Hematopoiesis initiates in the extraembryonic yolk sac. To isolate various types of precursor cells from this blood cell–forming tissue, yolk sac cells were immortalized by retroviral-mediated expression of the HOX11 homeobox-containing gene. Among the cell lines derived, some were able to spontaneously generate adherent stromal-like cells capable of taking up acetylated low-density lipoprotein, and they could be induced to form tubelike structures when cultured on Matrigel. Although these cell lines were negative for hematopoietic cell surface markers, they gave rise to hematopoietic colonies—containing cells belonging to the monocytic, megakaryocytic, and definitive erythroid lineages—when plated in methylcellulose medium supplemented with hematopoietic growth factors. Low amounts of Flk-1 mRNA could be detected in these cells, and they showed significant responsiveness to vascular endothelial growth factor, stem cell factor, basic fibroblast growth factor, and interleukin 6. They also expressed the transcription factors SCL, GATA2, GATA1, PU.1, and c-myb. These yolk sac–derived cell lines may represent a transitional stage of early hematopoietic development.
Introduction
Analyses of the early stages of murine embryonic development have shown that hematopoietic activity originates in the extraembryonic yolk sac and the aorta, gonads, and mesonephros. These locations remain the primary sites of hematopoiesis until approximately 12 postcoitum days (dpc) when the fetal liver becomes the predominant hematopoietic organ. In yolk sac, the earliest stage of hematopoietic development is localized in the blood islands, which consist of 2 lineages: a population of hematopoietic cells, surrounded by a layer of angioblasts. The simultaneous appearance of both hematopoietic and endothelial lineages in close proximity within the yolk sac blood islands indicates that hematopoietic and endothelial cells might be derived from the same precursor cell population.1
Further understanding of the origin of the hematopoietic lineage and the mechanisms involved in hematopoietic cell differentiation requires access to various precursor cell subsets. In the mouse, such studies are difficult as the embryo is extremely small at this time, hindering the ready isolation and characterization of transient cell populations. To circumvent this problem, we sought to establish permanent cell lines representative of the various types of precursors present in the yolk sac during this limited temporal period.HOX11, a homeobox-containing gene 2-4 having strong immortalizing potential for bone marrow5,6 as well as embryonic stem (ES) cell–derived7 8 hematopoietic cells, was used to transduce yolk sac cells by retroviral-mediated gene transfer. This approach resulted in immortalization of 2 distinct types of yolk sac cell populations.
Study design
Immortalization of yolk sac cells with a HOX11retroviral vector
Yolk sacs free of vitelline and umbilical arteries were carefully dissected from pregnant C57BL/6J mice at 9.0 to 9.5 dpc. They were rinsed 2 times in RPMI 1640 medium and dissociated into single cells by passing through 22-gauge needles. Cells were prestimulated in RPMI 1640 medium with 15% fetal bovine serum (FBS), interleukin 3 (IL-3; 20 ng/mL), IL-6 (50 ng/mL), and stem cell factor (SCF; 50 ng/mL) for 24 to 48 hours before being transduced with an MSCV-basedHOX11 retroviral vector5,7 8 in the presence of polybrene (8 μg/mL). Transduced cells from each single yolk sac were then cultured for 10 to 14 days in the medium containing IL-3, SCF, and IL-6 and suspension cells from each yolk sac were transferred into individual wells of 24-well plates. After the cells reached confluence, suspension cells from each well were sequentially transferred to new tissue culture plates thereafter. As subsequent tests showed that supplementation of IL-3 to the medium was sufficient for cell survival and proliferation, HOX11-immortalized yolk sac cell populations were routinely maintained in medium supplemented with this factor alone.
Hematopoietic differentiation of type I yolk sac precursor cells
Immortalized precursor cells (2000 cells/mL) were seeded into methylcellulose Iscove modified Dulbecco medium (IMDM) containing 30% FBS, 5% pokeweed mitogen-stimulated mouse spleen cell–conditioned medium, erythropoietin (2 U/mL), SCF (50 ng/mL), IL-3 (10 ng/mL), glutamine (10−4 M), β-mercaptoethanol (3.3 × 10−5 M), and hemin (100 μM) as we previously reported.9-11 After cultures were incubated at 37°C in a 5% CO2 moisture-saturated incubator for 10 to 12 days, all colonies (compact colonies) were harvested and dissociated into single cells by several passages through 22-gauge needles. Compact colony cells (5000 cells/mL) were then replated into the same culture system as the first stage. Progenitor cell–derived colonies consisting of various hematopoietic cell lineages were scored after 7 days of culture.
In vitro angiogenesis assay
For the in vitro angiogenesis assay,12 13Matrigel (BD Biosciences, Bedford, MA) containing a full combination of growth factors including vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) was thawed at 4°C overnight and wells of 24-well plates were coated with 300 μL liquid per well. The coated wells were then incubated at 37°C for 30 minutes and 2.5 × 104/mLHOX11-immortalized cells were seeded into each well in RPMI 1640 medium containing 15% FBS. After 3 to 4 days of incubation, morphologic changes of the cells were recorded under the microscope.
Results and discussion
To isolate precursor cells representative of various developmental stages from yolk sac, cell suspensions from individual yolk sacs at 9.0 to 9.5 dpc were transduced with a HOX11retroviral vector.5,7,8 As a result of HOX11expression, each yolk sac gave rise to a permanent cell line, whereas nontransduced yolk sac cells ceased growing after several passages. Interestingly, among the 34 cell populations immortalized, 8 (designated as type I) were capable of continuously producing both plastic-adherent, endothelial-like cells as well as nonadherent suspension cells in the liquid culture procedures described in “Study design.” This phenotype differs from that of theHOX11-immortalized bone marrow–derived5,6 and ES cell–derived hematopoietic cells7 obtained previously. The remaining 26 cell populations (designated as type II) contained strictly hematopoietic suspension cells that lacked this property. The immortalization procedure was repeated using 8.0 to 8.5 dpc and 10.0 to 10.5 dpc yolk sac cells, yielding similar results (data not shown).
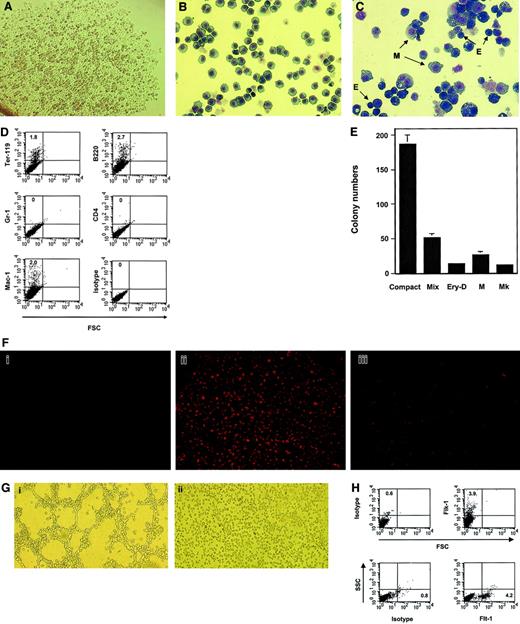
Single-cell cloning of all type I cell populations through limiting dilution resulted in a 60% ± 7% frequency of colony generation, all of which were composed of adherent, stromal-like cells as well as suspension cells (Figure 1A). Type I precursor cell populations were subsequently subcloned in the same manner. All subclones generated from 8 parental cell populations showed very similar morphology and biologic properties. Morphologic examination of cytospin samples revealed that they consisted of homogeneous populations of immature blastlike cells (Figure 1B).
Hematopoietic and endothelial-like potential of type I yolk sac precursor cell lines.
(A) Single type I yolk sac precursor cell–derived colony following limiting dilution. (B) Representative type I yolk sac precursor cells stained with the Hema 3 Stain Set (Biochemical Sciences, Swedesboro, NJ). (C) Cells were allowed to differentiate in hematopoietic growth factor–containing medium. Compact colonies derived were harvested and cytospin preparations were stained with the Hema 3 Stain Set. M indicates macrophage; E, definitive erythroid cells. (D) Compact colonies were harvested and subjected to FACS analyses with the indicated antibodies. (E) Cells from compact colonies were replated into the second-stage culture containing the same hematopoietic growth factors as the first stage. Secondary compact colonies and hematopoietic colonies generated from 7.5 × 103 primary compact colony cells were examined and scored after 7 to 10 days of incubation. Mix indicates mixed colony; Ery-D, definitive erythroid colony; M, macrophage colony; Mk, megakaryocyte colony; Compact, secondary compact colony. (F) Type I precursor cell lines were allowed to differentiate into confluent adherent cells in tissue culture plates. Suspension cells were washed off, and 10 μg/mL Ac-LDL labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (DiI-Ac-LDL; Biomedical Technologies, Stoughton, MA) was added to the cultures. Four hours later, medium was removed and the plates were washed 3 times with prewarmed phosphate-buffered saline. Cells were visualized under a fluorescence microscope. Fi, NIH3T3 cells (negative control); Fii, bone marrow–derived adherent (endothelial) cells (positive control); Fiii, adherent endothelial-like cells generated from type I precursor cells. (G) Matrigel assay: Gi, type I precursor cells; Gii, type II (hematopoietic) cells. (H) FACS analyses of type I precursor cell–derived adherent cells with the indicated antibodies. Shown in this figure are representatives of 2 (panels D,E, and H) to 4 (panels A-C, F, and G) type I precursor cell lines and 10 type II hematopoietic cell lines (panel G). Original magnifications: ×100 (A, F-G); × 200 (B-C).
Hematopoietic and endothelial-like potential of type I yolk sac precursor cell lines.
(A) Single type I yolk sac precursor cell–derived colony following limiting dilution. (B) Representative type I yolk sac precursor cells stained with the Hema 3 Stain Set (Biochemical Sciences, Swedesboro, NJ). (C) Cells were allowed to differentiate in hematopoietic growth factor–containing medium. Compact colonies derived were harvested and cytospin preparations were stained with the Hema 3 Stain Set. M indicates macrophage; E, definitive erythroid cells. (D) Compact colonies were harvested and subjected to FACS analyses with the indicated antibodies. (E) Cells from compact colonies were replated into the second-stage culture containing the same hematopoietic growth factors as the first stage. Secondary compact colonies and hematopoietic colonies generated from 7.5 × 103 primary compact colony cells were examined and scored after 7 to 10 days of incubation. Mix indicates mixed colony; Ery-D, definitive erythroid colony; M, macrophage colony; Mk, megakaryocyte colony; Compact, secondary compact colony. (F) Type I precursor cell lines were allowed to differentiate into confluent adherent cells in tissue culture plates. Suspension cells were washed off, and 10 μg/mL Ac-LDL labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (DiI-Ac-LDL; Biomedical Technologies, Stoughton, MA) was added to the cultures. Four hours later, medium was removed and the plates were washed 3 times with prewarmed phosphate-buffered saline. Cells were visualized under a fluorescence microscope. Fi, NIH3T3 cells (negative control); Fii, bone marrow–derived adherent (endothelial) cells (positive control); Fiii, adherent endothelial-like cells generated from type I precursor cells. (G) Matrigel assay: Gi, type I precursor cells; Gii, type II (hematopoietic) cells. (H) FACS analyses of type I precursor cell–derived adherent cells with the indicated antibodies. Shown in this figure are representatives of 2 (panels D,E, and H) to 4 (panels A-C, F, and G) type I precursor cell lines and 10 type II hematopoietic cell lines (panel G). Original magnifications: ×100 (A, F-G); × 200 (B-C).
The hematopoietic potential of type I precursor cells was first examined. After 10 to 12 days of culture, homogeneous compact colonies arose in the hematopoietic growth factor–containing methylcellulose cultures. Morphology of the cytospin preparations of the compact colonies showed a striking difference from that of the parental cell lines. The compact colonies were composed of differentiating hematopoietic cells corresponding to a variety of lineages, including definitive erythroid cells and macrophages, in addition to undifferentiated blastlike cells (Figure 1C). Fluorescence-activated cell sorting (FACS) analyses of hematopoietic lineage markers demonstrated that cells expressing Ter-119, Mac-1, and B220, but not Gr-1 or CD4, had developed within the compact colonies (Figure 1D), in contrast to the completely negative expression of these markers on the parental cell lines (see below). To better examine the hematopoietic differentiation potential of the precursor cells, a 2-stage culture protocol was used. Compact colonies generated during the first stage of culturing were harvested, dissociated, and replated under the same conditions. After a further 7 to 10 days of incubation, diverse colony types were observed. In addition to secondary compact colonies that were derived from the remaining undifferentiated or self-renewed precursor cells in the primary compact colonies, a variety of hematopoietic progenitor-derived colonies such as definitive erythroid colony–forming unit (Ery-D), macrophage colony-forming unit (CFU-M), and megakaryocyte colony-forming unit (CFU-Mk) colonies had formed (Figure 1E).
As the type I cell lines could spontaneously generate adherent cells in liquid culture, we next determined whether the adherent cells that arose might belong to the endothelial lineage. The adherent cells were examined for their ability to take up acetylated low-density lipoprotein (Ac-LDL), a characteristic of endothelial cells.14 15 In Figure 1F, it is shown that most adherent cells derived from the HOX11-immortalized precursor cells were capable of taking up fluorescenated Ac-LDL, DiI-Ac-LDL, even though the efficiency of dye uptake was lower than that of the positive control cells. To obtain further evidence in support of in vitro endothelial-like potential, the capacity of the precursor cell lines to form tubelike structures on basement membrane proteins was investigated. When type I precursor cells were seeded onto Matrigel-coated wells, a majority of the cells attached, aligned in tandem, and differentiated into tubelike or capillarylike structures (Figure 1Gi). In contrast, none of 10HOX11-immortalized type II (hematopoietic) yolk sac cell lines lacking the ability to form adherent cells in liquid culture exhibited this phenotype (Figure 1Gii). Additionally, even though complete endothelial development of type I precursor cells could not be demonstrated, perhaps due to a HOX11-mediated differentiation block as a consequence of immortalization, a low percentage (3%-5%) of cells positive for VEGF receptors 2 (Flk-1) and 1 (Flt-1) were detected within the adherent endothelial-like cell population by FACS analyses (Figure 1H). These observations suggest that type I yolk sac precursor cells immortalized by HOX11have a partial endothelial-like differentiation capacity.
Northern blot analyses showed that type I yolk sac precursor cells coexpressed transcription factors that play critical roles in early hematopoietic and endothelial development such as SCL, GATA1, GATA2, PU.1, and c-myb. Further characterization of the surface phenotypes demonstrated that these cell lines were negative for Sca-1, CD34, CD45, and CD18 plus all hematopoietic lineage markers tested (Ter-119, Gr-1, Mac-1, B220, CD3, and FcγRII), indicating that they do not represent committed primitive or mature hematopoietic cells. By contrast, HOX11-immortalized type II (hematopoietic) cell lines showed various levels of expression of Sca-1, c-kit, CD34, CD45, and CD18, depending on the stage at which they were immortalized (W.-M.Y. et al, unpublished results, February 2002). Interestingly, low amounts of Flk-1 mRNAs could be detected by Northern blotting (Figure 2A) in type I precursor cells. Notably, none of the type I cell lines examined3expressed the mesodermal marker geneBrachyury16-18 (Figure 2A), consistent with the interpretation that type I yolk sac precursor cells immortalized byHOX11 had already passed through this stage of embryonic development.
Transcription factor expression and growth factor responsiveness of type I yolk sac precursor cells.
(A) Total RNA (30 μg) was resolved in 1% agarose formaldehyde gels and transferred onto nylon membranes. The membranes were hybridized with α-32P-deoxycytidine triphosphate (dCTP)–labeled cDNA probes as indicated and the blots were visualized using a Storm860 phosphorimager (Molecular Dynamics, Sunnyvale, CA). Representative of the data obtained from 2 cell lines. (B) Colonies generated from 3000 cells in serum-free methylcellulose medium containing a serum substitute (BIT, from Stem Cell Technologies, Vancouver, BC, Canada) and VEGF (25 ng/mL), bFGF (50 ng/mL), IL-6 (25 ng/mL), SCF (25 ng/mL), or cytokine combinations. The colonies were scored after 14 days of incubation. Representative of 2 independent experiments with 3 cell lines.
Transcription factor expression and growth factor responsiveness of type I yolk sac precursor cells.
(A) Total RNA (30 μg) was resolved in 1% agarose formaldehyde gels and transferred onto nylon membranes. The membranes were hybridized with α-32P-deoxycytidine triphosphate (dCTP)–labeled cDNA probes as indicated and the blots were visualized using a Storm860 phosphorimager (Molecular Dynamics, Sunnyvale, CA). Representative of the data obtained from 2 cell lines. (B) Colonies generated from 3000 cells in serum-free methylcellulose medium containing a serum substitute (BIT, from Stem Cell Technologies, Vancouver, BC, Canada) and VEGF (25 ng/mL), bFGF (50 ng/mL), IL-6 (25 ng/mL), SCF (25 ng/mL), or cytokine combinations. The colonies were scored after 14 days of incubation. Representative of 2 independent experiments with 3 cell lines.
The growth responsiveness of type I yolk sac precursor cell lines to various cytokines was next tested. As shown in Figure 2B, bFGF, VEGF, SCF, and IL-6 individually and in combination significantly enhanced colony formation of the precursor cells in serum-substituted methylcellulose medium. Collectively, the morphologic, phenotypic, and functional properties of type I yolk sac cells immortalized byHOX11 suggest that they represent a transitional stage biased toward the hematopoietic lineage. However, these yolk sac–derived cell lines also demonstrated partial endothelial-like potential. Whether this property represents in vitro transdifferentiation or is indicative of a hemangioblast origin remains to be determined.
We thank Drs Christian Haudenschild, Daniel A. Lawrence, Grainne McMahon, Kevin D. Bunting, Dorothea Scandella, and Mehrdad Tondravi for technical assistance and helpful comments on this work.
Prepublished online as Blood First Edition Paper, July 5, 2002; DOI 10.1182/blood-2002-03-0937.
Supported by funds from the National Institutes of Health grants R01 HL68212-01A1 (to C.-K.Q.) and R01 HL66305 (to R.G.H.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Cheng-Kui Qu, Department of Hematopoiesis, Holland Laboratory, American Red Cross, 15601 Crabbs Branch Way, Rockville, MD 20855; e-mail: quc@usa.redcross.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal