Chronic active Epstein-Barr virus (CAEBV) infection syndrome is a heterogeneous EBV-related disorder characterized by chronic fatigue, fever, lymphadenopathy, and/or hepatosplenomegaly, associated with abnormal patterns of antibody to EBV. CAEBV can range from disabling mild/moderate forms to rapidly lethal disorders. Even patients with mild/moderate disease frequently suffer adverse effects from long-term anti-inflammatory agents and have a quality of life that progressively deteriorates. It is still unknown why these individuals are unable to produce an effective immune response to control EBV, and no effective treatment is currently available. Since ex vivo–expanded EBV-specific cytotoxic T lymphocytes (EBV-CTLs) can safely restore EBV-specific cellular immune responses in immunodeficient patients, we assessed the possibility that adoptive immunotherapy might also effectively treat CAEBV infection. Following stimulation with irradiated EBV-transformed lymphoblastoid cell lines (LCLs), EBV-CTLs were successfully generated from 8 of 8 patients with the mild/moderate form of CAEBV infection. These CTLs were predominantly CD3+ CD8+ cells and produced specific killing of the autologous LCLs. There were 5 patients with 1- to 12-year histories of disease who were treated with 1 to 4 injections of EBV-CTLs. Following infusion, there was resolution of fatigue and malaise, disappearance of fever, and regression of lymphadenopathy and splenomegaly. The pattern and titers of anti-EBV antibodies also normalized. No toxicity was observed. There were 4 patients who did not show any relapse of disease within 6 to 36 months follow-up; one patient had recurrence of fatigue and myalgia one year after CTL infusion. We suggest that adoptive immunotherapy with autologous EBV-CTLs may represent a safe and feasible alternative treatment for patients affected with mild/moderate CAEBV infection and that this approach should be evaluated in the more severe forms of the disease.
Introduction
More than 90% of adults have evidence of infection with Epstein-Barr virus (EBV).1 In the vast majority, infection is associated with a transient mild illness, which is more severe during primary infection of adults than of children. Although the virus persists in a latent state lifelong, there are rarely long-term sequelae.1,2 In a minority of patients, however, the acute phase of the illness is followed by a chronic disease.4-6 Mild/moderate chronic active Epstein-Barr virus (CAEBV) infection syndrome presents with fever, malaise, arthralgia, and myalgia with lymphadenopathy, persisting for at least 6 months, and is associated with abnormally high titers of antibodies to EBV-capsid antigen (VCA-IgG) and early antigen (EA-IgG), with little or no antibody to EBV nuclear antigens (EBNA).3-7 Affected individuals may also have measurable EBV early antigen (EA)– messenger-RNA (mRNA) or EBV-DNA in the peripheral blood, serum, or affected tissues.4,6,7 The life-threatening form of CAEBV is characterized by severe fevers, hepatosplenomegaly, and extensive lymphoadenopathy, followed by hepatic, cardiac, or pulmonary dysfunction. These patients have very high EBV-VCA titers and viral copy numbers in their peripheral blood. Although EBV usually infects B lymphocytes, in this most severe form of CAEBV, either the T-cell or natural killer cell compartment may also be involved to produce lethal lymphomas.8-11 The severe cases are more common in Japan, whereas mild-to-moderate forms are more common in the West.5,12 Even when classified as mild/moderate, CAEBV is a severely debilitating disorder and quality of life may be further diminished by the attendant depression and by the adverse effects of therapy with anti-inflammatory drugs (including steroids).4-7
The pathogenesis and etiology of CAEBV syndrome are not well characterized, and it is not clear whether the defect lies in the virus or in the host response.13-15 This lack of information has hampered the development of effective therapies. Sporadic clinical improvements have been reported after infusion of interleukin 2 (IL-2), high-dose immunoglobulin, antiviral drugs, or steroids, but these results have been hard to replicate.16,17 Hence, for the majority of patients affected by CAEBV, there is a progressive deterioration in quality of life, and if the disease is severe, death rapidly supervenes even after intervention with aggressive chemotherapy or bone marrow transplantation.9 18
We and other groups have demonstrated that infusion of EBV-specific cytotoxic T lymphocytes (CTLs) safely and effectively restores defective EBV immunity after hematopoietic stem cell and solid organ transplantation.19-24 In particular, we have been successful in the ex vivo generation of EBV-specific T cells from patients who lacked CTL function in vivo.21,25 Once returned to the patients, these ex vivo–generated cells produced the desired antiviral effector function.19-21 We have also been successful in generating autologous EBV-specific T cells from patients affected by Hodgkin disease or with congenital immunodeficiency, and in using these cells to control EBV-related diseases.26 27
We have now investigated whether EBV-CTLs can regularly be generated from patients with mild/moderate forms of CAEBV and if infusion of these cells affects the symptoms, physical signs, and serologic markers of the disorder.
Patients, materials, and methods
Study entry criteria
Diagnosis of active EBV infection was made on the basis of (1) fever, fatigue, and lymphadenopathy, and/or hepatosplenomegaly, that had persisted for more than 6 months in patients who also had (2) an abnormal pattern of anti-EBV antibodies (anti-VCA ≥ 1:640 and/or absent anti-EBNA) and/or (3) the presence of early antigen mRNA or levels of EBV-DNA in serum or plasma that were more than 2 SD from normal. The clinical profile of the 8 patients at the time of study entry is summarized in Table1.
Patient characteristics at the time of study entry
| Patient no. . | Sex . | Age, y . | Laboratory findings . | Clinical signs and symptoms . | Duration . | Treatment . | |||
|---|---|---|---|---|---|---|---|---|---|
| VCA . | EBNA . | EBV-DNA . | EA . | ||||||
| 1 | M | 27 | >640 | Negative | N/A | mRNA + | Continuous fever, severe fatigue, abdominal lymphadenopathy, splenomegaly | 12 y | Aspirin, IV IgG, steroids, acyclovir |
| 2 | F | 42 | >640 | Negative | <4 | ND | Fever, sleep disturbance, headaches, flulike malaise, myalgia, sore throat, paresthesias, reduced daily activity | 6 y | Acyclovir, IV IgG |
| 3 | M | 18 | >640 | Negative | 97 | ND | Severe fatigue, sleep disturbance, night sweats, intermittent lymphadenopathy | 4 y | Steroid |
| 4 | F | 45 | >640 | Negative | <4 | ND | Fatigue, headaches, arthralgia, night sweats, intermittent lymphadenopathy | 4 y | Acyclovir |
| 5 | F | 18 | >1280 | Negative | <4 | ND | Fatigue, malaise, oral and genitourinary ulcer, cognitive impairment, sleep disturbance | 1 y | Acyclovir |
| 6 | F | 2 | N/A | Negative | 400 | mRNA + | Persistent fever | 8 mo | Gancyclovir |
| 7 | F | 2 | N/A | Negative | >4000 | ND | Persistent fever, hepato- and splenomegaly | 6 mo | Acyclovir, gancyclovir, foscavir |
| 8 | F | 37 | N/A | Positive | N/A | >1:150 | Fatigue, cervical lymphadenopathy, headache, sore throat, night sweats | 6 mo | Acyclovir |
| Patient no. . | Sex . | Age, y . | Laboratory findings . | Clinical signs and symptoms . | Duration . | Treatment . | |||
|---|---|---|---|---|---|---|---|---|---|
| VCA . | EBNA . | EBV-DNA . | EA . | ||||||
| 1 | M | 27 | >640 | Negative | N/A | mRNA + | Continuous fever, severe fatigue, abdominal lymphadenopathy, splenomegaly | 12 y | Aspirin, IV IgG, steroids, acyclovir |
| 2 | F | 42 | >640 | Negative | <4 | ND | Fever, sleep disturbance, headaches, flulike malaise, myalgia, sore throat, paresthesias, reduced daily activity | 6 y | Acyclovir, IV IgG |
| 3 | M | 18 | >640 | Negative | 97 | ND | Severe fatigue, sleep disturbance, night sweats, intermittent lymphadenopathy | 4 y | Steroid |
| 4 | F | 45 | >640 | Negative | <4 | ND | Fatigue, headaches, arthralgia, night sweats, intermittent lymphadenopathy | 4 y | Acyclovir |
| 5 | F | 18 | >1280 | Negative | <4 | ND | Fatigue, malaise, oral and genitourinary ulcer, cognitive impairment, sleep disturbance | 1 y | Acyclovir |
| 6 | F | 2 | N/A | Negative | 400 | mRNA + | Persistent fever | 8 mo | Gancyclovir |
| 7 | F | 2 | N/A | Negative | >4000 | ND | Persistent fever, hepato- and splenomegaly | 6 mo | Acyclovir, gancyclovir, foscavir |
| 8 | F | 37 | N/A | Positive | N/A | >1:150 | Fatigue, cervical lymphadenopathy, headache, sore throat, night sweats | 6 mo | Acyclovir |
F indicates female; M, male; N/A, not available; ND, not done; VCA, anti–viral capsid antigen IgG (values detected in normal EBV-seropositive donors range from 1:60 to 1:320); EBNA, anti-EBV nuclear antigen IgG (values detected in normal EBV-seropositive donors range from 1:20 to 1:160); EBV-DNA, number of copies/μg of DNA in peripheral blood (the sensitivity of the real-time polymerase chain reaction is 4 copies/μg; values detected in normal EBV-seropositive donors range from 0 to 200 copies/μg of DNA); EA, early antigen; and IV IgG, intravenous immunoglobulin.
Patient details
Patient no. 1 was a 27-year-old man, presenting with a 12-year history of fever, chronic fatigue, multiple lymphadenopathy, and hepato/splenomegaly. Chronic administration of steroids and nonsteroidal anti-inflammatory drugs had produced only limited control of his symptoms, and he developed analgesic nephropathy necessitating hemodialysis. At this time, a diagnosis of CAEBV was made based on the presence of chronic fever and fatigue in association with lymphadenopathy and hepatosplenomegaly, positive EBV antigenemia, and reverse transcriptase–polymerase chain reaction (RT-PCR) for the early antigen mRNA (see “Study entry criteria” above). He then received acyclovir, with no discernible benefit, and was entered in the study.
Patient no. 2 was a 42-year-old woman with a 6-year history of chronic fatigue, dizziness, sore throat, tremors, headaches, photosensitivity, paresthesia, and significant reduced daily activity. No lymph node enlargement or organomegaly was detected, but over the previous 6 years the patient had had a progressive increase in her anti–viral capsid antigen (VCA) titer (from 1:40 to 1:640) and loss of her anti-EBNA antibody, with free EBV-DNA detected in her serum. She was enrolled in our study after failing to benefit from treatment with acyclovir or immunoglobulin.
Patient no. 3 was an 18-year-old man who was diagnosed with CAEBV based on the presence of severe fatigue, intermittent lymphadenopathy, and night sweats persisting for more than 4 years, associated with an anti-VCA titer persistently more than 1:640 and a low anti-EBNA titer (< 1:5). He had failed to respond to acyclovir.
Patient no. 4 was a 45-year-old woman presenting with 4-year history of fatigue, arthralgia, flulike symptoms, headaches, and intermittent lymphadenopathy. The anti-VCA titer and anti-EBNA antibodies were consistently more than 1:640 and negative, respectively.
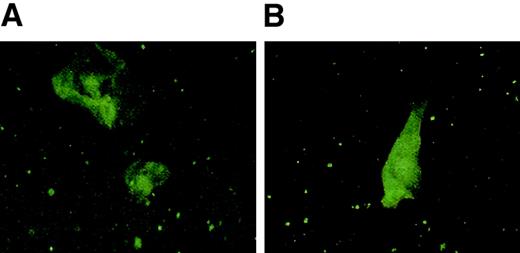
Patient no. 5 was an 18-year-old woman presenting with chronic fatigue and recurring genito-urinal and oral ulcerative lesions. These symptoms occurred after the diagnosis of primary EBV infection and had persisted for more than one year, despite acyclovir treatment. Anti-VCA antibody and anti-EBNA antibodies were persistently more than 1:1280 and negative, respectively. Biopsy of the oral and genital ulcerative lesions showed cells expressing both the early EBV-lytic antigen BZLF1 and the latent membrane protein antigen and LMP 1 respectively (Figure1).
EBV protein expression in cells from an ulcerative lesion from patient no. 5.
Epithelial cells obtained from a genital lesion from patient no. 5 were dried onto poly-L-lysine–coated slides and fixed in a chilled 1:1 mixture of methanol and acetone, blocked with 20% normal goat serum, stained with primary antibody for BZLF1 (Argene) or LMP-1 (mouse monoclonal OT22C), then stained with fluorescein isothiocyanate (FITC)–conjugated goat anti–mouse IgG. Slides were mounted with coverslips using Vectashield (Vector Laboratories) and then subjected to confocal microscopy. Panel A shows staining with BZLF1 and panel B shows staining with LMP-1.
EBV protein expression in cells from an ulcerative lesion from patient no. 5.
Epithelial cells obtained from a genital lesion from patient no. 5 were dried onto poly-L-lysine–coated slides and fixed in a chilled 1:1 mixture of methanol and acetone, blocked with 20% normal goat serum, stained with primary antibody for BZLF1 (Argene) or LMP-1 (mouse monoclonal OT22C), then stained with fluorescein isothiocyanate (FITC)–conjugated goat anti–mouse IgG. Slides were mounted with coverslips using Vectashield (Vector Laboratories) and then subjected to confocal microscopy. Panel A shows staining with BZLF1 and panel B shows staining with LMP-1.
Patient no. 6 was a 2-year-old girl who presented with fever persisting for more than 8 months associated with seropositivity for EA mRNA, negativity for anti-EBNA antibodies, and high peripheral blood EBV-DNA.
Patient no. 7 was a 2-year-old girl diagnosed with CAEBV based on the presence of persistent fever, hepato- and splenomegaly and high EBV-DNA titer for more than 6 months. She did not respond to antiviral treatment.
Patient no. 8 was a 37-year-old woman who developed fever, night sweats, cervical lymphadenopathy, and splenomegaly persisting for more than 6 months after her primary EBV infection. Despite antiviral treatment, her symptoms persisted and her anti-EA antibody titer remained over 1:150.
Samples
Peripheral blood mononuclear cells (PBMCs) were obtained after Ficoll (Lymphoprep medium 1.077; Life Technologies, Grand Island, NY) gradient centrifugation (400g for 40 minutes) and processed for spontaneous cell line outgrowth, EBV-transformed lymphoblastoid cell line (LCL) generation, and EBV-DNA analysis. The remaining cells were frozen for CTL production.
Detection of anti-VCA and anti-EBNA antibodies in serum and plasma
IgG anti–viral capsid antigen (VCA) and anti-EBNA antibodies were measured in serum or plasma, using routine enzyme-linked immunosorbent assays (ELISAs) (Department of Pathology, Texas Children's Hospital, Houston, TX) or by immunofluorescence staining.28 Using these approaches, the normal range detected in 35 normal EBV-seropositive donors varied from 1:80 to 1:320 for anti-VCA IgG and from 1:20 to 1:160 for anti-EBNA IgG.
Detection of EA mRNA
EA mRNA was evaluated by nested RT-PCR, as reported by Prang et al.29 Briefly, using the guanidinium isothiocyamate-phenol, RNA was extracted from peripheral blood B cells enriched after MACS separation, and reverse transcribed into cDNA. RT-PCRs and nested PCRs were performed using the primers reported.29 The specificity of the PCR was controlled by Southern blot as previously described.29 The quality of the RNA/cDNA preparation was controlled by PCR for the histone H3.3. P3HR1/16, an EBV-positive cell line, and BJAB, an EBV-negative cell line, were used as positive and negative controls, respectively.29
Detection of EBV-DNA in serum and PBMCs
Serum samples were processed as reported by Gan et al.30 Briefly, the pellet obtained after spinning serum at 50 000g for 60 minutes at 4°C (Beckman Ultracentrifuge in TLA Rotor 100.3) was digested for 3 hours at 37°C in the presence of 8 μL proteinase K (20 mg/mL) and stored at −20°C until PCR amplification of EBV-DNA was performed. For PBMCs, DNA was isolated from 3 to 5 × 106 cells using an anion exchange column (Qiagen). DNA (500 ng) was then tested by real-time PCR to quantitate EBV genome numbers, as previously reported.25 The sensitivity of the real-time PCR is 4 copies/μg DNA. Values detected in the peripheral blood of 38 normal EBV-seropositive donors ranged from less than 4 to 200 (median < 4) copies/μg DNA.
EBV-transformed B-LCLs
For LCL generation, 5 million fresh or frozen PBMCs were infected with concentrated supernatant from the B95-8 EBV-producer cell line, as previously reported.20,21 All cell lines were cultured in complete medium consisting of RPMI-1640 (Hyclone, Logan, UT) supplemented with 10% fetal bovine serum (FBS; Hyclone) and 2 mM L-glutamine (Gibco BRL). In patients 2, 3, 4, and 6, PBMCs were cultured alone or in the presence of cyclosporine A to determine whether spontaneous EBV-positive cell lines could be grown out in vitro from this group of patients.10 The cultures were examined for morphology and fed at weekly intervals.
Generation of EBV-specific T-cell lines
Polyclonal EBV-specific T-cell lines were generated as previously reported.20 Briefly, PBMCs (2 × 106 per well of a 24-well plate) were stimulated with autologous LCLs irradiated at 40 Gy at an effector-to-stimulator (E/S) ratio of 40:1. After 10 days, viable cells were restimulated with irradiated LCLs (at 4:1 E/S ratio) and 5 days later, 40 U/mL recombinant human interleukin 2 (rhIL-2; Proleukin, Chiron, Emeryville, CA) was added. Weekly restimulations with LCLs (at 4:1 E/S ratio) in the presence of IL-2 (40 U/mL) were then used to obtain sufficient cells for a minimum of least 3 infusions of 2 × 107 cells/m2. After expansion, the CTLs were examined for sterility, HLA antigen identity (using polymerase chain reaction–single-strand polymorphism DNA [PCR-SSP DN]–based procedures), immunophenotype, and EBV specificity, and then cryopreserved in 15% dimethyl sulfoxide (DMSO) (Sigma, St Louis, MO), 35% Hanks balanced salt solution (HBSS; Gibco) and 50% human serum albumin. All CTL and LCL lines were grown in the dedicated clinical cell culture facility in the Center for Cell and Gene Therapy at Baylor College of Medicine, following federally approved standard operating procedures. All reagents, supplies, and equipment were specifically designated for use in the facility with records kept regarding certificates of analysis and lot numbers.
Immunophenotyping
Cell surface phenotype was investigated using the following monoclonal antibodies: CD56 (Immunotech, Miami, FL), CD16, CD19, TCRαβ, TCRγδ, CD8, CD4, and CD3 (all from Becton Dickinson, San Jose, CA). Cells were analyzed with a FACSscan flow cytometer (Becton Dickinson). After external staining with CD8 or TCRγδ antigens, T-cell lines were fixed in paraformaldehyde (PFA) 4%, permeabilized with 0.1% saponin (Sigma), and stained for phycoerythrin (PE)–conjugated anti–granzyme A monoclonal antibody (CB-9; Pharmingen, San Jose, CA) or antiperforin monoclonal antibody (δG9; Pharmingen). Appropriate matched isotype controls (Becton Dickinson) were used in each experiment.
Chromium release assays
The cytotoxic activity of each CTL line was evaluated in a standard 4-hour 51Cr release assay, as previously described.31 Target cells included autologous LCLs, HLA antigen class I–mismatched LCLs, and the lymphokine-activated, killer cell–sensitive, T-cell lymphoma (HSB-2). HLA antigen class I and class II restricted killing was determined by preincubating target cells for 30 minutes with the monoclonal antibody W6/32 (Dako, Carpinteria, CA), which recognizes a monomorphic HLA antigen class I determinant, and the monoclonal antibody CR3/43 (Dako), which recognizes HLA-DR, -DP, and -DQ. To analyze the HLA antigen restricting alleles of the CTL lines, their cytotoxic activity was tested against a panel of LCLs sharing one or 2 HLA class I antigens. To analyze antigen specificity, autologous PHA blasts loaded with peptides representing known viral epitopes as well as HLA antigen–matched fibroblasts infected with vaccinia recombinants expressing the EBV latent antigens, EBNA 3A, 3B, and 3C, the lytic antigen BZLF1, or the control antigen, green fluorescent protein (GFP), were used as targets, as previously described.32
Adoptive transfer of EBV-specific CTLs and follow-up evaluation
Cells were thawed rapidly at 37°C and administered intravenously after premedication with antihistamine drugs. Patients were monitored for vital parameters before and immediately after the infusion. The first patient received CTLs at the Institute Charité in Berlin, Germany, with local internal review board (IRB) approval. He received 3 infusions of 1 × 107cytotoxic T cells/m2. The subsequent 4 patients were treated on a study approved by the Food and Drug Administration (FDA) and the Baylor College of Medicine IRB. The second patient received 4 injections of 2 × 107 cytotoxic T cells/m2, 6 months and then 3 months apart. The third and fourth patients received one infusion of 2 × 107 cytotoxic T cells/m2. The fifth patient received 2 infusions of 2 × 107 cytotoxic T cells/m2, 3 months apart.
To measure the effect of CTL infusion on the EBV-specific T precursor frequency, the number of cells producing interferon gamma (IFN-γ) in response to autologous LCL stimulation was assessed on PBMCs in toto, collected before and 2 or 4 weeks after CTL infusion, by Elispot assay, as reported previously with slight modifications.33 Briefly, MAHA S45 plates (Millipore) were coated with anti–IFN-γ antibody 1 DIK (Mabtech) overnight and blocked with complete medium for 1 hour at 37°C. Thawed PBMCs were added at doubling dilution from 1 × 105/well (3 replicates for each dilution) in the presence of autologous LCLs for 24 hours at 37°C, washed off, and then plates were incubated for 2 hours at 37°C with biotin anti–IFN-γ antibody 7-B6-1 (Mabtech). Appropriate controls consisting of PBMCs, LCLs, and medium alone were also plated and incubated with biotin anti–IFN-γ antibody 7-B6-1. Avidin peroxidase complex (Vector Laboratories, Burlingame, CA) was added for 1 hour at room temperature and spots developed with 3-amino-9-ethylcarbazole (AEC; Sigma) substrate mix. Spots were counted (Zellnet Consulting, New York, NY) and expressed as number of spots/106 cells when dilution was linear. A negligible number of spots (< 5) were produced by medium or LCLs or PBMCs alone.
Statistical analysis
A paired, 2-tailed Student t test (95% confidence interval) was used to determine the statistical significance of differences between samples. All data are represented as mean ± one standard deviation (SD).
Results
Characteristics of EBV-transformed B-cell lines from CAEBV patients
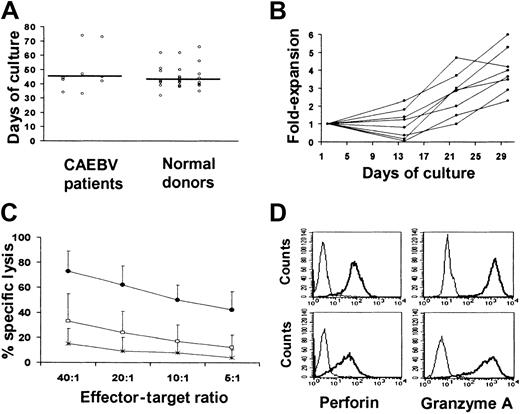
Spontaneous EBV transformation of B cells was not seen in any patient, even when cyclosporin A was added to the cell culture. Hence, EBV-transformed B LCLs were established using virus concentrated from a master cell bank of the B95-8 EBV producer cell line.31LCLs were successfully established (> 2 × 107 LCLs) from all 8 patients in a time frame similar to that required for LCL development from healthy individuals (median of 45 days versus 42 days) (Figure 2A).
Generation of lymphoblastoid cell lines and autologous EBV-specific CTLs from patients with persisting active EBV infection.
(A) The time required for the establishment of LCLs, using the B95-8 laboratory strain of EBV, from the 8 patients affected by CAEBV infection, was comparable to that required by a series of 36 healthy donors. (B) The kinetics of each CTL line generated from the 8 patients affected by CAEBV infection using weekly stimulations with autologous LCLs in the presence of IL-2. (C) The killing activity of the EBV-specific CTLs, as assessed in a standard chromium release assay. HBS-2 target cell lines (■), mismatched LCLs (*), and autologous LCLs (●) were labeled with 51Cr and incubated for 4 hours with CTL at the E/T ratios indicated. Killing of autologous LCLs was significantly higher compared with mismatched LCL and HSB-2, a LAK-sensitive EBV-negative T-cell lymphoma that provides a measure of lymphokine-activated killer cell–mediated killing. Means of the percent specific chromium release from target cells ± SD of the 8 CTL lines are presented. (D) The perforin (left) and granzyme A (right) expression of 2 representative CTL lines. CTLs were stained for intracellular perforin or granzyme using PE-labeled antiperforin or antigranzyme antibodies (thick line) versus isotype control (thin line) and analyzed by flow cytometry. Upper and lower panels represent the staining of a representative CD8+ CTL line and of the CD3+ TCRγδ+ cells generated from patient 2, respectively.
Generation of lymphoblastoid cell lines and autologous EBV-specific CTLs from patients with persisting active EBV infection.
(A) The time required for the establishment of LCLs, using the B95-8 laboratory strain of EBV, from the 8 patients affected by CAEBV infection, was comparable to that required by a series of 36 healthy donors. (B) The kinetics of each CTL line generated from the 8 patients affected by CAEBV infection using weekly stimulations with autologous LCLs in the presence of IL-2. (C) The killing activity of the EBV-specific CTLs, as assessed in a standard chromium release assay. HBS-2 target cell lines (■), mismatched LCLs (*), and autologous LCLs (●) were labeled with 51Cr and incubated for 4 hours with CTL at the E/T ratios indicated. Killing of autologous LCLs was significantly higher compared with mismatched LCL and HSB-2, a LAK-sensitive EBV-negative T-cell lymphoma that provides a measure of lymphokine-activated killer cell–mediated killing. Means of the percent specific chromium release from target cells ± SD of the 8 CTL lines are presented. (D) The perforin (left) and granzyme A (right) expression of 2 representative CTL lines. CTLs were stained for intracellular perforin or granzyme using PE-labeled antiperforin or antigranzyme antibodies (thick line) versus isotype control (thin line) and analyzed by flow cytometry. Upper and lower panels represent the staining of a representative CD8+ CTL line and of the CD3+ TCRγδ+ cells generated from patient 2, respectively.
Characteristics of EBV-specific CTLs from CAEBV patients
Growth rate.
EBV-specific CTLs were successfully generated from all 8 CAEBV patients. As shown in Figure 2B, the rate of CTL expansion obtained in these patients was comparable to the pattern observed with CTLs from more than 60 healthy allogeneic bone marrow donors, with a mean of 4-fold expansion after 3 stimulations (range, 2.3-6).21 31
Phenotype.
The majority of the CTL lines contained predominantly CD3+CD8+ T cells (mean 82%, range 50%-98%), whereas CD3+ CD4+ lymphocytes ranged from 2% to 36% of the total cells. In all but one T-cell line, more than 90% of cells were TCR αβ receptor positive. The exception was the CTL obtained from patient 2, which expressed γδ TCR on 35% of CD3+cells. Natural killer cells (defined as cells expressing CD56 and/or CD16 antigen but lacking CD3 expression) represented less than 2% of the population (range, 0%-2.2%). In 4 T-cell lines, 15% to 30% of the CD3+ cells had a lymphokine activated killer (LAK) cell phenotype, coexpressing the CD56 antigen. No CD19+ cells were detected, excluding the presence of surviving B cells in the infusion product (Table 2).
Immunophenotype of Epstein-Barr virus–specific cytotoxic T lymphocytes
| . | CD3+CD8+ . | CD3+CD4+ . | CD3+CD56+ . | CD3−CD56/16+ . | CD19+ . | TCR αβ . | TCR γδ . |
|---|---|---|---|---|---|---|---|
| Mean ± SD | 82 ± 18.8 | 16.4 ± 18.7 | 15.6 ± 13.4 | 1.2 ± 1.1 | 0 ± 0 | 93 ± 10.4 | 5.5 ± 11.9 |
| Range | 50-98 | 1.5-36 | 0.8-20 | 0-2.2 | 0 | 67-99 | 0.2-35 |
| . | CD3+CD8+ . | CD3+CD4+ . | CD3+CD56+ . | CD3−CD56/16+ . | CD19+ . | TCR αβ . | TCR γδ . |
|---|---|---|---|---|---|---|---|
| Mean ± SD | 82 ± 18.8 | 16.4 ± 18.7 | 15.6 ± 13.4 | 1.2 ± 1.1 | 0 ± 0 | 93 ± 10.4 | 5.5 ± 11.9 |
| Range | 50-98 | 1.5-36 | 0.8-20 | 0-2.2 | 0 | 67-99 | 0.2-35 |
Results are expressed as percentage of positive cells for surface markers by FACS analysis. The mean ± standard deviation and range of the 8 ex vivo–expanded, autologous, Epstein-Barr virus–specific T-cell lines are presented.
Cytotoxic activity.
In all cases CTL showed a significantly higher killing of the autologous LCLs (mean 62%, range 35%-80%, at an E/T cell ratio of 20:1) than of the HLA antigen mismatched LCLs (mean 9%, range 0%-33%, P < .001) or of HSB-2 (24%, range 0%-52%,P < .001) (Figure 2C). Moreover, killing was inhibited in the presence of the anti-HLA antigen class I monoclonal antibody (mean inhibition of 36%, range, 5%-51%, P < .001). There was no evidence of HLA class II antigen–restricted killing in any of the lines. No killing was observed against autologous PHA blasts, suggesting that autoreactive T cells had not been generated. Killing of target cells by these ex vivo–expanded EBV-specific CTLs was likely mediated through the perforin-granzyme pathway. Figure 1D shows that perforin and granzyme were expressed by both CD8+ cells (upper panels) and γδ-TCR, CD3+ CD8−CD4− effector cells expanded from patient 2 (lower panels).
Antigen specificity.
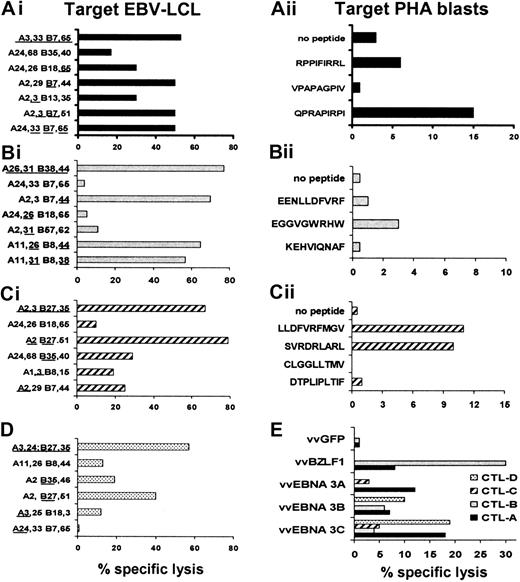
Most healthy individuals have immune responses directed against both EBV latent and lytic cycle proteins.34 It is possible that a defect in the CTL response to one or other groups of antigens may explain the imperfect control of viral replication in CAEBV patients. We therefore analyzed the antigen specificity of the CTL lines from 4 patients, determining the HLA antigen–restricted recognition of known EBV peptide epitopes. As shown in Figure3, CTLs from these patients recognized some of the predicted immunodominant EBV peptide epitopes as described for CTLs generated from healthy EBV-positive individuals.35 Moreover, lytic cycle antigen-specific CTLs were present in the CTL cultures, as demonstrated by lysis of fibroblasts infected with a vaccinia recombinant expressing BZLF 1 (Figure 3).
Characterization of EBV specificity of CTL lines expanded from 4 patients with CAEBV.
The EBV specificity of the T-cell lines generated from patients with persistent active EBV infection syndrome was determined by testing the cytotoxic activity of CTLs against a panel of LCL lines, EBV peptide-loaded autologous PHA blasts, or vaccinia virus–infected matched fibroblasts. Data from 20:1 E/T ratios are presented. Panels Ai, Bi, Ci, and D show the CTL recognition of autologous, HLA antigen–partially matched and HLA antigen–mismatched LCLs of 4 representative CTL lines. The CTL presented in panel Ai kills preferentially through B7, whereas intermediate cytotoxicity is directed against LCLs matched for A3 or B65. The CTL presented in panel Bi kills preferentially through B38 and B44, since no significant cytotoxic activity is presented against LCLs matched only for A31 or A26. Panel Ci shows that the T-cell line is prevalently B27-restricted, though A2-, A3-, and B35-restricted killing also occurs. CTLs presented in panel D also kill through B27. Once the HLA antigen restriction was established, the peptide epitope specificity was investigated on autologous or matched PHA blasts loaded with the peptides predicted from the literature (panels Aii, Bii, and C).35 49 The HLA antigen class I restriction and antigen location for the peptide epitopes used in the CTL assay are as follows: RPPIFIRRL (B7 EBNA 3A, coordinate 379-387), VPAPAGPIV (B7 EBNA 3C, coordinate 502-510), QPRAPIRPI (B7 EBNA 3A, coordinate 881-889), EENLLDFVRF (B44 EBNA 3C, coordinate 281-290), EGGVGWRHW (B44 EBNA 3C, coordinate 163-171), KEHVIQNAF (B44 EBNA 3C, coordinate 335-343), LLDFVRFMGV (A2 EBNA 3C, coordinate 284-293), SVRDRLARL (A2 EBNA 3A, coordinate 596-604), CLGGLLTMV (A2 LMP2a, coordinate 426-434), DTPLIPLTIF (A2 EBNA 2, coordinate 42-51). In panel E, these 4 lines (CTL line A, closed bar; CTL line B, gray; CTL line C, striped bar; CTL line D, dotted bar) were tested against HLA antigen–matched dermal fibroblasts infected with vaccinia virus recombinants expressing the EBNA 3A, B, and C genes or the lytic BZLF1 gene or GFP gene, as control.
Characterization of EBV specificity of CTL lines expanded from 4 patients with CAEBV.
The EBV specificity of the T-cell lines generated from patients with persistent active EBV infection syndrome was determined by testing the cytotoxic activity of CTLs against a panel of LCL lines, EBV peptide-loaded autologous PHA blasts, or vaccinia virus–infected matched fibroblasts. Data from 20:1 E/T ratios are presented. Panels Ai, Bi, Ci, and D show the CTL recognition of autologous, HLA antigen–partially matched and HLA antigen–mismatched LCLs of 4 representative CTL lines. The CTL presented in panel Ai kills preferentially through B7, whereas intermediate cytotoxicity is directed against LCLs matched for A3 or B65. The CTL presented in panel Bi kills preferentially through B38 and B44, since no significant cytotoxic activity is presented against LCLs matched only for A31 or A26. Panel Ci shows that the T-cell line is prevalently B27-restricted, though A2-, A3-, and B35-restricted killing also occurs. CTLs presented in panel D also kill through B27. Once the HLA antigen restriction was established, the peptide epitope specificity was investigated on autologous or matched PHA blasts loaded with the peptides predicted from the literature (panels Aii, Bii, and C).35 49 The HLA antigen class I restriction and antigen location for the peptide epitopes used in the CTL assay are as follows: RPPIFIRRL (B7 EBNA 3A, coordinate 379-387), VPAPAGPIV (B7 EBNA 3C, coordinate 502-510), QPRAPIRPI (B7 EBNA 3A, coordinate 881-889), EENLLDFVRF (B44 EBNA 3C, coordinate 281-290), EGGVGWRHW (B44 EBNA 3C, coordinate 163-171), KEHVIQNAF (B44 EBNA 3C, coordinate 335-343), LLDFVRFMGV (A2 EBNA 3C, coordinate 284-293), SVRDRLARL (A2 EBNA 3A, coordinate 596-604), CLGGLLTMV (A2 LMP2a, coordinate 426-434), DTPLIPLTIF (A2 EBNA 2, coordinate 42-51). In panel E, these 4 lines (CTL line A, closed bar; CTL line B, gray; CTL line C, striped bar; CTL line D, dotted bar) were tested against HLA antigen–matched dermal fibroblasts infected with vaccinia virus recombinants expressing the EBNA 3A, B, and C genes or the lytic BZLF1 gene or GFP gene, as control.
Infusion of EBV-specific CTLs
Ex vivo–expanded autologous EBV-specific CTLs were given to 5 of the above patients with CAEBV infection. CTL infusion was well tolerated by all, with no immediate or delayed adverse events.
In patient no. 1, the first dose of CTLs (1 × 107/m2) was followed within 10 days by disappearance of fever and reduction in lymphadenopathy. One month later his symptoms and signs recurred, although less severely, and he received 2 more infusions at the same dose, 2 weeks apart. These resulted in complete and sustained disappearance of fever, lymphadenopathy, hepatomegaly, and splenomegaly (from 1800 mL to < 400 mL). After more than 36 months, the patient remains in clinical remission, with no evidence of EA mRNA or antigenemia (Table3). He has returned to work after 5 years of absence, although his analgesic nephritis did not improve.
Virologic evaluation after CTL infusion
| Patient no. . | Virologic findings . | Before CTL infusion . | After CTL infusion3-150 . |
|---|---|---|---|
| 1 | EA mRNA | Positive | Negative |
| EBV-antigenemia | Positive | Negative | |
| Anti-VCA | >1:640 | 1:160 | |
| Anti-EBNA | Negative | N/A | |
| 2 | Anti-VCA | 1:640 | 1:80 |
| Anti-EBNA | Negative | 1:20 | |
| 3 | Anti-VCA | 1:640 | 1:80 |
| Anti-EBNA | 1:5 | 1:5 | |
| EBV-DNA | 97 | 0 | |
| 4 | Anti-VCA | 1:640 | 1:80 |
| Anti-EBNA | Negative | 1:40 | |
| 5 | Anti-VCA | 1:1280 | 1:160 |
| Anti-EBNA | Negative | 1:80 |
| Patient no. . | Virologic findings . | Before CTL infusion . | After CTL infusion3-150 . |
|---|---|---|---|
| 1 | EA mRNA | Positive | Negative |
| EBV-antigenemia | Positive | Negative | |
| Anti-VCA | >1:640 | 1:160 | |
| Anti-EBNA | Negative | N/A | |
| 2 | Anti-VCA | 1:640 | 1:80 |
| Anti-EBNA | Negative | 1:20 | |
| 3 | Anti-VCA | 1:640 | 1:80 |
| Anti-EBNA | 1:5 | 1:5 | |
| EBV-DNA | 97 | 0 | |
| 4 | Anti-VCA | 1:640 | 1:80 |
| Anti-EBNA | Negative | 1:40 | |
| 5 | Anti-VCA | 1:1280 | 1:160 |
| Anti-EBNA | Negative | 1:80 |
CTL indicates cytotoxic T lymphocytes; EA mRNA, early antigen messenger RNA; VCA, anti–viral capsid antigen (IgG); EBNA, anti-EBV nuclear antigen (IgG); and N/A, not available.
Evaluation performed 2 weeks after the adoptive transfer. Data were reconfirmed at the 4-week post-CTL infusion follow-up evaluation.
Patient no. 2 had no significant clinical improvement of symptoms after her first injection of 2 × 107 CTL/m2, although her anti-VCA titer declined from more than 1:640 to 1:80 and her anti-EBNA titer increased from undetectable to 1:20. The patient received 2 additional infusions of an equal number of CTLs 6 and 9 months after the first injection which resulted in a significant improvement of all symptoms in association with maintenance of her normalized viral serology (Table 3). A fourth injection was administered one year after the first infusion, though the patient had no symptoms and a normal performance status. After 2 months, she developed recurrence of fatigue and myalgia and was treated with anti–TNF-α antibody.
In patient no. 3, the symptoms of severe fatigue and sleep disturbance improved within 2 months of infusion (2 × 107CTL/m2) and at 10 months after infusion he remains well, has a normal sleep pattern, and is now employed full-time. His anti-VCA titer dropped from 1:640 to 1:80 within 2 weeks of the CTL infusion, and EBV-DNA completely disappeared from serum (Table 3).
In patient no. 4, fatigue improved within 3 months of CTL infusion (2 × 107/m2). Anti-EBNA antibodies became detectable and the high anti-VCA titer returned to normal (Table3). At 12 months follow-up, her clinical and laboratory findings remain stable.
Patient no. 5 had clinical resolution of EBV-positive oral and genital ulcers and decrease of the anti-VCA titer from 1:1280 to 1:160 after her first CTL injection (2 × 107/m2). A second infusion at the same dose, performed 3 months later, stabilized her virologic and clinical response. At a follow-up of 6 months after the first infusion, the patient has less fatigue and oral and genital lesions have not relapsed.
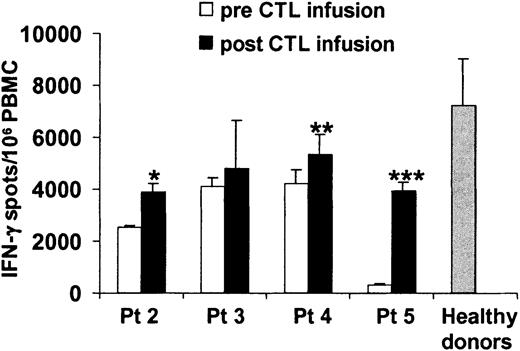
The immunologic status of patients 2, 3, 4, and 5 was assessed before and 2 to 4 weeks after the adoptive transfer by INF-γ Elispot assay. In all cases, an increase in the frequency of circulating EBV-specific T cells as measured by interferon-γ Elispot was demonstrated after CTL infusion (Figure4). However, the frequency of EBV precursors remains below the normal range observed in healthy donors.
Evaluation of the frequency of EBV-specific T cells in the peripheral blood of patients infected with CAEBV syndrome before and after adoptive immunotherapy.
The frequency of EBV-specific cells was determined by Elispot assay on PBMCs before (■) and after (▪) CTL infusion. Bars represent the mean numbers ± SD of interferon-gamma spot-forming cells per 106 PBMCs. Data from patient no. 1 were not available. ░ represents the mean ± SD of interferon-gamma spot-forming cells per 106 PBMCs observed in a representative series of normal healthy donors. * indicates P = .01; ** indicatesP = .03; *** indicates P = .002.
Evaluation of the frequency of EBV-specific T cells in the peripheral blood of patients infected with CAEBV syndrome before and after adoptive immunotherapy.
The frequency of EBV-specific cells was determined by Elispot assay on PBMCs before (■) and after (▪) CTL infusion. Bars represent the mean numbers ± SD of interferon-gamma spot-forming cells per 106 PBMCs. Data from patient no. 1 were not available. ░ represents the mean ± SD of interferon-gamma spot-forming cells per 106 PBMCs observed in a representative series of normal healthy donors. * indicates P = .01; ** indicatesP = .03; *** indicates P = .002.
Discussion
In this study we have demonstrated that we can generate EBV-specific CTLs from patients with mild/moderate CAEBV infection and that these CTLs have the same phenotype, function, and expansion kinetics as EBV-specific CTLs from healthy donors. CTL infusion appears safe and able to improve the clinical symptoms and signs related to chronic active EBV infection. The cell infusions also produce normalization of immune serology to the virus.
In principle, the CAEBV syndrome may be attributed either to abnormalities in the virus, or in the host response to infection. Although difficulties in establishing EBV-positive B-cell lines have been reported, those that have been obtained from patients with severe or mild forms of CAEBV infection express the same type III pattern of viral latency antigen expression as is observed in B-LCLs from healthy donors.36,37 This shows that the virus can establish latency in patient B cells, perhaps implying that the defect of CAEBV results from abnormalities in the host response to EBV. However, there may be other aspects of the virus-host relationship that are perturbed. While most CAEBV patients have normal neutralizing antibodies to EBV, they lack antibody to EBNA.38 This pattern is also characteristic of the response to EBV in patients with Burkitt lymphoma or AIDS, and further supports the concept of an immune defect.39,40 Such a defect could be relatively subtle and need not be truly EBV specific. For example, a primary defect of T cells has recently been demonstrated in patients with X-linked lymphoproliferative syndrome (XLP). These patients have a mutation in the signaling lymphocytic activation molecules (SLAM)–associated protein (SAP) gene that leads to a decreased ability to control virus-specific immune responses in general, including those directed to EBV.41 However, no such mutation has been found in patients with CAEBV.11,41 Alternatively, patients may have a disruption of Th1- and Th2-mediated immunity. It is generally thought that a Th1 response is important for the control of EBV, and the presence of circulating cytokines inhibitory to these cells may account for the increased risk of outgrowth by virus-infected B cells observed in both transplant recipients and patients with Hodgkin disease.42 In these patient groups, the cytokines IL-10 and IL-6 are present at the site of disease and in the circulation and may up-regulate IL-4 production by Th2 cells, which in turn suppresses activation of Th1 cells.43,44 We did not observe abnormal levels of hIL-10 or IL-6 in our own CAEBV patients (data not shown). However, increased levels of both these cytokines have been measured in plasma and serum samples from patients with acute EBV-induced mononucleosis, while viral IL-10 has been detected in many patients with CAEBV.45 Finally, it has been demonstrated in animal models that defects in the killing pathways of CTLs have a profound impact on homeostatic immune regulation during chronic infections.46 It is likely that the broad spectrum of clinical symptoms and signs of CAEBV can result from an equally broad spectrum of underlying immunologic disorders.47 The fact that signs and symptoms normalized after CTL infusion suggests that in some individuals the balance between cellular immunity and viral pathogenesis is disrupted and that an increase in cellular immunity may restore the balance in favor of a normal apathogenic latent state.
Our results have a number of implications for improving our understanding the pathobiology of CAEBV, at least of the mild/moderate forms common in the Western Hemisphere. The first is that there is clearly no gross loss of EBV-specific immunity. None of the individuals we studied had B cells that were able to form spontaneous LCL lines in the presence of autologous T cells. This result would be anticipated from an earlier study, which reported successful spontaneous outgrowth of EBV-positive B cells in just 30% of patients with CAEBV infection.35 The outgrowth of spontaneous LCLs in the absence of T-cell inhibitors is strongly associated with lymphoproliferative disease in hematopoietic stem cell transplant (SCT) recipients and indicates a gross lack of immune control of EBV-infected B cells.48 Hence, the lack of such spontaneous LCL outgrowth in our patients implies that immune control of infected B cells was intact, and indeed only one of our patients had an elevated virus load in peripheral blood.
The use of spontaneous EBV-transformed cell lines as antigen-presenting cells may expand CTLs that recognize the specific sequences of the endogenous virus.32 However, since we were unable to grow spontaneous EBV-infected cell lines from our cases we used the B95-8 strain of EBV to produce transformation. CTLs generated in response to the B95-8 strain of EBV should be effective in controlling wild-type EBV-infected cells, since EBV is a genetically stable virus and immunodominant epitopes appear to be conserved over time within an individual and between geographically isolated strains.35,49 Further, we have shown in more than 50 hematopoietic SCT recipients that B95-8 virus–specific CTLs are able to control endogenous strains of virus.21 Once infected with the B95-8 strain of virus, patient B cells showed no abnormalities. The time required for LCL establishment was comparable to that observed in healthy donors, as was the pattern of latency antigen expression (not shown). The rate of EBV-specific CTL expansion was also comparable to that observed in normal EBV-seropositive donors, and these ex vivo–expanded CTLs showed the typical coexpression of CD3 and CD8 antigens and specific HLA class I antigen–restricted cytotoxic activity against autologous LCLs previously observed in healthy donors.21 While patient CTLs recognized some of the immunodominant HLA antigen class I–restricted peptides predicted from studies performed with healthy donors, more work is required to determine if the patient CTL repertoire is appropriate or sufficient for the control of EBV.35,49 These essentially normal sets of ex vivo immune response data seem to refute the possibility that an irreversible defect in T-cell immunity is responsible for this illness. However, we have also shown that EBV-specific CTL lines with apparently normal function can readily be generated from patients who have developed EBV-positive posttransplantation lymphoproliferative disease and are evidently unable to control their EBV infection in vivo.25 In this context, removal of T lymphocytes from their immunosuppressive environment in vivo allows EBV-specific CTL precursors to expand and function normally.23-25 It remains to be determined whether or not such an inhibitory environment exists in CAEBV patients, and if it does whether it may be attributed to abnormal levels of inhibitory cytokine production.
As in other recipients of EBV-specific CTLs, administration of these cells to patients with CAEBV appeared safe. None of the patients had immediate or late adverse events due to CTL infusion. Clinical benefits represented by the resolution of symptoms (eg, fatigue, malaise, myalgia/arthralgia) and physical signs (eg, fever, ulceration, lymphadenopathy, and organomegaly) occurred in all and were associated with normalization of the serologic response to EBV antigens. Although patients often required more than one dose of CTLs, the total number of cells required to obtain a stable response was low, ranging from 2 to 6 × 107 CTL/m2. Our experience with gene-marked CTLs in SCT recipients has shown that such small numbers of cells can undergo substantial expansion in vivo and can persist long term (> 6 years).20 21 Massive expansion as seen in SCT recipients may not occur in patients with CAEBV for several reasons. First, inhibitory factors/cytokines may circulate in patients with CAEBV; second, unlike the SCT recipient, who has an empty niche for EBV-specific CTLs, the CAEBV patient already has circulating EBV-specific CTLs and homeostatic mechanisms may function to prevent expansion of the infused CTLs. Finally, the SCT recipient has a regenerating immune system that may be conducive to CTL expansion. Notwithstanding, the infused CTLs did increase the frequency of EBV-specific CTLs in CAEBV patients after infusion, but only gene-marking studies will determine if the infused CTLs expand in vivo. Long-term control of disease in all patients may require higher doses or boosting.
Although we do not know whether this same approach can successfully be used for patients with the progressive lethal forms of the disease,50 even patients with mild/moderate CAEBV infection often have a poor and progressively diminishing quality of life. The response of the patients and the persistence of the clinical, immunologic, and virologic effects suggest that autologous EBV-specific CTL infusions may represent an additional treatment option for this syndrome. However, since some of the reported responses such as fatigue and sleep disturbance were subjective, a controlled trial, with careful selection of the patients enrolled, is required to shed light on the utility of this approach.
We are grateful for the consistent and reliable support of Brian Newsom, Mike Cubbage, Tatiana Gotsolva, and April Durett of the Flow Cytometry Core Facility for FACS analyses. We thank Teresita Lopez and the QA/QC group of the GMP facility in the Center for Cell and Gene Therapy at Baylor College of Medicine for excellent technical assistance. We also thank Goley Richardson for nursing support and Shannon Inman for data management.
Prepublished online as Blood First Edition Paper, August 1, 2002; DOI 10.1182/blood-2002-01-0039.
Supported by National Institutes of Health grant CA61384, the Department of Pediatrics, Baylor College of Medicine and General Clinical Research Center (GCRC) at Baylor College of Medicine (RR00188). B.S. is a recipient of an Elizabeth Glaser Pediatric Research Foundation Clinical Investigator Fellowship. H.E.H. is a recipient of a Doris Duke distinguished clinical scientist award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Cliona M. Rooney, Center for Cell and Gene Therapy, 6621 Fannin St, MC 3-3320, Houston, TX 77030; e-mail:crooney@bcm.tmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal