Reoviruses infect cells that manifest an activated Ras-signaling pathway, and have been shown to effectively destroy many different types of neoplastic cells, including those derived from brain, breast, colon, ovaries, and prostate. In this study, we investigated the reovirus as a potential therapeutic agent against lymphoid malignancies. A total of 9 lymphoid cell lines and 27 primary human lymphoid malignancies, as well as normal lymphocytes and hematopoietic stem/progenitor cells, were tested for susceptibility to reovirus infection. For in vitro studies, the cells were challenged with reovirus (serotype 3 Dearing), and viral infection was assessed by cytopathic effects, viability, viral protein synthesis, and progeny virus production. We present evidence of efficient reovirus infection and cell lysis in the diffuse large B-cell lymphoma cell lines and Burkitt lymphoma cell lines Raji and CA46 but not Daudi, Ramos, or ST486. Moreover, when Raji and Daudi cell lines were grown subcutaneously in severe combined immunodeficient/nonobese diabetic (SCID/NOD) mice and subsequently injected with reovirus intratumorally or intravenously, significant regression was observed in the Raji-induced, but not the Daudi-induced, tumors, which is consistent with the in vitro results. Susceptibility to reovirus infection was also detected in 21 of the 27 primary lymphoid neoplasias tested but not in the normal lymphocytes or hematopoietic stem/progenitor cells. Our results suggest that reovirus may be an effective agent against several types of human lymphoid malignancies.
Introduction
The human reovirus, a naturally occurring double-stranded RNA virus, possesses impressive oncolytic properties. Our group has recently demonstrated the capacity of reovirus to destroy cells derived from human malignant gliomas and breast, colon, ovarian, pancreatic, and prostate carcinomas both in vitro and in vivo.1-4 Previous studies on parental NIH-3T3 and NIH-3T3 cells transformed with the oncogenes v-erbB, EGFR, sos,or ras (all activators of the Ras-signaling pathway) have shown the preference of reovirus to infect and kill cancer cells with an activated Ras or Ras-signaling pathway while sparing normal cells.5-8 Hence, mutations in ras itself, or in oncogenes signaling through Ras, could create an appropriate environment in the cell for reovirus replication, a situation that perhaps could be found in as many as 80% of human malignancies. In addition, unlike some other members of their family (eg, rotaviruses), reoviruses are not associated with any known human diseases and are considered nonpathogenic.9 Collectively, these properties offer hope for the use of reovirus as a novel anticancer agent. In this study, we evaluated reovirus as a potential antilymphoma therapeutic agent.
The lymphoid neoplasms, the second most rapidly increasing human malignancy within the last 20 years, represent a heterogeneous group of neoplasias consisting of a number of clinicopathological entities with various prognoses, treatments, and outcomes.10,11 While encouraging responses are often obtained with current treatment modalities, lymphomas are still the sixth-most- common cause of cancer deaths in the United States, and diseases such as low-grade non-Hodgkin lymphomas (NHLs) and chronic lymphocytic leukemia (CLL) remain incurable at present.12-15 Lymphomagenesis is often initiated and characterized by specific chromosomal rearrangements. These cytogenetic abnormalities, such as the t(8:14) and t(14:18) chromosome translocations, juxtapose oncogenes (c-myc andbcl-2 principally) to immunoglobulin enhancers and result in the deregulation of their expression.16,17 Additional alterations, such as trisomy of chromosome 12 in CLL, deletion of chromosome 13 (13q14), and others, have led to the discovery of the involvement of numerous proto-oncogenes in the neoplastic progression of lymphomas.18,19 Ras mutations, however, are believed to contribute rarely, if at all, to carcinogenesis in lymphoid malignancies. Studies have reported ras mutations in 15% of AIDS-related NHL, but a near-absence in follicular lymphomas (FLs), diffuse large B-cell lymphomas (DLBCLs), and CLL.20-22Nonetheless, activation of a Ras-dependent pathway may occur in certain lymphoid tumors that could render these neoplasms sensitive to reovirus oncolysis. For instance, Myc has been shown to cooperate with Ras in the transformation of B lymphocytes.23 Lymphoblasts infected with the Epstein-Barr virus (EBV) can show activation of the Ras pathway through the expression of the EBV-transforming latent membrane protein-1 (LMP-1).24 A number of CLL lymphoid cells express both the CD40 receptor and its ligand CD154, and an interaction between these 2 entities has been shown to stimulate p21ras.25,26 In addition, up to 46% of DLBCLs may have scattered mutation throughout the RhoH/TTF proto-oncogene, a recently discovered small guanosine triphosphate (GTP)–binding protein belonging to the Ras superfamily and expressed exclusively in hematopoietic tissues.27
Despite the fact that mutations in Ras itself are rarely implicated in lymphomagenesis, we hypothesized that a significant proportion of lymphoid malignancies may still be amenable to reovirus therapy. In this study, we used established human lymphoma cell lines, animal cancer models, and multiple primary human non-Hodgkin lymphoma and CLL specimens to examine the possibility of using reovirus against different types of lymphoid neoplasms.
Materials and methods
Cells and reovirus
Human Burkitt lymphoma cell lines (Raji, CA46, Daudi, Ramos, and ST486), obtained from American Type Culture Collection (ATCC; Manassas, VA), were maintained in RPMI containing 10% fetal bovine serum (FBS), 1% l-glutamine (Gibco/BRL, Rockville, MD), and antibiotics (Sigma, St Louis, MO). The diffuse large B-cell lymphomas (OCY-LY1, OCY-LY2, OCY-LY8, and OCY-LY10) described previously28 were initially propagated in Iscove modified Dulbecco medium (IMDM) supplemented with 20% human serum and antibiotics. Once exponential growth was achieved, the human serum was replaced by 20% FBS. Cells were kept at 37°C in a humidified 5% CO2 incubator.
Normal peripheral blood lymphocytes and bone marrow cells were obtained with the donors' consent and according to institutional ethical guidelines. Peripheral blood lymphocytes were separated by means of a Ficoll gradient, and bone marrow cells were processed according to the CD34+ progenitor cell isolation kit protocol (Miltenyi Biotec, Auburn, CA) as previously described.29 Cell fractions having a CD34+ cell purity of 90% or greater were used for subsequent experiments.
The Dearing strain of reovirus serotype 3 was propagated in L929 cells grown in suspension in Joklik modified Eagle medium (JMEM) containing 5% FBS. The virus was purified according to the protocol of Smith et al30 with the exception that β-mercaptoethanol was omitted from the extraction buffer. Dead virus was prepared by exposing the live virus to ultraviolet (UV) light for 45 minutes.
Reovirus infection of lymphoid cell lines in vitro
One million cells of each cell line described above were dispensed into 24-well plates and infected with reovirus at a multiplicity of infection (MOI) of 20 plaque-forming units (PFUs) per cell. For the trypan blue exclusion test, the cells were stained with 0.25% dye, and the remaining viable cells in 3 independent wells were counted on a hemacytometer. For metabolic labeling, [35S]-methionine was added to the culture medium for a period of 6 hours. After incubation, the medium was removed by centrifugation and the cells were lysed in lysis buffer (phosphate-buffered saline [PBS] containing 1% Triton X-100, 0.5% sodium deoxycholate, and 1 mM EDTA [ethylenediaminetetraacetic acid]). Lysates were cleared of debris by centrifugation and supernatants were stored at −70°C until use.
Polyclonal rabbit antireovirus serotype 3 serum was used for immunoprecipitation of [35S]-methionine–labeled reovirus proteins from cell lysates, as previously described.31Immunoprecipitated proteins were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE)32 followed by autoradiography.
Progeny virus
Three million cells grown in 6-well plates were infected with reovirus at an MOI of 20. After 45 minutes of binding at 4°C, the cells were returned to 37°C for 96 hours except for the time-point 0 samples, which were frozen immediately and stored at −70°C until use. Plates were then subjected to 3 rounds of freeze-thaw, and supernatants were used for plaque titration on L929 cells. All titration experiments were repeated in triplicate.
SCID/NOD mice studies
Six- to 8-week-old severe combined immunodeficient/nonobese diabetic (SCID/NOD) mice were obtained from the Cross Cancer Institute (Edmonton, AB, Canada) or The Jackson Laboratory (Bar Harbor, ME). The animals were maintained under specific pathogen-free conditions and treated according to a protocol approved by the University of Calgary Animal Care Committee (AB, Canada). As a subcutaneous xenograft model, 1.0 × 107 Raji or Daudi lymphoma cell lines in 100 μL PBS were subcutaneously injected in the hind flank of the mice. For intraneoplastic treatment, on day 0, reovirus was administrated intratumorally at 1.0 × 107PFUs of live reovirus (experimental group) or UV-inactivated reovirus (control group) in 50 μL PBS when palpable tumors were established. Two-dimensional tumor measurements were performed with calipers every other day for 30 days or until the animals showed severe complications due to excess tumor burden. For systemic treatment, on day 0, SCID/NOD mice bearing Raji tumors at the hind flank were injected intravenously into the tail vein with either 1 × 107 PFUs or 5 × 107 PFUs of live reovirus in 100 μL saline. Tumor size was measured every other day for a period of 20 days.
Histology and immunohistochemistry
Tumors (or remaining masses), taken from animals on day 20 after intravenous reovirus (or saline) injection, were fixed in 10% neutral buffered formalin and embedded in paraffin for histologic analysis. Sections were then immersed in xylene, followed by rehydration in decreasing concentrations of ethanol. For histopathologic examination, the sections were stained with hematoxylin and eosin (H&E). For immunohistochemistry (IH), endogenous peroxidase was inactivated in 3% hydrogen peroxide in methanol for 15 minutes. Sections were then incubated with primary rabbit antireovirus polyclonal antibody (1/1000 in PBS with 10% goat serum and 0.1% Triton X-100) partially purified by ammonium sulfate precipitation. Slides were washed in PBS and then subjected to avidin-biotin–horseradish peroxidase staining as recommended by the manufacturer (Vector, Burlingame, CA) and counterstained in hematoxylin. Cell lines and cell suspensions prepared from ex vivo samples infected for 48 hours with reovirus at an MOI of 20 PFUs per cell were fixed on slides with cytology fixative (Surgipath, Richmond, IL) and subjected to the same immunohistochemistry procedures.
Reovirus infection of primary human tumor samples
Surgical tumor biopsies and normal samples were kept in Dulbecco modified Eagle medium (DMEM) plus 20% FCS and 2 [time] antibiotics (Sigma). NHL and CLL specimens were received either as solid tumor specimens or as peripheral blood, bone marrow, or lymph node samples. Diagnosis was based on histopathology, immunohistochemistry, immunophenotypic studies (flow cytometry), and molecular analysis, all performed at Calgary Laboratory Services (AB, Canada). The cases were classified according to the World Health Organization (WHO) classification.33 Only tissue in excess of that needed for diagnostic purposes was used for the experiments, and all procedures were approved by the Human Ethics Committee at the University of Calgary. When solid tissue was obtained, tumors were washed in sterile PBS, mechanically disrupted with surgical blades, and filtered through a 100-μm Nylon cell strainer to make a single-cell suspension.
For flow cytometry analysis, 100 μL each cell suspension was washed in PBS before the addition of antibody (CD10, CD5, CD20) and 7-aminoactinomycin D (7AAD; Beckman Coulter, Hialeah, FL). Samples were incubated for 15 minutes at room temperature in the dark, washed, and resuspended in PBS. Then, 100 μL Flow Count beads (Beckman Coulter) were added to each tube before flow cytometric analysis on an EPICS XL (Beckman Coulter). Viable cells (7AAD−) were assessed for coexpression of CD20 and either CD5 (CLL samples) or CD10 (Burkitt lymphoma sample) and were counted by means of Flow Count beads.
Reovirus infection of normal lymphocytes and hematopoietic stem/progenitor cells
Normal purified lymphocytes and hematopoietic stem/progenitor CD34+ cells were washed twice in PBS prior to being placed in culture medium. The samples were then either mock-infected (control) or infected with reovirus (at an MOI of 20 PFUs per cell). Single-cell suspensions of lymphocytes and CD34+cells were radiolabeled with [35S]-methionine from 24 to 48 hours after infection and lysed. Whole-cell lysates were then analyzed directly by SDS-PAGE as described above. Infected and uninfected CD34+ cells were also plated in duplicate in methylcellulose colony assays as described previously,29and granulocyte erythroid macrophage megakaryocyte colony-forming units (CFU-GEMMs), granulocyte-macrophage CFUs (CFU-GMs), and erythroid burst-forming units (BFU-Es) were counted in an inverted microscope after 14-day culture at 37°C in 5% CO2.
Results
Various lymphoid cell lines are susceptible to reovirus infection in vitro
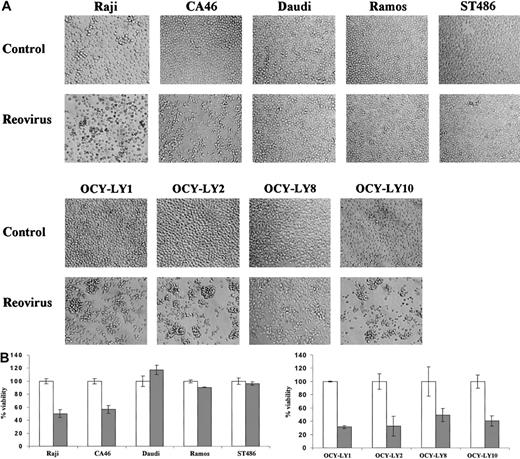
To determine the susceptibility of human lymphoma cell lines to reovirus, the Burkitt lymphoma EBV+ (Raji and Daudi) and EBV− (CA46, Ramos, and ST486) cell lines, as well as 4 diffuse large B-cell NHL cell lines (OCY-LY1, OCY-LY2, OCY-LY8, and OCY-LY10), were infected with reovirus at an MOI of 20 PFUs per cell. As shown in Figure 1A, no morphologic changes were observed in the cell lines Daudi, Ramos, or ST486 at 96 hours after infection. In contrast, the Raji and CA46 cell lines as well as all diffuse large B-cell NHLs infected with reovirus exhibited a granular phenotype, cell clumping, and loss of shape, characteristic of cytopathic effects. To assess whether these observed effects culminated in cell death, trypan blue exclusion was performed on each cell line at 96 hours after infection (Figure 1B). Viability was reduced in the diffuse large B-cell NHLs as well as in the Raji and CA46 cell lines (by 40% to 70%), while no change in viability was detected in the resistant cell lines.
Effect of reovirus on lymphoma cell lines in vitro.
(A) Reovirus-induced cytopathic effects in various lymphoma cell lines in vitro. Established human Burkitt lymphoma (Raji, CA46, Daudi, Ramos, and ST486) and diffuse large B-cell NHL (OCY-LY1, OCY-LY2, OCY-LY8, and OCY-LY10) cell lines were exposed to reovirus at an MOI of 20 PFUs per cell, and photomicrographs were taken at 96 hours after infection. Cytopathic effects are apparent in all diffuse large B-cell NHL as well as the Raji and CA46 cell lines, but not in Daudi, Ramos, or ST486 cells. Original magnification × 200. (B) Remaining viability. Viability of the same cell lines at 96 hours after infection was assessed by staining the cells with 0.25% trypan blue; unstained (viable) cells from 3 independent wells were counted by means of a hemocytometer. As expected, there is good correlation between cell death and manifestation of the cytopathic effects shown in Figure 1A. ■ indicates control; and ░, reovirus.
Effect of reovirus on lymphoma cell lines in vitro.
(A) Reovirus-induced cytopathic effects in various lymphoma cell lines in vitro. Established human Burkitt lymphoma (Raji, CA46, Daudi, Ramos, and ST486) and diffuse large B-cell NHL (OCY-LY1, OCY-LY2, OCY-LY8, and OCY-LY10) cell lines were exposed to reovirus at an MOI of 20 PFUs per cell, and photomicrographs were taken at 96 hours after infection. Cytopathic effects are apparent in all diffuse large B-cell NHL as well as the Raji and CA46 cell lines, but not in Daudi, Ramos, or ST486 cells. Original magnification × 200. (B) Remaining viability. Viability of the same cell lines at 96 hours after infection was assessed by staining the cells with 0.25% trypan blue; unstained (viable) cells from 3 independent wells were counted by means of a hemocytometer. As expected, there is good correlation between cell death and manifestation of the cytopathic effects shown in Figure 1A. ■ indicates control; and ░, reovirus.
To confirm that cell destruction was induced by reovirus replication, the infected cells were labeled with [35S]-methionine, followed by immunoprecipitation with antireovirus antibody for the detection of viral protein synthesis (Figure2A). Viral protein synthesis was evident in all the diffuse large B-cell NHL cell lines as well as in the Raji and CA46 cell lines, but was undetectable in the resistant cell lines (Daudi, Ramos, and ST486), even at 96 hours after infection. To determine whether reovirus protein synthesis in lymphoma cells is associated with progeny virus production, virus yields were determined at 96 hours after infection and compared with virus input at 0 hours (Figure 2B). Following the reovirus infection, susceptible cell lines Raji and CA46, as well as all the diffuse large B-cell lymphoma cell lines, produced a significant amount of progeny virions (15- to 110-fold) at 96 hours (P < .001), while the resistant cell lines showed little or no increase in virus progeny.
Reovirus replication in lymphoma cell lines in vitro.
(A) Reovirus protein synthesis in infected Burkitt lymphoma and diffuse large B-cell NHL cell lines. Established human Burkitt lymphoma (Raji, CA46, Daudi, Ramos, and ST486) and the diffuse large B-cell NHL (OCY-LY1, OCY-LY2, OCY-LY8, and OCY-LY10) cell lines were infected with reovirus. The cells were pulse-labeled with [35S]-methionine for 6 hours at 66 and 90 hours after infection for the Burkitt lymphomas and diffuse large B-cell lymphomas, respectively. Following labeling, the cells were harvested and lysed, and reovirus proteins were immunoprecipitated from part of the lysate by means of rabbit polyclonal antireovirus antibodies. Immunoprecipitates were then analyzed by SDS-PAGE. Reovirus protein synthesis is apparent in all diffuse large B-cell lymphomas as well as in the Raji and CA46 cell lines, but not the Daudi, Ramos, or ST486 cell lines. The 3 size classes of reovirus proteins (λ, μ, and ς) are indicated on the left. (B) Viral progeny. Established Burkitt lymphoma and diffuse large B-cell NHL cell lines were infected with reovirus. At 0 and 96 hours after infection, the cells were harvested and freeze-thawed 3 times, and the virus titer in the lysate was determined by plaque assay on L929 cells. Error bars indicate standard deviations of the means from 3 separate wells.
Reovirus replication in lymphoma cell lines in vitro.
(A) Reovirus protein synthesis in infected Burkitt lymphoma and diffuse large B-cell NHL cell lines. Established human Burkitt lymphoma (Raji, CA46, Daudi, Ramos, and ST486) and the diffuse large B-cell NHL (OCY-LY1, OCY-LY2, OCY-LY8, and OCY-LY10) cell lines were infected with reovirus. The cells were pulse-labeled with [35S]-methionine for 6 hours at 66 and 90 hours after infection for the Burkitt lymphomas and diffuse large B-cell lymphomas, respectively. Following labeling, the cells were harvested and lysed, and reovirus proteins were immunoprecipitated from part of the lysate by means of rabbit polyclonal antireovirus antibodies. Immunoprecipitates were then analyzed by SDS-PAGE. Reovirus protein synthesis is apparent in all diffuse large B-cell lymphomas as well as in the Raji and CA46 cell lines, but not the Daudi, Ramos, or ST486 cell lines. The 3 size classes of reovirus proteins (λ, μ, and ς) are indicated on the left. (B) Viral progeny. Established Burkitt lymphoma and diffuse large B-cell NHL cell lines were infected with reovirus. At 0 and 96 hours after infection, the cells were harvested and freeze-thawed 3 times, and the virus titer in the lysate was determined by plaque assay on L929 cells. Error bars indicate standard deviations of the means from 3 separate wells.
Regression of Raji tumors in mice treated with reovirus
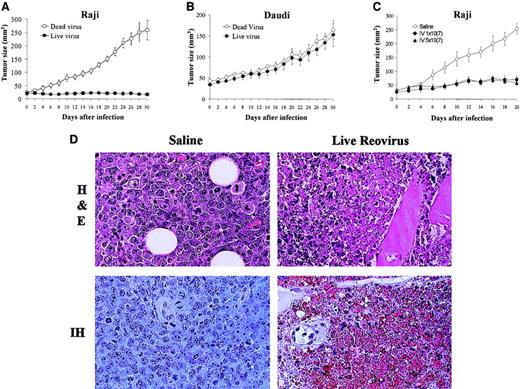
To assess whether the in vitro susceptibility to reovirus in the established lymphoma cell lines correlated with in vivo efficacy of reovirus in a xenograft model, we evaluated the antitumor effects of a single intratumoral reovirus injection on subcutaneous lymphoma tumors. We compared Raji cells that had shown permissiveness to in vitro reovirus infection with Daudi cells that were resistant. The results (Figure 3A) show that in live virus–treated mice, the Raji-derived tumor growth was significantly inhibited compared with control mice treated with UV-inactivated virus. In contrast, the Daudi tumors were completely resistant to reovirus therapy (Figure 3B), which was consistent with what was observed in vitro.
Effect of reovirus on lymphoid tumors in SCID/NOD mice.
(A-B) Intratumoral reovirus therapy of lymphoid tumors in SCID/NOD mice. SCID/NOD mice were subcutaneously implanted with 1 × 107 cells of either Raji (A) or Daudi (B) Burkitt lymphoma cell lines. Following the establishment of a palpable mass, the tumors received (on day 0) a single intratumoral injection of 1 × 107 PFUs of live reovirus (n = 8; ●) or UV-inactivated virus (n = 7; ○). Tumor growth was followed for a period of 30 days and measured 2-dimensionally with a caliper. (C) Intravenous administration of reovirus to SCID/NOD mice bearing Raji tumors. Animals bearing palpable subcutaneous Raji tumors were subjected (on day 0) to a single intravenous injection of 1 × 107 PFUs (n = 7) or 5 × 107 PFUs (n = 7) of reovirus (● and ▴, respectively). Control animals (n = 7) received saline injection (○). Tumor growth was followed for a period of 20 days and measured 2-dimensionally with a caliper. (D) H&E staining and IH of reovirus antigens in Raji tumors after intravenous reovirus treatment. H&E-stained section (original magnification × 400) shows necrosis of tumor cells 20 days after live reovirus treatment. IH-stained section (original magnification × 400) of remaining tumor cells stains positively for reovirus proteins (brown) while control tumor shows no staining.
Effect of reovirus on lymphoid tumors in SCID/NOD mice.
(A-B) Intratumoral reovirus therapy of lymphoid tumors in SCID/NOD mice. SCID/NOD mice were subcutaneously implanted with 1 × 107 cells of either Raji (A) or Daudi (B) Burkitt lymphoma cell lines. Following the establishment of a palpable mass, the tumors received (on day 0) a single intratumoral injection of 1 × 107 PFUs of live reovirus (n = 8; ●) or UV-inactivated virus (n = 7; ○). Tumor growth was followed for a period of 30 days and measured 2-dimensionally with a caliper. (C) Intravenous administration of reovirus to SCID/NOD mice bearing Raji tumors. Animals bearing palpable subcutaneous Raji tumors were subjected (on day 0) to a single intravenous injection of 1 × 107 PFUs (n = 7) or 5 × 107 PFUs (n = 7) of reovirus (● and ▴, respectively). Control animals (n = 7) received saline injection (○). Tumor growth was followed for a period of 20 days and measured 2-dimensionally with a caliper. (D) H&E staining and IH of reovirus antigens in Raji tumors after intravenous reovirus treatment. H&E-stained section (original magnification × 400) shows necrosis of tumor cells 20 days after live reovirus treatment. IH-stained section (original magnification × 400) of remaining tumor cells stains positively for reovirus proteins (brown) while control tumor shows no staining.
To determine if reovirus treatment is effective against tumors distal to the site of injection, Raji tumors were grown on the hind flank of SCID/NOD mice, and a single intravenous injection of reovirus of either 1 × 107 PFUs or 5 × 107 PFUs was administered to the mice in the tail vein. Both virus concentrations produced significant inhibition of tumor growth (Figure 3C) in comparison with saline-injected (control) animals. To confirm cell death and active viral replication in the tumor mass, H&E and immunohistochemical staining with antireovirus antibody were performed on paraffin-embedded sections taken on day 20 after injection (Figure 3D). H&E staining confirmed the killing of tumor cells from live virus–treated tumors, and IH staining with antireovirus antibody demonstrated active viral replication in the residual tumor cells.
Reovirus oncolysis of various primary lymphoma cells
The next step of our investigation was to determine whether reovirus oncolysis could also occur in primary samples of human lymphoid malignancy specimens. Single-cell suspensions were prepared from tissues, peripheral blood, bone marrow, or lymph node samples. They were then infected with reovirus and labeled with [35S]-methionine. At 48 hours after infection, cells were harvested, and lysates were prepared, immunoprecipitated with antireovirus antibody, and analyzed by SDS-PAGE. Since all our samples were heterogeneous, containing both neoplastic and reactive cells, it was important to determine whether reovirus protein synthesis occurred only in malignant cells. Accordingly, 3 samples of normal lymphocytes isolated from the peripheral blood, as well as 3 samples of hematopoietic stem/progenitor cells (CD34+ cells) isolated from the bone marrow of healthy individuals, were used as control. Representative data are shown in Figure4A. None of the normal controls showed permissiveness for reovirus replication, as evidenced by the lack of viral protein synthesis in these cells. In contrast, reovirus proteins (in 3 size groups, λ, μ, and ς) were easily detected in some, but not all, lymphomas (Figure 4A; Table 1).
Effect of reovirus on primary human lymphoid neoplasms.
CLL indicates chronic lymphocytic leukemia; DLCBL, diffuse large B-cell lymphoma; BL, Burkitt lymphoma; and PBL, peripheral blood lymphocytes from healthy donors. (A) Reovirus replication in normal cells (lymphocytes, CD34+ cells) and primary human lymphoid neoplasms. Normal lymphocytes, normal bone marrow stem cells, and cell suspensions from primary lymphoma tumors were infected with reovirus. The cells were labeled with [35S]-methionine from 24 to 48 hours after infection. The cells were then lysed, immunoprecipitated with antireovirus antibody, and analyzed by SDS-PAGE. (B) Flow cytometry analysis. Representative histograms of viable (7AAD−) CLL cells or Burkitt lymphoma cells after infection with 20 MOI of reovirus and staining with CD5-phycoerythrin (PE) and CD20–fluorescein isothiocyanate (FITC) (for CLL), or CD10-PE and CD20-FITC (for Burkitt lymphoma) are shown on the left. CLL cells coexpressing CD5 and CD20 were counted by means of Flow Count beads, and the results for 5 CLL samples are shown on the right. Burkitt lymphoma cells coexpressing CD10 and CD20 were counted similarly, and the results are shown. (C) Immunohistochemistry (IH) of reovirus antigens in virus-treated Raji and CLL cells. Cells were prepared and infected with reovirus as described in “Materials and methods.” Positive (brown) staining is observed in both Raji and CLL cells infected with reovirus, but not in the controls.
Effect of reovirus on primary human lymphoid neoplasms.
CLL indicates chronic lymphocytic leukemia; DLCBL, diffuse large B-cell lymphoma; BL, Burkitt lymphoma; and PBL, peripheral blood lymphocytes from healthy donors. (A) Reovirus replication in normal cells (lymphocytes, CD34+ cells) and primary human lymphoid neoplasms. Normal lymphocytes, normal bone marrow stem cells, and cell suspensions from primary lymphoma tumors were infected with reovirus. The cells were labeled with [35S]-methionine from 24 to 48 hours after infection. The cells were then lysed, immunoprecipitated with antireovirus antibody, and analyzed by SDS-PAGE. (B) Flow cytometry analysis. Representative histograms of viable (7AAD−) CLL cells or Burkitt lymphoma cells after infection with 20 MOI of reovirus and staining with CD5-phycoerythrin (PE) and CD20–fluorescein isothiocyanate (FITC) (for CLL), or CD10-PE and CD20-FITC (for Burkitt lymphoma) are shown on the left. CLL cells coexpressing CD5 and CD20 were counted by means of Flow Count beads, and the results for 5 CLL samples are shown on the right. Burkitt lymphoma cells coexpressing CD10 and CD20 were counted similarly, and the results are shown. (C) Immunohistochemistry (IH) of reovirus antigens in virus-treated Raji and CLL cells. Cells were prepared and infected with reovirus as described in “Materials and methods.” Positive (brown) staining is observed in both Raji and CLL cells infected with reovirus, but not in the controls.
Susceptibility to reovirus infection of ex vivo specimens of human non-Hodgkin lymphomas and chronic lymphocytic leukemias
| Diagnosis . | Lymphoma type . | Reovirus-susceptible samples, no. . | Reovirus-resistant samples, no. . |
|---|---|---|---|
| CLL | 15 | 0 | |
| NHL | Mantle cell | 1 | 0 |
| NHL | Burkitt lymphoma | 1 | 0 |
| NHL | Diffuse large B-cell | 2 | 0 |
| NHL | Small lymphocytic | 1 | 1 |
| NHL | Follicular, grade I | 1 | 0 |
| NHL | Follicular, grade II | 0 | 4 |
| NHL | Follicular, gade III | 0 | 1 |
| Diagnosis . | Lymphoma type . | Reovirus-susceptible samples, no. . | Reovirus-resistant samples, no. . |
|---|---|---|---|
| CLL | 15 | 0 | |
| NHL | Mantle cell | 1 | 0 |
| NHL | Burkitt lymphoma | 1 | 0 |
| NHL | Diffuse large B-cell | 2 | 0 |
| NHL | Small lymphocytic | 1 | 1 |
| NHL | Follicular, grade I | 1 | 0 |
| NHL | Follicular, grade II | 0 | 4 |
| NHL | Follicular, gade III | 0 | 1 |
To determine reovirus oncolysis of CLL cells, as well as a primary Burkitt lymphoma, the samples were infected with reovirus for 96 hours, and remaining cells were analyzed by flow cytometry for viability and cell surface marker profiles. Significant reduction in CD20+ and CD5+ CLL cells and CD20+and CD10+ Burkitt lymphoma cells was observed (Figure 4B). The use of cell surface markers confirmed that only the CLL cells or Burkitt lymphoma cells present in the samples were susceptible to reovirus infection. The presence of reovirus proteins was also assessed by immunohistochemistry; Figure 4C shows a representative primary CLL sample and Raji cells used as a positive control. This method could prove to be useful for the identification of reovirus-susceptible cells in a heterogeneous sample.
All together, we tested 27 primary B-cell type NHLs and CLLs (Table 1). Our results show that all B-cell CLL samples were susceptible to reovirus infection, whereas 5 of 6 follicular NHL samples were resistant. One of the 2 small lymphocytic NHLs was susceptible, as was 1 mantle cell NHL, a Burkitt lymphoma, and 2 diffuse large B-cell NHL samples tested.
Reovirus does not significantly affect clonogenic progenitors
To further determine whether normal human hematopoietic progenitors are susceptible to reovirus infection, CD34+cells treated with reovirus at an MOI of 20 PFUs per cell (or mock-treated) were plated in the methylcellulose clonogenic assay. The numbers of clonogenic CFU-GM, BFU-Es, and CFU-GEMM progenitors indicate that a significant proportion of human progenitors survived exposure to reovirus (Table 2).
Susceptibility of clonogenic CD34+ cells to reovirus infection
| Cells . | Control . | Infected . | P . |
|---|---|---|---|
| CFU-GM | 71.5 ± 15.5 | 56.0 ± 3.5 | .0989 |
| BFU-Es | 37.2 ± 20.8 | 26.8 ± 0.4 | .3560 |
| CFU-GEMM | 13.8 ± 2.5 | 12.8 ± 2.5 | .3390 |
| Cells . | Control . | Infected . | P . |
|---|---|---|---|
| CFU-GM | 71.5 ± 15.5 | 56.0 ± 3.5 | .0989 |
| BFU-Es | 37.2 ± 20.8 | 26.8 ± 0.4 | .3560 |
| CFU-GEMM | 13.8 ± 2.5 | 12.8 ± 2.5 | .3390 |
As described in “Materials and methods,” 1000 CD34+ cells per milliliter for CFU-GMs and BFU-Es, and 2000 CD34 cells per milliliter for CFU-GEMMs, were plated in methylcellulose clonogenic assays in duplicate. Data represent the means ± standard deviation (SD) of 2 separate experiments.
Discussion
Recently acquired knowledge of the pathobiology of lymphomas has significantly broadened our understanding of these diseases, providing new opportunities for future therapeutic approaches. Novel treatment methods include anti-CD20 (Rituximab) monoclonal antibody,34,35 radioimmunotherapy,36,37antitumor vaccines (developed by isolation of the specific idiotypic immunoglobin molecules from individual patients),38 gene therapy,39 and possibly oncolytic viruses.40As current forms of treatment involve considerable toxicities and increased risk of death due to incomplete responses and relapses after treatment,41 42 the development of new therapies is crucial for improving the survival and quality of life of patients with lymphomas.
Treatment of lymphoid malignancies with reovirus has the potential to be a safe and effective alternative to radiotherapy and/or multidrug chemotherapy. Reoviruses are common isolates of the respiratory and gastrointestinal (GI) tract of humans. They may cause mild upper respiratory and gastrointestinal illnesses in early childhood, but are not clearly associated with any known human diseases and are, therefore, considered nonpathogenic. Associations of reovirus with putative chronic hepatic infections or other diseases in adults have been reported, but none of these convincingly demonstrate that reovirus causes diseases in humans, especially in immunocompetent adults.9
Here we show for the first time that reovirus could represent a potent oncolytic agent that can eradicate some forms of human lymphoid malignancies. In our in vitro experiments, all diffuse large B-cell lymphoma cell lines examined supported reovirus replication, which resulted in subsequent lymphoma cell death. Cell lysis was also observed in the Burkitt lymphoma cell lines Raji and CA46. However, other lymphoma lines, such as Daudi, Ramos, and ST486, were resistant to reovirus infection. Interestingly, a comparable pattern of infectability and resistance to oncolytic herpes simplex virus-1 (HSV-1) is also observed in these cell lines (T.A., P.W.K.L., unpublished observations, 2002). Since oncogenes in the Ras-signaling pathway dictate host-cell permissiveness for both reovirus and HSV-1,43 differences in the activation of this pathway must exist between these cell lines. The resistance in Burkitt lymphoma cells may prove important in the attempt to characterize the molecular basis of reovirus (and HSV-1) oncolysis, and is currently under investigation.
In our in vivo experiments, Raji (but not Daudi) tumor xenografts grown in SCID/NOD mice underwent regression upon treatment with live reovirus. This was not observed when UV-inactivated reovirus was used, suggesting that virus replication is important for tumor regression. We have also obtained evidence that reovirus is effective in killing tumors that are remote from the injection site using intravenous injections of reovirus into SCID/NOD mice bearing subcutaneous Raji tumors. This raises the possibility of using reovirus to target malignant lymphomas that are disseminated at the time of diagnosis.
Our investigations of ex vivo primary tumor cells derived from different lymphoproliferative disorders revealed surprising results. Reovirus susceptibility was evident in all B-cell CLL (15 of 15) samples tested while 5 of 6 follicular lymphomas remained resistant to reovirus infection. In addition, 1 of 2 small lymphocytic lymphomas was also resistant, whereas a mantle cell NHL, a Burkitt lymphoma, and 2 diffuse large B-cell NHLs showed reovirus protein expression after in vitro infection. As mutations of ras rarely occur in lymphomas, differences in the activation of the Ras pathway must exist between the diverse types of lymphoid malignancies. In CLL, trisomy of chromosome 12 (where the K-ras proto-oncogene, as well as the gene encoding for the insulinlike growth factor receptor 1, is located) is a common alteration in this disease.19,44,45 Amplification or mutation of crucial genes, resulting from this cytogenetic abnormality, may result in the activation of Ras (or the Ras-signaling pathway) in CLLs and confer a favorable environment for reovirus to replicate. Furthermore, the Ras pathway in CLL cells may be stimulated in an autocrine manner by expression of both the CD40 receptor and its ligand CD154 involved in B-cell proliferation and survival.25,26 Susceptibility of Burkitt lymphomas and some types of NHLs may be due to the presence of the Epstein-Barr virus LMP-1 transforming protein that has been shown to mimic or cooperate with CD40 and could lead to activation of the Ras pathway.24,46,47 Moreover, Myc deregulation, frequently observed in lymphoid disorders, has been shown to collaborate with Ras in the transformation of B lymphocytes.23 Myc overexpression is likely to be involved in the reovirus susceptibility of lymphomas.
It is interesting that 5 specimens of FLs remained resistant to reovirus infection. FLs are slow-growing tumors characterized by the presence of the t(14;18) translocation, which is present in 95% of the cases, and results in the amplification of the antiapoptosis gene bcl-2.17 The Ras-signaling pathway in these cells may not yet be activated at this stage of the disease. However, FLs can progress to DLBCL characterized by more aggressive behavior.33 It is likely that in DLBCL the Ras-signaling pathway is activated. Our observation that the 4 DLBCL cell lines tested and 2 ex vivo DLBCL samples are all permissive to reovirus is congruent with this notion. A recently isolated proto-oncogene, RhoH/TTF, encoding a novel Rho GTP-binding protein exclusively expressed in hematopoietic tissues, was reported to be aberrant in 46% of DLBCLs.27 Activation of the Ras pathway by RhoH/TTF could lead to susceptibility to reovirus infection. Nonetheless, the involvement of this gene in reovirus oncolysis of DLBCL as well as its status in other lymphoid malignancies needs to be investigated further.
Our study included cell lines derived from aggressive B-cell lymphomas while the majority of ex vivo specimens were obtained from patients with CLL. The reason for this imbalance is that cell lines are usually derived from aggressive lymphomas, whereas ex vivo samples from indolent lymphoid malignancies can easily be obtained from peripheral blood. Samples derived from human tumors, peripheral blood, and bone marrow are composed of a mixture of neoplastic and nonneoplastic cells, and it is uncertain which cells in these samples are infected by reovirus. Our observation that normal peripheral blood lymphocytes and hematopoietic stem/progenitor cells derived from normal bone marrow are resistant to reovirus further suggests that only neoplastic lymphoid cells are targets for this virus. Analysis of 5 CLL samples and a Burkitt lymphoma sample by flow cytometry demonstrated that the neoplastic cells, but not nonneoplastic lymphocytes, are affected by reovirus infections. These findings are congruent with the notion that reovirus oncolysis is restricted to neoplastic cells. The reason for the variability between the CLL samples in susceptibility to reovirus is unclear at present and will be investigated further. Moreover, given our observation that multipotential hematopoietic progenitor cells were not killed after exposure to reovirus and remained clonogenic, the differential susceptibility of neoplastic versus normal cells should be exploited further. For example, in vitro purging protocols could be developed for the treatment of certain types of NHL and/or CLL.
It was reported recently that an antilymphoma effect was also observed with the use of live attenuated measles virus.40Interestingly, regression of human lymphoma xenografts in immunodeficient mice induced by measles virus infection was detected in the Raji cell line, the same line that we used for our animal studies. It would be important to evaluate whether measles virus injected into the same types of lymphoid malignancies and cell lines as used in this study exhibits a pattern of infectability similar to that detected with reovirus. This could further confirm the involvement of oncogenes in Ras signaling in dictating the permissiveness of host cells to infection by oncolytic viruses. Finally, we suggest that the methods used in this study to determine the infectability of primary lymphoid malignancies by reovirus could be used for preclinical screening for a potential reovirus therapy. Further studies are needed to evaluate the efficacy of this potential treatment and to elucidate the molecular mechanisms underlying reovirus oncolysis of lymphoid malignancies.
The authors are grateful to Dr Hans Messner and Nazir Jamal (Ontario Cancer Institute, Toronto, Canada) for providing the diffuse large B-cell lymphoma cell lines (OCY-LY1, OCY-LY2, OCY-LY8, and OCY-LY10) and to Leanne Fong for performing immunohistochemistry.
Prepublished online as Blood First Edition Paper, July 25, 2002; DOI 10.1182/blood-2002-02-0503.
Supported by grants to A.E.K. from the Alberta Cancer Board (no. R1-170) and Leukemia Research Fund of Canada (The United Food and Commercial Workers Union Award).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anna E. Kossakowska, Dept of Pathology, Foothills Hospital, 1403 29th St NW, Calgary, AB, Canada T2N 2T9; e-mail: anna.kossakowska@cls.ab.ca.

![Fig. 2. Reovirus replication in lymphoma cell lines in vitro. / (A) Reovirus protein synthesis in infected Burkitt lymphoma and diffuse large B-cell NHL cell lines. Established human Burkitt lymphoma (Raji, CA46, Daudi, Ramos, and ST486) and the diffuse large B-cell NHL (OCY-LY1, OCY-LY2, OCY-LY8, and OCY-LY10) cell lines were infected with reovirus. The cells were pulse-labeled with [35S]-methionine for 6 hours at 66 and 90 hours after infection for the Burkitt lymphomas and diffuse large B-cell lymphomas, respectively. Following labeling, the cells were harvested and lysed, and reovirus proteins were immunoprecipitated from part of the lysate by means of rabbit polyclonal antireovirus antibodies. Immunoprecipitates were then analyzed by SDS-PAGE. Reovirus protein synthesis is apparent in all diffuse large B-cell lymphomas as well as in the Raji and CA46 cell lines, but not the Daudi, Ramos, or ST486 cell lines. The 3 size classes of reovirus proteins (λ, μ, and ς) are indicated on the left. (B) Viral progeny. Established Burkitt lymphoma and diffuse large B-cell NHL cell lines were infected with reovirus. At 0 and 96 hours after infection, the cells were harvested and freeze-thawed 3 times, and the virus titer in the lysate was determined by plaque assay on L929 cells. Error bars indicate standard deviations of the means from 3 separate wells.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-02-0503/5/m_h82323490002.jpeg?Expires=1769095500&Signature=0AnVd5ipuuvEgS-zinY0aol7IuRwtdUZTwe8uVH9lH~TBk2iH9nXoA0v9H3YSOXc11SnYT2LNyVsWeiKWdudaeXwdOxr9oIX7PhDRaH6jUKh75gCuMKHZftfqEI2a0kjY14EVW0k0ja-4GF8m4HpnEgXCbisARRFES7C-ifdvWemOT8e7gYC6xgvtUyJwyTZDO1zwecBi0H7wzk1lgSF1zKMU86adDIbijYrKfAGWz~hajFOOB41T-P9hSdqT2FA0l3D-LyjEjrJkGXhz5CmRyCb-~6z~v0Ss8-cqAj3VUGCRX~YQ-6dox-Y31DcHRtvsCf9VnxrxRKTpNWokqyTuw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. Effect of reovirus on primary human lymphoid neoplasms. / CLL indicates chronic lymphocytic leukemia; DLCBL, diffuse large B-cell lymphoma; BL, Burkitt lymphoma; and PBL, peripheral blood lymphocytes from healthy donors. (A) Reovirus replication in normal cells (lymphocytes, CD34+ cells) and primary human lymphoid neoplasms. Normal lymphocytes, normal bone marrow stem cells, and cell suspensions from primary lymphoma tumors were infected with reovirus. The cells were labeled with [35S]-methionine from 24 to 48 hours after infection. The cells were then lysed, immunoprecipitated with antireovirus antibody, and analyzed by SDS-PAGE. (B) Flow cytometry analysis. Representative histograms of viable (7AAD−) CLL cells or Burkitt lymphoma cells after infection with 20 MOI of reovirus and staining with CD5-phycoerythrin (PE) and CD20–fluorescein isothiocyanate (FITC) (for CLL), or CD10-PE and CD20-FITC (for Burkitt lymphoma) are shown on the left. CLL cells coexpressing CD5 and CD20 were counted by means of Flow Count beads, and the results for 5 CLL samples are shown on the right. Burkitt lymphoma cells coexpressing CD10 and CD20 were counted similarly, and the results are shown. (C) Immunohistochemistry (IH) of reovirus antigens in virus-treated Raji and CLL cells. Cells were prepared and infected with reovirus as described in “Materials and methods.” Positive (brown) staining is observed in both Raji and CLL cells infected with reovirus, but not in the controls.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/12/10.1182_blood-2002-02-0503/5/m_h82323490004.jpeg?Expires=1769095500&Signature=QVjgeOpD5DtL8ivre7GV8JEUrdvZ9VuBocqo0JUFrjq6mUvAwU~SJ2yF9vn~05lPR~3VrU1AziDlIx8BTFj58l81IiGAvB4zdVrvzSMpTNP~eq94k1ZP9sRwAtadqe20FqArcdgwd~fduAWYP4mvopfn-CF0KRe2IGZTo-iAjkpEBjo8oY0KzG-A8RBLIxF9X3q5L-Elg5R3tXX0dsEgtvTMcKZ6il8fHzJ4XbtqmCaYCNSC5SvobnZG5wjmuDB-d7eatCVk1Hntwz~QhqzB9Pr47wQDpAofae1EeC9HBJfGhtfI3UQUFRdXcjp12CjG2FLLN~QedmEK6CFuyrl~6A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal