Little is known about the distribution in normal cells of CLIP-170, a linkage mediator between endocytic vesicles and microtubules, and restin, a splice variant encoded by the same gene and marker for Hodgkin and Reed-Sternberg (HRS) cells of Hodgkin disease. Although only trace amounts of CLIP-170/restin are present in peripheral blood mononuclear cell subpopulations, monocyte-derived dendritic cells (DCs) and interleukin-4 (IL-4) + CD40L-activated B cells express high levels of CLIP-170/restin. CLIP-170/restin colocalizes preferentially with membranes of intermediate macropinocytic vesicles, suggesting a new function of CLIP-170/restin in the trafficking of macropinosomes to the cytoskeleton, which is a crucial step in antigen presentation. The strong expression of CLIP-170/restin in HRS cells, DCs, and activated B cells underscores their functional similarities supporting a function-based concept of HRS cells as professional antigen-presenting cells.
Introduction
Dendritic cells (DCs) are a unique class of leukocytes.1,2 Their primary function is to capture and process antigens for presentation to T cells, a task at which they are superior to other antigen-presenting cells (APCs).3 For antigen uptake DCs are equipped with specialized endocytic receptors, engulfment mechanisms, and processing compartments.4,5 The high rate of antigen uptake reflects the high macropinocytic activity of these cells. Macropinocytosis is a fluid-phase endocytotic process for the engulfment of solutes as well as small molecular compounds. Although endocytosis through cell surface receptors uses primarily clathrin-mediated systems, macropinosomes are a distinct class of non–clathrin-coated endocytic vesicles that function independently of receptors. Macropinocytosis occurs constitutively in all cells at low levels, but it is strongly up-regulated in certain cell types after appropriate stimulation (eg, with growth factors or phorbol esters). Macropinosomes are heterogeneous in size and originate from membrane ruffles at the cell margins by folding back on themselves. The fate of endocytic vesicles has been intensively studied. Although a proportion of solute is rapidly returned to the cell surface, another significant proportion is delivered to the lysosomal compartment after passing through early and late endosomes, thus intermingling with the major histocompatibility complex (MHC) class II–enriched compartment (MIIC) to load MHC II molecules.6,7 The classical tubular lysosome represents the terminal compartment of the endocytic pathway. Recent data suggest that macropinocytosis driven by constitutive membrane ruffling activity of murine bone marrow–derived dendritic cells also leads to the release of endocytosed material into the cytosol, thus mediating the presentation of exogenous antigens on MHC I molecules.8 9 Ultrastructural details of the mechanisms involved in this process are still under investigation. Although the initial phase of macropinocytosis is actin dependent, the subsequent transport of vesicles is associated with microtubules. However, it is still unknown how the attachment of macropinosomes to cytoskeletal structures is accomplished.
One of the best-studied proteins involved in the binding of endocytic vesicles to the cytoskeleton is CLIP-170. CLIP-170 was originally isolated from HeLa cells by Rickard and Kreis10,11 and was shown to be a cytoplasmic linker protein mediating the interaction of endosomal intermediates, which traffic from early endosomes to late endosomes/lysosomes and bind to microtubules.12 13Although the linking of endosomes to microtubules has been demonstrated by in vitro and in vivo studies, colocalization of endosomes with CLIP-170 has not been convincingly demonstrated thus far.
Independent of the discovery of CLIP-170, restin (Reed-Sternberg intermediate filament-associated protein), which is derived from a splice variant of the same gene, was cloned from the cell line U937 and was shown by immunohistologic studies to be highly expressed in Hodgkin lymphoma–derived cell lines, in Hodgkin and Reed-Sternberg (HRS) cells of Hodgkin disease of different histologic subtypes, and in large-cell anaplastic lymphoma.14-16 Significant expression in normal cells was not described. Restin differs from CLIP-170 merely by the insertion of a 35-amino acid stretch located in the center of the molecule and is encoded by a differentially spliced exon flanked by large introns. We came across CLIP-170/restin when we identified it as an autoantigen expressed by HRS cells during our analysis of the autologous humoral immune response of patients with Hodgkin disease against their tumor by SEREX, the serological analysis of antigens by recombinant expression cloning.17 The more detailed analysis of this study reveals that DCs are the only subpopulation of fresh cells in the peripheral blood with a significant CLIP-170/restin expression, but CLIP-170/restin is up-regulated in the prime professional APCs—that is, DCs and activated B cells. CLIP-170/restin colocalizes in particular with late endosomes during the process of macropinocytosis. The observation that similar and extraordinary amounts of protein are found in APCs and in HRS cells, for which the CLIP-170 variant restin had originally been described as a specific marker, is consistent with other findings that demonstrate phenotypic and functional similarities between HRS cells and professional APCs.
Materials and methods
The study was approved by the local ethical review board (Ethikkommission der Ärztekammer des Saarlandes). Recombinant DNA work was done with the official permission and according to the rules of the State Government of Saarland. Informed consent was obtained from healthy volunteers for blood donations.
Cell culture
Hodgkin cell lines L540, L428,18HDLM-2,19 and KMH-220 and the anaplastic large cell line Karpas 299 21 have been described previously. A Chinese hamster ovary (CHO) cell line transformed with a nearly full-length restin cDNA14 was kindly provided by Dr Juergen Brueggen. All cell lines were maintained in RPMI 1640 medium (HyClone, Cramlington, United Kingdom) supplemented with 2 mM L-glutamine, 1% pyruvate, streptomycin/penicillin, and 10% fetal calf serum (FCS; PAA Laboratories, Linz, Austria). Monocyte-derived DCs were maintained under serum-free conditions in AIM V medium (Gibco, Karlsruhe, Germany). Cells were grown in a 37°C incubator containing a 95% air/5% CO2 atmosphere.
Antibodies and antisera
The monoclonal antibodies 4D3 (IgG) and 2D6 (IgM) have been described previously11 and were kindly provided by Dr Thomas Kreis. The epitope of 4D3 was mapped to the region between amino acids 965 and 1050, whereas 2D6 recognizes the C-terminal part (amino acid 1238 to the end) of CLIP-170. Secondary antibodies were purchased from Sigma (Munich, Germany). Anti-CD3, anti-CD11c, anti-CD14, anti-CD16, and anti-CD19 were obtained from Behring (Liederbach, Germany), anti-CD40 was purchased from PharMingen (Heidelberg, Germany), and anti-CD1a and anti-CD83 were purchased from Immunotech (Hamburg, Germany). Polyclonal anti-restin serum was obtained by immunization of rabbits with His-tagged recombinant protein. The His-tag protein, representing the N-terminal 662 amino acid fragment of restin, was expressed and purified using the pQE system (Qiagen, Hilden, Germany) according to the manufacturer's instructions.
Separation of cells by magnetic cell sorting
For the enrichment of different subpopulations, low-density peripheral mononuclear cells were labeled with antibodies coupled to iron beads (Miltenyi, Bergisch Gladbach, Germany) and were separated by a magnetic cell sorting system (MACS; Miltenyi). CD3+ T lymphocytes and CD14+ monocytes were separated by single-step enrichment with anti-CD3 or anti-CD14 antibodies, respectively. For B or natural killer (NK) cell enrichment, peripheral blood mononuclear cells (PBMCs) were depleted of T lymphocytes and monocytes with anti-CD3 and anti-CD14 before positive selection with anti-CD19 or anti-CD16 beads. Blood dendritic cells were obtained by negative sorting with anti-CD3, anti-CD14, anti-CD16, and anti-CD19 antibodies followed by enrichment with anti-CD83 (Dianova, Hamburg, Germany). All fractions obtained were assessed by subsequent flow-cytometric analysis. Flow cytometry was used to analyze the purity of cells separated by magnetic cell sorting and purified subpopulations before and after stimulation (see below). Cells were directly stained with phycoerythrin (PE)– or fluorescein isothiocyanate (FITC)–labeled monoclonal mouse antibodies for different markers or by a combination of marker-specific monoclonal antibodies with an affinity-purified, isotype-specific, labeled secondary goat antimouse antibody. Samples were analyzed on a FACScan (Becton Dickinson, Heidelberg, Germany). Exclusion of dead cells was ensured by forward/side light scatter analysis and by propidium iodide staining.
Generation of monocyte-derived dendritic cells
Dendritic cells were generated as previously described.22 Low-density PBMCs were obtained from heparinized blood of healthy adult volunteers by density-gradient centrifugation according to the standard Ficoll-Hypaque method (Pharmacia, Uppsala, Sweden). Then 5 × 106 cells/mL were incubated for 45 minutes in 6-well culture dishes (Nunc, Wiesbaden, Germany) in AIM V (Gibco). Supernatants and nonadherent cells were discarded carefully, and adherent cells were cultured with 1000 U/mL interleukin-4 (IL-4) (Genzyme, Rüsselsheim, Germany) and 1000 U/mL granulocyte macrophage–colony-stimulating factor (GM-CSF; Genzyme) for 7 days. During the time-course of cytokine-mediated differentiation from monocytes to dendritic cells, samples were taken at different time points and were frozen in RPMI 1640/10% fetal calf serum (FCS) with 10% dimethyl sulfoxide (DMSO) for subsequent Western blot analysis. Maturation of DCs was induced on day 6 by supplementation with 1000 U/mL tumor necrosis factor-α (TNF-α) and 10 000 U/mL IL-1β or alternatively by CD40 cross-linking for at least 24 hours by culturing on NIH-3T3 cells stably transfected with CD40 ligand.
Stimulation of T and B cells
After magnetic cell sorting (see above), purified T cells (5 × 106 cells/mL) were incubated for 24 hours in 24-well plates that had been coated with monoclonal anti-CD3 and anti-CD28 antibodies in medium containing 50 U/mL IL-2 (Roche, Grenzach, Germany). Stimulated T cells were then transferred to uncoated wells, and half the IL-2–containing culture medium was replaced every 2 days. B cells (5 × 106 cells/mL) were stimulated by incubation in culture medium supplemented with IL-4 (500 U/mL; Biomol, Hamburg, Germany) and CD40L (100 ng/mL; Biomol), with half the supplemented medium replaced every 2 days. Samples of the unstimulated and stimulated T and B cells were taken every 2 days during the 10-day cultures and were centrifuged, and the cell pellets were stored at −80°C until analysis by Western blot or reverse transcription–polymerase chain reaction (RT-PCR), respectively.
Western blot analysis
Affinity-purified rabbit antiserum and monoclonal antibodies were used to detect CLIP-170/restin protein expression in cell lysates. Samples of 20 μg cell lysate were mixed with 2× sodium dodecyl sulfate (SDS) buffer (0.1 M Tris-HCl, pH 6.8, 0.2 M dithiothreitol, 4% SDS, 0.2% bromophenol blue, and 20% glycerol) together with protease inhibitors (20 mM benzamidine HCl, 100 mM ε-aminocapronic acid, 40 mM iodoacetamide, 200 mg/100 mL leupeptin, 0.1 μg/mL aprotinin, 10 μg/mL soybean trypsin inhibitor, 2 mM phenylmethylsulfonyl fluoride [PMSF]) electrophoresed in 10% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and then blotted onto nylon membranes (Schleicher & Schuell, Dassel, Germany) by semidry transfer (Bio-Rad Laboratories, Munich, Germany). After blocking with 5% low-fat milk in Tris-buffered saline (TBS) for 1 hour, the membranes were incubated with 1:500 diluted rabbit antirestin serum and for 1 hour with alkaline phosphatase–conjugated mouse anti–rabbit IgG (Dianova). After each incubation step the membranes were washed extensively in TBS and 0.01% Tween 20. Visualization of positive reactions was performed by staining with 5-bromo-4-chloro-3-indolyl phosphate (BCIP) and nitroblue tetrazolium (NBT) according to the manufacturer's instructions (Bio-Rad).
RT-PCR analysis of CLIP-170/restin expression
To distinguish between the 2 splice variants, a PCR assay was established for the detection of restin and CLIP-170 transcripts that takes advantage of the fact that restin contains a 105-bp 35–amino acid insertion in its rod domain, which is absent from CLIP-170. Total cellular RNA was extracted from bulk PBMCs, cultured monocytes, and DCs as described above. cDNA synthesis was performed in a total volume of 20 μL using 10 μg total RNA as a template with dT18oligonucleotides (2.5 mM) and random hexamers (10 pM) for priming and 150 U Superscript-RT (Life Technology, Karlsruhe, Germany) for reverse transcription. cDNA thus obtained was tested for integrity by amplification of β-actin transcripts in a 25-cycle PCR reaction. Sense primer A (5′-ACA TGA ACC AGC ATG TCC TGG AAT TGG A-3′) and antisense primer B (5′-AGT GAC ATG TCC ACA TCC CCA GCA GGC-3′) were used for PCR amplification, yielding a 307-bp product if CLIP-170 was amplified but a 412-bp product if restin was amplified. Additionally, restin-specific PCR was performed by using primer C (5′-GAG CAT GCC CGC ATT AAG GAG CTT GAA-3′), which resides in the restin-specific region, together with primer B. PCR was conducted at 95°C for 1 minute, 64°C for 1 minute, and 72°C for 2 minutes for 25 cycles, with a final extension at 72°C for 10 minutes. Twenty-five cycles of amplification were performed to assess the products of PCR with primers A and B in the linear phase of amplification and thus gave semiquantitative information about the ratio between CLIP-170 and restin. All samples were reassessed for restin expression by PCR with primers B and C under standard conditions.
Immunofluorescence analysis
For immunofluorescence analysis cells were grown in 8-chamber slides coated with poly-L-lysine (Sigma, Munich, Germany). Before staining, cells were fixed with phosphate-buffered saline (PBS)/paraformaldehyde (1%) and were permeabilized in PBS/saponin (0.1%). Staining of fixed cells was performed with 2D6 or 4D3 antibody followed by an isotype-specific FITC- or CY3-labeled secondary antibody. For immunofluorescence analysis, cell nuclei were counterstained with DAPI (for staining with CY3-labeled antibodies) or propidium iodide (PI) (for staining with FITC-labeled antibodies) and were embedded in antifade (DAKO). Samples were viewed using a conventional fluorescence microscope (Olympus) or a confocal laser scanning microscope (LSM2; Zeiss, Oberkochen, Germany).
Macropinocytosis assay
Macropinosomes of monocyte-derived dendritic cells and Hodgkin cell lines were labeled by pulsing with fixable FITC-dextran and were identified by their phase brightness. DCs on coverslips were washed with PBS at 37°C and were incubated for 3 minutes with PBS containing 1 mg/mL Texas red (TR)–dextran or FITC-dextran, Mr 70 000 (Molecular Probes, Eugene, OR). At the end of the pulse, cells were washed with ice-cold PBS and subsequently were chased for 0, 5, 10, 30, and 60 minutes in medium at 37°C. Incubations were terminated by the removal of warm medium and substitution with ice-cold PBS/NaN3. Cells were fixed for 10 minutes at room temperature with PBS/1% paraformaldehyde. On reconstitution at 4°C overnight in PBS/0.1% saponin/0.01% NaN3, staining for CLIP-170 was performed with monoclonal antibody 2D6 or polyclonal CLIP-170/restin–specific rabbit antiserum diluted in PBS/saponin 0.1%/0.01% NaN3using FITC-labeled anti–mouse IgM or CY-3–labeled anti–rabbit IgG as a secondary antibody. Stained cells were analyzed by conventional and confocal fluorescence microscopy for colocalization of macropinosomes and CLIP-170/restin.
Results
Expression of CLIP-170/restin in fresh human PBMCs
For the identification of cellular subpopulations with high CLIP-170/restin expression, PBMCs were fractionated using magnetic cell sorting before expression analysis by Western blot. Flow cytometric analysis of the cells yielded by sorting revealed a purity of at least 90% for each distinct cell population, as demonstrated by positivity for CD3, CD19, and CD14, respectively. CD16+ NK cells were enriched to 85% purity (data not shown). Peripheral blood dendritic cells, which constitutively represent 0.1% of the whole PBMC population, could be enriched up to 60% by the above-described protocol. They appeared as strongly HLA-DR–positive cells with the typical dendritic cell–like morphology.
Cell pellets were obtained from Hodgkin-derived cell lines, Epstein-Barr virus (EBV)–transformed lymphoblastoid cell lines (LCLs), bulk PBMCs, and cellular subpopulations enriched by magnetic cell sorting and from monocytes, T cells, and B cells before and after stimulation. Lysates from these pellets were separated on polyacrylamide gels under denaturing conditions, blotted onto nitrocellulose membranes, and immunostained with the affinity-purified polyclonal restin antiserum for Western blot analysis. Only trace amounts of protein were detected in bulk PBMCs and in samples enriched for CD3+, CD19+, CD16+, and CD14+ cells.
To check for a constitutive expression of CLIP-170/restin, DCs freshly isolated from the circulating peripheral blood were studied. The DC population obtained by magnetic-assisted sorting showed a strong expression of CD80, CD86, and HLA-DR as determined by flow cytometry but was negative for CD14. Immunofluorescence microscopy of these cells revealed a subcellular localization of CLIP-170/restin identical to the one observed in cultured mature monocyte-derived DCs (see below). The levels of CLIP-170/restin found in DCs were similar to the high levels of the protein observed in the Hodgkin-derived cell lines KMH-2, HDLM-2, L428, and L540.
Expression of CLIP-170/restin in stimulated human PBMCs
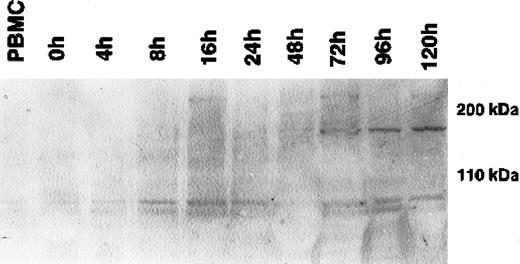
During cytokine-induced differentiation of monocytes into DCs, as confirmed by the expression of CD1a and CD11b and the up-regulation of HLA-DR in flow cytometry, a single 170-kDa band appeared after 72 hours of incubation with IL-4 and GM-CSF (Figure1). The maximum expression was reached after 120 hours of cytokine-supplemented culture. Expression at the mRNA level, as determined by RT-PCR, correlated strictly with the expression of CLIP-170/restin protein demonstrated by immunofluorescence. CD40L-mediated or TNF-α– and IL-1β–induced maturation of dendritic cells had no significant effect on CLIP-170/restin expression levels, as analyzed in independent experiments (not shown).
Western blot analysis of the kinetics of CLIP-170/restin expression during the differentiation of adherent monocytes into dendritic cells.
CLIP-170/restin protein is not detectable using a polyclonal rabbit anti–CLIP-170/restin antiserum in freshly isolated monocytes, but it appears after 72 hours of culture in IL-4– and GM-CSF–supplemented medium and reaches a maximum at day 5.
Western blot analysis of the kinetics of CLIP-170/restin expression during the differentiation of adherent monocytes into dendritic cells.
CLIP-170/restin protein is not detectable using a polyclonal rabbit anti–CLIP-170/restin antiserum in freshly isolated monocytes, but it appears after 72 hours of culture in IL-4– and GM-CSF–supplemented medium and reaches a maximum at day 5.
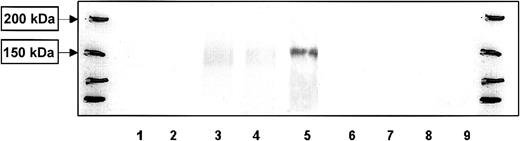
Similar to monocyte-derived DCs, purified B cells expressed CLIP-170/restin after stimulation with IL-4 and CD40 ligand (CD40L). Maximal expression was reached after 4 days and was maintained thereafter (Figure 2). In contrast, T cells stimulated with CD3 and CD28 did not express CLIP-170/restin over a period of observation of 10 days in culture (Figure 2).
Western blot analysis of the kinetics of CLIP-170/restin expression during the stimulation of peripheral blood B and T cells.
CLIP-170/restin protein is not detectable using a polyclonal rabbit anti–CLIP-170/restin antiserum in freshly isolated B cells, but it appears after 4 days of culture in IL-4– and CD40L-supplemented medium, whereas CD3 and CD28 activated T cells remain negative. Lane 1, fresh B cells; 2, B cells after 2 days of stimulation with IL4 + CD40 L; 3, B cells after 4 days of stimulation with IL4 + CD40 L; 4, B cells after 10 days of stimulation with IL4 + CD40 L; 5, Hodgkin-derived cell line L540; 6, fresh T cells; 7, T cells 2 days after stimulation with anti-CD3 and anti-CD28; 8, T cells 4 days after stimulation with anti-CD3 and anti-CD28; and 9, T cells 10 days after stimulation with anti-CD3 and anti-CD28. The first and last lanes represent molecular weight markers.
Western blot analysis of the kinetics of CLIP-170/restin expression during the stimulation of peripheral blood B and T cells.
CLIP-170/restin protein is not detectable using a polyclonal rabbit anti–CLIP-170/restin antiserum in freshly isolated B cells, but it appears after 4 days of culture in IL-4– and CD40L-supplemented medium, whereas CD3 and CD28 activated T cells remain negative. Lane 1, fresh B cells; 2, B cells after 2 days of stimulation with IL4 + CD40 L; 3, B cells after 4 days of stimulation with IL4 + CD40 L; 4, B cells after 10 days of stimulation with IL4 + CD40 L; 5, Hodgkin-derived cell line L540; 6, fresh T cells; 7, T cells 2 days after stimulation with anti-CD3 and anti-CD28; 8, T cells 4 days after stimulation with anti-CD3 and anti-CD28; and 9, T cells 10 days after stimulation with anti-CD3 and anti-CD28. The first and last lanes represent molecular weight markers.
Subcellular localization of CLIP-170/restin in cultured dendritic cells
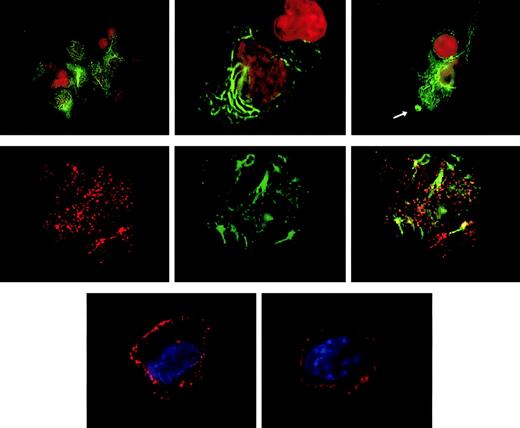
Targeting of the CLIP-170/restin in DCs was analyzed by conventional immunofluorescence microscopy and confocal laser scanning analysis of cells after staining with 2D6 monoclonal antibody or specific polyclonal rabbit antiserum. Besides the typical distribution known from HeLa cells, in which CLIP-170/restin appears in numerous small cytoplasmic patches that represent microtubule plus ends,12,23,24 CLIP-170/restin appeared in cultured DCs in numerous large, submembranous patches predominantly beneath membrane-ruffling sites. In addition, CLIP-170/restin appeared in a varying (10%-60%) proportion of immature DCs as thick bundles of cytoplasmic rings and rods, a pattern found otherwise only in CLIP-170/restin–transfected cells that have very high protein levels (Figure 3, top row). Double staining revealed that CLIP-170/restin aggregates colocalize particularly with microtubule bundles, indicating that CLIP-170/restin is involved in microtubule organization (Figure 2, middle row). Maturation of DCs resulted in a reorganization of the subcellular distribution of CLIP-170: the centripetal cytoplasmic staining resolved, and CLIP-170/restin became restricted to submembranous regions corresponding to the microtubule plus ends23 24 (Figure 3, bottom row).
Subcellular localization of CLIP-170/restin in immature and mature dendritic cells.
(Top row) In immature DCs, CLIP-170/restin appears in numerous large, submembranous and cytoplasmic patches predominantly beneath membrane ruffling sites (arrow). Moreover, in a large proportion of DCs, the protein occurs in thick and branching cytoplasmic bundles, a pattern previously reported exclusively for CLIP-170–cDNA-transfected cells that express very high amounts of the protein. Staining was performed using the CLIP-170/restin-specific monoclonal antibody 2D6 (green). Nuclei were visualized using PI (red). (Middle row) Double staining with 2D6 (red on the left) and microtubule antibodies (green in the middle) demonstrates that CLIP-170/restin aggregates colocalize preferentially with microtubule bundles, indicating the involvement of CLIP-170/restin in microtubule organization (right). (Bottom row) Maturation of DCs with different stimuli leads to dramatic reorganization of CLIP-170/restin with nearly exclusive submembranous localization and loss of centripetal cytoplasmic patches and bundles.
Subcellular localization of CLIP-170/restin in immature and mature dendritic cells.
(Top row) In immature DCs, CLIP-170/restin appears in numerous large, submembranous and cytoplasmic patches predominantly beneath membrane ruffling sites (arrow). Moreover, in a large proportion of DCs, the protein occurs in thick and branching cytoplasmic bundles, a pattern previously reported exclusively for CLIP-170–cDNA-transfected cells that express very high amounts of the protein. Staining was performed using the CLIP-170/restin-specific monoclonal antibody 2D6 (green). Nuclei were visualized using PI (red). (Middle row) Double staining with 2D6 (red on the left) and microtubule antibodies (green in the middle) demonstrates that CLIP-170/restin aggregates colocalize preferentially with microtubule bundles, indicating the involvement of CLIP-170/restin in microtubule organization (right). (Bottom row) Maturation of DCs with different stimuli leads to dramatic reorganization of CLIP-170/restin with nearly exclusive submembranous localization and loss of centripetal cytoplasmic patches and bundles.
Differential PCR analysis of CLIP-170 and restin
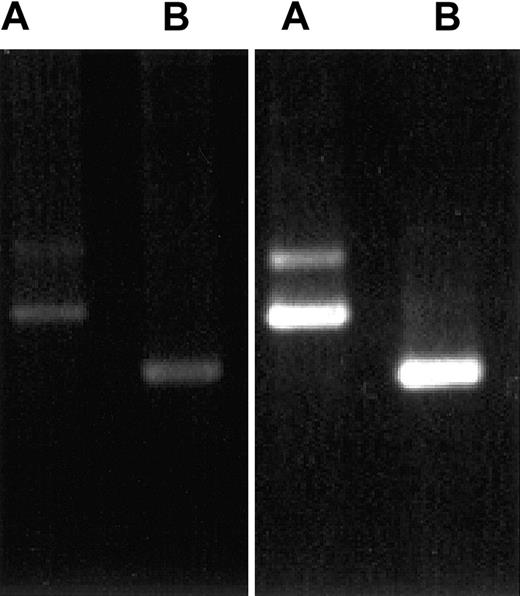
Because CLIP-170 and restin differ in a stretch of 35 amino acids that cannot be distinguished by antiserum and available monoclonal antibodies, we investigated the differential expression of CLIP-170 and restin by a specifically designed PCR. The PCR procedure used for this investigation allowed for the differentiation between both transcripts and a semiquantitative estimation of their expression levels. For transcript expression analysis, RNA extracted from PBMCs and immature DCs was used for RT-PCR with transcript-specific oligonucleotides. Strong signals specific for both splice variants were observed in monocyte-derived DCs (Figure 4) and IL-4/CD40L–stimulated B cells, but only faint signals were observed in PBMCs.
Demonstration of CLIP-170 and restin expression by differential PCR.
PCR protocols distinguishing between restin and CLIP-170 either because of length differences of their products (A) or by amplifying only restin, but not CLIP-170 (B), reveal that both variants are weakly expressed in PBMCs (left gel) but are strongly expressed in DCs (monocytes after 90 hours of culture in IL-4 and GM-CSF; right gel).
Demonstration of CLIP-170 and restin expression by differential PCR.
PCR protocols distinguishing between restin and CLIP-170 either because of length differences of their products (A) or by amplifying only restin, but not CLIP-170 (B), reveal that both variants are weakly expressed in PBMCs (left gel) but are strongly expressed in DCs (monocytes after 90 hours of culture in IL-4 and GM-CSF; right gel).
Involvement of CLIP-170/restin in macropinocytosis
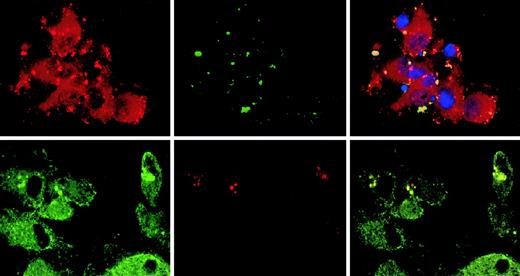
The observation that CLIP-170/restin accumulates in plasma membrane regions with ruffling and protrusions prompted us to investigate its involvement in the uptake and targeting of macropinosomes. Colocalization of CLIP-170/restin with macropinosomes was studied in DCs by confocal microscopy subsequent to dye-labeled, dextran pulse-chase experiments. CLIP-170/restin was not only observed at sites of membrane ruffling, it covered the whole bottom of the calixlike membrane foldings that initiate macropinocytic engulfment of particles. Although only a small proportion of newly forming macropinosomes colocalized with CLIP-170/restin, their frequency increased with time and reached a maximum of approximately 60% to 80% after 10 minutes, indicating that most intermediate/late endosomes associate with CLIP-170/restin (Figure5).
Association of CLIP-170/restin with macropinosomes.
Monocyte-derived DCs pulsed with labeled dextran particles for 5 minutes to visualize macropinocytosis were stained for CLIP-170/restin after a 10-minute chase. (Top row) Immunofluorescence analysis of FITC-dextran–pulsed DCs (green) stained with a polyclonal rabbit anti–CLIP-170/restin serum (red) combined with DAPI labeling of nuclei (blue) reveals colocalization (yellow) of macropinosomes with regions of CLIP-170/restin enrichment. (Bottom row) Using confocal laser microscopy of DCs stained with monoclonal 2D6 antibody combined with FITC-conjugated secondary antibody (green) after pulsing with Texas Red dextran (red), the association of CLIP-170/restin with macropinosomes was confirmed.
Association of CLIP-170/restin with macropinosomes.
Monocyte-derived DCs pulsed with labeled dextran particles for 5 minutes to visualize macropinocytosis were stained for CLIP-170/restin after a 10-minute chase. (Top row) Immunofluorescence analysis of FITC-dextran–pulsed DCs (green) stained with a polyclonal rabbit anti–CLIP-170/restin serum (red) combined with DAPI labeling of nuclei (blue) reveals colocalization (yellow) of macropinosomes with regions of CLIP-170/restin enrichment. (Bottom row) Using confocal laser microscopy of DCs stained with monoclonal 2D6 antibody combined with FITC-conjugated secondary antibody (green) after pulsing with Texas Red dextran (red), the association of CLIP-170/restin with macropinosomes was confirmed.
Discussion
Our study on the expression of CLIP-170/restin in normal human cells has yielded 3 major results. First, it is the first study to describe a high-level expression of CLIP-170/restin in DCs and activated B cells, the prime professional APCs. Second, it describes for the first time the subcellular distribution of this molecule in normal cells. Third, it provides evidence that the high-level expression level of CLIP-170/restin is associated with a hitherto unknown function of this molecule—that is, its involvement in macropinocytosis and, hence, in antigen presentation. Finally, our results suggest a function-based concept of Hodgkin and Reed-Sternberg cells, the still enigmatic neoplastic cells in Hodgkin disease.
Many of the specialized functions of professional APCs require the reorganization of the cytoskeleton. Therefore, the identification of cytoskeleton elements that are DC specific, such as the p55 actin-bundling protein fascin,25,26 is of considerable importance for the understanding of the intercellular interactions and the antigen-uptake mechanisms in these cells. CLIP-170 has been proposed by Kreis and colleagues to mediate endocytic vesicle–microtubule interactions by capturing peripheral endosomes and facilitating their translocation by motor molecules.12,27 Preferential colocalization of CLIP-170/restin with membranes of intermediate macropinocytic vesicles in DCs suggests that the subcellular organization of CLIP-170/restin in DC enables these cells to execute a crucial part in the process of antigen presentation, the hallmark function of DC—the uptake and transport of antigens by macropinocytosis. In contrast, we have not observed a high rate of colocalization of the protein with TR-transferrin–labeled endosomes, which represents the clathrin-dependent internalization pathway of micropinocytosis.4
The initial phase of macropinocytosis, including membrane ruffling, is an actin-dependent process,28 whereas the further centripetal transport of macropinosomes depends on an appropriate microtubule function.7 CLIP-170 has been shown recently to colocalize extensively with cytoplasmic dynein,29 the major microtubule motor protein involved in the centripetal organelle movement. The dynein activator dynactin colocalizes at microtubule plus ends with CLIP-170/restin in a way that depends on the putative cargo-binding domain of CLIP-170/restin.30 This may indicate a role of CLIP-170/restin in the macropinosome cargo loading of the dynein transport system. Dynein itself has been shown to participate in the transport of endocytosed material to lysosomes because reagents that disturb the cytoplasmic dynein function abrogate the fusion of early and late endosomal compartments.31 32CLIP-170 binding to macropinosomes in the intermediate phase of the endosome transport may therefore determine the targeting of these vesicles in APCs. This would be consistent with our observation that the colocalization of CLIP-170/restin with macropinosomes reaches a maximum after 10 minutes of engulfment of solutes. CLIP-170/restin may thus participate in membrane sorting at the crossroad between lysosome targeting and MIIC delivery. According to this view, macropinosomes not associated with CLIP-170 could become excluded from the lysosome transport pathway and deliver their solutes to MIIC compartments or, alternatively, back to the cell surface.
Our observation of high-level expression of CLIP-170/restin in DCs is also consistent with the data described by Bilbe et al14for restin. Restin was originally detected by a monoclonal antibody raised against the U937 cell line, which is derived from a case of malignant histiocytosis, a clinical entity thought to be a malignant proliferation of cells of histiocytic or Langerhans cell lineages.33
Although DCs are the only freshly isolated cells that express CLIP-170/restin, high-level expression of this molecule can be induced in monocyte-derived DCs after stimulation with IL-4 and GM-CSF and in B cells on activation with IL-4 and CD40L, but not in stimulated T cells. Such an activation of B cells is accompanied by the transient formation of antigen-processing compartments, enabling antigen-specific B cells to become effective APCs.34 Our data suggest that the high-level expression of CLIP-170/restin is not only part of the dramatic cytoskeletal changes that regulate the morphology of B cells on stimulation with IL-4 and CD40L,35 it might play a key role in the acquisition of the functional capacity of professional APCs by stimulated B cells.
The immunohistologic profile of HRS cells has long been known to be variable and is therefore of little help in assigning a defined lineage to the HRS cells.36 Similarly, though investigations of the antigen receptor at the molecular levels suggest that most HRS cells are of a B-cell–derived genetic origin,37,38 recent reports have demonstrated that HRS cells might have genotypes other than that of B cells—for example, T cells and null cells.39,40 Like HRS cells, dendritic cells can be derived from different progenitor cells, such as myeloid cells,41CD4+CD3−CD11c, so-called plasmacytoid T cells,42 and CD19+ pro–B cells.43 Interestingly, during the phenotypic transition to DCs, these progenitor cells acquire typical DC markers such as high-density HLA class II molecule expression and high levels of CD80, CD86, and CD83, and they lose their respective typical lineage markers such as CD19 in B cells and CD14 in monocyte-derived DCs. It is conceivable that in a similar scenario, HRS cells acquire the phenotype and functions of professional APCs during or after the transformation of their progenitor cells, resulting in DC-like cells irrespective of their cellular origin. The loss of typical lineage markers during this transformation process makes it difficult to trace the origin of the neoplastic HRS cells to a defined lineage.
Our demonstration that CLIP-170/restin is involved in characteristic functions of professional APCs argues in favor of HRS cells beingfunctionally professional APCs. Assuming that the phenotype (rather than the genotype) of the neoplastic HRS cells determines the morphologic characteristics of the neoplastic cells and the clinical features of Hodgkin disease, a concept that looks at neoplastic cells in Hodgkin disease from a function-based rather than a lineage-oriented perspective could reconcile the diverging immunophenotypic and genetic findings in HRS cells. Such a concept provides a unifying view of the HRS cells as professional APCs and can explain the shared morphologic and clinical features of Hodgkin disease despite the reported genotypic and lineage differences of the neoplastic HRS cells.
We thank Michael Becker for excellent technical assistance.
Supported by grant Pf 135/6-1 from Deutsche Forschungsgemeinschaft (M.P.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael Pfreundschuh, Innere Medizin I, Universität des Saarlandes, D-66421 Homburg, Germany; e-mail:michael.pfreundschuh@uniklinik-saarland.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal