Bone marrow (BM)–derived dendritic cells (DCs) cultured in granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 4 (IL-4) have been used to generate antitumor immune responses. The cytokine Flt3 ligand (Flt3L) also has been shown to generate BM DCs. We sought to determine if DCs generated by using Flt3L then matured with lipopolysaccharide (LPS) could lead to DCs with in vivo anti–acute myelogenous leukemia (anti-AML) activity. LPS and tumor necrosis factor α (TNF-α) are effective agents for maturing DCs; however, they have potential in vivo toxicities. Cytosine-phosphorothioate-guanine oligodeoxynucleotides (CpGs) are considered relatively nontoxic, potent activators of DC function and maturation in vitro and in vivo. We investigated whether CpGs would be comparable to TNF-α or LPS for the maturation of GM-CSF/IL-4–generated DCs. DCs cultured with GM-CSF/IL-4 and matured with TNF-α, LPS, or CpG produced a greater allogeneic T-cell response compared with Flt3L/LPS-generated DCs. All 4 distinct DC types were pulsed with AML-lysate and administered before tumor challenge produced an increase in the total number of splenic anti-AML–specific cytotoxic T-lymphocyte precursors and led to significantly (P ≤ .0001) improved survival compared with nonvaccinated controls. GM-CSF/IL-4/LPS was superior to Flt3L/LPS for generating anti-AML effects in vivo. Whereas TNF-α was comparable to LPS in conferring on GM-CSF/IL-4 DCs anti-AML effects in vivo, CpGs were superior to LPS. These data have important clinical implications and are the first to show that Flt3L-generated DCs can provide antitumor protection and that nontoxic agents such as CpGs and Flt3L may be useful in the clinical development of DC vaccines.
Introduction
Dendritic cells (DCs) are the most potent antigen-presenting cell (APC) and have been used to present tumor-specific antigens as tumor vaccines.1-9 DC vaccines are being tested as a means of providing antitumor immune response in patients.4-9 The maturational state of DCs affects the ability of the cells to uptake, process, and present tumor antigens; migrate to lymph nodes that drain tumor sites; and ultimately stimulate a potent T-cell–mediated antitumor response.10 Immature DCs more effectively capture and process antigen than mature DCs. In addition, immature DCs appear to more effectively travel to lymph nodes where, having undergone maturation and up-regulation of costimulatory molecules in vivo, these now mature DCs can generate a T-cell response.10-15 The maturational stage of the DCs ultimately may have significant effects on their ability to function as vehicles for antitumor immunotherapy.
Different methods have been described for the ex vivo generation of mature DCs from bone marrow (BM), peripheral blood, or splenic progenitor cells. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 4 (IL-4) are commonly used together to generate immature DCs from both murine and human progenitor cells.16 The in vivo administration of the cytokine Flt3 ligand (Flt3L) has been shown to stimulate the proliferation of hematopoietic progenitors in mice, primates, and humans.17,18 It also greatly increases the numbers of functionally active DCs in BM, gastrointestinal lymphoid tissue, liver, lymph nodes, lung, peripheral blood, peritoneal cavity, spleen, and thymus.19,20 We have shown that Flt3L has potent antileukemia effects when administered in vivo as a single agent to naive mice and mice receiving bone marrow transplants.21Brasel et al22 described the use of Flt3L to generate DCs from BM progenitors by culturing with the cytokine for 8 days. These DCs expressed high levels of costimulatory and major histocompatibility complex (MHC) molecules, stimulated a potent allogeneic T-cell response, and were able to process and present protein antigen to antigen-specific CD4+ T cells. These data suggest that the use of Flt3L in BM progenitor cultures may be a more simplified and potent method of generating potent DCs ex vivo. The in vivo function of Flt3L-generated DCs has not been previously reported.
Cytosine-phosphorothioate-guanine oligodeoxynucleotides (CpGs) are synthetic oligodeoxynucleotides that mimic immunostimulatory bacterial DNA.23,24 They are unmethylated DNA sequences containing characteristic CpG dinucleotides in particular base contexts.23-25 These CpG motifs are thought to be responsible for initiating a potent immune response in mice, primates, and humans.26-28 CpGs have been shown to activate APCs (particularly tissue DCs), leading to up-regulation of costimulatory molecules necessary for T-cell activation and conversion to effector cytotoxic T lymphocytes (CTLs), and CpGs also stimulate DC secretion of proinflammatory cytokines, particularly interferon-γ (IFN-γ), IL-12, IL-6, and tumor necrosis factor α (TNF-α) necessary for potent immune responses.23,29-31 CpGs have been shown to generate mature DCs from human progenitor cells and murine tissue progenitors in vitro and may be a promising new agent for use in the ex vivo generation and maturation of DCs.32-34 Recently, we have shown that CpGs have potent antileukemia effects when administered as a single agent in naive mice and mice receiving bone marrow transplants.35
Previously, we have shown that TNF-α–matured, lysate-pulsed DCs provide substantial antileukemia responses in a murine model.21 To determine if maturing DCs using the new agent CpG are effective in producing an antitumor vaccination response in murine acute myelogenous leukemia (AML), we compared DCs generated by using GM-CSF and IL-4 and then matured with TNF-α, LPS, or CpG. We further sought to determine if DCs generated by culturing BM in the presence of Flt3L, matured with LPS, and pulsed with AML lysate would provide comparable anti-AML effect to DCs generated by using GM-CSF and IL-4. Data presented indicate that GM-CSF/IL-4 DCs pulsed with tumor antigens and matured with CpGs are a highly effective means of generating anti-AML vaccine responses after in vivo adoptive transfer into naive mice.
Materials and methods
Mice
C57BL/6 and BALB/c, female mice, aged 5 to 6 weeks were obtained from The National Institutes of Health (Bethesda, MD), housed in a specific pathogen-free environment, and fed ad libitum. C57BL/6 mice were used for in vitro DC generation and in vivo antitumor immune response evaluation between 8 and 10 weeks of age. BALB/c mice were used for in vitro assays between 8 to 12 weeks of age.
Cells and cell culture reagents
The AML cell line, C1498 (MHC I+, II−) was maintained in RPMI-1640 culture medium supplemented with 1 mM penicillin/streptomycin, 2 mM L-glutamine, and 10% fetal bovine serum (FBS; HyClone, Ogden, UT). The cells were maintained at 37°C and 5% CO2. C1498 cells grown in AIM-V serum-free media (Life Technologies, Carlsbad, CA) were used to generate tumor cell lysate by subjecting AML cells suspended in phosphate-buffered saline (PBS) through 4 freeze/thaw cycles in liquid nitrogen and a 37°C water bath. The cell viability was evaluated by trypan blue exclusion, and no viable cells were present at the completion of 4 cycles. Lysates were frozen at −80°C until use. Prior to use, lysates were thawed and centrifuged at 1200 rpm, and the supernatant was used as the source of tumor antigen.
Generation of DCs from BM progenitors
BM was harvested from the long bones of the femur, tibia, and fibula of mice as previously described.36 Red cells were lysed by ammonium chloride incubation, and the single cell suspension was depleted of mature T cells, B cells, granulocytes, and IAb+ (MHC II+) cells by using the following antibody cocktail followed by complement lysis. The antibody cocktail contained the following antibodies: anti–Thy 1.2 (30-H-12), anti-B220 (RA3-6B2), anti–Gr-1 (RA-8C5), and IAb (AF6-120.1.2; American Type Culture Collection [ATCC], Manassas, VA). The DC progenitors were then incubated at 1 × 106 cells/mL Dulbecco modified Eagle medium (DMEM)–complete media with cytokine for 5 to 7 days at 37°C and 10% CO2 in 6-well plates with 3 mL per well. Four distinct cytokine combinations were used to generate DCs from marrow precursors. The first combination used GM-CSF (R&D Systems, Minneapolis, MN) 1000 U/mL and IL-4 (Shering-Plough, Kenilworth, NJ) 1000 U/mL for 5 days followed by 2 further days in culture with TNF-α (4 ng/mL; R&D Systems) according to our previously published studies20 (method 1). On the basis of literature,37 we chose a second approach to generate mature DCs by using GM-CSF (R&D Systems) 150 U/mL and IL-4 (Shering-Plough) 75 U/mL for 7 days followed by 2 further days in culture with LPS (0.1 μg/mL; Sigma, St Louis, MO) (method 2), or CpG oligodeoxynucleotide (CpG 2006; 2 μg/mL) (Coley Pharmaceutical Group, Wellesley, MA) (method 3). CpG 2006 has been previously shown to effectively mature human peripheral blood progenitors into DCs.32 Cytokines were replenished on day 3-4 by removing two thirds of the media and replenishing with fresh media supplemented with cytokine. Nonadherent and loosely adherent cells were removed by pipetting on day 4-5 and replated with fresh cytokine-containing media in new 6-well plates. Cells were again replated with fresh cytokine-containing media when the maturational cytokine (TNF-α, LPS, or CPG) was added on days 7 to 9. DCs generated using GM-CSF and IL-4 and matured with TNF-α were compared in parallel cultures using the 2 concentrations of GM-CSF and IL-4; there were no differences in the morphology and phenotype of the cells either before or after TNF-α (data not shown).
For the fourth approach, Flt3L was used as a single cytokine to generate immature DCs. BM progenitors depleted of red blood cells but not mature cells were incubated with Flt3L (200 ng/mL; Immunex, Seattle, WA) for 9 days. Final maturation was accomplished by the addition of LPS (1 μg/mL; Sigma) for the final 24 hours of culture as previously described 22 (method 4).
Phenotypic evaluation of DCs
The cell surface antigen expression of the DCs was compared by using flow cytometry of cells on days 7 to 9 of incubation after DCs were pulsed with tumor antigen. There was no phenotypic difference detected between DCs evaluated prior to pulsing with tumor lysate and after an 18-hour incubation with lysate (data not shown). Cells were washed and incubated with α-FCR (CD16/CD32) (2.4G2) (Pharmingen, San Diego, CA) at 4°C for 10 minutes to block nonspecific binding of fluorochromes. The following directly conjugated antibodies (Pharmingen) were incubated with DCs at 4°C for 30 minutes: CD8α–fluorescein isothiocyanate (FITC), CD4-FITC, NK1.1-phycoerythrin (PE), B220-PE, CD11b-PE, CD80-FITC, CD86-FITC, CD-40–FITC, H2Kb-PE, IAb-FITC, and CD11c-PE. Cells were washed and analyzed using the FACS Caliber (Becton-Dickinson, San Diego, CA) and were gated and analyzed using forward and side-scatter plots on 10 000 live events.
Allogeneic T-cell response
The ability of the DCs to generate a potent allogeneic T-cell stimulatory response was evaluated using BALB/c T cells isolated from the lymph nodes of normal mice. The T cells were plated at 2 × 105 cells per well and stimulated with graded concentrations of irradiated (30 Gy, Cs source) DCs in 96-well plates. Cells were labeled with tritiated thymidine (1 μCi/well [0.037 MBq/well]) for approximately 18 hours prior to harvesting. Cells were harvested on days 1 to 6, and tritiated thymidine incorporation was calculated as counts per minute with the use of a Beta Counter (Packard Instruments, Downers Grove, IL) in the absence of scintillant application that typically yields counts per minute of 5% to 10% of those with scintillant. Supernatant was obtained from cell cultures prior to the addition of tritiated thymidine and analyzed for Th1 (IL-2, IFN-γ) and Th2 (IL-10) cytokines by enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN). The sensitivity of the assay was 3.0 pg/mL for IL-2, 2.0 pg/mL for IFN-γ, and 4 pg/mL for IL-10.
In vivo AML protection
DCs were generated as described and pulsed with C1498 lysate at a ratio of 3:1 (lysate cell equivalents/DC) on days 7 to 9 of culture and incubated in 6-well plates for 18 hours. DCs were washed 3 times in PBS and resuspended in sterile PBS for injection into mice. Mice (n = 5-10) were vaccinated 14 and 7 days prior to tumor challenge with 0.5 × 106 DCs that were injected into the lateral tail vein at a DC dose and schedule previously determined to provide a high degree of protection to a uniformly lethal dose of C1498 (1-2 × 105 cells). Mice were then challenged on day 0 with a supralethal dose of C1498 tumor cells (1-2 × 106cells per mouse) injected intravenously to facilitate comparisons of the degree of AML protection.
In vivo CTLp generation
The cytotoxic T-lymphocyte precursor (CTLp) frequency was analyzed by limiting dilution as previously described.38Spleens from mice (n = 3 per group) that received DCs and nonvaccinated controls were harvested on day 0, a single cell suspension was obtained by passing the cells through a wire mesh, and red cells were lysed by using ammonium chloride. Graded concentrations of splenocytes (1 × 105 − 3 × 102) (30 replicates per dilution) were stimulated with 1 × 104irradiated (100 Gy, Cs source) C1498 tumor cells per well and IL-2 (20 U/mL; Amgen, Thousand Oaks, CA) in 96-well plates for 7 days. Tritiated thymidine-labeled C1498 tumor cells (1 × 104 per well) were then added to the cultures and incubated for 4 hours at 37°C. Parallel dilutions were compared for each group with the irrelevant tritium-labeled tumor targets YAC-1 (H2a) lymphoma cells and B16F10 (H2b) melanoma cells. Cytotoxicity was assessed by the JAM assay39 that measures the level of tritium retained by cells that have not undergone lysis. The CTLp frequency was calculated as previously described by means of Poisson-distribution statistics.40 Results are expressed as the CTLp frequency per spleen, which takes into account the variability in spleen size with vaccination.
Statistics
The Kaplan-Meier product-limit method was used to calculate survival rates. Differences between groups were determined using the generalized Wilcoxon test. Survival data are also presented as median survival time (MST), the point in time at which half of the mice remain alive.
Results
Similar morphology and phenotype of DCs generated using GM-CSF/IL-4 matured with LPS, CpG, or TNF-α and DCs generated using Flt3L/LPS
Previous studies from our laboratory demonstrated that DCs generated using GM-CSF/IL-4 and TNF-α, a protocol also used for human DC generation in clinical trials, provided substantial anti-AML protective responses in naive and BM transplant-treated mice.21 Labeur et al,37 using a murine model of squamous cell carcinoma, suggested that DCs matured with LPS were more potent in vivo, produced higher levels of IL-12, and led to greater CTL responses compared with DCs matured with TNF-α. Comparing TNF-α–matured DCs to CpG 1826–matured DCs, Brunner et al41 showed that DCs matured with this CpG had superior IL-12 production, greater induction of alloreactive T-cell responses, and improved in vivo antitumor effects in a murine model of colon cancer. Because LPS maturation cannot be used clinically owing to its potential severe systemic toxicities, we sought to determine whether CpG may provide similar DC maturation properties and provide comparable or superior in vivo anti-AML responses compared with those already observed with TNF-α–matured DCs. Using a different approach, Brasel et al22 described the in vitro generation of DCs using Flt3L and LPS. Therefore, we sought to determine if these Flt3L-generated DCs had similar anti-AML effects in vivo.
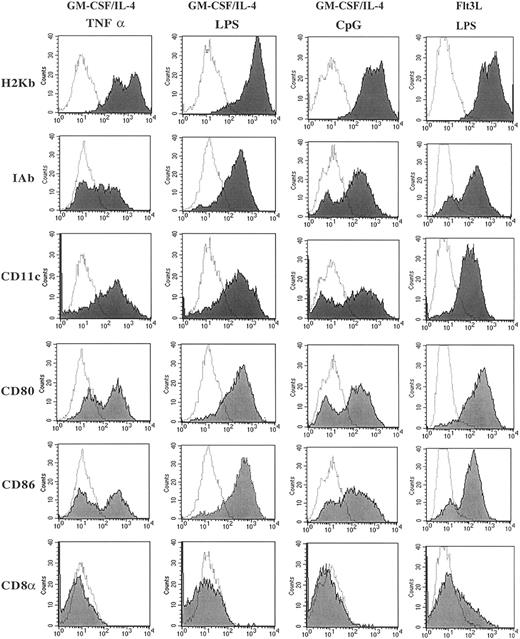
Comparing the several methods of DC generation from murine BM precursor cells, all 4 distinct methods generated cells with morphology consistent with that described for DCs with large dendrite projections (data not shown). Consistent with our previous data,22 the DCs generated using Flt3L/LPS were smaller and had fewer dendrites compared with DCs generated using GM-CSF/IL-4 and matured with TNF-α, LPS, or CpG (data not shown). Flow cytometry was used to evaluate the cell surface expression of markers typically present on DCs. Figure1 illustrates these results for MHC class I (H2Kb), MHC class II (IAb), CD80 (B7-1), CD86 (B7-2), CD11c, and CD8α. Cells generated by GM-CSF/IL-4 and then matured with LPS, CpG, or TNF-α had high levels of expression of MHC class I and class II, CD11c and the costimulatory molecules CD80 and CD86, and no expression of CD8α. Maturation with LPS reproducibly led to the highest expression of CD80, CD86, and IAb. The surface expression of CD4, CD8, B220, and NK1.1 were less than 1% on cells matured with LPS, CpG, or TNF-α. The phenotype of the cells generated used Flt3L/LPS also demonstrated high levels of expression of MHC class I and class II, CD11c, and the costimulatory molecules CD80 and CD86 as previously described.22 In contrast to the DCs cultured with GM-CSF/IL-4, the cells generated in the presence of Flt3L/LPS had a population of cells that expressed CD8α (15%-25% of the cells). This population coexpressed CD11b, CD80, CD86, CD40, and IAb and also lacked T-cell–receptor α/β (TCRα/β) expression, indicating that CD8α is present on the DC population and not present on a T-cell population that had been expanded in vitro. Our phenotype data are consistent with that already published for Flt3L-generated DCs, suggesting a distinct population of DCs that coexpresses CD8α.22
Phenotypic characterization of DCs cultured in GM-CSF/IL-4 and matured using CpG, TNF-α, or LPS and DCs cultured with Flt3L and matured using LPS.
Cultured DCs were harvested and washed with 2% FBS/PBS, blocked with αFCR, and incubated with directly conjugated fluorescent antibody for 30 minutes. Live cells were gated by using forward and side scatter dot plots, and 10 000 live events were analyzed for each sample. Data are presented as solid histograms for the expression of the phenotypic marker noted on the left for each preparation method; GM-CSF/IL-4 matured with TNF-α, LPS, or CpG 2006 or Flt3L matured with LPS. The dotted histogram represents the background fluorescence of the isotype control. Data presented are representative of 3 independent experiments.
Phenotypic characterization of DCs cultured in GM-CSF/IL-4 and matured using CpG, TNF-α, or LPS and DCs cultured with Flt3L and matured using LPS.
Cultured DCs were harvested and washed with 2% FBS/PBS, blocked with αFCR, and incubated with directly conjugated fluorescent antibody for 30 minutes. Live cells were gated by using forward and side scatter dot plots, and 10 000 live events were analyzed for each sample. Data are presented as solid histograms for the expression of the phenotypic marker noted on the left for each preparation method; GM-CSF/IL-4 matured with TNF-α, LPS, or CpG 2006 or Flt3L matured with LPS. The dotted histogram represents the background fluorescence of the isotype control. Data presented are representative of 3 independent experiments.
GM-CSF/IL-4– and Flt3L-generated DCs stimulate potent alloreactive responses that are comparable with TNF-α, LPS, or CpG maturation agents
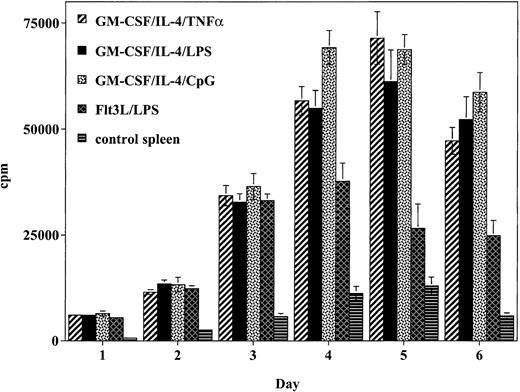
To determine the relative ability of the cultured DCs to stimulate a naive alloreactive T-cell response in vitro, T cells were incubated with graded concentrations of irradiated DCs (Figure2). Cells generated using GM-CSF/IL-4 and matured with TNF-α produced a potent allogeneic T-cell response that peaked on day 5 and was 5.5-fold higher than spleen cell control responses. This response was comparable to the alloreactive response of DCs generated using GM-CSF/IL-4 and matured with either LPS or CpG. Whereas DCs generated using Flt3L/LPS generated a strong allogeneic T-cell response, this response was weaker and less sustained compared with the response observed with the DCs generated using GM-CSF/IL-4 and matured with TNF-α, LPS, or CpG.
DCs matured with CpG, TNF-α, or LPS stimulate the proliferation of allogeneic T cells to a greater degree than Flt3L/LPS-matured DCs.
Cultured DCs were added to lymph node preparations from naive balb/c mice. Data presented represent the response of 1 × 105balb/c responder cells: 1 × 104 DCs (10:1 responder-to-stimulator ratio) expressed as mean cpm ± SD of 6 replicate wells per DC preparation method. Similar results were obtained in 2 independent experiments.
DCs matured with CpG, TNF-α, or LPS stimulate the proliferation of allogeneic T cells to a greater degree than Flt3L/LPS-matured DCs.
Cultured DCs were added to lymph node preparations from naive balb/c mice. Data presented represent the response of 1 × 105balb/c responder cells: 1 × 104 DCs (10:1 responder-to-stimulator ratio) expressed as mean cpm ± SD of 6 replicate wells per DC preparation method. Similar results were obtained in 2 independent experiments.
Because Flt3L-generated DCs have a greater population of CD8α+ DCs, it is possible that these Flt3L DC cultures produce cytokines that skew the response more toward a Th2 response that may down-regulate the alloresponse than GM-CSF/IL-4–cultured DCs. To determine if this difference in alloresponsiveness could be related to differences in cytokines produced by the different cultures, cytokine levels present in the supernatants of the cultures were evaluated on days 2, 4, and 6. There was no difference in the Th1 cytokine (IL-2 and IFN-γ) levels detected in the supernatants of any of the cultures on the days evaluated (data not shown). However, the IL-10 level in the cultures with the Flt3L/LPS-generated cells was higher on day 4 (168 pg/mL) compared with the level in cultures with GM-CSF/IL-4–generated DCs matured with LPS (11 pg/mL), CpG (40 pg/mL), or TNF-α (29 pg/mL). As we have previously shown in an allogeneic culture system, this higher IL-10 level may contribute to the lower peak response seen in the cultures with the Flt3L-generated DCs, suggesting a potential inhibitory effect modulating the response.42
DCs generated using GM-CSF/IL-4 and matured with TNF-α or LPS have comparable in vivo anti-AML effects
Previous data from our laboratory have shown that DCs generated using GM-CSF/IL-4, matured with TNF-α, and pulsed with AML lysate provide substantial antitumor protection when administered to naive mice or to mice receiving BM transplants 14 and 7 days prior to a uniformly lethal C1498 dose (1-2 × 105).21Labeur et al37 suggested that DCs matured with LPS provide greater in vivo antitumor protection compared with DCs matured with TNF-α. For in vivo comparative studies, a supralethal dose (2 × 106 cells/mouse) of C1498 cells was administered. The minimal lethal dose of C1498 is 1 × 105 cells per mouse. We chose a tumor dose more than 10-fold higher than is the minimal required to be able to clearly discern any differences in protective effect that may not be evident at a lower tumor cell dose.
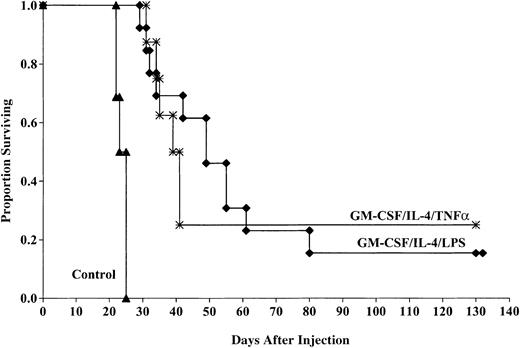
Aggregate data from 2 experiments indicate that the cohorts of mice vaccinated with DCs matured with TNF-α had a significant survival advantage compared with those of control mice after challenge with 2 × 106 tumor cells (25% compared with 0%,P < .0001; Figure 3). The MSTs were also improved for mice receiving TNF-α–matured DCs compared with controls (41 versus 25 days). This response was comparable to that seen in mice that received DCs generated with GM-CSF/IL-4 and matured with LPS (15% surviving compared with 0% in controls, P < .0001) with a MST of 53.3 days. There was no significant (P = 0.3) survival difference between cohorts of mice that received TNF-α– or LPS-matured DCs despite the longer MST of mice that received LPS-matured DCs.
LPS- and TNF-α–matured DCs cultured in GM-CSF/IL-4 provide comparable protection against murine AML challenge.
DCs were cultured and matured with either LPS or TNF-α and pulsed with AML (C1498) cell lysate for 18 hours then administered intravenously (0.5 × 106 cells/mouse) 14 and 7 days prior to tumor challenge with 2 × 106 C1498 cells/mouse. The mice receiving DC vaccination had significantly improved survival compared with nonvaccinated controls (P < .0001). There was no significant difference (P = .3) between mice receiving LPS- or TNF-α–matured DCs. Data are pooled results of 2 independent experiments (13 total/group).
LPS- and TNF-α–matured DCs cultured in GM-CSF/IL-4 provide comparable protection against murine AML challenge.
DCs were cultured and matured with either LPS or TNF-α and pulsed with AML (C1498) cell lysate for 18 hours then administered intravenously (0.5 × 106 cells/mouse) 14 and 7 days prior to tumor challenge with 2 × 106 C1498 cells/mouse. The mice receiving DC vaccination had significantly improved survival compared with nonvaccinated controls (P < .0001). There was no significant difference (P = .3) between mice receiving LPS- or TNF-α–matured DCs. Data are pooled results of 2 independent experiments (13 total/group).
DCs matured with CpGs provide superior in vivo anti-AML protective response compared with DCs generated with GM-CSF/IL-4 and matured with LPS
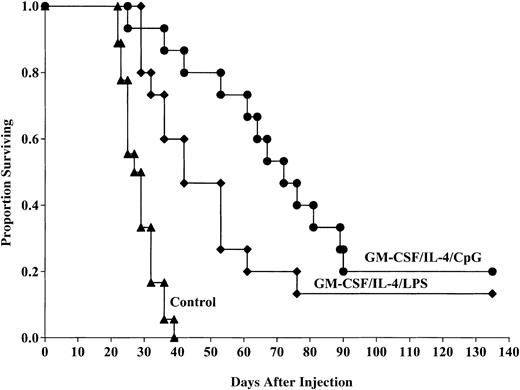
Because the MST of mice that received GM-CSF/IL-4, LPS-matured DCs was longer than mice that received DCs matured with TNF-α, we chose to directly compare LPS-induced DC maturation to that using CpG for in vivo protective responses. Pooled data from 2 replicate experiments indicated that DCs generated using GM-CSF/IL-4, then matured with CpG 2006, and pulsed with C1498 lysate provided a significant survival advantage compared with control mice after lethal tumor challenge (20% compared with 0%, P ≤ .0001; Figure4). The survival advantage provided by DCs matured with CpG was significantly improved compared with the survival of mice administered GM-CSF/IL-4/LPS–matured DCs (20% versus 13% survival, respectively, P = .02). The MST of mice that received CpG-matured DCs was longer than mice that received LPS-matured DCs (73.9 versus 49.98 days, respectively). These results indicate that CpG-matured DCs provide superior antitumor responses to LPS-matured DCs in vivo and prolong the MST. Because LPS- and TNF-α–matured DCs provide similar antitumor response, we conclude that CpG-matured DCs are superior to either LPS- or TNF-α–matured DCs for providing in vivo antitumor protective responses.
CpG-matured DCs cultured in GM-CSF/IL-4 provide superior protection against murine AML challenge compared with LPS-matured DCs.
DCs were cultured and matured with either LPS or CpG 2006 and pulsed with AML (C1498) cell lysate for 18 hours then administered intravenously (0.5 × 106 cells/mouse) 14 and 7 days prior to tumor challenge with 2 × 106 C1498 cells/mouse. The mice receiving DC vaccination had significantly improved survival compared with nonvaccinated controls (P ≤ .0001). The survival advantage provided by DCs matured with CpG was significantly improved compared with the survival of mice administered GM-CSF/IL-4/LPS–matured DCs (20% versus 13% survival, respectively,P = .02). The MST of mice that received CpG-matured DCs was longer than mice that received LPS-matured DCs (73.9 versus 49.98 days, respectively). Data are pooled results of 2 independent experiments (15 total mice/group).
CpG-matured DCs cultured in GM-CSF/IL-4 provide superior protection against murine AML challenge compared with LPS-matured DCs.
DCs were cultured and matured with either LPS or CpG 2006 and pulsed with AML (C1498) cell lysate for 18 hours then administered intravenously (0.5 × 106 cells/mouse) 14 and 7 days prior to tumor challenge with 2 × 106 C1498 cells/mouse. The mice receiving DC vaccination had significantly improved survival compared with nonvaccinated controls (P ≤ .0001). The survival advantage provided by DCs matured with CpG was significantly improved compared with the survival of mice administered GM-CSF/IL-4/LPS–matured DCs (20% versus 13% survival, respectively,P = .02). The MST of mice that received CpG-matured DCs was longer than mice that received LPS-matured DCs (73.9 versus 49.98 days, respectively). Data are pooled results of 2 independent experiments (15 total mice/group).
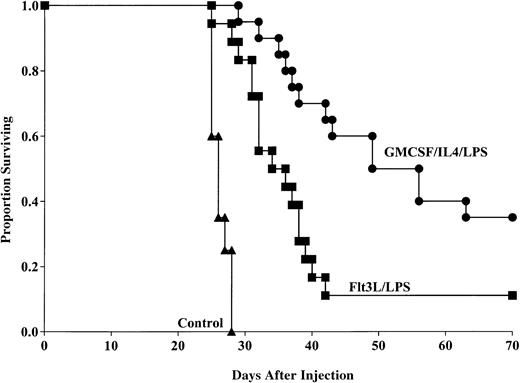
Flt3L generates DCs capable of providing anti-AML protective response
To determine whether Flt3L-generated, LPS-matured DCs could provide anti-AML protection when pulsed with C1498 lysate, mice were administered this DC population 14 and 7 days prior to lethal (1 × 106) tumor challenge. Aggregate data from 2 replicate experiments indicated that mice that received the Flt3L/LPS-generated DCs had significantly longer survival compared with nonvaccinated mice after tumor challenge (11% versus 0% surviving, respectively, P < .0001; Figure5) with MSTs of 36 and 26.3 days, respectively. Moreover, mice that received the GM-CSF/IL-4–generated DCs had significantly improved survival compared with mice that received DCs generated using Flt3L (35% versus 11% survival, respectively, P = .002) with MSTs of 56 and 36 days, respectively. These data indicate that DCs generated by either GM-CSF/IL-4 or Flt3L are capable of providing tumor protection to murine AML, but DCs cultured with GM-CSF/IL-4 are superior to cells cultured with Flt3L in this model system.
GM-CSF/IL-4/LPS–matured DCs provide superior protection against AML compared with DCs generated using Flt3L and matured with LPS.
DCs were generated using GM-CSF/IL-4 or Flt3L and matured with LPS. DCs were then pulsed with AML (C1498) cell lysate for 18 hours then administered intravenously (0.5 × 106 cells/mouse) 14 and 7 days prior to tumor challenge with 1 × 106 C1498 cells/mouse. Mice receiving either DC vaccination had significantly improved survival compared with nonvaccinated controls (P < .0001). Mice receiving GM-CSF/IL-4/LPS–generated DCs had significantly improved survival compared with mice that were vaccinated with DCs generated using Flt3L and LPS (P = .002). Data are pooled results of 2 independent experiments (20 total mice/group).
GM-CSF/IL-4/LPS–matured DCs provide superior protection against AML compared with DCs generated using Flt3L and matured with LPS.
DCs were generated using GM-CSF/IL-4 or Flt3L and matured with LPS. DCs were then pulsed with AML (C1498) cell lysate for 18 hours then administered intravenously (0.5 × 106 cells/mouse) 14 and 7 days prior to tumor challenge with 1 × 106 C1498 cells/mouse. Mice receiving either DC vaccination had significantly improved survival compared with nonvaccinated controls (P < .0001). Mice receiving GM-CSF/IL-4/LPS–generated DCs had significantly improved survival compared with mice that were vaccinated with DCs generated using Flt3L and LPS (P = .002). Data are pooled results of 2 independent experiments (20 total mice/group).
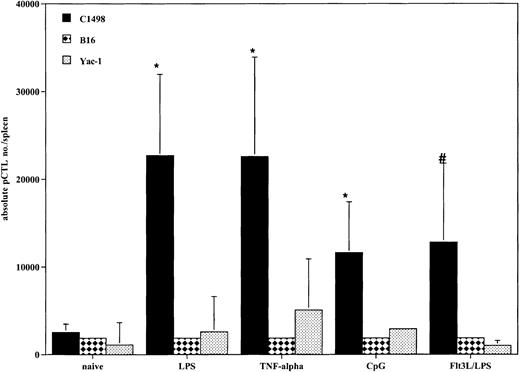
All DC-treated mice generated tumor-specific CTLps in response to vaccination
Because all cohorts of mice that received DCs prior to tumor challenge had improved survival compared with controls, we determined if there was evidence that a tumor-specific response was achieved in vivo at the time of systemic AML challenge. Tumor-specific CTLps were detectable in all mice that received DC vaccinations (Figure6). All cohorts of DC-treated mice had an increase in the absolute number of tumor-specific splenic CTLps compared with naive mice. There were no substantial differences among the 4 different propagation methods. These responses were specific for C1498, as there was minimal response to the irrelevant tumor targets B16 and Yac-1. The lack of response to Yac-1 cells indicates that the responding cells were not natural killer (NK) cells, as Yac-1 is sensitive to NK killing.
DCs generated with GM-CSF/IL-4 and matured with TNF-α, LPS, or CpG and DCs generated with Flt3L and matured with LPS produce AML-specific CTLp cells in vivo.
Cohorts of 8 to 10 mice were injected intravenously with DCs pulsed with AML (C1498) lysate (0.5 × 106 cells/mouse) 14 and 7 days prior to tumor challenge on day 0. On day 0, the 3 representative mice per group were killed, and the spleens were harvested for splenic C1498-reactive CTLp frequency estimate. The mean total splenic anti-AML CTLp number ± SEM is shown on the y-axis for each of the 4 groups and naive control. All methods of DC propagation and in vivo vaccination generated greater numbers of tumor-specific CTLps compared with naive controls (*P < .05,#P = .07). There were no substantial differences among the 4 different propagation methods. The irrelevant tumor controls, B16F10 and Yac-1, failed to stimulate a CTLp response, demonstrating tumor specificity in the DC vaccinated mice.
DCs generated with GM-CSF/IL-4 and matured with TNF-α, LPS, or CpG and DCs generated with Flt3L and matured with LPS produce AML-specific CTLp cells in vivo.
Cohorts of 8 to 10 mice were injected intravenously with DCs pulsed with AML (C1498) lysate (0.5 × 106 cells/mouse) 14 and 7 days prior to tumor challenge on day 0. On day 0, the 3 representative mice per group were killed, and the spleens were harvested for splenic C1498-reactive CTLp frequency estimate. The mean total splenic anti-AML CTLp number ± SEM is shown on the y-axis for each of the 4 groups and naive control. All methods of DC propagation and in vivo vaccination generated greater numbers of tumor-specific CTLps compared with naive controls (*P < .05,#P = .07). There were no substantial differences among the 4 different propagation methods. The irrelevant tumor controls, B16F10 and Yac-1, failed to stimulate a CTLp response, demonstrating tumor specificity in the DC vaccinated mice.
Discussion
Our study is the first comparative study evaluating the phenotype and in vivo biologic function of GM-CSF/IL-4–generated, TNF-α–, LPS-, or CpG-matured and Flt3L-generated, LPS-matured DCs. We present data that CpGs are superior to TNF-α or LPS for maturing GM-CSF/IL-4–generated BM-derived DCs in providing anti-AML protective effects via vaccination. GM-CSF/IL-4–cultured TNF-α–matured DCs are currently in clinical trial and could be considered the “gold standard” for propagation of DCs from BM progenitors. Both Flt3L and CpG are in clinical trials as single agent therapies to induce tumor immune responses in vivo. Our data indicate that CpG maturation of ex vivo–generated DCs warrants testing in humans as a potential antitumor vaccination strategy and suggests that Flt3L-propagated, LPS-matured DCs may be less effective in generating anti-AML response in vivo.
CpGs have been shown to induce potent DC-mediated responses when these agents have been given in vivo.32,33 We have previously shown that the systemic administration of CpG as a single agent leads to both protective and therapeutic anti-AML responses.21 The effect of CpGs on activating DCs leads to up-regulation of costimulatory molecules on the surface of DCs and increased cytokine production that further enhances the ability of DCs to stimulate T-cell responses. Hartmann et al32 have reported that CpGs are able to generate and mature DCs from human peripheral blood progenitors. Others have shown that DCs can be generated by using murine endothelial progenitors in the presence of CpGs that then leads to DCs with the characteristics of tissue Langerhans cell.33,34 Our data show that DCs cultured with GM-CSF/IL-4 and matured with CpG leads to comparable costimulatory molecule expression to that seen on DCs matured with LPS and TNF-α. CpG-matured DCs stimulated a potent allogeneic T-cell response that was comparable to the T-cell responses stimulated by LPS- and TNF-α–matured DCs. These in vitro data are consistent with published data using CpGs to mature DCs from human and mouse progenitors.32,33,41 Our data show that CpG-matured DCs provided superior protection against AML challenge compared with LPS or TNF-α, both of which have been used as potent stimulators of DC maturation.37 41 The clinical importance of this finding is that CpGs may be able to replace TNF-α or LPS for the ex vivo generation of DCs. LPS is not approved for clinical use and as such, even though it is a very potent stimulator of DC maturation, is it not an option for clinical development because of toxicity concerns. TNF-α is a commercially available cytokine that is relatively expensive to produce in large quantities and can have substantial side effects when injected into humans. A major advantage of CpGs is that they are inexpensive to produce in large quantities and currently are in human clinical trials. Given that DCs matured using CpGs appear to be superior to LPS- or TNF-α–matured DCs for inducing an in vivo anti-AML response, CpGs may be important for the production of DCs for use in clinical trials.
LPS and CpG mediate their effects on DC activation via binding to distinct Toll-like receptors (TLRs) on the surface of the DC (specifically TLR4 and TLR9, respectively).43,44 The exact downstream signaling responses are not clearly understood, but both use the MyD88 adapter protein for signaling and up-regulation of NFκB.45,46 The shared intracellular signaling pathways that are triggered by LPS and CpG may explain their very similar effects on DC maturation. There may, however, be differences that are not yet elucidated, as LPS and CpG do have distinct receptors, and there may be other downstream signaling events. Our studies were conducted using CpG 2006. Different CpGs have variable immunostimulatory responses based on the structure of the flanking sequences and number of CG repeats.47 Therefore, extrapolation of the data from our studies to other CpGs is not possible. It is possible that the variable immunostimulatory responses observed with different CpGs is related to differences in signaling through TLR9.44 In human DCs, TLR9 is only expressed on the subset of DCs referred to as plasmacytoid pre-DCs.48,49 Myeloid DCs express other TLRs, predominantly TLR4, but lack TLR9 expression.48 A murine equivalent of the human plasmacytoid DC has recently been described.50-52 It is possible that CpG is maturing a subset of murine DCs or DC precursors that preferentially express TLR9 at different stages of maturation as is seen in human DCs. Studies are ongoing to determine if CpG maturation of the ex vivo–generated DCs is dependent on TLR9 in the murine model system.
Flt3L is an effective cytokine for generating immature DCs in vivo and has been used to generate antitumor immune responses in a variety of tumor models.53-57 In human phase I clinical trials, the administration of Flt3L has led to substantial increases in peripheral blood monocytes and circulating DCs, resulting in increased DCs at tumor sites as well as increased DCs available for leukapheresis and vaccine generation.58,59 Clinical and cellular antitumor responses have been observed in up to 50% of the patients treated with Flt3L-generated DC vaccines.59 Because immature DCs are thought not to be as efficient at presenting antigen to T cells, it may be more beneficial to generate immature DCs ex vivo using Flt3L and mature them to obtain maximal antitumor responses. Brasel et al22 described the use of Flt3L, as a single cytokine, for the ex vivo generation of BM-derived DCs. We have used this method and further expanded the data by using in vivo studies in a murine model of AML. Our flow cytometry data are consistent with that of Brasel et al,22 indicating a population of DCs that coexpress CD8α differing from the GM-CSF– and IL-4–generated DCs that lacked CD8α expression. This population of CD8α+ DCs also coexpressed CD11b and the costimulatory molecules CD80 and CD86 as well as MHC class I and II molecules. It has been reported that the DCs generated in vivo by the systemic administration of Flt3L have high levels of CD8α expression when isolated from the spleen or lymph nodes and have been found to have a lymphoid DC phenotype.18-20,22 It has been proposed that these lymphoid DCs may not be as effective at capturing, processing, and presenting tumor antigen.10 It has also been recently suggested that Flt3L-cultured DCs may lead to the generation of a DC population (prelymphoid or plasmacytoid) that is suppressed by GM-CSF.60 The Flt3L-generated DCs were not as potent at generating an in vitro allogeneic T-cell response compared with DCs produced by the GM-CSF/IL-4 methods. Even though the DCs generated using Flt3L provided substantial anti-AML protection compared with control mice, this protection was inferior to that produced by GM-CSF/IL-4/LPS–matured DCs. It is unclear as to the reasons for these differences. The CD8α expression may denote a subpopulation of DCs that have an inhibitory or regulatory rather than a stimulatory role.61,62 Evidence from the in vitro allogenic T-cell stimulation studies indicate that more IL-10 was produced by the T cells at earlier time points in the cultures containing Flt3L-generated DCs, suggesting that the Flt3L-generated DCs may also stimulate an inhibitory or modulating Th2 response. Another possibility is that the CD8α expression by the DCs represents an activation state of these cells as proposed by Merad et al63 who reported that CD8α is expressed on all DCs (“myeloid and lymphoid”) on migration to the lymph node. They suggested that the expression of CD8α was not lineage related but activation related. It has also been recently shown that splenic lymphoid progenitors and either lymphoid or myeloid BM progenitors are capable of generating both CD8α+ and CD8α− DC populations, further supporting that CD8α expression on DCs is not lineage related but possibly related to a maturational or activation state.64 65 We did not detect expression of CD8α on the DCs generated using GM-CSF/IL-4 and matured with CpG, LPS, or TNF-α, but low-level expression cannot be excluded as sorted populations were not evaluated.
Several studies have been published on the use of DC vaccines for the protection from recurrence and treatment of solid tumors.1-9 We have previously published the successful prevention of AML after bone marrow transplantation by using DCs pulsed with AML lysate.21 These previous studies used DCs generated using GM-CSF/IL-4 and matured with TNF-α and demonstrated initial antitumor protective responses as well as protection from lethal rechallenge long term. We now show that CpGs can be substituted for TNF-α in this culture system resulting in improved survival in mice administered the CpG matured DC vaccine. We have also previously shown that DC vaccination is superior to the systemic administration of Flt3L for the prevention of AML progression.21 As Flt3L expands the immature population of DCs after in vivo administration, it may be necessary to mature these DCs ex vivo to obtain maximal antitumor response. Our data indicate that Flt3L may be an effective agent for the ex vivo or in vivo generation of DCs for use in the prevention of AML, but this protection may not be as good as that attained with GM-CSF/IL-4–generated DCs.
In conclusion, LPS has been used to mature DCs in vitro and is a potent stimulator of immune responses in vivo. The clinical use of LPS in humans is limited because of its risk for inducing substantial toxicity. Our data indicate that DCs generated using GM-CSF/IL-4 and CpG produce DCs that are comparable to DCs generated using LPS or TNF-α in vitro and are capable of providing superior protection against AML in vivo. Our data also indicate that Flt3L, as a single agent used in culture with BM progenitors, produces DCs capable of generating an anti-AML response. However, this response is not as robust as that seen with vaccination using GM-CSF/IL-4–generated DCs. Both CpGs and Flt3L are agents currently in human clinical trials and may be excellent agents to consider for use in the generation of DCs for clinical use in antitumor vaccine trials. Our data may have substantial implications on the relative efficacy of methods used for the ex vivo generation of DCs for future human trials.
We thank Micki Diers and John Vaala for their technical assistance.
Prepublished online as Blood First Edition Paper, August 8, 2002; DOI 10.1182/blood-2002-04-1063.
Supported in part by grants from the Children's Cancer Research Fund, Viking Children's Fund, Coley Pharmaceutical Group, and grants R01 CA-72669 and RO1 CA-85922 from the National Institutes of Health.
A.M.K. has a commercial interest in the Coley Pharmaceutical Group and the CpGs used are products of Coley Pharmaceutical Inc. K.B. has a commercial interest in the Immunex Corporation and Flt3L is a product of the Immunex Corporation (now Amgen).
B.J.W. and N.N. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Brenda J. Weigel, University of Minnesota Cancer Center, Department of Pediatrics, Division of Pediatric Hematology/Oncology and Blood & Marrow Transplant, MMC 366, 420 Delaware St SE, Minneapolis, MN 55455; e-mail:weige007@tc.umn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal