We have previously shown that bivalent human γ1 CD3 monoclonal antibody (mAb) is ineffective at mediating lysis of human T cells with human complement. In this paper we have used genetic engineering and sulfur chemistry to prepare 2 types of human γ1 CD3 mAb dimer, with the aim of improving complement lysis activity. The IgG molecules forming the dimers were linked together at their C-termini by stable bismaleimide thioether bridges. The first dimer was composed of 2 bivalent mAb molecules. This dimer proved incapable of lysing human T-cell blasts with human, rabbit, or guinea pig complement. The second dimer consisted of 2 molecules of a monovalent derivative (possessing a single Fab domain) of the bivalent mAb. This dimer was highly lytic with human complement, with a lytic titer 64-fold greater than that of the nondimerized monovalent mAb. The maximum level of lysis of human T-cell blasts achieved with this monovalent mAb dimer was equal to that obtained with the therapeutic antilymphocyte mAb alemtuzumab, but its lytic titer was 4-fold greater. The monovalent mAb dimer was also found to be lytic in the presence of rabbit and guinea pig complement. Dimerization of monovalent antibodies may provide a general strategy for improving the cytolytic activity of other mAbs that are normally unable to induce lysis with complement. The monovalent CD3 mAb dimer may have potential for development as an agent for immunotherapy of T-cell leukemia.
Introduction
Monoclonal antibodies (mAbs) specific for cell surface antigens can be used to target unwanted cell types in vivo for destruction by immune effector mechanisms.1,2 Although the most important factor determining an antibody's in vivo cytolytic potency is its interaction with Fc receptor–bearing cells, complement lysis can also play a significant role.3 Any modification that can be made to a mAb to improve complement lysis activity is likely to have a beneficial effect on its overall cytolytic activity, as long as its interaction with Fc receptors is not compromised.
Human or humanized mAbs rather than rodent mAbs are the preferred choice for human mAb therapy.4,5 They minimize the likelihood of eliciting an antiglobulin response in the patient, which may block the effect of the therapeutic antibody.5Consequently, when aiming to maximize cytolytic activity, attention has focused on mAbs of the human IgG1 and 3 subclasses, because these subclasses are able to recruit both complement and cytotoxic FcγR-bearing cells.6 However, in the case of complement, the efficiency of cytolysis is not solely governed by mAb heavy-chain isotype. The properties of the target cell surface antigen also play a role.7-9 Some antigens, such as surface immunoglobulin on B lymphocytes,10 CD33 on myeloid cells,11 and CD3 on T lymphocytes,12 redistribute and modulate when cross-linked by normal bivalent mAbs. Antibodies to such antigens are generally poor inducers of complement lysis, but the problem can be overcome to some extent using enzymatic or genetic engineering techniques to remove or inactivate one of the Fab domains of the mAb.1,13-15 This renders the mAb monovalent, preventing it from cross-linking the target antigen. This strategy has been shown to improve complement lysis activity, but the drawback is that it also reduces mAb-binding avidity by a factor of 6,15significantly increasing the concentration of mAb that must be used (or dose that must be given therapeutically) to achieve effective coating of the target cell.
Several groups of researchers have shown that tail-to-tail dimerization of bivalent humanized IgG1 mAbs (mediated by intermolecular disulfide bonds formed between cysteine residues, introduced into position 444 of the heavy chain by genetic engineering) can give dramatic (100- to 200-fold) improvements in complement lysis activity.16-18Although encouraging, some caution is needed in judging the implications of these studies for human antibody therapy. In 2 of the studies,16,17 using mAbs specific for a hapten and CD33, respectively, nonhuman sources of complement were used in the cell lysis assays. The importance of this is underlined by the observation that no lysis was observed with the CD33 mAb dimer when tested with human complement in the same assay. In a third study,18dimers of the highly lytic CD52 mAb alemtuzumab (Campath-1H) only showed enhanced complement lysis activity when tested against a cell line expressing abnormally low levels of the target antigen.
In this report, we describe the production and characterization of dimers of bivalent and monovalent humanized IgG1 mAbs specific for the human CD3 antigen. The dimers were created using a bismaleimide cross-linker to form a nonreducible intermolecular bridge between cysteine residues introduced at position 444 of the heavy chains of the monomers. When tested for their lytic activity on human T-cell blasts using serum from the T-cell donor as the source of complement, the monomers and dimers of the bivalent CD3 mAb gave no lysis. Conversely, the dimers of the monovalent mAb gave more lysis than the benchmark CD52 mAb alemtuzumab, an improvement of approximately 64-fold over the nondimerized monovalent mAb. Dimerization of monovalent mAbs may provide a strategy for improving the cytolytic activity of antibodies specific for other complement-resistant cell surface antigens.
Materials and methods
Cloning and expression of the CD3 mAb H- and L-chain genes
The humanization of the H- and L-chain genes of the CD3 mAb YTH 12.5 (human γ1 G1m (1,17) λ Kern−Oz−), and the construction of the gene encoding the N-terminally truncated human γ1 H chain (tH) polypeptide have been described elsewhere.15 cDNA versions of the genes were used for antibody production in this present study. A second version of the H-chain cDNA was created, in which the serine codon (TCT) at position 444 (numbering based on human myeloma protein EU)19 was changed to cysteine (TGC). The Ser444Cys mutation, along with a Cys233Ser substitution (Kabat numbering),19 were also introduced into the tH-chain gene. In total, 4 different immunoglobulin genes were produced (H, L, HCys444, and tHCys444), encoding the 4 different polypeptides that are components of the bivalent and monovalent CD3 mAb monomers illustrated in Figure1A.
Structures of the humanized (γ1, λ) CD3 mAb monomers and dimers and the reaction scheme for their preparation.
(A) WT indicates bivalent mAb not carrying the Ser444Cys mutation; H, heavy-chain polypeptide; L, light-chain polypeptide; HCys444, heavy-chain polypeptide carrying the Ser444Cys mutation; tHCys444, N-terminally truncated heavy-chain carrying the Cys233Ser and Ser444Cys double mutation; mal,N,N′-1,2-phenylenedimaleimide cross-linkage. (B) Outline of the stages involved in the dimerization procedure. DTT indicates dithiothreitol; Py-SS-Py, 2,2′-dipyridyl disulphide; o-PDM,N,N′-1,2-phenylenedimaleimide; G, blocking groups such as glutathione.
Structures of the humanized (γ1, λ) CD3 mAb monomers and dimers and the reaction scheme for their preparation.
(A) WT indicates bivalent mAb not carrying the Ser444Cys mutation; H, heavy-chain polypeptide; L, light-chain polypeptide; HCys444, heavy-chain polypeptide carrying the Ser444Cys mutation; tHCys444, N-terminally truncated heavy-chain carrying the Cys233Ser and Ser444Cys double mutation; mal,N,N′-1,2-phenylenedimaleimide cross-linkage. (B) Outline of the stages involved in the dimerization procedure. DTT indicates dithiothreitol; Py-SS-Py, 2,2′-dipyridyl disulphide; o-PDM,N,N′-1,2-phenylenedimaleimide; G, blocking groups such as glutathione.
The vectors PEE13 and PEE6 (Lonza Biologics PLC, Slough, England)20 were used for immunoglobulin expression. The L-chain gene was inserted between the HindIII andEcoRI sites of PEE13. The H, HCys444, and tHCys444 genes were separately inserted between theHindIII and EcoRI sites of PEE6. PEE13/PEE6 combination vectors carrying either the L + H, or L + HCys444 genes were then produced.
Murine NS0 cells were transfected20 with either the L + H combination vector, the L + HCys444combination vector, or a mixture of the L + H combination vector and the PEE6-tHCys444 vector to produce cell lines constitutively expressing wild-type bivalent, bivalentCys444, or monovalentCys444 γ1 CD3 mAbs, respectively. Colonies of transfected cells were screened for human IgG secretion by enzyme-linked immunosorbent assay (ELISA); colonies producing monovalent mAbs were identified by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).15
Purification and dimerization of antibodies
The CD3 mAbs were concentrated and purified from culture supernatant by protein A-Sepharose affinity chromatography.21
Homodimers of mAbs carrying the Cys444 mutation were prepared using a variation of the protocols described by Glennie et al,22 Stevenson et al,23 and Stalteri and Mather,24 as outlined in Figure 1B. BivalentCys444 CD3 mAb at a protein concentration of approximately 2 mg/mL was reduced by the addition of dithiothreitol (DTT; Sigma, code D9779, Poole, England) to a final concentration of 20 mM (Figure 1B, step 1), followed by incubation for 20 minutes at 37°C and 40 minutes at 25°C. The DTT was removed by dialysis against phosphate-buffered saline (PBS) + 1.0 mM EDTA (ethylenediaminetetra-acetic acid), then the reduced interchain disulfide bonds were reconstituted by treatment with 2,2′-dipyridyl disulfide (Py-SS-Py; Sigma, code D5767) as follows. Dimethylformamide (DMF, Sigma, code D8654) was first added to the dialyzed antibody to a final concentration of 15% vol/vol. Py-SS-Py from a 20-mM stock solution in anhydrous DMF (Aldrich, code 22705-6, Poole, England) was also added, to a final molar concentration of 0.7 times the molar concentration of free sulfhydryl (-SH) groups in the reduced antibody (estimated as 10 for the bivalentCys444 mAb). The mixture was incubated at 37°C for 1 hour, then cooled on ice for 15 minutes. A one-tenth volume of ice-cold 0.5 M sodium acetate, pH 4.6, was added to adjust the pH of the solution to 4.7; this was followed by DTT to a final concentration of 1.0 mM, and incubation was continued at 4°C for 1 hour. Under these conditions DTT selectively reduces cysteine-SS-pyridyl disulfide bonds (Cys-SS-Py) bonds, while leaving the re-formed interchain disulfide bonds intact. The antibody was dialyzed as before, then put through a repeat round of disulfide bond reconstitution with Py-SS-Py (but not DTT reduction) followed by dialysis, to ensure that the inter–H-chain disulfide bonds in the IgG hinge had re-formed. At this point the antibody was reconcentrated to approximately 2 mg/mL using a Millipore ultrafree centrifugal device (Fisher, code FDR-582-050R, Loughborough, England), and stored at −80°C prior to the final steps in the cross-linking procedure.
To begin the final steps in the dimerization process, up to 15 mg antibody, which had been through the disulfide bond reduction and reconstitution process, was thawed and again selectively reduced with DTT at pH 4.7 as described earlier. The DTT was removed by dialysis against 50 mM sodium acetate, 1 mM EDTA, pH 4.6, at 4°C for 4 hours, followed by dialysis against the same buffer at pH 5.3 at 4°C, overnight. (All sodium acetate buffer used for these dialysis steps, and for the buffer exchange on Sephadex G25, see below, were degassed under vacuum then purged with N2 gas before use.) The mAb solution was then split into 2 portions in the ratio of 0.55:0.45 and held on ice. The bismaleimide cross-linking reagent N, N′-1,2-phenylenedimaleimide (o-PDM, Aldrich, code 10459-0) dissolved in anhydrous DMF was added to the larger portion of antibody to give final concentrations of o-PDM and DMF of 4.5 mM and 25% vol/vol, respectively. After 40 minutes on ice followed by centrifugation at 12 000 rpm for 2 minutes at 4°C, this sample was buffer-exchanged into 20 mM sodium acetate, 5 mM EDTA, using an XK16/40 column of Sephadex G25 (Pharmacia, Bucks, England) equipped with a cooling mantle of circulating water kept at 0°C. This relatively long column of Sephadex G25 (40 cm) was necessary to ensure complete removal of surplus N, N′-1,2-phenylenedimaleimide24 and was run at 12 mL/min to achieve a fast and efficient buffer exchange. Fractions containing antibody were identified by OD 280 measurement and collected on ice, pooled, and then mixed with the remaining portion of reduced, dialyzed antibody. The reaction mixture was reconcentrated as before to approximately 2 mg/mL, then incubated on a rotary shaker at 4°C for 3 days to allow dimers to form. At the end of this timeN-acetyl-l-cysteine (Sigma, code A8199) was added at a final concentration of 1 mM to block any free maleimide groups, and the mixture was held at room temperature for 10 minutes.
The dimers were separated from nondimerized monomers by gel filtration on an XK26/100 column of Superdex 200 pg (Pharmacia, code 17-1043-01) equilibrated with 25 mM Tris (tris(hydroxymethyl)aminomethane), pH 7.5, 0.5 M NaCl, 0.1% wt/vol betaine, 2 mM EDTA, and 0.05% sodium azide. Dimer and monomer peak fractions were separately pooled and concentrated to approximately 1 mg/mL. They were then dialyzed extensively against PBS, filter sterilized, and stored at −80°C prior to assessment for antigen binding and cell lysis activity (see “Competitive binding assay” and “Complement lysis assay”).
The monovalentCys444 mAb was dimerized in essentially the same way as the bivalent mAb, except for the first stage. The protein A–purified immunoglobulin from the NS0 cells transfected with the combination of H, L, and tHCys444 genes actually contains 3 species of molecules due to the random association of H- and tH-chain polypeptides within the cell.15 These species are bivalent CD3 mAb (∼170 kDa), monovalent CD3 mAb (∼120 kDa), and Fc molecules (∼70 kDa), consisting of 2H + 2L, H + L + tHCys444, and 2tHCys444 polypeptides, respectively. Prior to dimerization, the monovalent species first had to be separated from the other 2. This was achieved by gel filtration using the system described above for separation of the bivalent mAb monomers and dimers. Before loading the protein A–purified antibody onto the column, it was first reduced with 20 mM DTT as described in the first step of the bivalent mAb dimerization procedure. After gel filtration, the purified, reduced monovalent mAb fraction collected from the column was then immediately treated with Py-SS-Py in the disulfide bond reconstitution step, without prior dialysis into PBS. When calculating the quantity of Py-SS-Py to use, the number of -SH groups in the monovalentCys444 mAb was assumed to be 7.
The buffers used in the above procedures were prepared with deionized water containing < 0.12 endotoxin units (EU)/mL as measured using a Limulus amoebocyte assay (Sigma, code 210-A1). Final antibody preparations contained 1 to 3 × 10−3 EU/mg mAb.
Competitive binding assay
The relative binding avidity of the CD3 mAb monomers and dimers were assessed in a competitive binding assay using cells from a human T-cell leukemia cell line (HPBALL), and 1 μg/mL biotinylated wild-type bivalent humanized CD3 mAb as the labeled antibody, as described previously.15 The control mAb alemtuzumab25 (humanized γ1 G1m (1,17) κ, CD52 mAb) was kindly provided by Dr G. Hale (Therapeutic Antibody Centre, Heddington, Oxford, England).
Assessment of CD3 antigen modulation
Human T-cell blasts1 were incubated with a saturating concentration (3 μg/mL) of test CD3 antibody (or nonmodulating control mAb, alemtuzumab) in culture medium at 37°C in a CO2 incubator. At 6 time points between 1 and 24 hours after beginning the incubation, samples of cells were taken and prepared for fluorescence-activated cell sorting (FACS) analysis to assess the surface expression of CD3 antigen. This was done by rinsing the cells in FACS diluent (PBS with 1% bovine serum albumin [BSA], 5% heat-inactivated normal rabbit serum, 0.1% sodium azide), then staining them for 1 hour at 4°C with 1 μg/mL R-phycoerythrin–labeled goat antihuman IgG (Sigma, code P 9170) in FACS diluent. The mean fluorescence of approximately 5000 cells/sample was then determined using a Becton Dickinson FACScan (Oxford, England). To indicate the maximum level of staining that could be produced by the test CD3 mAbs in the absence of modulation, T-cell blasts were also incubated with each mAb (3 μg/mL) for 1 hour at 37°C in cell culture medium in the presence of 0.1% sodium azide to minimize modulation. These cells were then also stained with R-phycoerythrin–labeled goat antihuman IgG and analyzed by FACS.
Complement lysis assay
Human T-cell blasts1 were labeled with51Cr, washed, then added (2 × 105 cells/well in a 25-μL volume) to 50-μL dilutions of test mAb in a 96-well round-bottomed microtray. After mixing, 25 μL defibrinated homologous human serum was added to each well as a source of human complement, and the plate mixed again. Alternatively, 25 μL freshly reconstituted lyophilized rabbit HLA-ABC serum (Sigma, code S7764) or guinea pig serum (Sigma, code S1639) was used as a source of rabbit or guinea pig complement, respectively. Diluent (Iscove modified Dulbecco medium [IMDM]; Gibco BRL, Paisley, Scotland; code 42200-048 containing 1% BSA) was used in “no antibody” and “no complement” control wells. The plate was incubated at 37°C for 1 hour; then the extent of 51Cr release was determined by gamma counting.
Antibody-dependent cellular cytotoxicity
Human CD56+ natural killer (NK) cells were isolated from peripheral blood mononuclear cells (PBMCs) using CD56 antibody (Becton-Dickinson, code 347740) and M-450 Dynabeads (Dynal, code 110.01; Bromborough, England) as described by Naume et al.26 The CD56+ cells were cultured for 15 days as described by Miller et al.27 On day 10 the small percentage of CD56+ CD3+ cells that copurified with the CD56+ CD3− cells were removed by complement lysis using 5 μg/mL of the monovalent γ1 CD3 mAb dimer, and homologous human serum at a final concentration of 33% vol/vol. After 1 hour at 37°C the cells were washed and returned to culture. On day 15 the cells were harvested for use as effector cells in antibody-dependent cellular cytotoxicity (ADCC) assays.
Target T cells were freshly isolated from the NK cell donor on the day of the assay. PBMCs isolated on lymphoprep gradients were passed down a nylon wool column and then depleted of CD56+ cells using magnetic beads. This population was typically 65% CD3+ and nearly 100% CD52+. The lymphocytes were labeled with51Cr (Amersham, code CJS4; Bucks, England), washed, then incubated with dilutions of test mAb (in RPMI 1640 + 1% BSA) in a 96-well round-bottomed microtray (2 × 104 target cells/well). After 90 minutes the cells were washed 3 times to remove nonbound mAb, then effector cells were added (effector-target [E/T] ratio of 5:1). After a further 4 hours at 37°C the extent of51Cr release was determined.
Results
Dimerization of antibodies using the Shopes mutation (Ser444Cys)
The C-terminally linked IgG dimers that have been described previously16-18 have been held together by disulfide bonds, formed between cysteine residues introduced at H-chain position 444. In this study the strategy of introducing a cysteine at position 444 was combined with the use of a bismaleimide cross-linking reagent. The aim was to produce dimers held together by a nonreducible covalent linkage, the stability of which has been confirmed in vivo.28 Two types of bismaleimide-linked dimer were assembled from a humanized IgG1 mAb specific for the human CD3 antigen (Figure 1). The first (the bivalentCys444 dimer) was composed of 2 bivalent IgG molecules, each with 2 Fab domains. The second (the monovalentCys444 dimer) consisted of 2 monovalent IgG molecules, each possessing only a single Fab domain.
The monovalent IgG molecules used to make the second dimer were generated using a “truncated H-chain” strategy. This enabled IgG carrying only one Cys444 per molecule to be produced, preventing the formation of high-order multimers during the dimerization process.
The 2 dimers, along with their respective nondimerized monomers (bivalentCys444 monomer and monovalentCys444monomer) and the original wild-type humanized IgG1 CD3 mAb (bivalent WT monomer) were analyzed for purity, antigen-binding avidity, and in vitro cytolytic activity.
Analysis of cross-linked CD3 antibodies by SDS-PAGE
The SDS-PAGE performed under nonreducing conditions (Figure2) revealed multiple bands in each sample. The major band in each lane is the relevant mAb monomer or dimer. The minor 70-kDa band in the monovalentCys444monomer and dimer preparations (lanes 3 and 4, respectively) is probably residual Fc protein produced by the monovalent mAb cell line that was not separated from the monovalent antibody (by the gel filtration step), prior to the beginning of the dimerization process. The minor 140-kDa band in the monovalentCys444 dimer preparation (lane 4) is probably some of this Fc protein that has cross-linked to produce Fc dimers. Some of the other minor bands with molecular weights smaller than the major bands represent partially dissociated IgG molecules, present because a proportion of the IgG in the purified mAb preparations exists naturally in a partially reduced state. This is normal and is seen in mAb that has not been through the dimerization process (the BMWT mAb, lane 5).
SDS-PAGE analysis of CD3 mAb monomers and dimers performed under nonreducing conditions.
A 1.5-μg quantity of each sample was run on a 5% to 18% gradient polyacrylamide gradient gel possessing a 4% stacking gel, under nonreducing conditions. The gel was stained with Coomassie blue. Lane 1 (BM), bivalentCys444 monomer; lane 2 (BD), bivalentCys444 dimer; lane 3 (MM), monovalentCys444 monomer; lane 4 (MM), monovalentCys444 dimer; lane 5 (BMWT), bivalentWT monomer; MWM, molecular weight markers. The positions of the bands corresponding to the antibody monomers and dimers are illustrated on the gel.
SDS-PAGE analysis of CD3 mAb monomers and dimers performed under nonreducing conditions.
A 1.5-μg quantity of each sample was run on a 5% to 18% gradient polyacrylamide gradient gel possessing a 4% stacking gel, under nonreducing conditions. The gel was stained with Coomassie blue. Lane 1 (BM), bivalentCys444 monomer; lane 2 (BD), bivalentCys444 dimer; lane 3 (MM), monovalentCys444 monomer; lane 4 (MM), monovalentCys444 dimer; lane 5 (BMWT), bivalentWT monomer; MWM, molecular weight markers. The positions of the bands corresponding to the antibody monomers and dimers are illustrated on the gel.
Other bands of greater size than the major bands are also present in some of the preparations. In the bivalentCys444 dimer sample (lane 2), 2 minor bands are visible above the main dimer band. It is not clear what these particular 2 bands are, but one possibility is that they represent species of dimer in which the mAb molecules are held together by only 1, rather than 2, bismaleimide linkages. The existence of single-linkage dimers in this preparation was suggested by SDS-PAGE analysis under reducing conditions (not shown), where a faint band of non–cross-linked H chains was visible. Under nonreducing conditions, the 2 faint high-molecular-weight bands in the bivalentCys444 monomer preparation indicate that it is contaminated by a small quantity of bivalentCys444 dimer from the gel filtration procedure (lane 1). Likewise, the monovalentCys444 monomer contains a small amount of its corresponding dimer (lane 3).
When SDS-polyacrylamide gels were run under reducing conditions (not shown), HCys444 and tHCys444 polypeptide dimers could be seen in the dimerized mAbs, confirming the nonreducible nature of the bismaleimide cross-linkage. HCys444 polypeptide dimers could also be seen in the bivalentCys444 monomer preparation, indicating that many of the monomer molecules have an intramolecular bismaleimide cross-linkage. This linkage is probably between the 2 Cys444 residues rather than between 2 of the hinge -SH groups, because corresponding tHCys444polypeptide dimers were absent in the monovalentCys444monomer preparation (the monovalentCys444 mAb has the same potential to form intramolecular linkages in the hinge as the bivalentCys444 mAb, but because it only has a single Cys444 residue, the formation of a linkage between the C-termini of its H and tH polypeptides is not possible).
The effect of cross-linking on CD3 antibody-binding avidities
The relative binding avidities of the CD3 mAb monomers and dimers were compared by measuring their ability to block the binding of biotinylated wild-type CD3 mAb to cells expressing the human CD3 antigen (Figure 3). The results obtained with the bivalentCys444 monomer and bivalentWTmonomer suggest that the chemical manipulations involved in the cross-linking procedure may have slightly reduced the antibody's binding activity (by a factor of 1.3). Dimerization of the bivalentCys444 mAb produced only a 1.3-fold rise in avidity; this is in line with the increases reported by others16,17 for disulfide bond–linked bivalent mAb dimers. The avidity of the monovalentCys444 monomer was 4.7-fold less than that of the bivalentCys444 monomer on a microgram per milliliter basis; this is equivalent to a 3.5-fold difference on a molar Fab basis. This difference is not as great as the 6-fold difference reported previously between monovalent and bivalent CD3 mAbs.15 It is probably due to the presence of traces of monovalentCys444 dimer protein as seen by SDS-PAGE. The presence of small amounts of dimerized antibody would increase the apparent avidity of the monovalentCys444 monomer preparation. Dimerization of the monovalentCys444 mAb increased its avidity by a factor of at least 2.8 (possibly more, if the estimate for the monovalentCys444 monomer was too high). This raised its avidity to just under that of the bivalentCys444 monomer (1.2-fold less on a molar Fab basis).
Comparison of relative binding avidities of cross-linked CD3 antibodies.
(■, BM), bivalentCys444 monomer; (▵, BD), bivalentCys444 dimer; (○, MM), monovalentCys444 monomer; (×, MD), monovalentCys444 dimer; (+, BMWT), bivalentWT monomer; (⋄, alem), CD52 mAb alemtuzumab. Fluorescence values obtained in the absence of competitor mAb (♦, 100% fluorescence), or in the absence of competitor mAb and biotinylated CD3 mAb (▪, 0% fluorescence) are indicated. Each point is the mean of duplicate tests.
Comparison of relative binding avidities of cross-linked CD3 antibodies.
(■, BM), bivalentCys444 monomer; (▵, BD), bivalentCys444 dimer; (○, MM), monovalentCys444 monomer; (×, MD), monovalentCys444 dimer; (+, BMWT), bivalentWT monomer; (⋄, alem), CD52 mAb alemtuzumab. Fluorescence values obtained in the absence of competitor mAb (♦, 100% fluorescence), or in the absence of competitor mAb and biotinylated CD3 mAb (▪, 0% fluorescence) are indicated. Each point is the mean of duplicate tests.
Antibody dimers modulate CD3 antigen expression
The relative antigen-modulating activities of the dimerized CD3 mAbs was assessed by incubating human T-cell blasts in saturating concentrations of the mAbs over a 24-hour period. Cell samples were taken at different time points to determine the level of cell surface CD3 antigen expression. The results (Figure4) indicated that the modulating activity of the monovalentCys444 dimer was very similar to that of the bivalentWT monomer; both reduced the level of CD3 antigen on the surface of the T-cell blasts by three fourths after 24 hours. The bivalentCys444 dimer modulated the CD3 antigen to a similar extent, but its initial modulation rate was more rapid. The mAb alemtuzumab, which was used as a nonmodulating control antibody, caused little change in the cell surface level of its target antigen CD52, except in the first 1-hour period of the experiment. The brighter fluorescence given by this antibody is due to the fact that CD52 is expressed at a higher level on the cell surface compared to CD3.8
Modulation of CD3 antigen expression on T cells after incubation with CD3 antibodies.
(♦, BMWT), bivalentWT monomer; (▵, BD), bivalentCys444 dimer; (×, MD), monovalentCys444 dimer; (⋄, alem), CD52 mAb alemtuzumab. Each point is the mean of duplicate samples. The mean fluorescence values have been corrected using a no first antibody control.
Modulation of CD3 antigen expression on T cells after incubation with CD3 antibodies.
(♦, BMWT), bivalentWT monomer; (▵, BD), bivalentCys444 dimer; (×, MD), monovalentCys444 dimer; (⋄, alem), CD52 mAb alemtuzumab. Each point is the mean of duplicate samples. The mean fluorescence values have been corrected using a no first antibody control.
Complement-mediated lytic activities of the CD3 antibody dimers
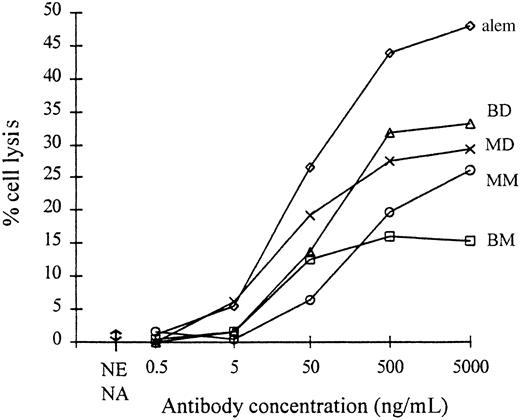
The pattern of complement lysis (Figure5) observed with the monovalent and bivalent monomers using human complement was the same as that reported previously.15 The bivalentWT monomer and bivalentCys444 monomer gave no lysis, whereas the monovalentCys444 monomer produced 22% lysis at a concentration of 25 μg/mL, a lytic titer 16-fold lower than that seen with the benchmark CD52 mAb, alemtuzumab. Some of the activity of the monovalentCys444 monomer preparation is probably due to the presence of the dimer contaminant seen in SDS-PAGE.
Lysis of human T-cell blasts with human complement.
(♦, BMWT), bivalentWT monomer; (■, BM), bivalentCys444 monomer; (▵, BD), bivalentCys444 dimer; (○, MM), monovalentCys444 monomer; (×, MD), monovalentCys444 dimer; (⋄, alem), CD52 mAb alemtuzumab. Each point is the mean of duplicate tests. The range of lysis observed with all mAbs at their maximum test concentration in the absence of complement is indicated (NC).
Lysis of human T-cell blasts with human complement.
(♦, BMWT), bivalentWT monomer; (■, BM), bivalentCys444 monomer; (▵, BD), bivalentCys444 dimer; (○, MM), monovalentCys444 monomer; (×, MD), monovalentCys444 dimer; (⋄, alem), CD52 mAb alemtuzumab. Each point is the mean of duplicate tests. The range of lysis observed with all mAbs at their maximum test concentration in the absence of complement is indicated (NC).
In contrast to previous studies which described dimerized bivalent mAbs specific for a hapten and CD33,16,17 no improvement in complement lysis activity was observed with dimerized bivalent CD3 mAb. One possible reason for the difference (other than the different target antigens involved) was that the previous studies used nonhuman sources of complement. Human cells are generally more resistant to lysis by human complement because they are protected by anticomplement defense proteins, such as CD59, which inhibit the formation of homologous complement membrane attack complexes.29 To explore this possibility, we also tested the lytic activity of the dimerized bivalent mAb using rabbit serum and guinea pig serum as sources of complement, but the results were again negative (data not shown).
The complement lysis result obtained with the monovalentCys444 dimer was completely different. Although its binding avidity was slightly less than that of the bivalentCys444 dimer, the maximum level of lysis produced with human complement was equal to the maximum produced by alemtuzumab, and its lytic titer was 4-fold greater (Figure 5). Dimerization of the monovalentCys444 CD3 mAb had therefore produced a 64-fold increase in lytic titer. This was consistently observed in 4 lysis experiments. Interestingly, when rabbit complement was used (data not shown) the difference between the monovalentCys444 dimer and alemtuzumab increased to 16-fold, but when guinea pig complement was used there was a marked reversal in the relative lytic activities of the 2 antibodies (alemtuzumab 60% lysis, monovalentCys444 dimer 10% lysis, at 12.5 μg/mL mAb with guinea pig complement). Despite the poor level of lysis seen with the monovalentCys444 dimer with guinea pig complement, it was the only form of CD3 mAb to show any activity with this complement type.
The complement source-dependent difference in the relative lytic activities of alemtuzumab and the monovalentCys444 dimer suggests that the activity of complement from different species is influenced to different extents by the antigen specificity of the activating antibody. It implies that an antibody with an antigenic specificity that works well with one species of complement will not necessarily be good with another. This is an important consideration if antibodies are being selected for therapeutic applications based on their lytic activity with nonhuman complement.
CD3 antibody dimers are effective in ADCC
When tested in an ADCC assay (Figure6), the monovalentCys444dimer and the bivalentCys444 dimer produced similar levels of cytotoxicity, giving levels of lysis above those of their nondimerized counterparts. This indicates that the use of the bismaleimide cross-linker has not destroyed the ability of dimers to interact with FcγR-bearing cytotoxic cells, an important feature because it correlates strongly with in vivo cell depletion activity.3 Whereas complement-dependent lysis was detected down to a concentration of 200 ng/mL monovalentCys444dimer, ADCC was detectable at concentrations of CD3 mAb dimers as low as 5 to 50 ng/mL (compare Figures 5 and 6).
ADCC of human PBMCs by homologous CD56+effector cells.
(■, BM), bivalentCys444 monomer; (▵, BD), bivalentCys444 dimer; (○, MM), monovalentCys444 monomer; (×, MD), monovalentCys444 dimer; (⋄, alem), CD52 mAb alemtuzumab. Each point is the mean of duplicate tests. The range of lysis observed with all mAbs at their maximum test concentration in the absence of effector cells (NE) and the level of lysis given by effector cells in the absence of mAb (NA) are indicated.
ADCC of human PBMCs by homologous CD56+effector cells.
(■, BM), bivalentCys444 monomer; (▵, BD), bivalentCys444 dimer; (○, MM), monovalentCys444 monomer; (×, MD), monovalentCys444 dimer; (⋄, alem), CD52 mAb alemtuzumab. Each point is the mean of duplicate tests. The range of lysis observed with all mAbs at their maximum test concentration in the absence of effector cells (NE) and the level of lysis given by effector cells in the absence of mAb (NA) are indicated.
Discussion
The reason the monovalentCys444 dimer is so active in complement lysis while the bivalentCys444 dimer is ineffective is not clear. One hypothesis used in the past to explain the superior performance of (nondimerized) monovalent CD3 mAbs compared to bivalent CD3 mAbs is that monovalent mAbs induce less antigenic modulation. However, the monovalentCys444 dimer has 2 Fab domains and is able to cross-link antigen on the cell surface, and we have found that it modulates antigen to a similar extent as the original nonlytic bivalentWT monomer. If reduced modulation was the only mechanism behind the superior lytic activity of monovalent CD3 mAbs, dimerization would be predicted to inhibit their complement lysis activity. This was not the case.
Tail-to-tail antibody dimers have been proposed to form naturally (via noncovalent interactions) after the binding of IgG to cell surface antigens,30 facilitating C1q binding. This idea is supported by the studies of Shopes16 and Caron et al,17 in which human IgG1 dimers formed artificially using engineered disulfide binds were found to have much higher lytic activity than their monomeric counterparts. A second hypothesis that has been used to explain the superior lytic activity of monovalent compared to bivalent CD3 mAbs is that monovalent binding to the cell surface might permit greater mobility in the Fc region, making it easier for adjacent Fc regions to interact and achieve a configuration favorable for complement activation.15 31 If binding antigen by both Fab's inhibits suitable Fc interactions in bivalent CD3 mAbs, then prefixing their Fc regions in a favorable configuration (ie, by covalent tail-to-tail dimerization) prior to reaction with antigen would be predicted to overcome the problem. However, the bivalentCys444 dimer did not show improved lytic activity. This observation also suggests that the cross-linking of the 2 Fc regions in the monovalentCys444 dimer is not the only factor responsible for its potency.
One major difference in the structure of the 2 CD3 mAb dimers is that the monovalentCys444 dimer is held together by a single cross-link, whereas the bivalentCys444 dimer has the potential to be held together by 2. It is unlikely that this could be solely responsible for their different lytic activities, because SDS-PAGE carried out under reducing conditions (not shown) indicated that a proportion of the dimers in the bivalentCys444 dimer preparation are in fact only held together by one intermolecular cross-linkage. Therefore, if the single cross-link was the determining factor for the observed lytic differences, some lytic activity would probably have been seen with this dimer. Nevertheless, it could make a beneficial contribution to dimer flexibility by permitting a greater degree of bending and rotation of the dimer.
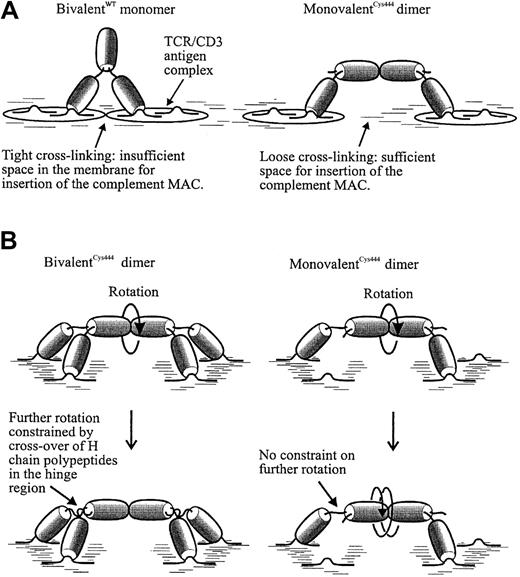
Perhaps the important factor influencing the outcome of complement activation by γ1 CD3 mAbs is the distance or freedom of movement between adjacent cross-linked CD3 antigen complexes, rather than the occurrence or absence of antigen cross-linking per se. The 2 antigen-binding sites on the monovalentCys444 dimer are further apart than those on the bivalentCys444 monomer. Furthermore, the monovalentCys444 dimer is potentially very flexible. When both Fab domains of this dimer are bound to a cell surface, the Fc domains should still be totally free to rotate around their longitudinal axes. This is not true for the bivalent mAb or its dimer, where crossover of the H-chain polypeptides between the hinge and the 2 Fab domains will restrict Fc rotation. These features of the monovalentCys444 dimer (illustrated in Figure7) may give the complement membrane attack complex easier access to the cell membrane. Some support for this idea is given by previous work1 showing that monovalent and bivalent CD3 mAbs (of the rat γ2b isotype) fix similar levels of C1q and cell-bound C3. This indicates that their complement-activating properties are closely matched and indirectly implies that a later step in the complement cascade is the limiting factor. A correlation between segmental flexibility and the complement-fixing activity of antibodies has been suggested in the past.31
Models to explain the improved lytic activity of the monovalentCys444 dimer (MD).
(A) Representation of the different cross-linked antigen lattices that might be formed on the cell surface by wild-type bivalent and dimerized monovalent antibody. MAC indicates membrane attack complex. (B) Probable differences in the relative rotational freedom of the Fc regions of the bivalent and monovalent antibody dimers, when bound to CD3 antigen on a cell surface.
Models to explain the improved lytic activity of the monovalentCys444 dimer (MD).
(A) Representation of the different cross-linked antigen lattices that might be formed on the cell surface by wild-type bivalent and dimerized monovalent antibody. MAC indicates membrane attack complex. (B) Probable differences in the relative rotational freedom of the Fc regions of the bivalent and monovalent antibody dimers, when bound to CD3 antigen on a cell surface.
In conclusion, we have shown that if bivalent γ1 mAb monomers and dimers of a particular antigenic specificity are unable to mediate complement lysis of target cells, it is possible to achieve efficient lysis using dimers of a monovalent derivative of the mAb. Effective lysis of human T cells has also been achieved using an antigen that is less abundantly expressed than the CD52 antigen recognized by the highly lytic alemtuzumab antibody. These observations create opportunities to improve the lytic function of other bivalent monoclonal antibodies with poor lytic abilities. The results also provide an insight into why cells may be resistant to lysis by mAbs to certain antigens. The next step is to determine whether or not the enhanced in vitro complement lysis activity of the monovalentCys444 dimer translates into superior cell-depleting activity in vivo. The stability of the bismaleimide cross-linker used to make the dimers should allow this assessment to be made.
The cross-linking protocol was developed based on initial work by Dr Sonya Patterson. Dr B. Shenton is acknowledged for help with FACS analysis. The γ region genes of the humanized YTH 12.5 mAbs were kindly provided by Prof H. Waldmann (University of Oxford, England).
Prepublished online as Blood First Edition Paper, August 1, 2002; DOI 10.1182/blood-2002-03-0731.
Supported by the Leukaemia Research Fund, London, England.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Soren U. Nielsen, Department of Microbiology and Immunology, University of Newcastle upon Tyne, Medical School, Framlington Pl, Newcastle upon Tyne, England, NE2 4HH; e-mail:s.u.nielsen@ncl.ac.uk.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal