Specifically, transplant patients exhibit a paucity of memory B cells10-12 and an abnormally low accumulation of somatic mutations in their Ig variable region genes.12-14 Because memory cell formation and somatic hypermutation result from T helper cell–driven germinal center (GC) reactions,15 it is likely that T cell–B cell collaboration or GC formation remains impaired for extended periods. The exact nature of this impairment is unknown, but it has been attributed to GVHD or pretransplantation conditioning.16
In addition to malignancies and blood disorders, stem cell transplantation is used to treat genetic diseases, especially those of the immune system. Severe combined immune deficiency (SCID) is a primary immune deficiency that results from a variety of inherited mutations.17,18 SCID patients typically lack T cells and natural killer (NK) cells, although most contain normal numbers of B cells.17,18 BM or peripheral stem cell transplantation is necessary early in life to restore the immune system and rescue the patient. Because SCID recipients typically receive modest or no pretransplantation conditioning, normal T-cell and B-cell numbers can appear within months of transplantation.19,20In spite of this, many SCID recipients of haploidentical stem cells exhibit prolonged humoral deficiency.19-25 Several studies have demonstrated that humoral dysfunction is attributed to the lack of donor B-cell engraftment.21,23-25 This suggests that host B cells in SCID patients are dysfunctional or only partially functional and may not be able to appropriately respond to donor T-cell signals. The reason for poor donor B-cell engraftment is unclear, but it may reflect the inability of donor B-cell precursors to compete for space with the host B-cell pool.26
An alternative to postnatal transplantation for the treatment of immune deficiencies is the administration of allogeneic BM or stem cells in utero. In theory, in utero transplantation (IUT) offers a number of potential advantages.26 Introduction of normal stem cells during gestation should allow for restoration of immune function earlier in life compared with postnatal transplantation. In addition, the immature fetal environment may offer a less competitive setting for engraftment of donor stem cells, especially those leading to the B-lymphocyte lineage. Finally, introduction of allogeneic BM in utero may foster immune tolerance and minimize the possibility of GVHD.
IUT has proven successful for the treatment of genetic deficiencies in several mouse models. In mice with defects in metabolism,27,28 growth factors,29,30 or lymphocyte production,31-33 IUT provided clear benefit. In particular, administration of fully allogeneic stem cells to SCID mice at days 13 to 16 of gestation resulted in stem cell engraftment, multilineage reconstitution, and restoration of T-cell and B-cell populations.31-33 Analysis of allogeneic IUT SCID mice revealed donor B cells in BM, spleen, and lymph nodes and both CD4+ and CD8+ T cells in the thymus and peripheral lymphoid organs.31,32 Importantly, GVHD was minimal in these recipients and tolerance to both donor and host major histocompatibility complex (MHC) was documented using mixed lymphocyte reaction (MLR) assays.31 32 These findings underscore the potential advantages of IUT as a corrective therapy.
We examined B-cell reconstitution and function in SCID mice administered allogeneic BM in utero. Multiparameter flow cytometry was used to assess the distribution of B-cell subsets in BM, spleen, and peritoneal cavity (PC). Functional competence of engrafted B cells was determined by measuring antigen-specific Ab responses after T cell–independent (TI) and T cell–dependent (TD) challenge and enumerating GC B cells. Similar experiments were performed with normal adult mice conditioned with total body irradiation (TBI) and administered allogeneic BM. The results demonstrated reconstitution of all B-cell subsets in the BM and periphery of recipient mice. Basal Ig levels and Ab responses after TI antigen challenge were also restored. Upon TD immunization, isotype switching was found to be diminished in recipients of allogeneic BM, as was the formation of GC. Thus, although the data show that allogeneic IUT provides complete restoration of the B-cell compartment in SCID mice, certain components of the humoral response remain abnormal.
Materials and methods
Mice
C57BL/6 (H2b) and BALB/c (H2d) mice were obtained from the National Institutes of Health (Bethesda, MD). BALB/c-SCID mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Adult mice used as a source of adult BM and as postnatal recipients were at least 6 weeks of age. All mice were housed in microisolator cages under specific pathogen-free conditions in the animal care unit of either the University of Minnesota or the University of Iowa.
In utero transplantation
The IUT technique has been previously described.31Briefly, BM single-cell suspensions were obtained from the hind leg bones and filtered through nylon mesh (Nitex HC3-41, Tetko, Chicago, IL). In some groups, BM T cells were depleted by incubation with anti-Thy1.2 (30-H-12) and baby rabbit complement. Four ×106 T cell–depleted (TCD) or non-TCD BM cells were injected in 5- to 10-μL volume into pregnant females at day 15 or 16 of gestation. The pregnant mice were anesthetized, a midline ventral abdominal incision was introduced, and the uterine horns were exposed. BM cells were injected intraperitoneally into each fetus by means of hand-drawn glass pipettes, muscle layers were sutured, and the skin was closed with metal surgical clips. High-level chimeric mice with no evidence of GVHD, as measured by normal weight gain and the maintenance of peripheral B cells, were used for study starting at 12 weeks of age. Donor-cell chimerism in the allogeneic IUT groups, assessed by anti-H2b staining of peripheral blood, averaged 85%, with a range of 56% to 100%.
BM transplantation following TBI
Adult BALB/c mice were conditioned with 600 cGy TBI from an x-ray source and 5 × 106 C57BL/6 TCD BM cells were injected intravenously 24 hours later. TBI mice with no evidence of GVHD were used for study after 12 weeks of rest.
Flow cytometric reagents
The following monoclonal antibodies (mAbs) were prepared by 50% ammonium sulfate precipitation from serum-free (HB101) culture supernatants: 6B2, a rat IgG anti–mouse B220 (CD45R); b7-6, a rat IgG anti–mouse IgM; M1/69, a rat IgG anti–mouse heat-stable antigen (Ag) (HSA; CD24); B3B4, a rat IgG anti–mouse CD23; 7E9, a rat IgG anti–mouse CD21/35; 53.7.313, a rat IgG anti–mouse CD5; BP-1, a mouse IgG anti–mouse Ly-51; and EH-144, a mouse IgG anti–mouse H2Kb. Chromatographically purified rat and mouse IgG was purchased from Jackson ImmunoResearch (West Grove, PA) and used for isotype controls. The various Abs were biotin, fluorescein isothiocyanate (FITC), or cyanine 5.18, conjugated using standard protocols. Phycoerythrin (PE)–streptavidin was purchased from Southern Biotechnology Associates (Birmingham, AL). FITC–peanut agglutinin (PNA), specific for terminal galactosyl (β-1,3)N-acetylgalactoseamine residues, was obtained from Vector Laboratories (Burlingame, CA).
Flow cytometric analysis
BM from both hind legs, spleen, and peritoneal lavage cells were harvested and washed in balanced salt solution (BSS). Mononuclear cells were isolated by density centrifugation over FicoLite-LM (Atlanta Biologicals, Norcross, GA) followed by further washing in BSS. Cells (5 × 105) were suspended in staining buffer (BSS supplemented with 5% bovine calf serum and 0.1% NaN3) and incubated with biotin-, FITC-, and cyanine 5.18–conjugated reagents in the presence of 25 μg 2.4G2 (anti-CD16/32) and 10 μL normal rat serum. After washing in staining buffer, cells were further incubated with PE-streptavidin, washed, and suspended in fixative (1% formaldehyde in 1.25 × phosphate-buffered saline [PBS]). Stained cells were run on a FACSVantage SE flow cytometer (Becton Dickinson, Mountain View, CA) equipped with a primary argon ion laser and a rhodamine 6G CR559 dye head laser (Coherent, Palo Alto, CA) pumped by a second argon ion laser. A minimum of 30 000 events was collected per sample. Low angle and orthogonal light scatter were used to exclude dead cells and debris, and electronic compensation was used to correct for spectral overlap between FITC and PE. Data were analyzed on a VAX station 3200 computer equipped with DESK FACS analysis software (kindly provided by Wayne Moore, Stanford University, Stanford, CA).
Immunization
Mice were immunized intraperitoneally with 25 μg of TNP (2,4,6 trinitrophenyl)–Ficoll (Biosearch Technologies, Novato, CA) or with 0.2 mL of 10% v/v sheep red blood cells (SRBCs) (Colorado Serum Company, Denver, CO). Alternatively, mice were injected subcutaneously with 50 μg KLH (Calbiochem, La Jolla, CA) in complete Freund adjuvant (CFA; Sigma Chemical, St Louis, MO), followed 2 weeks later by a second injection in incomplete Freund adjuvant (IFA; Sigma Chemical). At designated time points, mice immunized with TNP-Ficoll or KLH were anesthetized and blood was drawn by retro-orbital bleeds. Blood samples were clotted and separated serum was stored at −70°C until enzyme-linked immunosorbent assay (ELISA). Spleens from mice immunized with SRBC were obtained 8 days after challenge and analyzed by flow cytometry for GC B-cell content.
ELISA determinations
Baseline total IgM and IgG serum levels were assessed as follows: 96-well ELISA plates were coated with goat anti-IgG (1μg/mL; Rockland, Gilbertsville, PA) or goat anti-IgM (2 μg/mL; Sigma Chemical Co) Ab in 0.1M NaHCO3 buffer (pH 9.2). Wells were blocked with 1% bovine serum albumen–phosphate-buffered saline (BSA-PBS). Sera were serially diluted from 16 000-fold to 256 000-fold in 0.25% BSA-PBS and added to the wells. After incubation and washing, horseradish peroxidase (HRP)–conjugated secondary Abs (Southern Biotechnology Associates) specific for the different Ig isotypes (goat anti-IgM, goat anti-IgG1, goat anti-IgG2b) were added to the wells. After further incubation, O-phenylenediamine dihydrochloride (Sigma Chemical Co) was added as substrate in 0.06 M citric acid buffer (pH 5.0) containing 0.015% H2O2. The reaction was stopped with 2 N H2SO4 and the colorimetric product was read at 490 nm (Model 550 Microplate Reader, Bio-Rad Laboratories, Hercules, CA) with background subtraction at 650 nm. Ig concentrations were calculated from standard curves generated with mouse IgM (Rockland) and IgG (Southern Biotechnology Associates) standards using Microplate Manager software (Bio-Rad Laboratories). All washes between steps were done with 0.05% Tween 20–PBS.
Anti-KLH levels were determined in a similar manner, with the following modifications: Diluted serum samples were incubated in wells that had been coated with KLH at 5 μg/mL (Calbiochem). HRP-conjugated secondary antibodies were used as above. Values were calculated using standard curves generated from wells coated with serially diluted isotype controls and developed with the anti-isotype reagents.
Following TNP-Ficoll immunization, IgM and IgG3 anti-TNP levels were determined as follows: 96-well ELISA plates were coated with isotype-specific capture Abs at a concentration of 1 to 5 μg/mL in buffer consisting of 0.05 M Tris (tris(hydroxymethyl)aminomethane; pH 9.5) and 0.2% NaN3. The capture Abs utilized were goat anti–mouse IgM (Jackson ImmunoResearch) and goat anti–mouse IgG3 (Southern Biotechnology Associates). Coated plates were blocked with 5% dry milk–PBS. Control mAbs (for standard curves) and serum samples appropriately diluted in 5% dry milk–PBS were added and similarly incubated. After washing, 0.5 μg TNP–chicken gamma globulin–biotin diluted in 5% dry milk–PBS was added to each well, and the plates were further incubated. Alkaline phosphatase streptavidin (0.3 μg; Zymed, San Francisco, CA) diluted in 5% dry milk–PBS was added after washing. Substrate (0.3 mg; Sigma Chemical Co) diluted in substrate buffer consisting of 0.05 M Na2CO3 and 1 × 10−3 M MgCl2. 6H2O in H2O (pH 9.8) was added to each well. The reaction was stopped by addition of 0.2% NaOH, and absorbance was measured at a dual wavelength of 405 and 540 nm with a Microplate Autoreader EL311 (Bio-Tek Instruments, Winooski, VT). All washes between steps were done with 0.05% Tween 20–PBS. Ab concentrations were determined from standard curves with DeltaSOFT software (Bio-Tek Instruments). The control mAbs utilized for standard curves were 4G2F8 (a mouse IgM anti-TNP mAb) and 8-11 (a mouse IgG3 anti-TNP mAb). The anti-TNP–specific mAbs were affinity purified by passage of hybridoma culture supernatants over TNP–bovine gamma globulin–Sepharose 6B followed by elution with TNP-glycine (Sigma Chemical Co).
Statistical analysis
Where indicated, one-way analysis of variance (ANOVA) followed by the Tukey-Kramer multiple comparisons posttest was used to determine significance.
Results
B-cell subsets in reconstituted mice
In previous work, SCID mice given allogeneic TCD or non-TCD BM in utero exhibited total B-cell reconstitution that was similar to that of adult control mice.32 Because B-cell engraftment in that study was assessed using only a pan–B cell marker and functional capacity was not determined, the focus of the present work was to further assess the B-cell compartment in IUT SCID recipients. Accordingly, multicolor flow cytometry was utilized to determine fine subset analysis of the B-cell compartment, and TI and TD antigen challenge was used to measure the functional competence of engrafted B cells.
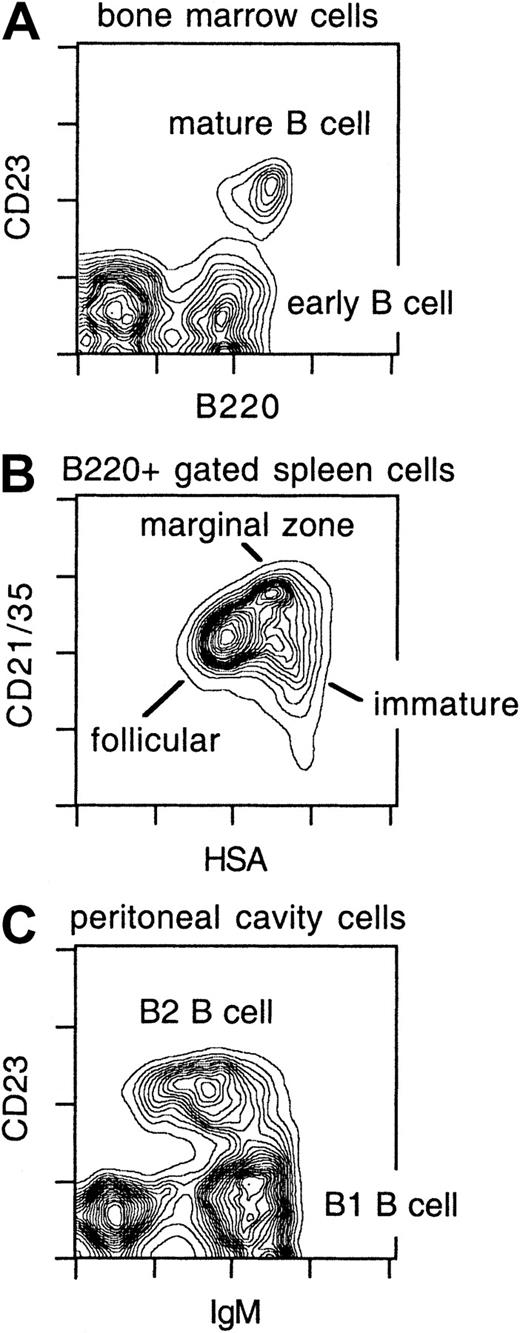
In initial experiments, subsets of B cells were enumerated in the BM, spleen, and PC of IUT, TBI, and control mice. B cells mature in the BM and develop in a highly regulated manner, reflecting genetic recombination events at the heavy and light chain immunoglobulin loci.34,35 These stages can be defined by surface markers and grouped into early and mature B-cell subsets by the combination of mAbs specific for B220 (CD45R) and CD23 (Figure1A). In the adult spleen, 3 major B-cell subsets are normally found.34,35 These are the immature, follicular, and marginal zone (MZ) B-cell populations, and they are delineated by a combination of the B220, HSA, and CD21/35 markers (Figure 1B). Immature B cells are a small population in the adult spleen and represent recent BM immigrants completing maturation and incorporation into the peripheral B-cell pool. Follicular and MZ B cells are long-lived mature B-cell subsets that contribute to both TI and TD responses. B1 and B2 cells compose the 2 prominent B-cell subsets in the PC.34 35 As illustrated in Figure 1C, these are distinguished by anti-IgM and anti-CD23 mAbs. B1 B cells are a self-renewing population with the capacity to produce low-affinity polyreactive Abs. B2 B cells exhibit characteristics similar to follicular B cells in the secondary lymphoid organs.
B-cell subsets in normal mice.
BM, spleen, and PC lavage cells from C57BL/6 mice were harvested and stained with anti-B220 and anti-CD23 mAbs (BM); anti-B220, anti-CD21/35, and anti-HSA mAbs (spleen); or anti-IgM and anti-CD23 mAbs (PC) and analyzed by flow cytometry. The spleen CD21/35 vs HSA contour plot (panel B) is derived from B220+gated cells.
B-cell subsets in normal mice.
BM, spleen, and PC lavage cells from C57BL/6 mice were harvested and stained with anti-B220 and anti-CD23 mAbs (BM); anti-B220, anti-CD21/35, and anti-HSA mAbs (spleen); or anti-IgM and anti-CD23 mAbs (PC) and analyzed by flow cytometry. The spleen CD21/35 vs HSA contour plot (panel B) is derived from B220+gated cells.
The various B-cell subsets were examined in BALB/c SCID mice receiving allogeneic (C57BL/6) TCD or non-TCD BM in utero. BALB/c SCID mice given congenic BM in utero served as positive control for B-cell reconstitution after IUT. In addition, adult BALB/c mice conditioned with TBI and administered allogeneic TCD BM were tested. TBI mice given allogeneic grafts achieve excellent engraftment without GVHD.32 This group was included, as it represents the more common regimen of ablative conditioning followed by allogeneic BM transplantation. Postnatal SCID mice reconstituted with allogeneic BM were not pursued because levels of engraftment similar to those of IUT SCID mice are routinely associated with GVHD and profound B-cell lymphopenia.32 Normal adult C57BL/6 (donor) and BALB/c (recipient) mice were analyzed to provide control baseline values.
In Table 1, total B-cell recoveries from BM and spleen for each of the control and experimental groups are listed, along with average B-cell frequencies. The results indicate excellent B-cell engraftment, although some variation is observed. In particular, SCID mice receiving TCD BM exhibited lower total B-cell recoveries, consistent with previous observations.32 This likely reflects the ability of donor T cells to facilitate engraftment of adult BM-derived stem cells in the fetal environment. Frequencies of the major B-cell subsets in BM, spleen, and PC for each of the groups were determined by flow cytometric analysis (as defined in Figure 1) and are summarized in Table 2. Examination of the BM revealed normal proportions of early and mature B-cell subsets in all transplant groups. In addition, fine subset analysis of the early (B220+CD23−) population with BP-1 (anti–Ly-51) mAb demonstrated normal distribution of the pro- and pre-B-cell subsets (data not shown). These data indicate that in both IUT and TBI mice, allogeneic stem cells seed the BM and give rise to long-term B-cell lymphopoiesis. Dissection of the splenic B-cell compartment demonstrated that the immature, follicular, and MZ B-cell subsets were reconstituted in all groups. The presence of immature B cells in the spleen is consistent with ongoing B-cell development in the BM. Importantly, the presence of mature follicular and MZ B cells suggests that incorporation of maturing B cells into peripheral pools occurred appropriately, even in an allogeneic setting. Table 2 reveals some group-to-group variation in the percentages of the 3 subsets. The reason for this is not readily apparent since mechanisms that regulate proportions of splenic B-cell subsets are presently not understood. The low frequency of MZ B cells in the TBI group is noteworthy, however, and may reflect irradiation-induced damage to the splenic stroma. Examination of PC B cells shows that both B1 and B2 populations were present in all mice. As summarized in Table 2, mean percentages of B1 and B2 B cells were not markedly different among the 3 IUT categories, and were similar to those in normal C57BL/6 and BALB/c mice. In data not shown, the majority of the B1 B-cell compartment consisted of B1b (CD5−) B cells, although B1a B cells (CD5+) were present. TBI mice again represent an exception, in that the frequency of B1 B cells in the PC was low. Finally, B-cell reconstitution in lymph nodes was normal in all transplant groups (data not shown). Thus, in utero reconstitution of SCID mice with either TCD or non-TCD allogeneic BM resulted in the normal selection and expansion of all peripheral B-cell subsets. Adult TBI mice given allogeneic TCD BM also displayed a full complement of peripheral B-cell subsets, although splenic MZ and peritoneal B1 B cells were present in lower frequencies.
Total B-cell recovery in IUT, TBI, and control mice
| Mouse group* . | Total BM B cells (× 106) . | BM B-cell frequency, % . | Total splenic B cells (× 106) . | Splenic B-cell frequency, % . |
|---|---|---|---|---|
| C57B1/6 control | 3.2 ± 0.9 | 21.1 ± 5.0 | 47.6 ± 18.3 | 56.0 ± 9.7 |
| BALB/c control | 2.1 ± 0.8 | 16.2 ± 3.7 | 54.4 ± 9.2 | 57.6 ± 3.5 |
| Congenic IUT | 1.1 ± 0.6 | 11.6 ± 4.4 | 37.6 ± 19.9 | 44.2 ± 7.2 |
| Allo NTCD IUT | 3.7 ± 2.6 | 16.9 ± 5.2 | 45.4 ± 10.5 | 55.9 ± 6.8 |
| Allo TCD IUT | 1.6 ± 1.7 | 13.8 ± 13.1 | 25.8 ± 9.1 | 43.6 ± 9.1 |
| Allo TBI | 3.3 ± 2.2 | 19.3 ± 4.4 | 38.3 ± 10.3 | 58.8 ± 3.3 |
| Mouse group* . | Total BM B cells (× 106) . | BM B-cell frequency, % . | Total splenic B cells (× 106) . | Splenic B-cell frequency, % . |
|---|---|---|---|---|
| C57B1/6 control | 3.2 ± 0.9 | 21.1 ± 5.0 | 47.6 ± 18.3 | 56.0 ± 9.7 |
| BALB/c control | 2.1 ± 0.8 | 16.2 ± 3.7 | 54.4 ± 9.2 | 57.6 ± 3.5 |
| Congenic IUT | 1.1 ± 0.6 | 11.6 ± 4.4 | 37.6 ± 19.9 | 44.2 ± 7.2 |
| Allo NTCD IUT | 3.7 ± 2.6 | 16.9 ± 5.2 | 45.4 ± 10.5 | 55.9 ± 6.8 |
| Allo TCD IUT | 1.6 ± 1.7 | 13.8 ± 13.1 | 25.8 ± 9.1 | 43.6 ± 9.1 |
| Allo TBI | 3.3 ± 2.2 | 19.3 ± 4.4 | 38.3 ± 10.3 | 58.8 ± 3.3 |
BM and spleen cells were harvested and spun over FicoLite-LM to isolate viable mononuclear cells. After cell counts, cell suspensions were stained with anti-B220 mAb, followed by flow cytometric analysis. Percentage of B220+ cells was determined by software gating. Values are mean ± SD. IUT mice were examined starting at 12 weeks of age; TBI mice were tested starting at 12 weeks after transplantation. Allo NTCD indicates recipients of allogeneic non-TCD BM; allo TCD, recipients of allogeneic TCD BM.
n values for each group range from 6 to 11.
B-cell subset frequencies in IUT, TBI, and control mice
| Mouse group* . | Percent of total B cells ± SD . | ||||||
|---|---|---|---|---|---|---|---|
| BM early . | BM mature . | Spleen follicular . | Spleen MZ . | Spleen immature . | PC B1 . | PC B2 . | |
| C57B1/6 control | 71.8 ± 14.3 | 28.2 ± 14.3 | 68.5 ± 8.4 | 23.4 ± 11.0 | 8.1 ± 4.4 | 65.5 ± 20.1 | 34.5 ± 20.1 |
| BALB/c control | 68.8 ± 8.1 | 31.2 ± 8.1 | 80.7 ± 3.0 | 16.2 ± 3.7 | 3.2 ± 1.0 | 55.3 ± 13.2 | 44.7 ± 13.2 |
| Congenic IUT | 58.5 ± 16.7 | 41.5 ± 16.7 | 69.5 ± 7.3 | 26.3 ± 8.5 | 4.2 ± 1.7 | 59.6 ± 16.2 | 40.4 ± 16.2 |
| Allo NTCD IUT | 58.6 ± 13.6 | 41.4 ± 13.6 | 65.4 ± 9.1 | 26.9 ± 11.5 | 7.6 ± 2.8 | 54.8 ± 20.2 | 45.2 ± 20.2 |
| Allo TCD IUT | 63.9 ± 14.3 | 36.1 ± 14.3 | 57.8 ± 10.5 | 36.1 ± 8.3 | 6.1 ± 4.9 | 56.5 ± 18.0 | 43.5 ± 18.0 |
| Allo TBI | 58.5 ± 14.3 | 41.5 ± 14.3 | 79.7 ± 6.8 | 10.5 ± 2.9 | 9.8 ± 6.4 | 38.2 ± 18.3 | 61.8 ± 18.3 |
| Mouse group* . | Percent of total B cells ± SD . | ||||||
|---|---|---|---|---|---|---|---|
| BM early . | BM mature . | Spleen follicular . | Spleen MZ . | Spleen immature . | PC B1 . | PC B2 . | |
| C57B1/6 control | 71.8 ± 14.3 | 28.2 ± 14.3 | 68.5 ± 8.4 | 23.4 ± 11.0 | 8.1 ± 4.4 | 65.5 ± 20.1 | 34.5 ± 20.1 |
| BALB/c control | 68.8 ± 8.1 | 31.2 ± 8.1 | 80.7 ± 3.0 | 16.2 ± 3.7 | 3.2 ± 1.0 | 55.3 ± 13.2 | 44.7 ± 13.2 |
| Congenic IUT | 58.5 ± 16.7 | 41.5 ± 16.7 | 69.5 ± 7.3 | 26.3 ± 8.5 | 4.2 ± 1.7 | 59.6 ± 16.2 | 40.4 ± 16.2 |
| Allo NTCD IUT | 58.6 ± 13.6 | 41.4 ± 13.6 | 65.4 ± 9.1 | 26.9 ± 11.5 | 7.6 ± 2.8 | 54.8 ± 20.2 | 45.2 ± 20.2 |
| Allo TCD IUT | 63.9 ± 14.3 | 36.1 ± 14.3 | 57.8 ± 10.5 | 36.1 ± 8.3 | 6.1 ± 4.9 | 56.5 ± 18.0 | 43.5 ± 18.0 |
| Allo TBI | 58.5 ± 14.3 | 41.5 ± 14.3 | 79.7 ± 6.8 | 10.5 ± 2.9 | 9.8 ± 6.4 | 38.2 ± 18.3 | 61.8 ± 18.3 |
BM and spleen B-cell percentages are presented as a proportion of total B220+ cells, and PC B-cell percentages as a proportion of total IgM+ cells. Values are mean ± SD. IUT mice were examined starting at 12 weeks of age. TBI mice were tested starting at 12 weeks after transplantation. B-cell subsets were defined as follows: BM early indicates B220+CD23−; BM mature, B220+CD23+; spleen follicular, B220+CD21/35intHSAlow; spleen MZ, B220+CD21/35highHSAint; spleen immature, B220+CD21/35lowHSAhigh; PC B1, IgM+CD23−; and PC B2, IgM+CD23+.
n values for each group range from 6 to 11.
Baseline Ig levels in reconstituted mice
Although donor B-cell reconstitution was observed in SCID and TBI recipients of allogeneic BM, it was not clear whether these cells were functionally competent. Thus, experiments were performed to test the capacity of engrafted B cells to produce Ig under a variety of conditions. As an initial measure of humoral competence, baseline levels of serum IgM, IgG1, and IgG2b were determined in all groups. (IgG2a was not measured because C57BL/6 mice do not have the γ2a constant region gene). The presence of serum Ig is a result of both TI (IgM and a portion of IgG) and TD (a portion of IgG) Ab responses.36 37 The results, summarized in Table3, demonstrate significant levels of serum IgM and IgG in all mice that received transplants. The mechanisms that regulate production and homeostasis of serum Ig were thus operative in both IUT and adult TBI mice.
Baseline serum Ig levels (μg/mL) in IUT, TBI, and control mice
| Mouse group3-150 . | IgM . | IgG1 . | IgG2b . |
|---|---|---|---|
| C57B1/6 control | 1435 ± 544 | 409 ± 83 | 342 ± 166 |
| BALB/c control | 550 ± 396 | 974 ± 872 | 653 ± 682 |
| Congenic IUT | 2305 ± 906 | 510 ± 158 | 125 ± 43 |
| Allo NTCD IUT | 2181 ± 1068 | 652 ± 747 | 711 ± 515 |
| Allo TCD IUT | 2042 ± 975 | 377 ± 258 | 1186 ± 445 |
| Allo TBI | 1712 ± 966 | 876 ± 577 | 928 ± 657 |
| Mouse group3-150 . | IgM . | IgG1 . | IgG2b . |
|---|---|---|---|
| C57B1/6 control | 1435 ± 544 | 409 ± 83 | 342 ± 166 |
| BALB/c control | 550 ± 396 | 974 ± 872 | 653 ± 682 |
| Congenic IUT | 2305 ± 906 | 510 ± 158 | 125 ± 43 |
| Allo NTCD IUT | 2181 ± 1068 | 652 ± 747 | 711 ± 515 |
| Allo TCD IUT | 2042 ± 975 | 377 ± 258 | 1186 ± 445 |
| Allo TBI | 1712 ± 966 | 876 ± 577 | 928 ± 657 |
Total IgM and IgG levels were determined by sandwich ELISA, using sera obtained from unimmunized mice. Values are mean ± SD. IUT mice were examined starting at 12 weeks of age. TBI mice were tested starting at 12 weeks after transplantation.
n values for each group range from 13 to 16.
TI responses in reconstituted mice
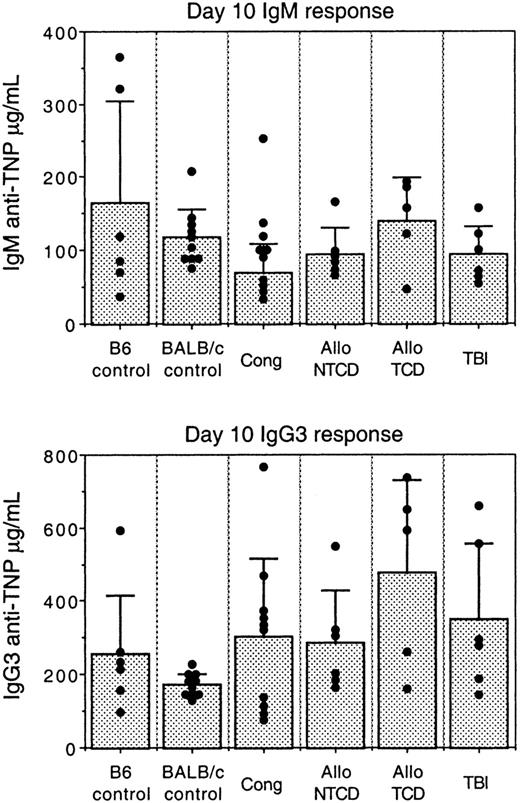
In order to assess the ability of reconstituted B cells to respond to antigenic challenge, mice were first immunized with TNP-Ficoll, a prototypic TI antigen. TI antigens invoke an Ab response in the absence of classic CD4+ T-cell help and induce high levels of IgM and IgG3 antibodies in the mouse.38 It has been proposed that MZ and B1 B cells produce the majority of Abs subsequent to TI challenge.39 Figure 2 shows serum IgM and IgG3 anti-TNP Ab levels present 10 days after immunization. This time point represents the peak of the Ab response following TNP-Ficoll administration. It is clear that all transplant groups were fully competent to produce IgM and IgG3 Abs after TI challenge. Importantly, these responses were comparable to those of normal mice, indicating that engrafted B cells, especially those belonging to the MZ and B1 B-cell subsets, were capable of responding to foreign challenge. The responses in adult TBI mice are also of interest, given the low frequencies of MZ and B1 B cells in this group. Although these B cells were not at optimal levels, their numbers were evidently sufficient to produce strong Ab responses to TNP-Ficoll.
Comparison of TI Ab responses in IUT, TBI, and control mice.
Mice were immunized intraperitoneally with 25 μg of TNP-Ficoll. Sera were obtained 10 days after immunization and tested for IgM and IgG3 anti-TNP levels by sandwich ELISA. Each dot represents an individual mouse. Bar graphs represent average Ab levels ± SD. IUT mice were immunized starting at 12 weeks of age. TBI mice were challenged starting at 12 weeks after transplantation.
Comparison of TI Ab responses in IUT, TBI, and control mice.
Mice were immunized intraperitoneally with 25 μg of TNP-Ficoll. Sera were obtained 10 days after immunization and tested for IgM and IgG3 anti-TNP levels by sandwich ELISA. Each dot represents an individual mouse. Bar graphs represent average Ab levels ± SD. IUT mice were immunized starting at 12 weeks of age. TBI mice were challenged starting at 12 weeks after transplantation.
TD responses in reconstituted mice
The induction of Ab responses to TD antigens, as opposed to TI antigens, is more complex and requires the participation of CD4+ helper T cells.40 TD responses are characterized by formation of GC, in which extensive isotype switching, affinity maturation, and memory cell production occurs.15To determine the capacity of mouse transplant recipients to generate TD humoral responses, KLH was administered in Freund adjuvant (at day 0 in CFA and at day 14 in IFA). In addition to IgM, TD antigens in Freund adjuvant elicit IgG1 and IgG2 Abs. Blood was drawn 7 days after the second immunization and tested for IgM, IgG1, and IgG2b anti-KLH Ab levels by ELISA. As shown in Table 4, all mice generated equivalent IgM Ab levels. As expected, C57BL/6 and BALB/c controls and SCID mice given congenic BM in utero produced strong IgG1 Ab titers and smaller, albeit significant, IgG2b levels. Of interest, mice reconstituted with allogeneic BM in utero displayed a poor ability to generate switched Ab responses, with a particular lesion in IgG1. IgG2b levels were not as severely affected but were variably reduced. Adult TBI mice engrafted with allogeneic BM also showed a modest depression of IgG1 Ab titers, although IgG2 levels were normal.
Anti-KLH Ab levels (μg/mL) in IUT, TBI and control mice
| Mouse group4-150 . | IgM . | IgG1 . | IgG2b . |
|---|---|---|---|
| C57Bl/6 control | 34 ± 35 | 427 ± 333 | 32 ± 50 |
| BALB/c control | 65 ± 34 | 933 ± 266 | 93 ± 115 |
| Congenic IUT | 13 ± 8 | 539 ± 478 | 7 ± 10 |
| Allo NTCD IUT | 25 ± 21 | 39 ± 77 | 2 ± 3 |
| Allo TCD IUT | 25 ± 21 | 26 ± 33 | 21 ± 31 |
| Allo TBI | 42 ± 38 | 208 ± 110 | 96 ± 82 |
| Mouse group4-150 . | IgM . | IgG1 . | IgG2b . |
|---|---|---|---|
| C57Bl/6 control | 34 ± 35 | 427 ± 333 | 32 ± 50 |
| BALB/c control | 65 ± 34 | 933 ± 266 | 93 ± 115 |
| Congenic IUT | 13 ± 8 | 539 ± 478 | 7 ± 10 |
| Allo NTCD IUT | 25 ± 21 | 39 ± 77 | 2 ± 3 |
| Allo TCD IUT | 25 ± 21 | 26 ± 33 | 21 ± 31 |
| Allo TBI | 42 ± 38 | 208 ± 110 | 96 ± 82 |
Mice were immunized with KLH in CFA at day 0 and with KLH in IFA at day 14. Sera were obtained 7 days after the second immunization and anti-KLH Ab levels were determined by sandwich ELISA. Values are mean ± SD. IUT mice were immunized starting at 12 weeks of age. TBI mice were challenged starting at 12 weeks after transplantation.
n values for each group range from 13 to 16.
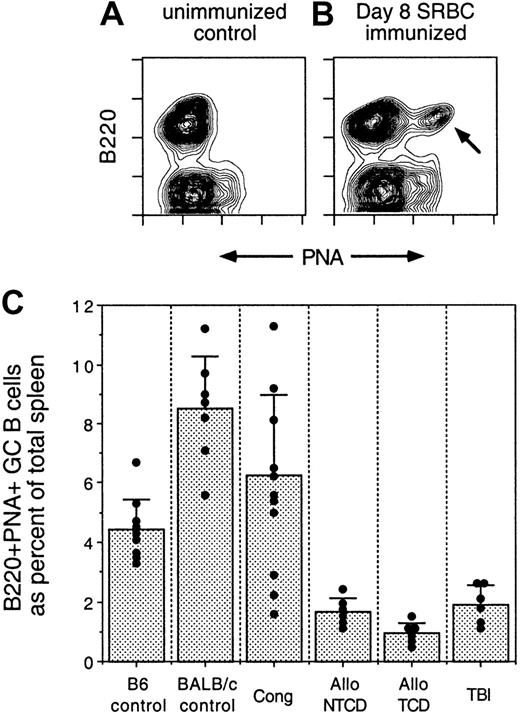
Because induction of GC is a central event in TD responses, IUT SCID mice were tested for their capacity to induce GC reactions. Both control mice and transplant recipients were immunized with SRBCs and spleens were harvested 8 days after challenge. SRBCs were chosen for these experiments because they induce robust GC formation.41 In normal mice, SRBC-induced GC B cells peak at days 6 to 8 after injection and can be detected flow cytometrically by the combination of anti-B220 mAb and PNA.41 As illustrated in panels A and B of Figure3, GC B cells are B220+PNAhigh and are present only after immunization. In Figure 3C, the capacity of the various groups to generate splenic GC responses after SRBC immunization is shown. Control mice and IUT mice administered congenic BM displayed expected levels of GC B cells. Of interest, recipients of allogeneic BM in utero displayed GC responses that were significantly reduced compared with those of donor control mice. GC B-cell levels in allogeneic IUT mice were reduced regardless of age (even in mice 6 months or 1 year of age), indicating that delayed reconstitution of lymphoid elements did not account for the poor response. Similarly, GC responses in TBI mice were suboptimal compared with those of the donor control mice. These data are consistent with those in Table 4, indicating a general lesion in TD Ab responses.
Comparison of GC formation in IUT, TBI, and control mice.
(A) Splenocytes from an unimmunized C57BL/6 mouse were stained with anti-B220 mAb and PNA, followed by flow cytometric analysis. (B) Splenocytes from a C57BL/6 mouse immunized intraperitoneally with SRBC 8 days previously were stained with anti-B220 mAb and PNA, followed by flow cytometric analysis. Arrow designates the GC B-cell population. (C) IUT, TBI, and control mice were immunized intraperitoneally with SRBC. Spleens were harvested 8 days after immunization and stained with anti-B220 mAb and PNA, followed by flow cytometric analysis. Percentages of GC (B220+PNAhigh) B cells, as a proportion of total splenic mononuclear cells, were determined by software gating. Each dot represents an individual mouse. Bar graphs represent average GC B-cell frequencies ± SD. Compared with the responses of C57Bl/6 mice (donor strain), Allo NTCD IUT, Allo TCD IUT, and TBI GC responses were significantly reduced (P < .001). IUT mice were immunized starting at 12 weeks of age. Some of the allo IUT mice were challenged at intermediate (20-30 weeks of age) and late (58 weeks of age) time points. TBI mice were challenged starting at 12 weeks after transplantation.
Comparison of GC formation in IUT, TBI, and control mice.
(A) Splenocytes from an unimmunized C57BL/6 mouse were stained with anti-B220 mAb and PNA, followed by flow cytometric analysis. (B) Splenocytes from a C57BL/6 mouse immunized intraperitoneally with SRBC 8 days previously were stained with anti-B220 mAb and PNA, followed by flow cytometric analysis. Arrow designates the GC B-cell population. (C) IUT, TBI, and control mice were immunized intraperitoneally with SRBC. Spleens were harvested 8 days after immunization and stained with anti-B220 mAb and PNA, followed by flow cytometric analysis. Percentages of GC (B220+PNAhigh) B cells, as a proportion of total splenic mononuclear cells, were determined by software gating. Each dot represents an individual mouse. Bar graphs represent average GC B-cell frequencies ± SD. Compared with the responses of C57Bl/6 mice (donor strain), Allo NTCD IUT, Allo TCD IUT, and TBI GC responses were significantly reduced (P < .001). IUT mice were immunized starting at 12 weeks of age. Some of the allo IUT mice were challenged at intermediate (20-30 weeks of age) and late (58 weeks of age) time points. TBI mice were challenged starting at 12 weeks after transplantation.
Discussion
Previous work exploring transplantation of allogeneic BM into either SCID recipients in utero or adult mice after TBI demonstrated normal levels of total B cells in the peripheral lymphoid organs.31-33 Although engraftment of B cells appeared normal, the humoral competence of these animals was not tested. In the present studies, therefore, we dissected the B-cell compartment of reconstituted mice by multiparameter flow cytometry and tested the ability of these mice to produce Abs under both TI and TD conditions. The results demonstrated complete restoration of all B-cell subsets in both IUT and TBI mice and return of full TI Ab production, but only partial recovery of isotype switching and GC formation after TD Ag challenge. The importance of these findings rests with the potential of IUT to restore all lymphocyte subsets, including B cells, in patients with primary immune deficiencies. Equally important, the results showed that engraftment of allogeneic donor B cells after IUT allowed for partial recovery of humoral competence. Although TD responses were still suboptimal, allogeneic IUT may provide SCID patients with sufficient humoral capacity to better resist infection. Finally, the observation that IUT and TBI recipients of allogeneic BM exhibit poor isotype switching and GC formation offers a model with which persistent humoral defects that can occur after postnatal BM transplantation in humans can be investigated.10-14
Allogeneic BM administered to BALB/c SCID mice in utero allowed for complete reconstitution of all B-cell subsets in the BM and periphery. This included all developmental subsets in the BM, immature, follicular, and MZ B cells in the spleen and B1 and B2 B cells in the PC. Except for a modest reduction in total numbers of engrafted B cells in mice given TCD BM, the presence or absence of mature T cells in the donor inoculum did not affect the proportions of B-cell subsets in the fully mature mouse. These observations suggest that in IUT SCID recipients, adult allogeneic BM is capable of restoring normal B-cell lymphopoiesis, incorporation of B cells into the peripheral pool, and long-term survival.
Administration of C57BL/6 TCD BM to adult BALB/c mice given TBI also resulted in reconstitution of all developmental and peripheral B-cell subsets. Two differences were noted between these recipients and IUT engrafted mice. Although present, the proportion of splenic MZ B cells was lower in TBI animals. This may reflect irradiation-induced damage to the splenic stroma and the inability to properly organize the reconstituting spleen. TBI mice also displayed lower levels of B1 B cells in the PC. This observation may be explained by the poor ability of adult BM to completely restore B1a B cells when transferred into the postnatal host environment.42
In addition to testing for physical reconstitution of the B-cell compartment, it was equally important to examine the functional capacity of engrafted B cells. The results demonstrated restoration of normal baseline Ig serum levels as well as the ability to respond after TI challenge. In mice that underwent transplantation and were immunized with TNP-Ficoll, normal IgM and IgG3 anti-TNP Abs were observed. This strongly suggests that allogeneic B cells engrafted in IUT SCID mice or adult TBI mice have a normal capacity for activation and differentiation. When we tested the ability of donor B cells to respond to TD challenge, however, both IUT and TBI mice demonstrated suboptimal responses compared with normal C57BL/6 and BALB/c mice, as well as SCID mice reconstituted in utero with congenic BM. Although IgM anti-KLH Ab titers were normal, IgG levels were variably reduced in recipients of allogeneic BM. Consistent with this observation, IUT and TBI mice showed poor GC formation upon immunization with SRBCs. In both cases TD responses were not totally absent, but were clearly compromised.
As opposed to TI antigens, TD responses demand a greater number of participating cell types in order to effect optimal Ab production. Most important is the activation of antigen-specific CD4+ T helper cells, which in turn drive the activation of B cells and promote GC formation.15,40 It is presently understood that professional antigen-presenting cells (most likely dendritic cells [DCs]) are essential for the activation of CD4+ T cells in secondary lymphoid organs.43 Once generated, CD4+ T helper cells activate B cells, through a well-described series of steps, and further regulate B-cell proliferation and differentiation.40 Given the importance of T helper cells in this process, one can envision a number of scenarios leading to limiting numbers of these cells and a failure to optimally induce TD responses. Because all B cells in IUT and TBI mice receiving C57BL/6 BM express MHC class II molecules of the H2b haplotype, activation of these cells during a TD response requires H2b-restricted CD4+ T helper cells. The presence of H2b-restricted CD4+ T cells in the periphery depends in turn, upon their positive selection by H2b MHC class II+ restricting elements in the thymus. Most studies using radiation BM chimeras have suggested that thymic epithelium mediates positive selection of T cells.44 Should this be the case, most peripheral T helper cells would be H2d-restricted in mouse recipients of BM transplants and would offer little or no help to the donor B cells. Recent reports have demonstrated, however, that donor-derived hematopoietic cells are capable of taking up residence in the thymus45,46 and, importantly, mediating positive selection.46-48 Accordingly, recipients of allogeneic BM may contain a mixture of H2b- and H2d- restricted CD4+ T cells, a notion supported by the observation that peripheral T cells in IUT and TBI mice are tolerant of both donor and host MHC.32 At present, it is unclear whether T cells restricted to one or the other haplotype dominate. If H2b-restricted T cells are limiting, one would anticipate suboptimal TD responses.
Even if sufficient numbers of H2b-restricted CD4+ T cells are present, the availability of activated T helper cells could be restricted by a paucity of peripheral H2b+ DCs. This would also lead to limited B-cell responses and would explain the observed results. As discussed above, activation of CD4+ T cells in secondary lymphoid organs is mediated by DCs. Because DCs are derived from BM, one would anticipate the availability H2b+ DCs in the periphery of IUT and TBI mice. Whether sufficient donor-derived DCs reside in the periphery of mice that underwent transplantation remains to be verified. Assuming that H2b-restricted CD4+ T cells are available and become activated by sufficient numbers of H2b+ DCs in IUT and TBI mice, one could further explain suboptimal TD Ab responses by a defect in T helper cell activity. Should donor-derived T cells lack either costimulatory activity (eg, CD40 ligand expression) or cytokine production (eg, IL-4 or IFN-γ), TD Ab responses would be altered.
As opposed to lesions in the T-cell or DC compartments, suboptimal TD responses could reflect defects in follicular dendritic cells (FDCs). GC development and maintenance requires the presence of mature FDCs.15 Although controversial, evidence points to a nonhematopoietic origin for FDCs,49 and accordingly, these cells could be the target of a graft-versus-host reaction after allogeneic BM transplantation. In human BM transplant patients with GVHD, it has been demonstrated that lymph node FDCs and accompanying B-cell clusters are significantly reduced.16 Although IUT and TBI mice did not have any clinical evidence of GVHD, subclinical anti–host responses cannot be ruled out. Thus, should FDCs be exquisitely sensitive to GVHD, their numbers in peripheral lymphoid organs could be reduced, resulting in compromised GC responses. Preliminary experiments suggest this possibility to be unlikely, as staining of splenic sections from immunized IUT mice with anti-IgD mAb and FDCM1 mAb50 demonstrated normal follicular organization and FDC networks (data not shown). Finally, the observed lesion in TD responses might coincide with an inability of donor-derived B cells to respond to helper activity. This is unlikely, however, given the normal levels of serum Ig in unimmunized IUT and TBI mice and normal titers of IgM and IgG Ab after TNP-Ficoll challenge. These latter results suggest that engrafted donor B cells are capable of normal activation, isotype switching, and differentiation to Ab-forming cells.
The capacity of allogeneic BM to fully restore the B-cell compartment in SCID mice after IUT contrasts with a recent report describing in utero transplantation of BM-derived haploidentical stem cells into a human X-linked SCID patient.51 At birth, as well as at 3 and 6 months of age, the patient exhibited high levels of donor T-cell engraftment. However, donor B cells were found to constitute less than 1% of peripheral B cells at these times.51 The reason for poor donor B-cell engraftment in this patient is not clear, but it may reflect insufficient numbers of transplanted B-cell progenitors. It is important to note, however, that murine SCID (defect in DNA-dependent protein kinase) and human X-linked SCID (mutation in IL-2 receptor γ chain) result in differing degrees of immune deficiency. Whereas both T cells and B cells are absent in SCID mice, only the former are missing in patients with X-linked SCID. Accordingly, minimal B-cell engraftment in the X-linked SCID patient after IUT could reflect the inability of donor precursors to compete for space with the host B-cell pool. In this regard, the murine SCID model is a more severe form of immune deficiency and may best represent human genetic diseases where both T-cell and B-cell development are impaired.
In summary, the present study demonstrated the effectiveness of allogeneic IUT in restoring the entire B-cell compartment in SCID mice. These data complement and extend previous work showing full reconstitution of CD4+ and CD8+ T cells in SCID mice receiving transplants in utero with allogeneic BM.32The engrafted B cells were further shown to be functional by virtue of normal baseline serum Ig levels and strong Ab responses after TI antigen challenge. Of interest, TD responses after KLH or SRBC immunization were found to be suboptimal in spite of normal CD4+ T-cell and B-cell numbers. Specifically, isotype switching and GC formation were reduced in all recipients of allogeneic BM. This finding is noteworthy, as it may be related to low numbers of memory B cells and a paucity of variable region somatic mutations observed in long-term human BM transplant patients.10-14Because GCs are required for memory cell formation and somatic hypermutation, a similar GC defect in human recipients of allogeneic BM could account for these observations. Future work will thus focus on revealing the basis for suboptimal TD responses in reconstituted mice.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-04-1232.
Supported by National Institutes of Health grants R01 HL49997, R01 HL52952, and R01 AI31265.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Thomas J. Waldschmidt, Department of Pathology, University of Iowa College of Medicine, Iowa City, IA 52246; e-mail: thomas-waldschmidt@uiowa.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal