Using the allo-restricted T-cell approach to circumvent tolerance, we have previously identified a cytotoxic T-lymphocyte (CTL) epitope in the transcription factor Wilms tumor antigen 1 (WT1) presented by HLA-A0201 (A2) class I molecules. Here we describe an additional A2-presented epitope and show that CTLs against both epitopes kill WT1-expressing leukemia cell lines. Colony-forming assays demonstrated that both types of CTL killed CD34+progenitor cells from A2+ leukemia patients, but not from A2+ healthy individuals. The long-term culture-initiating cell (LTC-IC) assay was used to analyze the killing activity of WT1-specific CTLs against the more immature fraction of CD34+ cells. The CTLs killed LTC-ICs of patients with chronic myelogenous leukemia (CML), whereas the function of normal CD34+ progenitor/stem cells was not inhibited. Together, the data show that CTLs specific for 2 distinct peptide epitopes of WT1 can discriminate between normal and leukemia LTC-ICs, suggesting that such CTLs have the potential to selectively kill CML progenitor/stem cells.
Introduction
Wilms tumor antigen 1 (WT1) is a transcription factor essential for embryonic development, but after birth low-level expression is restricted to few cell types including hemopoietic stem cells, myoepithelial progenitor cells, renal podocytes, and some cells in testis and ovary.1-5 Recent studies have demonstrated that WT1 is overexpressed in several types of leukemia and that overexpression may be required for the uncontrolled proliferation and defective differentiation of leukemic cells.6-8
Tumor elimination is most effectively accomplished by high-avidity antigen-specific cytotoxic T lymphocytes (CTLs).9However, the described low-level expression of WT1 in normal cells would be expected to cause partial or complete tolerance of high-avidity CTLs. In the past, we have developed the allo-restricted CTL approach as a tool to circumvent immunologic tolerance to proteins expressed in normal cells.10 Using this approach we have recently generated high-avidity allo-restricted CTLs against an HLA-A0201 (A2)–presented, WT1-derived peptide, called WT126. We now describe high-avidity allo-restricted CTLs specific for a new A2-presented epitope, WT235, and show that CTLs against both epitopes can recognize immature CD34+ cells from patients with chronic myelogenous leukemia (CML).
Study design
Cell lines
Myeloid leukemia lines BV173, LAMA81, U937, SD-1, BAF-3, KU-812, 697, and T2 cells (deficient in transporter associated with antigen processing [TAP] and thus incapable of endogenous peptide loading) were maintained in RPMI plus 10% fetal calf serum (FCS).
Allo-HLA–restricted CTL lines
Allo-restricted CTLs were first stimulated in bulk cultures with peptide-loaded, TAP-deficient cells, followed by plating in 96-well plates under limiting dilution conditions to identify and expand peptide-specific CTL lines.11 The WT235-specific CTL line 4 expanded best and was used in all experiments. The51Cr-release assays were done as previously described.11
CFU-GM and LTC-IC assays
The local ethics committee approved the use of clinical samples and patient consent was obtained. Normal CD34+ cells were purified from adult bone marrow (n = 1), mobilized peripheral blood, or cord blood (n = 7). Using the MiniMACS system (Miltenyi Biotec, Bisley, United Kingdom), leukemic CD34+ cells were purified from peripheral blood of CML patients in chronic phase who were not treated with interferon-α for at least 3 months. The cell purity was assessed by fluorescence-activated cell sorting (FACS) staining with fluorochrome-labeled anti-CD34 antibodies (clone 581, BD-Pharmingen, Oxford, United Kingdom) and ranged from 80% to 99%. Cells were cryopreserved and defrosted for each experiment. Granulocyte-macrophage colony-forming unit (CFU-GM) assays were performed as previously described.11 Cells were assayed for long-term culture-initiating cells (LTC-ICs) in a 5-week 2-stage culture at 33°C in LTC medium (Iscove modified Dulbecco medium [IMDM] plus 10% FCS, 10% horse serum, and 10−6mol/L hydrocortisone). M2-10B4 cells (kindly donated by C. Eaves, Vancouver, BC, Canada) were used as feeder cells. Cultures were fed weekly for 5 weeks and LTC-ICs were determined as total CFU-GM output.
Results and discussion
Identification of WT235 as a CTL epitope naturally presented by HLA-A2
Using the T2 whole cell–binding assay, we found that the peptide WT235 (CMTWNQMNL; single-letter amino acid codes) showed similar A2-binding activity as the previously published WT126 peptide (RMFPNAPYL; data not shown). We explored whether the WT235 peptide could stimulate specific CTLs in vitro, using responder lymphocytes from healthy individuals. We have chosen the allo-restricted approach because of concerns that self-restricted T cells may be devoid of high-avidity CTLs due to tolerance.
Figure 1A shows the specificity of 3 allo-restricted CTLs raised against WT235 peptides. The killing of TAP-deficient T2 targets was dependent on T2 coating with WT235 peptides, whereas coating HLA-A2–binding control peptides did not result in CTL killing. Next, we tested CTLs raised against the WT235 and the previously described WT126 epitope11 against T2 target cells coated with WT235 peptide and WT126 peptide, to show that there was no cross-recognition of the 2 WT1-derived peptide epitopes (Figure 1B). The peptide titration curve suggested that WT235-specific CTLs were high avidity because they recognized low picomolar peptide concentrations (Figure 1C).
Specificity of allo-restricted CTLs generated against the WT235 peptide.
(A) Killing of T2 cells (HLA-A2+, TAP−) coated with WT235 peptides (open symbols) and an A2-binding control peptide from the E7 protein of human papilloma virus (solid symbols). Three allo-resticted CTL lines (line 2, 4, 12) were tested in this experiment, and line 4, which expanded best, was used in all subsequent experiments. (B) Killing of T2 targets coated with WT125 or WT235 peptides using the WT235 CTL line 4 (squares) or a previously described allo-restricted CTL line 77 (round symbols) raised against the WT126 peptide (see Gao et al11). (C) Killing activity of WT235 CTL line 4 against T2 loaded with decreasing amounts of WT235 peptide. (D) RT-PCR of a panel of leukemia lines. Shown is RT-PCR amplification of WT1 RNA and the housekeeping abl RNA of 8 leukemia lines. The lymphoblastoid cell line C1R-A2 served as negative control for WT1 expression. RT-PCR with intron-spanning primers of WT1 and abl was performed as described previously.11 (E) Killing of a panel of leukemia lines by the WT235-specific CTL line 4 and by the WT126-specific CTL line 77 (F). The leukemia cell lines LAMA81, BV173, 697, and BAF-3 were HLA-A2+, and SD-1, U937, and KU-812 were HLA-A2− (determined by FACS staining with 2 A2-specific antibodies). In panel E, T2 cells were coated with WT235 peptide and in panel F with WT126 peptide. (G) Killing activity of the WT235-specific line 4 against the lymphoblastoid cell line C1R-A2 in the presence and absence of WT235 peptides. (H) Killing activity of the WT235-specific line 4 against purified normal CD34+ cells or CML patient-derived CD34+ cells. CML patients 1, 3, and 4 were HLA-A2+ and CML patient 2 was A2−.
Specificity of allo-restricted CTLs generated against the WT235 peptide.
(A) Killing of T2 cells (HLA-A2+, TAP−) coated with WT235 peptides (open symbols) and an A2-binding control peptide from the E7 protein of human papilloma virus (solid symbols). Three allo-resticted CTL lines (line 2, 4, 12) were tested in this experiment, and line 4, which expanded best, was used in all subsequent experiments. (B) Killing of T2 targets coated with WT125 or WT235 peptides using the WT235 CTL line 4 (squares) or a previously described allo-restricted CTL line 77 (round symbols) raised against the WT126 peptide (see Gao et al11). (C) Killing activity of WT235 CTL line 4 against T2 loaded with decreasing amounts of WT235 peptide. (D) RT-PCR of a panel of leukemia lines. Shown is RT-PCR amplification of WT1 RNA and the housekeeping abl RNA of 8 leukemia lines. The lymphoblastoid cell line C1R-A2 served as negative control for WT1 expression. RT-PCR with intron-spanning primers of WT1 and abl was performed as described previously.11 (E) Killing of a panel of leukemia lines by the WT235-specific CTL line 4 and by the WT126-specific CTL line 77 (F). The leukemia cell lines LAMA81, BV173, 697, and BAF-3 were HLA-A2+, and SD-1, U937, and KU-812 were HLA-A2− (determined by FACS staining with 2 A2-specific antibodies). In panel E, T2 cells were coated with WT235 peptide and in panel F with WT126 peptide. (G) Killing activity of the WT235-specific line 4 against the lymphoblastoid cell line C1R-A2 in the presence and absence of WT235 peptides. (H) Killing activity of the WT235-specific line 4 against purified normal CD34+ cells or CML patient-derived CD34+ cells. CML patients 1, 3, and 4 were HLA-A2+ and CML patient 2 was A2−.
Next, the CTLs were tested against a panel of leukemia cell lines. Reverse transcription-polymerase chain reaction (RT-PCR) analysis showed that all leukemia cell lines expressed WT1 RNA endogenously (Figure 1D). Figure 1E shows that the WT235-specific CTLs killed leukemia cell lines, provided they were A2+. The WT126-specific CTLs were tested in the same experiment against the same panel of leukemia cell lines (Figure 1F). The killing specificity of the 2 CTL populations was similar except for one interesting exception. The WT1-expressing, A2+ leukemia cell line 697 was killed by WT126-specific CTLs but not by WT235-specific CTLs. The possibility that a WT1 mutation or an intracellular processing defect in 697 cells fails to produce WT235 peptides without affecting the production of the WT126 epitope needs to be explored further.
WT235-specific CTLs failed to kill the A2+ lymphoblastoid cell line C1R-A2, indicating that WT1− hematopoietic cells do not express any CTL-recognized peptides and were killed only after coating with WT235 peptides (Figure 1G). Finally, we tested the killing activity of WT235-specific CTLs against fresh CD34+ cells isolated from a healthy donor and from patients with CML. The results indicated that normal A2+ CD34+ cells were not recognized by WT235-specific CTLs, whereas low-level killing was observed against CD34+ cells from A2+ CML patients, but not against A2− CML samples. The low level of killing indicated that only a subpopulation of CD34 cells was recognized by the CTLs, which has been observed previously with WT126-specific CTLs,11 and is probably due to heterogeneity of WT1 expression among CD34+ cells.
WT235-specific CTLs inhibit colony formation (CFU-GMs) and LTC-ICs from CML cells
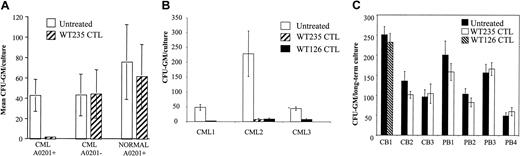
Purified CD34+ cells from patients with chronic-phase CML were exposed to WT235-specific CTLs for 4 hours and then plated in the CFU-GM assay. Colony formation was inhibited by 95% to 100% in the 6 A2+ CML patients analyzed (Figure2A). In contrast, there was no significant inhibition of colony formation when A2− CML cells or A2+ CD34+ cells from healthy donors were treated with CTLs.
Effect of CTL specific for WT235 and WT126 on progenitor/stem cells.
(A) Analysis of CTL-mediated inhibition of colony formation (CFU-GM) of CD34+ cells purified from CML patients and healthy donors. Purified CD34+ cells were cocultured with WT235-specific CTLs for 4 hours or cultured for 4 hours in the absence of CTLs, followed by triplicate plating of 3000 CD34+ cells in methylcellulose medium supplemented with stem cell factor, interleukin 3 (IL-3), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and granulocyte colony-stimulating factor (G-CSF). After 14 days the CFU-GMs were determined as means of triplicate cultures. The graph shows the means and SD of 15 independent experiments with CD34+ from 6 HLA-A2+ CML patients, 5 A2+ healthy donors, and 4 HLA-A2− CML patients. (B,C) Analysis of CTL-mediated inhibition of LTC-ICs of CD34+ cells purified from CML patients (B) and normal cord blood (CB) or mobilized peripheral blood (PB; C). CD34+cells were mock treated or treated with WT126-specific CTLs (CML nos. 2 and 3; CB no.1) or WT235-specific CTLs (CML nos. 1 and 2; CB nos. 2 and 3; PB nos. 1-4) for 4 hours, followed by culture for 5 weeks on irradiated M2-10B4 feeder cells. The assay was carried out in quadruplicate cultures to reduce culture variation. Cells from adherent and nonadherent fraction of the cultures were pooled at week 5, and 20% of the cells were plated in a CFU-GM assay in triplicate. The results are expressed as CFU-GM output (means ± SD).
Effect of CTL specific for WT235 and WT126 on progenitor/stem cells.
(A) Analysis of CTL-mediated inhibition of colony formation (CFU-GM) of CD34+ cells purified from CML patients and healthy donors. Purified CD34+ cells were cocultured with WT235-specific CTLs for 4 hours or cultured for 4 hours in the absence of CTLs, followed by triplicate plating of 3000 CD34+ cells in methylcellulose medium supplemented with stem cell factor, interleukin 3 (IL-3), IL-6, granulocyte-macrophage colony-stimulating factor (GM-CSF), and granulocyte colony-stimulating factor (G-CSF). After 14 days the CFU-GMs were determined as means of triplicate cultures. The graph shows the means and SD of 15 independent experiments with CD34+ from 6 HLA-A2+ CML patients, 5 A2+ healthy donors, and 4 HLA-A2− CML patients. (B,C) Analysis of CTL-mediated inhibition of LTC-ICs of CD34+ cells purified from CML patients (B) and normal cord blood (CB) or mobilized peripheral blood (PB; C). CD34+cells were mock treated or treated with WT126-specific CTLs (CML nos. 2 and 3; CB no.1) or WT235-specific CTLs (CML nos. 1 and 2; CB nos. 2 and 3; PB nos. 1-4) for 4 hours, followed by culture for 5 weeks on irradiated M2-10B4 feeder cells. The assay was carried out in quadruplicate cultures to reduce culture variation. Cells from adherent and nonadherent fraction of the cultures were pooled at week 5, and 20% of the cells were plated in a CFU-GM assay in triplicate. The results are expressed as CFU-GM output (means ± SD).
These results are analogous to those achieved by Molldrem et al using CTLs against proteinase-3, which inhibited the CFU-GM activity of bone marrow cells from CML patients expressing elevated levels of proteinase-3, without affecting CFU-GMs of normal bone marrow.12 However, proteinase-3 is a differentiation antigen that is expressed in hematopoietic cells of the myeloid lineage. Thus, CTLs would be expected to be effective in the killing of committed CFU-GM progenitors and mature myeloid cells, but less effective against more immature cells.
We used the LTC-IC assay to test if WT1-specific CTLs can recognize immature cells present in the CD34+ population from CML patients. In 3 independent experiments, 86% to 100% inhibition of colony formation was observed after treating CML CD34+cells with CTLs specific for the WT126 or WT235 epitopes (Figure 2B). In contrast, 7 independent experiments showed that the same CTLs did not inhibit the LTC-IC activity of normal A2+CD34+ cells (Figure 2C).
A study in patients with acute myelogenous leukemia (AML) showed that leukemia cells with the immature CD34+HLA-DR−phenotype expressed significantly more WT1 than leukemia cells with the more mature CD34+HLA-DR+phenotype.7 This study also showed that immature CD34+ cells from leukemia patients expressed at least 10-fold more WT1 than equivalent normal CD34+ cells. This may explain why WT1-specific CTLs can selectively kill immature CD34+ cells from patients with CML.
We have not found any other examples in the literature showing selective CTL killing of LTC-ICs of leukemia patients. Furthermore, a comparison of the CTL-mediated CFU-GM inhibition described here and published results with the bcr/abl tyrosine kinase inhibitor imatinib13 indicates that the CTLs are far superior in their ability to discriminate between leukemic and normal progenitor cells. Together, the LTC-IC and CFU-GM data suggest that WT1-specific CTLs are probably the most promising reagents to attack selectively leukemia progenitor/stem cells without causing damage to normal CD34+ cells.
The HLA-mismatch between CTL donor and recipient, which may lead to the rejection of infused CTLs, is a major drawback of immunotherapy with allo-restricted CTLs in patients who did not previously receive an allogeneic stem cell transplant. These limitations can be overcome using T-cell receptor (TCR)–based gene transfer. We have recently shown that retroviral transduction can be used to transfer TCRs into human CD8 T cells and that the transduced cells displayed the same specificity as the CTL from which the TCR was isolated.14 This strategy should allow us to equip autologous human CD8 T cells with the specificity of TCRs derived from allo-restricted CTLs.
We thank Dr F. Ramirez for helpful discussion, and Prof R. Lechler for critical review of the manuscript.
Supported by grants from the Leukemia Research Fund (to I.B. and L.G.) and a grant from the Medical Research Council (to S.P.).
All authors reviewed the manuscript and agree with its content and with the submission to Blood. I.B. and L.G. equally contributed to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hans J. Stauss, Department of Immunology, Faculty of Medicine, Imperial College, Hammersmith Hospital, Du Cane Road, London W12 0NN, United Kingdom; e-mail:h.stauss@ic.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal