Translocations involving the immunoglobulin heavy-chain switch region and fibroblast growth factor receptor 3 (FGFR3) are identified in 10% to 15% of patients with myeloma. In previous research we overexpressed FGFR3 or the constitutively active FGFR3-TD mutant in an interleukin-6 (IL-6)–dependent murine myeloma cell line, B9. FGFR3-enhanced IL-6 responsiveness increased phosphorylation of STAT3 and up-regulated Bcl-xL. Since Bcl-xL was up-regulated, we have tested FGFR3-expressing B9 cells for chemotherapy sensitivity. FGFR3 expression did not alter sensitivity to melphalan or doxorubicin. In contrast, B9 cells overexpressing FGFR3 were resistant to treatment with dexamethasone, a phenomenon successfully reversed using a Bcl-xL antisense oligonucleotide. These data demonstrate that the overexpression of FGFR3 in B9 cells confers resistance to dexamethasone but not to anthracyclines or alkylating agents, at least in part through the up-regulation of Bcl-xL. This finding has potential implications for the use of chemotherapy in t(4;14)-positive myeloma.
Introduction
Translocations involving the immunoglobulin heavy (IgH)–chain switch region, and a recurring series of translocation partners, are now known to occur in patients with multiple myeloma (MM).1-5 One of these, a karyotypically silent t(4;14)(p16;q32.3) translocation, is identified in approximately 15% of MM patients.4-9 This translocation has been associated with aberrant fibroblast growth factor receptor 3 (FGFR3) expression and subsequent FGFR3 mutations that render the generated protein constitutively active.7,9 Activating mutations of FGFR3 have been reported in 10% of myeloma patients who are t(4;14) positive and in approximately 2% of myeloma patients overall.10
We transduced the interleukin-6 (IL-6)–dependent murine myeloma cell line, B9, with retroviruses expressing functional wild-type or constitutively active FGFR3.11 Overexpression of wild-type and mutant FGFR3 genes resulted in decreased apoptosis, increased proliferative response to IL-6, and, at high levels of FGFR3 expression, IL-6 independence.11 B9 clones ectopically expressing FGFR3 also displayed increased phosphorylation of signal transducer and activator of transcription 3 (STAT3) and higher levels of Bcl-xL expression than controls. Levels of both proteins remained consistently high, even after cytokine withdrawal.11 Overexpression of Bcl-xL has been suggested as a marker of chemoresistant disease.12Furthermore, Bcl-xL expression in malignant plasma cells has been shown to strongly correlate with a decreased response rate to chemotherapeutic agents,12 and it has been demonstrated to play an important role in protection from apoptosis by growth factor starvation of myeloma cells.13 Therefore, we hypothesized that the up-regulation of Bcl-xL by FGFR3 may also be sufficient to confer resistance to common chemotherapy agents in malignant plasma cells.
Study design
Cell lines, retroviral vectors, and tissue culture conditions
The B9 cell lines, viruses, and tissue culture conditions used in these experiments have been previously described.11 For IL-6 rescue experiments, cell lines were washed 3 times with 1× phosphate-buffered saline to remove any residual IL-6 and were cultured in Iscove modified Dulbecco medium (IMDM) supplemented with 5% fetal calf serum (FCS) and penicillin–streptomycin (Gibco, Gaithersburg, MD). Twenty-four hours after IL-6 withdrawal, the media were supplemented with 2% IL-6–conditioned media. Human myeloma cell lines (OPM1, OPM2, LP1, UTMC2, KHM11, KMS11, and H929) were grown in IMDM supplemented with 5% FCS and penicillin–streptomycin.
Antisense preparation and treatment
The 2′-O-methoxyethyl chimeric phosphorothioate oligodeoxynucleotides used in this study were synthesized by Sigma (Milwaukee, WI). The Bcl-xL antisense 5′-CTGGATCCAAGGCTCTAGGT-3′ and a control nonsense sequence, oligo 5′-CTGGATCCAAGGATCGAGGT-3′, which does not recognize any known gene, have been previously reported and characterized.14 DMRIE-C (Life Technologies, Rockville, MD) was used to increase oligo uptake of cells. Transfection protocols were performed according to the manufacturer's instructions.
Cell viability
To examine the effect of common chemotherapeutic drugs on FGFR3-expressing cells, therapeutic agents were added at increasing concentrations to the culture media containing B9-MINV (vector control), B9-WT (wild-type FGFR3), or B9-TD (activated mutant FGFR3) cells. Cells were analyzed 72 hours after incubation using an Annexin V assay kit (PharMingen, San Diego, CA), and the results were obtained by flow cytometry. MTT assays were also used according to the manufacturer's instructions (Promega, Madison, WI).
Western blots
Lysates were created and analyzed using Western blotting techniques according to previously published procedures.15Membranes were probed with antibodies to Bcl-xL and FGFR3 (C15) (Santa Cruz Biotechnology, CA). Goat anti–rabbit IgG horseradish peroxidase (PharMingen) was used as the secondary antibody, and blots were developed by enhanced chemiluminescence according to the manufacturer's instructions.
Results and discussion
To determine whether FGFR3 overexpression could confer chemoresistance, we used 3 cell lines previously generated in our laboratory.11 All cell lines were derived from the murine IL-6–dependent myeloma cell line B9. These cells were infected with an empty retrovirus vector control (B9MINV) or the same retrovirus vector containing either the wild-type human FGFR3 gene (B9-WT) or a constitutively active form of FGFR3 found in thanatophoric dysplasia (B9-TD). We treated all 3 cell lines with chemotherapeutic drugs commonly used for the treatment of MM. Following exposure to melphalan (Mel) or doxorubicin (Dox), all cell lines responded in a similar manner, with significant cell death at relatively low levels of drug (Figure 1A).
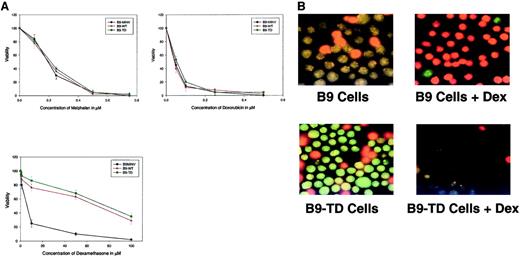
Treatment of FGFR3-transduced cells with melphalan, doxorubicin, and dexamethasone.
(A) B9MINV, B9-WT, and B9-TD cells were induced with various concentrations of drug and analyzed using an MTT assay kit 72 hours after induction. Elevated expression of FGFR3 confers resistance to dexamethasone. (B) Ethidium bromide/acridine orange–stained (green represents viable cells) B9MINV and B9-TD cells either untreated or treated with 10 μM Dex and rescued with IL-6. Only B9MINV cells are sensitive to dexamethasone treatment.

Treatment of FGFR3-transduced cells with melphalan, doxorubicin, and dexamethasone.
(A) B9MINV, B9-WT, and B9-TD cells were induced with various concentrations of drug and analyzed using an MTT assay kit 72 hours after induction. Elevated expression of FGFR3 confers resistance to dexamethasone. (B) Ethidium bromide/acridine orange–stained (green represents viable cells) B9MINV and B9-TD cells either untreated or treated with 10 μM Dex and rescued with IL-6. Only B9MINV cells are sensitive to dexamethasone treatment.
In contrast, when treated with dexamethasone (Dex), all cell lines were resistant (data not shown). Because B9 cells are IL-6 dependent, we believed that the observed Dex resistance likely reflected protective factors that were up-regulated by IL-6 and that such effects obscured any role for FGFR3. Therefore, we starved B9 cells of IL-6 for 24 hours in the presence of Dex and then rescued surviving cells with IL-6. Following Dex treatment, cells ectopically expressing FGFR3 remained resistant to Dex, whereas parental and vector-only control cells were now sensitive to Dex-induced cell death (Figure 1A). Staining with acridine orange and ethidium bromide visually demonstrates an increase in cell viability in Dex-treated FGFR3-expressing cells (Figure 1B).
Because Dex resistance appears to be correlated with FGFR3 expression levels, we next examined the sensitivity of B9 clones expressing high or low levels of wild-type FGFR3 with corresponding variable Bcl-xL levels (Figure 2A). When treated with Dex, IL-6–starved high FGFR3-expressing B9 clones remained drug resistant, whereas the low FGFR3-expressing cells behaved in a pattern more indicative of the B9MINV controls (Figure 2B). Therefore, it appears that Dex resistance in B9 cells is, at least in part, related to FGFR3 expression levels.
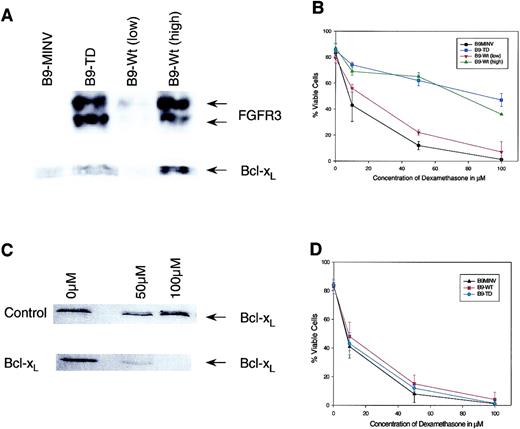
FGFR3-overexpressing cells are resistant to dexamethasone because of Bcl-xL up-regulation.
(A) Western blot analysis of high- and low-expressing FGFR3 clones. (B) Treatment of high and low FGFR3-transduced cells with dexamethasone rescued with IL-6 and analyzed using Annexin V assay kit at 72 hours after treatment. This demonstrates a correlation between FGFR3 and Bcl-xL levels and dexamethasone resistance. (C) Western blot analysis of B9-TD following treatment with control and antisense oligonucleotides demonstrates successful down-regulation of Bcl-xL. (D) B9MINV, B9-WT, and B9-TD cells treated with various concentrations of dexamethasone following Bcl-xL antisense treatment and analyzed using Annexin V assay kit 72 hours after treatment. The protective effect of FGFR3 expression is abrogated.

FGFR3-overexpressing cells are resistant to dexamethasone because of Bcl-xL up-regulation.
(A) Western blot analysis of high- and low-expressing FGFR3 clones. (B) Treatment of high and low FGFR3-transduced cells with dexamethasone rescued with IL-6 and analyzed using Annexin V assay kit at 72 hours after treatment. This demonstrates a correlation between FGFR3 and Bcl-xL levels and dexamethasone resistance. (C) Western blot analysis of B9-TD following treatment with control and antisense oligonucleotides demonstrates successful down-regulation of Bcl-xL. (D) B9MINV, B9-WT, and B9-TD cells treated with various concentrations of dexamethasone following Bcl-xL antisense treatment and analyzed using Annexin V assay kit 72 hours after treatment. The protective effect of FGFR3 expression is abrogated.
In an earlier paper, we demonstrated that cells that overexpress the wild-type or the constitutively active FGFR3 gene maintain high levels of Bcl-xL during IL-6 withdrawal.11 Thus, we hypothesized that Dex resistance reflects the maintenance of Bcl-xL levels after IL-6 withdrawal. To examine this hypothesis, B9-FGFR3 cells were subsequently pretreated with a Bcl-xL antisense oligonucleotide 24 hours before the addition of Dex. Under these conditions, we demonstrated that the previously resistant FGFR3 cells became sensitive to Dex (Figure 2C-D). These data demonstrate that FGFR3-overexpressing cells become chemoresistant to Dex, at least in part through the up-regulation of Bcl-xL.
IL-6–induced Dex resistance has also recently been reported in ANBL6 (an IL-6–dependent human myeloma cell line), in that IL-6 is able to protect ANBL6 cells from Dex- and Mel-, but not Dox-, induced apoptosis.16 Changes in ras or p53 expression can also alter the ANBL6 response to IL-6.16 In this regard, FGFR3 has been reported to be a target for activating mutations that enable FGFR3 to play a ras-like role in tumor progression.17Mutations of ras and FGFR3 in human myeloma also appear mutually exclusive, and both ras and FGFR3 signaling lead to mitogen-activated protein kinase activation.17 Thus, FGFR3 and ras appear to alter IL-6 responsiveness and potentially confer Dex resistance.
We next investigated several human IL-6–independent myeloma cell lines with known FGFR3 translocations. Four of 7 t(4;14) cell lines tested were resistant to Dex (OPM1, OPM2, KHM11, and H929), even at high levels of the drug (data not shown). The 3 cell lines that were Dex sensitive were retested following stimulation with FGF ligand. FGF treatment abrogated Dex sensitivity in 2 (LP1 and UTMC2) of the 3 FGFR3-positive cell lines assayed in this manner (data not shown), but not in the cell line KMS11. Thus, FGFR3 stimulation reverses Dex sensitivity in at least some human myeloma cell lines. Studies in primary myeloma cells will be required to confirm this observation.
These results support the need to define the prognostic significance of IgH translocations in myeloma and suggest that tailored therapeutic approaches may be required. The correlation between FGFR3 translocations and clinical resistance to dexamethasone has yet to be determined.
Prepublished online as Blood First Edition Paper, July 25, 2002; DOI 10.1182/blood-2002-02-0608.
Supported by funding from the National Cancer Institute of Canada, the McCarty Cancer Foundation, the Myeloma Research Foundation, and the ABC group.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
A. K. Stewart, Department of Medical Oncology, 5th Floor, Room 126, The Princess Margaret Hospital, 610 University Ave, Toronto, ON, Canada M5G 2M9; e-mail:kstewart@uhnres.utoronto.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal