Internal tandem duplication (ITD) mutations of the juxtamembrane domain–coding sequence of the FLT3 gene are found in up to 34% of patients with acute myeloid leukemia (AML) and are associated with a poor prognosis. FLT3/ITDs result in constitutive activation of the tyrosine kinase domain and transform growth factor–dependent cell lines. FLT3 activation leads to antiapoptotic and proliferative signals, but little is known about the impact of FLT3/ITDs on differentiation. This study was designed to investigate the effect of FLT3/ITD expression on the differentiation of the 32Dcl3 (32D) myeloblastic cell line to neutrophils in response to granulocyte colony-stimulating factor (G-CSF). Expression of FLT3/ITD completely blocked morphologic differentiation and induction of myeloperoxidase (MPO), lysozyme, and CCAAT/enhancer-binding protein ε (C/EBPε) in response to G-CSF. Wild-type FLT3 and vector-transfected 32D cells were able to differentiate, although the maturation of FLT3-transfected cells was delayed by FLT3 ligand (FL) stimulation. CEP-701, a potent FLT3 tyrosine kinase inhibitor, overcame the morphologic block in differentiation caused by FLT3/ITD expression and allowed G-CSF induction of myeloid maturation markers. These findings suggest that blocking differentiation may be one of the mechanisms by which FLT3/ITDs contribute to leukemogenesis. CEP-701 and other FLT3 inhibitors may be useful for overcoming the block to differentiation (as well as the block to apoptosis) in the leukemic cells of patients with AML.
Introduction
Proliferation and differentiation of normal hematopoietic cells are partly regulated by growth factors, acting through specific receptors.1 These receptors are divided into a number of families by sequence homologies and structural characteristics. FLT3 is a member of the class III receptor tyrosine kinase family that also includes KIT and FMS, 2 receptors with important roles in hematopoiesis. FLT3 is preferentially expressed on hematopoietic stem/progenitor cells and plays a role in both differentiation and proliferation.2
FLT3 is expressed in most cases of acute myeloid leukemia (AML) and acute B-lineage leukemia (ALL).3-5 Internal tandem duplication (ITD) mutations of the juxtamembrane domain–coding sequence of the FLT3 gene have been identified in 17% to 34% of AML patients and 5% of myelodysplastic syndrome (MDS) patients.6,7 The ITDs range in size from 18 to 174 bp and are always in frame. They are often accompanied by inserted sequences. The result is an addition of repeated and novel amino acid sequences to the juxtamembrane domain of the FLT3 receptor. In vitro studies have shown that FLT3/ITD receptors dimerize in a ligand-independent manner, leading to autophosphorylation of the receptor through constitutive activation of the tyrosine kinase domain.8 Constitutive activation leads to autonomous, cytokine-independent growth with subsequent transformation of cells.9
FLT3/ITDs are associated with leukocytosis in AML and with leukemic transformation of myelodysplasia.10-12 In most studies of both children and adults with AML, the presence of FLT3/ITDs has been associated with poor prognosis.13,14 The activation of wild-type FLT3 receptor by FLT3 ligand (FL) also stimulates the proliferation of primary AML cells.15 Thus, evidence strongly suggests that activation of FLT3 plays an important role in the pathogenesis of leukemia.
Both uncontrolled proliferation and a block in differentiation characterize acute leukemia. The role of FLT3/ITDs in giving a proliferative advantage to cells has been previously reported.16,17 However, the possible role of FLT3/ITDs in blocking hematopoietic differentiation has not been studied. In this report, we investigate the effect of FLT3/ITD expression on the differentiation of 32D cells. 32D cells are a diploid, interleukin 3 (IL-3)–dependent, nonleukemic, murine myeloid cell line.18 Upon transfer from IL-3 to granulocyte colony-stimulating factor (G-CSF) , this cell line mimics the normal program of neutrophil differentiation. In this study, we found that FLT3/ITD expression blocks the ability of 32D cells to differentiate. As FLT3/ITDs depend on their intrinsically activated tyrosine kinase activity for signaling, this activity might be targeted to overcome the block to differentiation. We investigated this possibility by using CEP-701, a potent FLT3 tyrosine kinase inhibitor.19 20 CEP-701 is relatively selective for FLT3, as it has no activity against KIT, FMS, PDGFR, BCR-ABL, insulin receptor, or PKC at doses more than 10-fold higher than the IC50 (50% inhibitory concentration) for FLT3.
Materials and Methods
Reagents
Recombinant murine IL-3 was purchased from Pepro Tech (Rocky Hill, NJ). Recombinant human FL was kindly provided by Imclone Systems (New York, NY). Rabbit antihuman FLT3 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal mouse antiphosphotyrosine antibody 4G10 and recombinant protein A-agarose were purchased from Upstate Biotechnology (Lake Placid, NY). Horseradish peroxidase–conjugated secondary antibody and the enhanced chemiluminescence (ECL) detection system were from Amersham (Arlington Heights, IL). CEP-701 was provided by Cephalon (West Chester, PA).
Cells
The IL-3–dependent murine myeloid cell line 32Dcl3 (referred to hereafter as “32D cells”) was used throughout these studies. Cells were cultured in RPMI 1640, supplemented with 1 ng/mL recombinant mouse IL-3, 10% heat-inactivated fetal calf serum (FCS), and antibiotics (penicillin and streptomycin), at 37°C with 5% CO2. For the differentiation assay, 32D cells were washed twice with phosphate-buffered saline (PBS) and then transferred to medium containing 20 ng/mL G-CSF (Amgen, Thousand Oaks, CA). FL was used at 100 ng/mL where indicated in the differentiation assay and was added fresh to cultures every other day.
DNA constructs and retroviral transfection
Fragments including the internal tandem duplicated region of mutated FLT3 were amplified from RNA isolated from bone marrow samples from AML patients at our institution by reverse transcription–polymerase chain reaction (RT-PCR). The ITD fragments were sequenced and used to replace the corresponding wild-type region in full-length FLT3 cDNA. Full-length cDNA coding for either a FLT3/ITD or a wild-type FLT3 was cloned into the pBabePuro retroviral vector.21 This ITD resulted in an addition of 6 amino acids, RTDFRE, after amino acid No. 596.22 Retroviral constructs were transfected into φCRE packaging cells by transient transfection, and pooled transfectants were then selected in 2 μg/mL puromycin.23For retroviral transduction, φCRE packaging lines were irradiated to 3000 cGy and cocultured with 32D cells for 48 hours with 8 μg/mL Polybrene. Stable transfectants were then selected by limiting dilution in 96-well plates with 2 μg/mL puromycin.
Immunoprecipitation and Western blot analysis
Cells were washed twice with ice-cold PBS and lysed for 30 minutes in ice-cold NP-40 lysis buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 100 mM NaF, 10% glycerol, 1% NP-40, and 10 mM EDTA [ethylenediaminetetraacetic acid]) containing protease and phosphatase inhibitors (2 mM sodium orthovanadate, 50 μg/mL antipain, 5 μg/mL aprotinin, 1 μg/mL leupeptin and 10 μg/mL phenylmethylsulfony fluoride; Sigma, St Louis, MO). Clarified lysates (500 μg) were incubated with rabbit polyclonal antibody to human FLT3 and with protein A-agarose at 4°C. The immunoprecipitates were washed 3 times with ice-cold TBS-T (10 mM Tris-HCl, pH 7.4, 100 mM NaCl, 0.1% Tween-20) resuspended in sodium dodecyl sulfate (SDS) sample buffer, heated, and separated by SDS polyacrylamide gel electrophoresis (SDS-PAGE) in 8% gels. Gels were blotted onto nylon membrane (Millipore, Bedford, MA) and stained with the indicated antibody. Antibody binding was detected by incubation with a horseradish peroxidase–conjugated secondary antibody, followed by chemiluminescence detection.
Morphologic differentiation and proliferation assays
Morphologic differentiation was assessed by cytospin, followed by Wright-Giemsa staining and examination under × 100 microscopy.
To determine cellular proliferation, cells were seeded at an initial density of 2 × 105/mL in 10% FCS/RPMI medium supplemented with various concentrations of CEP-701 and cultured for up to 11 days. The medium was replenished every 2 days, and the cell densities were adjusted to 2 × 105/mL. Viable cells were determined on the basis of trypan blue exclusion at intervals.
Northern blot analysis
Total RNA was extracted from 1 × 107 cells by means of RNeasy columns (Qiagen, Valencia, CA). The RNA samples (15 μg/lane) were separated on 1% formaldehyde-denaturing agarose gels and transferred to nylon membranes (NEN, Boston, MA). cDNAs of myeloperoxidase (MPO), lysozyme, c-myc, c-myb, C/EBPε, and actin were used as probes. All probes were labeled with32P-dCTP by means of a random primer labeling kit (Stratagene, Cedar Creek, TX). Probes were hybridized for 16 hours to blots in 0.3× SSC (1 × SSC = 0.15 M NaCl + 0.15 M Na citrate), 50% formamide, 0.4% SDS, 2× Denhardt reagent, 0.125 mg/mL salmon sperm DNA, at 42°C. The membranes were washed twice with 2× SSC/0.1% SDS for 30 minutes at 42°C and then exposed to XAR film (Kodak, Rochester, NY) at −80°C for 2 to 24 hours.
Apoptosis assessment by annexin V–PE staining
Evaluation of apoptosis was performed by annexin V/7-AAD staining according to the recommendations of the manufacturer (Becton Dickinson, San Jose, CA). After CEP-701 treatment, 5 × 105 cells were washed with cold PBS and resuspended in 100 μL of binding buffer. Following incubation with 5 μL of annexin V–PE and 5 μL of 7-AAD in the dark for 15 minutes at room temperature, cells were analyzed by flow cytometry (FACSort, Becton Dickinson) with Cell Quest software (Becton Dickinson). Compensation was performed with samples stained with either annexin V–PE or 7-AAD alone.
Results
FLT3/ITD is constitutively autophosphorylated in 32D cells and transforms 32D cells to IL-3–independent growth
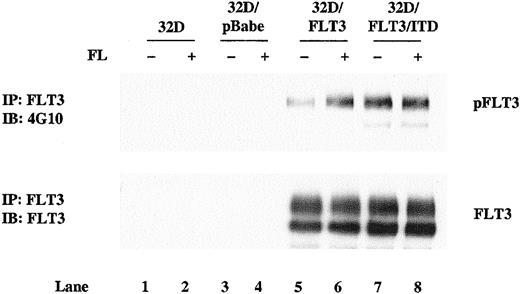
To investigate the effects of FLT3/ITD expression on granulocytic differentiation, we transfected 32D cells with pBabePuro retroviral vectors expressing either FLT3/ITD or wild-type FLT3. pBabePuro vector was also introduced into 32D cells as a control. Stable expression of FLT3/ITD and FLT3 was confirmed by Western blotting of cell lines cloned by limiting dilution. Clones expressing comparable levels of FLT3/ITD or FLT3 were selected to allow a direct comparison of the effects of the different constructs (Figure1, bottom panel, lanes 5-8). Wild-type FLT3–transfected 32D cells showed little phosphorylation, but phosphorylation was greatly stimulated by addition of FL (Figure 1, top panel, lane 5 and 6). In contrast, the ITD mutant was tyrosine phosphorylated in the absence of added FL (Figure 1 top panel, lanes 7 and 8). 32D/FLT3/ITD cells proliferated without the addition of IL-3 or FL, whereas 32D/FLT3 and pBabePuro vector–transfected 32D cells required IL-3 for their growth. These data confirm a previous report that in transfected 32D cells, FLT3/ITD is constitutively activated and transforms cells to IL-3 independence.17
Ligand-independent phosphorylation of FLT3/ITD in 32D cells.
Total cellular protein extracts derived from 1 × 107parental 32D cells and 32D subclones transduced with the pBabePuro vector, pBabePuro-FLT3, and pBabePuro-FLT3/ITD were immunoprecipitated with anti-FLT3 antibody. Cells were incubated without (lanes 1, 3, 5, 7) and with (lanes 2, 4, 6, 8) FL (100 ng/mL) for 5 minutes before extracts were made. The immunoprecipitates were resolved by 8% SDS-PAGE and subjected to immunoblot analysis with antiphosphotyrosine antibody 4G10 (top panel). The same membrane was stripped and reprobed with anti-FLT3 antibody (bottom panel).
Ligand-independent phosphorylation of FLT3/ITD in 32D cells.
Total cellular protein extracts derived from 1 × 107parental 32D cells and 32D subclones transduced with the pBabePuro vector, pBabePuro-FLT3, and pBabePuro-FLT3/ITD were immunoprecipitated with anti-FLT3 antibody. Cells were incubated without (lanes 1, 3, 5, 7) and with (lanes 2, 4, 6, 8) FL (100 ng/mL) for 5 minutes before extracts were made. The immunoprecipitates were resolved by 8% SDS-PAGE and subjected to immunoblot analysis with antiphosphotyrosine antibody 4G10 (top panel). The same membrane was stripped and reprobed with anti-FLT3 antibody (bottom panel).
FLT3/ITD blocks 32D cells from morphologic and functional differentiation to granulocytes
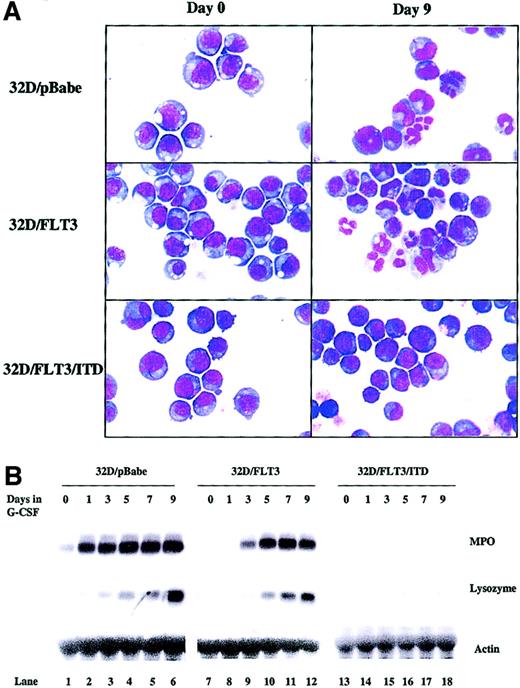
The course of granulopoiesis in 32D cells progresses as follows: undifferentiated blasts become promyelocytes, myelocytes, metamyelocytes, and, finally, mature granulocytes. To examine the effect of FLT3/ITD expression on the process of 32D cell differentiation, we exposed 32D cells transfected with FLT3/ITD, FLT3, or pBabePuro to G-CSF (20 ng/mL) for 9 days. The morphology of the cells was assessed daily by cytospin followed by staining with Wright-Giemsa and analysis by light microscopy. Cells were grouped into 3 categories: myeloblasts, intermediate cells (including promyelocytes, myelocytes, and metamyelocytes), and mature granulocytes. When 32D/FLT3 or 32D/pBabe cells were transferred to medium containing G-CSF, they began to differentiate by day 3, and by day 9 most of the cells showed some degree of granulocytic differentiation (Figure2A and Table1). In contrast, 32D/FLT3/ITD cells maintained their immature blastlike morphologic features after 9 days of treatment with G-CSF (Figure 2A and Table 1) and after even longer periods of treatment (data not shown). These results were also observed when other clones expressing the same constructs were examined (data not shown).
FLT3/ITD blocks 32D cell morphologic and functional differentiation to granulocytes.
32D/pBabe, 32D/FLT3, and 32D/FLT3/ITD cells were washed and transferred from medium containing IL-3 (1 ng/mL) to medium containing G-CSF (20 ng/mL) for 9 days. Every other day, fresh G-CSF–containing medium was added to cells to replace the old medium. (A) Cytospins were prepared on days 0 and 9. The cytospins were subjected to Wright-Giemsa staining followed by light microscopy. Original magnification, × 100. (B) Total cellular RNA from 32D/pBabe (lanes 1-6), 32D/FLT3 (lanes 7-12), and 32D/FLT3/ITD (lanes 13-18) cells was prepared from 1 × 107cells after 0, 1, 3, 5, 7, and 9 days in G-CSF. RNA (15 μg) from each time point was then subjected to Northern blotting with MPO, lysozyme, and actin cDNA probes.
FLT3/ITD blocks 32D cell morphologic and functional differentiation to granulocytes.
32D/pBabe, 32D/FLT3, and 32D/FLT3/ITD cells were washed and transferred from medium containing IL-3 (1 ng/mL) to medium containing G-CSF (20 ng/mL) for 9 days. Every other day, fresh G-CSF–containing medium was added to cells to replace the old medium. (A) Cytospins were prepared on days 0 and 9. The cytospins were subjected to Wright-Giemsa staining followed by light microscopy. Original magnification, × 100. (B) Total cellular RNA from 32D/pBabe (lanes 1-6), 32D/FLT3 (lanes 7-12), and 32D/FLT3/ITD (lanes 13-18) cells was prepared from 1 × 107cells after 0, 1, 3, 5, 7, and 9 days in G-CSF. RNA (15 μg) from each time point was then subjected to Northern blotting with MPO, lysozyme, and actin cDNA probes.
Differentiation of 32Dcl3 cells is blocked by expression of FLT3/ITD
| Cells . | Day 0 . | Day 9 . | ||||
|---|---|---|---|---|---|---|
| 32D/pBabe . | 32D/FLT3 . | 32D/FLT3/ITD . | 32D/pBabe . | 32D/FLT3 . | 32DFLT3/ITD . | |
| Myeloblasts, % | 81.3 ± 2.5 | 85.7 ± 3.8 | 82.3 ± 4.5 | 14.3 ± 2.5 | 11.3 ± 3.2 | 85.3 ± 5.0 |
| Intermediates, % | 18.7 ± 2.5 | 14.3 ± 3.8 | 17.7 ± 4.5 | 64 ± 3.6 | 66.7 ± 4.5 | 14.7 ± 5.0 |
| Granulocytes, % | 0 | 0 | 0 | 21.7 ± 4.0 | 14.7 ± 5.0 | 0 |
| Cells . | Day 0 . | Day 9 . | ||||
|---|---|---|---|---|---|---|
| 32D/pBabe . | 32D/FLT3 . | 32D/FLT3/ITD . | 32D/pBabe . | 32D/FLT3 . | 32DFLT3/ITD . | |
| Myeloblasts, % | 81.3 ± 2.5 | 85.7 ± 3.8 | 82.3 ± 4.5 | 14.3 ± 2.5 | 11.3 ± 3.2 | 85.3 ± 5.0 |
| Intermediates, % | 18.7 ± 2.5 | 14.3 ± 3.8 | 17.7 ± 4.5 | 64 ± 3.6 | 66.7 ± 4.5 | 14.7 ± 5.0 |
| Granulocytes, % | 0 | 0 | 0 | 21.7 ± 4.0 | 14.7 ± 5.0 | 0 |
Differential counts of 32D/pBabe, 32D/FLT3, and 32D/FLT3/ITD cell cultures induced by G-CSF (20 ng/mL) were performed on days 0 and 9.
Values are the mean percentages, ± SD, of cells from 3 independent counts examined by light microscopy after Wright-Giemsa staining of the cytospins. “Intermediates” include promyelocytes, myelocytes, and metamyelocytes.
We also examined the question of whether or not the morphologic maturation observed in transfected 32D cells was consistent with functional maturation. MPO and lysozyme are markers of early and late 32D granulocytic differentiation, respectively.24 The expression of these genes by transfected 32D cell lines was evaluated by Northern blotting. G-CSF strongly induced both MPO and lysozyme expression in 32D/FLT3 and 32D/pBabe cells, with a time course that paralleled that of morphologic differentiation (Figure 2B, lanes 1-12). In contrast, G-CSF was unable to induce either MPO or lysozyme expression in 32D/FLT3/ITD cells (Figure 2B, lanes 13-18). These results strongly suggest that FLT3/ITD blocks the G-CSF–stimulated differentiation of 32D cells.
FL stimulation slows the differentiation of 32D cells expressing wild-type FLT3
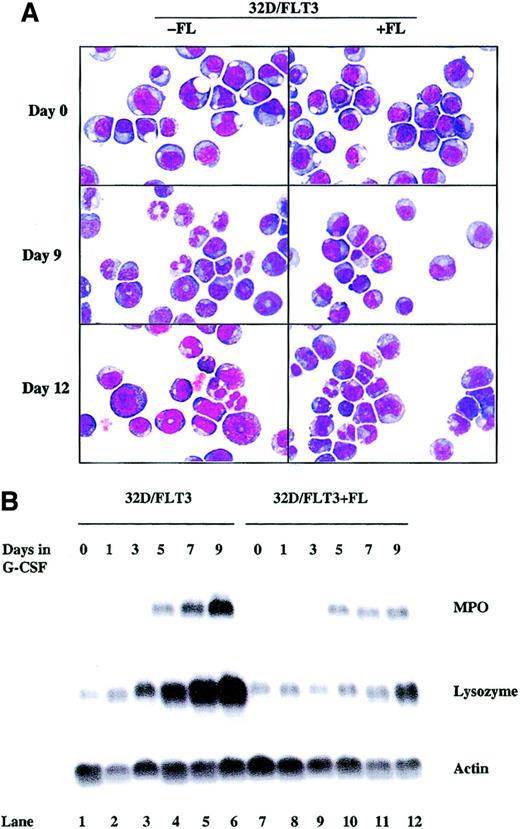
Wild-type FLT3 receptor was fully activated (as assessed by resultant autophosphorylation) only upon stimulation with FL (Figure1). To examine the effect of maximal stimulation of wild-type FLT3 on the granulocytic maturation of 32D cells, we exposed wild-type FLT3–expressing 32D cells to both FL (100 ng/mL) and G-CSF (20 ng/mL). When FL was added, 32D/FLT3 cells displayed a lower percentage of differentiating cells than the control group stimulated with G-CSF alone (48% vs 86% by day 9; Figure 3A, Table 2). The cell differentiation was only slowed, not absolutely blocked, as 82% of the cells showed signs of differentiation by day 12. We also examined MPO and lysozyme expression in 32D/FLT3 cells treated with G-CSF with and without FL. MPO and lysozyme were induced in both of the groups but the levels of induction in the FL-treated group were lower than the levels induced by G-CSF alone (Figure 3B, lanes 9-12 vs lanes 3-6). These data indicate that FL signaling through FLT3 slows the differentiation of 32D/FLT3 cells but is unable, even with maximal stimulation, to equal the effect of FLT3/ITD in blocking differentiation.
FL stimulation slows the differentiation of 32D cells expressing wild-type FLT3.
32D/FLT3 cells were washed and transferred from medium containing IL-3 to medium containing G-CSF (20 ng/mL) with and without FL (100 ng/mL) and cultured for 12 days. Fresh medium with G-CSF with and without FL was used to replace the old medium every 48 hours. (A) Cytospins were prepared on days 0, 9, and 12. The morphologic features of the cells were visualized by means of Wright-Giemsa staining followed by light microscopy. Original magnification, × 100. (B) Total cellular RNA was prepared from 1 × 107 32D/FLT3 cells after 0, 1, 3, 5, 7, and 9 days in medium containing G-CSF without FL (lanes 1-6) or with FL (100 ng/mL; lanes 7-12). RNA (15 μg) was then subjected to Northern blotting with MPO, lysozyme, and actin cDNA probes.
FL stimulation slows the differentiation of 32D cells expressing wild-type FLT3.
32D/FLT3 cells were washed and transferred from medium containing IL-3 to medium containing G-CSF (20 ng/mL) with and without FL (100 ng/mL) and cultured for 12 days. Fresh medium with G-CSF with and without FL was used to replace the old medium every 48 hours. (A) Cytospins were prepared on days 0, 9, and 12. The morphologic features of the cells were visualized by means of Wright-Giemsa staining followed by light microscopy. Original magnification, × 100. (B) Total cellular RNA was prepared from 1 × 107 32D/FLT3 cells after 0, 1, 3, 5, 7, and 9 days in medium containing G-CSF without FL (lanes 1-6) or with FL (100 ng/mL; lanes 7-12). RNA (15 μg) was then subjected to Northern blotting with MPO, lysozyme, and actin cDNA probes.
FL stimulation slows, but does not block, differentiation of 32D/FLT3 cells
| Cells . | G-CSF . | G-CSF+FL . | ||||
|---|---|---|---|---|---|---|
| Day 0 . | Day 9 . | Day 12 . | Day 0 . | Day 9 . | Day 12 . | |
| Myeloblasts, % | 82.7 ± 5.0 | 13.7 ± 2.5 | 14.2 ± 1.2 | 83.7 ± 3.2 | 51.7 ± 2.5 | 17.5 ± 0.5 |
| Intermediates, % | 17.3 ± 5.0 | 63.7 ± 4.6 | 56.8 ± 2.8 | 16.3 ± 3.2 | 46.3 ± 3.8 | 59.7 ± 6.2 |
| Granulocytes, % | 0 | 22.6 ± 2.5 | 29.0 ± 2.8 | 0 | 2.0 ± 1.0 | 22.8 ± 6.6 |
| Cells . | G-CSF . | G-CSF+FL . | ||||
|---|---|---|---|---|---|---|
| Day 0 . | Day 9 . | Day 12 . | Day 0 . | Day 9 . | Day 12 . | |
| Myeloblasts, % | 82.7 ± 5.0 | 13.7 ± 2.5 | 14.2 ± 1.2 | 83.7 ± 3.2 | 51.7 ± 2.5 | 17.5 ± 0.5 |
| Intermediates, % | 17.3 ± 5.0 | 63.7 ± 4.6 | 56.8 ± 2.8 | 16.3 ± 3.2 | 46.3 ± 3.8 | 59.7 ± 6.2 |
| Granulocytes, % | 0 | 22.6 ± 2.5 | 29.0 ± 2.8 | 0 | 2.0 ± 1.0 | 22.8 ± 6.6 |
Differential counts of 32D/FLT3 cell cultures induced by G-CSF (20 ng/mL) with and without FL (100 ng/mL) were performed on days 0, 9, and 12.
Values are the mean percentages, ± SD, of cells from 3 independent counts examined by light microscopy after Wright-Giemsa staining of the cytospins. “Intermediates” include promyelocytes, myelocytes, and metamyelocytes.
CEP-701 inhibits the kinase activity of FLT3/ITD and the proliferation of 32D/FLT3/ITD cells
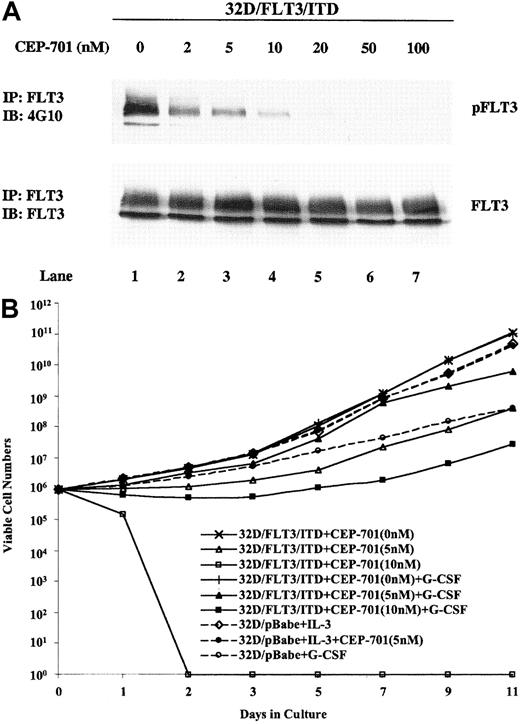
CEP-701 is an indolocarbazole derivative that is very potent in inhibiting FLT3 in BaF3/FLT3/ITD cell lines as well as primary AML samples expressing FLT3/ITDs.19 When used to treat 32D/FLT3/ITD cells, CEP-701 potently suppressed the phosphorylation of mutant FLT3 with an IC50 of approximately 2 nM (Figure4A, top panel). The level of FLT3 expression did not change during treatment with CEP-701 (Figure 4A, bottom panel). To directly evaluate the growth-inhibitory effect of CEP-701 on 32D/FLT3/ITD cells, we counted viable cells in a hemacytometer after increasing time of exposure to the drug. CEP-701 inhibited proliferation and induced cytotoxicity in 32D/FLT3/ITD cells exposed to concentrations over 10 nM (Figure 4B). G-CSF addition partially overcame these inhibitory activities. In the presence of G-CSF and 5 nM CEP-701, 32D/FLT3/ITD cells still grew well, although growth was slightly slower than in the absence of CEP-701. At 5 nM, CEP-701 significantly inhibited the phosphorylation of FLT3/ITD (Figure4A); thus, 5 nM was chosen as the concentration to be used in all subsequent experiments. Under the same conditions of exposure, CEP-701 did not affect the growth of 32D/pBabe cells in the presence of IL-3. G-CSF treatment of 32D/pBabe cells induced differentiation and thus did inhibit the proliferative rate of these cells. It is noteworthy that the inhibitory effect of 5 nM CEP-701 on the proliferation of 32D/FLT3/ITD cells in G-CSF was less than the inhibitory effect of G-CSF alone on the proliferation of 32D/pBabe cells.
CEP-701 inhibits the kinase activity of FLT3/ITD and the proliferation of 32D/FLT3/ITD cells.
(A) 32D/FLT3/ITD cells were treated with CEP-701 at increasing concentrations for 1 hour at 37°C. Cell lysates were immunoprecipitated with anti-FLT3 antibody and immunoblotted with antiphosphotyrosine antibody (top panel). The same membrane was then stripped and probed with anti-FLT3 antibody (bottom panel). (B) Effects of CEP-701 on the growth of 32D/FLT3/ITD cells and 32D/pBabe cells were evaluated by counting the number of viable cells for 11 days. 32D/FLT3/ITD cells were seeded at an initial density of 2 × 105/mL in 10% FCS/RPMI medium supplemented with various concentrations of CEP-701 with and without G-CSF (20 ng/mL). 32D/pBabe cells were seeded at the same density in medium containing IL-3 (1 ng/mL) either with or without CEP-701 (5 nM), or G-CSF. The medium was replenished every other day, and the cell densities were adjusted to 2 × 105/mL. Viable cells were counted on the basis of trypan blue exclusion. Results shown are the means from triplicate assays.
CEP-701 inhibits the kinase activity of FLT3/ITD and the proliferation of 32D/FLT3/ITD cells.
(A) 32D/FLT3/ITD cells were treated with CEP-701 at increasing concentrations for 1 hour at 37°C. Cell lysates were immunoprecipitated with anti-FLT3 antibody and immunoblotted with antiphosphotyrosine antibody (top panel). The same membrane was then stripped and probed with anti-FLT3 antibody (bottom panel). (B) Effects of CEP-701 on the growth of 32D/FLT3/ITD cells and 32D/pBabe cells were evaluated by counting the number of viable cells for 11 days. 32D/FLT3/ITD cells were seeded at an initial density of 2 × 105/mL in 10% FCS/RPMI medium supplemented with various concentrations of CEP-701 with and without G-CSF (20 ng/mL). 32D/pBabe cells were seeded at the same density in medium containing IL-3 (1 ng/mL) either with or without CEP-701 (5 nM), or G-CSF. The medium was replenished every other day, and the cell densities were adjusted to 2 × 105/mL. Viable cells were counted on the basis of trypan blue exclusion. Results shown are the means from triplicate assays.
CEP-701 overcomes the FLT3/ITD–mediated block to differentiation in 32D/FLT3/ITD cells
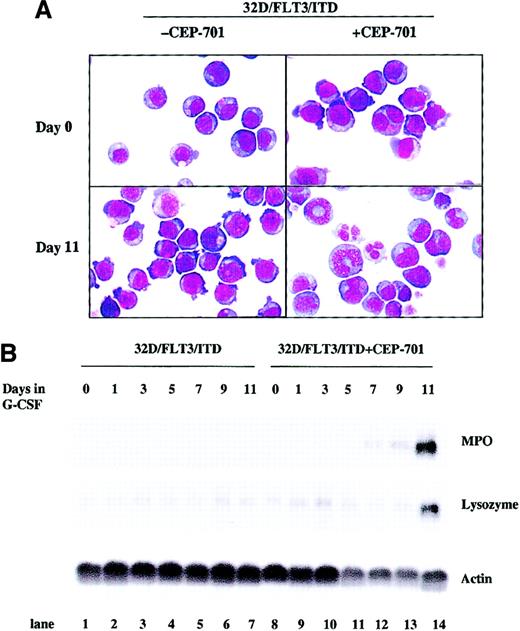
Inhibition of constitutively activated FLT3/ITD tyrosine kinase activity might overcome the block to differentiation in 32D/FLT3/ITD cells. To explore this possibility, we cultured 32D/FLT3/ITD cells with 5 nM CEP-701 for 11 days in the presence of G-CSF. Induction of differentiation was assayed by quantitating morphologic changes daily by light microscopy and by measuring the expression of MPO and lysozyme by Nothern blotting. Morphologic analysis demonstrated that most 32D/FLT3/ITD cells underwent differentiation by day 11 in the presence of CEP-701 and G-CSF, in marked contrast to 32D/FLT3/ITD cells treated with G-CSF alone (85% vs 14%; Figure 5A and Table 3). CEP-701 treatment also overcame the block to induction of MPO and lysozyme expression in the 32D/FLT3/ITD cells treated with G-CSF (Figure 5B, lanes 8-14 vs lanes 1-7).
CEP-701 overcomes the FLT3/ITD-mediated block of differentiation in 32D/FLT3/ITD cells.
32D/FLT3/ITD cells were treated with or without CEP-701 (5 nM) in the presence of G-CSF (20 ng/mL) for 11 days. Every other day, fresh medium containing G-CSF with or without CEP-701 was added to cells to replace the old medium. (A) Cytospins were prepared on days 0 and 11. The morphologic features of the cells were visualized by means of Wright-Giemsa staining followed by light microscopy. Original magnification, × 100. (B) Total cellular RNA was prepared from 1 × 107 cells on days 0, 1, 3, 5, 7, 9, and 11 of culture without (lanes 1-7) or with (lanes 8-14) CEP-701. RNA (15 μg from each sample) was then subjected to Northern blotting with MPO, lysozyme, and actin cDNA probes.
CEP-701 overcomes the FLT3/ITD-mediated block of differentiation in 32D/FLT3/ITD cells.
32D/FLT3/ITD cells were treated with or without CEP-701 (5 nM) in the presence of G-CSF (20 ng/mL) for 11 days. Every other day, fresh medium containing G-CSF with or without CEP-701 was added to cells to replace the old medium. (A) Cytospins were prepared on days 0 and 11. The morphologic features of the cells were visualized by means of Wright-Giemsa staining followed by light microscopy. Original magnification, × 100. (B) Total cellular RNA was prepared from 1 × 107 cells on days 0, 1, 3, 5, 7, 9, and 11 of culture without (lanes 1-7) or with (lanes 8-14) CEP-701. RNA (15 μg from each sample) was then subjected to Northern blotting with MPO, lysozyme, and actin cDNA probes.
CEP-701 overcomes the FLT3/ITD-mediated block of differentiation in 32D cells
| Cells . | G-CSF . | G-CSF + CEP-701 . | ||
|---|---|---|---|---|
| Day 0 . | Day 11 . | Day 0 . | day 11 . | |
| Myeloblasts, % | 84.7 ± 3.8 | 85.7 ± 5.9 | 88.0 ± 3.0 | 14.7 ± 4.7 |
| Intermediates, % | 15.3 ± 3.8 | 14.3 ± 5.9 | 12.0 ± 3.0 | 66.7 ± 5.5 |
| Granulocytes, % | 0 | 0 | 0 | 18.7 ± 3.5 |
| Cells . | G-CSF . | G-CSF + CEP-701 . | ||
|---|---|---|---|---|
| Day 0 . | Day 11 . | Day 0 . | day 11 . | |
| Myeloblasts, % | 84.7 ± 3.8 | 85.7 ± 5.9 | 88.0 ± 3.0 | 14.7 ± 4.7 |
| Intermediates, % | 15.3 ± 3.8 | 14.3 ± 5.9 | 12.0 ± 3.0 | 66.7 ± 5.5 |
| Granulocytes, % | 0 | 0 | 0 | 18.7 ± 3.5 |
Differential counts of 32D/FLT3/ITD cell cultures treated with G-CSF (20 ng/mL) with and without CEP-701 (5 nM) were performed on days 0 and 11.
Values are the mean percentages, ± SD, of cells from 3 independent counts examined by light microscopy after Wright-Giemsa staining of the cytospins. “Intermediates” include promyelocytes, myelocytes, and metamyelocytes.
To rule out any direct effects CEP-701 might have on differentiation, we cultured 32D/FLT3/ITD cells with 5 nM CEP-701 for 11 days and assessed differentiation by the same functional and morphologic differentiation assays. CEP-701 alone was unable to induce MPO or lysozyme expression, measured by Northern blotting, and no phenotypic changes associated with myeloid differentiation were observed by light microscopy (data not shown). The same result was observed for 32D/pBabe cells grown in IL-3 medium with 5 nM CEP-701 (data not shown).
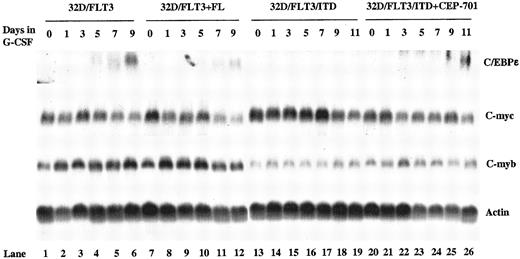
CCAAT/enhancer-binding protein ε (C/EBPε) is a transcription factor whose expression is restricted to maturing myeloid cells, and it is an important regulator of myelopoiesis.25 26 The mechanism by which FLT3/ITD expression blocks differentiation could be through inhibition of genes critical for differentiation. We examined the expression of C/EBPε mRNA in FLT3/ITD- and FLT3-transfected 32D cells. In 32D/FLT3/ITD cells, in contrast to 32D/FLT3 cells, induction of C/EBPε expression by G-CSF was completely blocked (Figure6, lanes 1-6 vs lanes 13-19). However, when 32D/FLT3/ITD cells were exposed to both CEP-701 and G-CSF, C/EBPε expression was observed by days 9 and 11 (Figure 6, lanes 25 and 26). Induction of C/EBPε by G-CSF in 32D/FLT3 cells was somewhat decreased when the cells were stimulated with FL (Figure 6, lanes 10-12 vs lanes 4-6). This decrease is consistent with the delayed differentiation seen earlier in 32D/FLT3 cells stimulated with FL. C/EBPε expression was not induced by CEP-701 in 32D/FlT3/ITD or 32D/pBabe cells in the absence of G-CSF (data not shown).
Regulation of C/EBPε, c-myc, and c-myb in 32D/FLT3 and 32D/FLT3/ITD cells.
32D/FLT3 cells were washed and transferred from medium containing IL-3 to medium containing G-CSF (20 ng/mL) without (lanes 1-6) or with (lanes 7-12) FL (100 ng/mL) and cultured for 9 days. 32D/FLT3/ITD cells were treated without (lanes 13-19) or with (lanes 20-26) CEP-701 (5 nM) in the presence of G-CSF (20 ng/mL) for 11 days. Every other day the medium was replaced. Total cellular RNA was extracted from 1 × 107 32D/FLT3 cells on days 0, 1, 3, 5, 7, and 9. Total cellular RNA was also prepared from 1 × 107 32D/FLT3/ITD cells on days 0, 1, 3, 5, 7, 9, and 11. RNA (15 μg from each sample) was then subjected to Northern blotting with C/EBPε, c-myc, c-myb, and actin cDNA probes.
Regulation of C/EBPε, c-myc, and c-myb in 32D/FLT3 and 32D/FLT3/ITD cells.
32D/FLT3 cells were washed and transferred from medium containing IL-3 to medium containing G-CSF (20 ng/mL) without (lanes 1-6) or with (lanes 7-12) FL (100 ng/mL) and cultured for 9 days. 32D/FLT3/ITD cells were treated without (lanes 13-19) or with (lanes 20-26) CEP-701 (5 nM) in the presence of G-CSF (20 ng/mL) for 11 days. Every other day the medium was replaced. Total cellular RNA was extracted from 1 × 107 32D/FLT3 cells on days 0, 1, 3, 5, 7, and 9. Total cellular RNA was also prepared from 1 × 107 32D/FLT3/ITD cells on days 0, 1, 3, 5, 7, 9, and 11. RNA (15 μg from each sample) was then subjected to Northern blotting with C/EBPε, c-myc, c-myb, and actin cDNA probes.
c-myc and c-myb are important regulators of cellular proliferation. Overexpression of c-myc blocks differentiation in many cell types.27-29 In particular, c-myc and c-myb overexpression in 32D cells has previously been shown to block differentiation normally induced by G-CSF.30 31 FLT3/ITD expression could block differentiation of 32D cells by increasing the expression of c-myc and/or c-myb. To investigate this possibility, we examined the expression of the c-myc and c-myb genes in 32D/FLT3/ITD and 32D/FLT3 cells. However, neither FL stimulation of 32D/FLT3 cells nor FLT3/ITD expression increased c-myc or c-myb RNA levels (Figure 6, lanes 7-12 and 13-19). Consistent with this finding, CEP-701 did not decrease the level of expression of these genes during differentiation of 32D/FLT3/ITD cells in G-CSF (Figure 6, lanes 20-26). Visual results were confirmed by densitometry followed by quantitation with National Institutes of Health (NIH) Image 1.62 (data not shown).
Increases in cell death occur during differentiation of 32D/FLT3/ITD and 32D/pBabe cells
To assess the effect of CEP-701 on apoptosis of 32D/FLT3/ITD cells, we cultured cells in G-CSF with and without CEP-701 (5nM) for up to 11 days. Apoptosis was assessed by fluorescence-activated cell sorter (FACS) analysis after staining with annexin V–PE and 7-AAD at intervals. Early apoptotic cells were defined as those staining positive for annexin and negative for 7-AAD; late apoptotic or necrotic cells were defined as those staining positive for both; live cells were defined as those staining negative for both. Over a time course of 11 days, CEP-701 resulted in an increasing fraction of early and late apoptotic cells in 32D/FLT3/ITD cells in the presence of G-CSF (from 11.2% on day 0 to 36.5% by day 11; Table4). A similar increase in the fraction of apoptotic cells was observed during differentiation of 32D/pBabe cells induced by G-CSF (from 6.6% on day 0 to 42.7% by day 11; Table 4). In contrast, G-CSF alone was unable to induce apoptosis in 32D/FLT3/ITD cells in the absence of CEP-701. Additionally, in the presence of IL-3, CEP-701 did not induce apoptosis in 32D/pBabe cells.
Cell death occurs during the differentiation of 32D/FLT3/ITD and 32D/pBabe cells
| . | Day 0, % . | Day 1, % . | Day 11, % . |
|---|---|---|---|
| 32D/FLT3/ITD | 11.2 ± 2.5 | 11.1 ± 1.7 | 8.8 ± 0.6 |
| 32D/FLT3/ITD+G-CSF | 11.2 ± 2.5 | 8.6 ± 1.3 | 9.9 ± 0.4 |
| 32D/FLT3/ITD+G-CSF+CEP-701 | 11.2 ± 2.5 | 18.5 ± 1.1 | 36.5 ± 2.9 |
| 32D/pBabe+IL-3 | 6.6 ± 1.0 | 9.5 ± 0.3 | 11.8 ± 0.6 |
| 32D/pBabe+IL-3+CEP-701 | 6.6 ± 1.0 | 5.9 ± 0.3 | 6.1 ± 0.5 |
| 32D/pBabe+G-CSF | 6.6 ± 1.0 | 16.8 ± 1.6 | 42.7 ± 0.3 |
| . | Day 0, % . | Day 1, % . | Day 11, % . |
|---|---|---|---|
| 32D/FLT3/ITD | 11.2 ± 2.5 | 11.1 ± 1.7 | 8.8 ± 0.6 |
| 32D/FLT3/ITD+G-CSF | 11.2 ± 2.5 | 8.6 ± 1.3 | 9.9 ± 0.4 |
| 32D/FLT3/ITD+G-CSF+CEP-701 | 11.2 ± 2.5 | 18.5 ± 1.1 | 36.5 ± 2.9 |
| 32D/pBabe+IL-3 | 6.6 ± 1.0 | 9.5 ± 0.3 | 11.8 ± 0.6 |
| 32D/pBabe+IL-3+CEP-701 | 6.6 ± 1.0 | 5.9 ± 0.3 | 6.1 ± 0.5 |
| 32D/pBabe+G-CSF | 6.6 ± 1.0 | 16.8 ± 1.6 | 42.7 ± 0.3 |
32D/FLT3/ITD cells were cultured in medium with or without G-CSF (20 ng/mL) in the presence or absence of CEP-701 (5 nM). 32D/pBabe cells were cultured either in medium containing IL-3 (1 ng/mL) with and without CEP-701 (5 nM) or in medium containing G-CSF (20 ng/mL). Early and late apoptotic cells were detected by annexin V/7-AAD staining followed by flow cytometry on days 0, 1, and 11.
Values are the mean percentages, ± SD, of cells from triplicate experiments.
Discussion
In normal hematopoiesis, myeloid progenitor cells proceed through a series of steps resulting in terminally differentiated cells.32-34 AML is characterized by the uncontrolled proliferation of myeloid cells that accumulate at different stages, where their further differentiation is blocked. FLT3 activation mutations are genetic alterations that occur frequently in AML.35 The presence of an FLT3/ITD mutation results in increased leukocytosis and a higher relapse rate in patients expressing such a mutation.10,14,36 Identifying the impact that FLT3/ITDs have on normal myelopoiesis is essential to understanding this common subset of AML. FLT3/ITDs have been shown to induce factor-independent growth of 32D cells.17 In this study, we have demonstrated that FLT3/ITD expression also blocks the differentiation of 32D cells in response to G-CSF.
FL stimulation of 32D/FLT3 cells slowed, but was unable to completely block, differentiation. FLT3/ITDs are characterized by constitutive dimerization and subsequent autophosphorylation of tyrosine residues in the absence of FL.8 Whether FLT3/ITDs block differentiation by expressing a higher level of kinase activity than FL-stimulated FLT3 or through qualitative differences in signaling remains an important area for further investigation. Several differences in signaling have been seen between wild-type FLT3 and FLT3/ITD mutant receptors.16,17 Constitutively activated FLT3 causes strong phosphorylation and activation of STAT5. In contrast, wild-type FLT3 stimulated with FL causes lesser, transient activation of STAT5.16,37 STAT proteins are known to be activated in a number of primary leukemias and cell lines.38-42 STAT5 phosphorylation thus could be the intracellular signal that accounts for the difference in blocking differentiation between ITD mutation and wild-type FLT3. Supporting this possibility, the introduction of v-Src into 32D cells results in constitutive STAT activation and a corresponding block in G-CSF–mediated differentiation.43 Of note, exogenous IL-3 or transfection with BCR-ABL also prevents 32D differentiation in response to G-CSF.18 44 Whether each of these genes uses the same pathway in blocking differentiation is not known.
c-myc and c-myb can serve as negative regulators of differentiation because forced expression of these genes blocks differentiation in several cell types.45 46 However, there is no significant difference between FLT3/ITD– and FLT3–transfected 32D cells in c-myc and c-myb RNA expression levels mediated by G-CSF treatment. Thus, neither of these factors is likely to contribute to the differentiation block caused by FLT3/ITD expression. Inhibition of C/EBPε induction was observed as a consequence of FLT3/ITD expression. However, C/EBPε is normally induced relatively late in the process of myeloid differentiation and thus this block is likely a consequence, rather than the cause, of inhibited differentiation.
Targeted inhibition of FLT3/ITD tyrosine kinase activity might overcome the resultant differentiation block, as well as the other functions of constitutively activated FLT3, in AML. CEP-701 is a member of a family of synthetic derivatives of the indolocarbazole K252a. These drugs inhibit kinase activity by acting as competitive inhibitors of adenosine triphosphate (ATP) binding to the active site of the kinase domain.47,48 CEP-701 inhibits the phosphorylation of FLT3 with an IC50 of approximately 2 nM in 32D/FLT3/ITD cells. In comparison, STI571 inhibits BCR-ABL with an IC50of 100 to 300 nM.49 CEP-701 has very little activity against the other class III receptor tyrosine kinase family members, KIT, FMS and PDGFR, until levels greater than 500 nM are reached. Thus, CEP-701 is a potent and relatively selective inhibitor of FLT3.19 CEP-701 inhibition of FLT3 overcomes the differentiation block caused by expression of FLT3/ITD in 32D cells, as demonstrated by morphologic changes and induction of differentiation markers. Interestingly, differentiation was somewhat slowed, as indicated by the delayed induction of MPO, lysozyme, and C/EBPε in 32D/FLT3/ITD cells when compared with 32D/FLT3 and 32D/pBabe cells. One explanation for this finding is that CEP-701 does not completely inhibit the tyrosine kinase activity of FLT3/ITD at a concentration of 5 nM (Figure 4A). Evidence for this hypothesis is provided by the observation that FL stimulation, which activates the FLT3 receptor, also slows the differentiation of 32D/FLT3 cells (Figure 3A-B).
Hematopoietic cells have 3 major fates: proliferation, differentiation, and cell death.50 These processes are closely intertwined. For example, under normal circumstances, cell proliferation and cell death are carefully balanced. Induction of differentiation is generally associated with a loss of proliferative capacity, and cell death eventually follows terminal maturation. This relationship is also observed in leukemia. For example, all-trans retinoic acid (ATRA) induces differentiation and subsequent apoptosis of acute promyelocytic leukemic cells.51 In this study, a similar induction of apoptosis occurred during the differentiation of 32D/pBabe and 32D/FLT3/ITD cells.
In summary, this study implies that FLT3/ITD mutations occurring in patients with AML may also contribute to leukemogenesis by blocking differentiation. Inhibition of FLT3 kinase activity may overcome the block to differentiation in myeloid cells and thus may improve the outcome of AML patients expressing FLT3/ITD. It will be interesting to see if FLT3 inhibition overcomes blocked differentiation in patients with AML now that these inhibitors have entered clinical trials.
The authors thank Dr Qianfei Wang for his helpful suggestions and technical assistance.
Prepublished online as Blood First Edition Paper, August 1, 2002; DOI 10.1182/blood-2002-03-0936.
Supported by grants from the National Institutes of Health (CA 70970 and CA 91177; D.S.); the Leukemia and Lymphoma Society of America (D.S., A.D.F.); Cephalon, Inc (D.S.); and the Children's Cancer Foundation (D.S., A.D.F.). A.D.F. is a Leukemia and Lymphoma Society Scholar.
D.S. is a paid consultant to Cephalon, Inc, which provided partial funding for the studies described in this report. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its conflict-of-interest policies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Donald Small, Bunting Blaustein Cancer Research Bldg, Room 251, 1650 Orleans St, Baltimore, MD 21231-1000; e-mail: donsmall@jhmi.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal