The urokinase receptor (urokinase plasminogen activator receptor; uPAR) regulates monocyte adhesion by direct binding to vitronectin and by forming complexes with integrins. Therefore, possible up-regulation of uPAR in acute myocardial infarction (AMI) may affect monocyte adhesion. In 20 patients with AMI, uPAR surface expression (measured by flow cytometry) was increased compared with that in patients with chronic stable angina (mean ± SD fluorescence, 179 ± 96 vs 80 ± 53; P = .002). Expression of uPAR correlated with activation of β2-integrins lymphocyte function–associated antigen 1 (LFA-1) and macrophage antigen 1 (Mac-1), measured by using monoclonal antibodies (mAbs) 24 and CBRM1/5. Isolated mononuclear cells (MNCs) from patients with AMI showed enhanced adhesiveness to human umbilical vein endothelial cells (HUVECs), to fibrinogen (Mac-1 ligand), and to vitronectin (uPAR ligand). Excessive adhesion of MNCs to HUVECs was inhibited by mAbs anti-CD18 (84%), anti-CD11a (51%), and anti-CD11b (57%), indicating a major contribution of LFA-1 and Mac-1. The mAb anti-uPAR R3 blocked adhesion of cells from patients with AMI to vitronectin (95%) but also β2-integrin–mediated adhesion to fibrinogen (79%) and HUVECs (66%). Incubation of monocytic MonoMac6 cells with plasma from patients with AMI enhanced uPAR messenger RNA expression and cell adhesion to HUVECs. Thus, released soluble factors may contribute to enhanced monocyte adhesion in AMI. Mouse pre-B lymphocytes (BAF3 cells) transfected with various amounts of uPAR complementary DNA showed a strong correlation of uPAR expression with β2-integrin–dependent adhesion to intercellular adhesion molecule 1, thus providing evidence for the functional relevance of uPAR up-regulation in an isolated in vitro system. In conclusion, we found that uPAR expression is elevated on monocytes in AMI and contributes to enhanced cell adhesion. Thus, uPAR may be a novel target for prevention of unwanted monocyte recruitment as part of inflammatory cardiovascular processes.
Introduction
Activated monocytes play a key role in the pathophysiology of atherosclerosis and acute myocardial infarction (AMI).1 To exert their local inflammatory potential, monocytes must attach to the vessel wall and emigrate to the site of inflammation. The complexity of this process requires the concerted interaction of adhesion receptors and their respective coreceptors or counter-receptors.2 The urokinase receptor (urokinase plasminogen activator receptor [uPAR], CD87) regulates monocyte adhesion by direct binding to the extracellular matrix protein vitronectin3,4 and by regulating the function of the β2-integrins lymphocyte function–associated antigen 1 (LFA-1; αLβ2, CD11a/CD18) and macrophage antigen 1 (Mac-1; αMβ2, CD11b/CD18).5,6 The uPAR, a 55- to 60-kDa protein that is linked to the cell surface by means of a glycolipid anchor, is known to physically associate in cis with various receptors, such as LFA-1 and Mac-1, under certain conditions.7 In inflammatory situations, binding of LFA-1 and Mac-1 to their endothelial counter-receptor intercellular adhesion molecule 1 (ICAM-1) is essential for monocyte recruitment to the subendothelial space. Absence of uPAR inhibited leukocyte adhesion in vitro5,6,8and granulocyte/monocyte transmigration in a murine model of peritonitis.9 Consistently, mice deficient in uPAR show decreased leukocyte defense against pulmonary bacterial infections.10 Nevertheless, it is not known whether up-regulation of uPAR on blood cells above physiologic levels alters their adhesive behavior.
In AMI, proinflammatory cytokines such as interleukin-1β (IL-1β), IL-6, IL-8, and tumor necrosis factor α are secreted into the plasma.11 These factors promote monocyte recruitment to the subendothelial space and induce up-regulation of uPAR on monocytic cells in vitro.12 In vivo, uPAR up-regulation on monocytes has been observed in patients with multiple sclerosis13 and in healthy volunteers who received an intravenous injection of endotoxin,14 findings suggestive of a modulation of uPAR in vivo under inflammatory conditions. Nevertheless, an effect of pathophysiologic changes in uPAR expression on cell function has not been demonstrated yet. Thus, we studied monocyte expression of uPAR in patients with AMI and its functional effects on cell adhesion.
Patients, materials, and methods
Reagents
Phorbol 12-myristate 13-acetate (PMA) and human fibrinogen were from Sigma (Munich, Germany). Murine ICAM-1–Fc and human ICAM-1 were from R&D Systems (Minneapolis, MN). Recombinant phosphatidylinositol-specific phospholipase C (pi-PLC) was from Oxford Glyco Systems (Abingdon, United Kingdom [UK]). Vitronectin was purified from human plasma as described previously.15Monoclonal antibodies (mAbs) anti-uPAR R3 (blocking) and R4 (nonblocking) were provided by Dr G. Hoyer-Hansen (Copenhagen, Denmark); mAb anti-CD11b (Bear1) was from Immunotech (Hamburg, Germany); and mAb CBRM1/5, which recognizes only the activated epitope of CD11b and blocks Mac-1–dependent adhesion,16 was provided by Dr T. Springer (Boston, MA). The mAb 24, which detects another activation-dependent epitope on LFA-1 and Mac-1, was a gift from Dr N. Hogg (London, UK).17 Blocking mAb antihuman CD18 (IB4) was from Ancell (Bayport, MN). Blocking mAb antihuman CD11a (L15) was provided by Dr C. Figdor (Nijmegen, The Netherlands). Murine antihuman IgG2a (Sigma) was used as an isotype-matched control antibody. Blocking mAb antimouse CD18 (GAME46) was from Pharmingen (Hamburg, Germany).
Patients
The study group comprised 20 patients presenting with AMI (Killip class I-II) within 24 hours after the onset of pain. The diagnosis was based on a history of prolonged ischemic chest pain, notable ST-segment elevations, and elevation in creatine kinase level above 240 U/mL with a concomitant rise in MB isoenzyme. We included patients who met the first criterion and at least one of the other criteria. Blood samples were obtained on admission before revascularization by stent placement. Twenty patients with chronic stable angina (CSA) and angiographically proven coronary disease and 20 healthy volunteers recruited from the hospital staff were studied as control groups. Patients with noncardiac diseases that may have affected results, such as inflammatory disorders, cancer, and infection, were excluded. The baseline clinical, demographic, and angiographic data in the 2 patient groups are shown in Table1. Approval was obtained from the institutional ethical committee of Technische Universität München for these studies. Informed consent was provided according to the Declaration of Helsinki.
Baseline characteristics of the study population
| Characteristic . | AMI group (n = 20) . | CSA group (n = 20) . |
|---|---|---|
| Mean age, y (range) | 61.5 (42-80) | 63.5 (38-83) |
| Sex: M/F | 17/3 | 15/5 |
| Hypercholesterolemia | 11 | 15 |
| Arterial hypertension | 12 | 14 |
| Smoker | 7 | 5 |
| Diabetes mellitus | 3 | 5 |
| One-vessel disease | 5 | 4 |
| Two-vessel disease | 8 | 5 |
| Three-vessel disease | 7 | 11 |
| Mean ± SD time from onset of pain to blood sampling (h) | 14 ± 6 | — |
| Mean ± SD peak creatine kinase level (U/L) | 1249 ± 1135 | — |
| Target vessel | ||
| LAD | 10 | — |
| LCx | 5 | — |
| RCA | 5 | — |
| Venous bypass graft | 0 | — |
| Characteristic . | AMI group (n = 20) . | CSA group (n = 20) . |
|---|---|---|
| Mean age, y (range) | 61.5 (42-80) | 63.5 (38-83) |
| Sex: M/F | 17/3 | 15/5 |
| Hypercholesterolemia | 11 | 15 |
| Arterial hypertension | 12 | 14 |
| Smoker | 7 | 5 |
| Diabetes mellitus | 3 | 5 |
| One-vessel disease | 5 | 4 |
| Two-vessel disease | 8 | 5 |
| Three-vessel disease | 7 | 11 |
| Mean ± SD time from onset of pain to blood sampling (h) | 14 ± 6 | — |
| Mean ± SD peak creatine kinase level (U/L) | 1249 ± 1135 | — |
| Target vessel | ||
| LAD | 10 | — |
| LCx | 5 | — |
| RCA | 5 | — |
| Venous bypass graft | 0 | — |
Values are numbers of patients unless otherwise indicated.
LAD indicates left anterior descending coronary artery; LCx, left circumflex coronary artery; and RCA, right coronary artery.
Cells
Mononuclear cells (MNCs) were isolated from patients by Ficoll gradient centrifugation (20 minutes at 4°C at 800g). Human umbilical vein endothelial cells (HUVECs; PromoCell, Heidelberg, Germany) were cultured (2 to 4 passages) in low-serum endothelial cell growth medium (PromoCell) on gelatin-coated tissue-culture plastic. BAF3 cells (mouse pre-B lymphocytes), which do not express uPAR, were from the American Type Culture Collection (Rockville, MD). The BAF3 cells were cultured in RPMI 1640 containing 10% (vol/vol) fetal-calf serum (FCS), 100 U/mL penicillin, 100 μg/mL streptomycin (Gibco, Grand Island, NY), and 2 ng/mL IL-3 (Stratham Biotech, Hannover, Germany) and were stably transfected with uPAR complementary DNA (cDNA) by electroporation (BioRad, Munich, Germany). Cells were selected in the presence of 1.0 mg/mL G418 (Calbiochem, Bad Soden, Germany) and characterized for uPAR expression by flow cytometry and Western blot analysis. Nontransfected cells and 4 clones showing different expression of uPAR were used for subsequent experiments. Human monocytic MonoMac6 cells18 were cultured in VLE–RPMI-1640 (Biochrom, Berlin, Germany) containing 10% low-toxin FCS (Clonetics, Taufkirchen, Germany).
Adhesion assays
Cell-to-cell adhesion.
HUVECs were seeded on gelatin-coated, 96-well plates for 48 hours. Confluency was confirmed by microscopical inspection before each experiment. Freshly isolated MNCs or MonoMac6 cells were washed twice in adhesion medium (25 mM serum-free RPMI 1640 and HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid]) and given various pretreatments. Cells (7 × 105/mL) were coincubated with HUVEC monolayers in the absence or presence of blocking or control mAbs for 30 minutes (37°C, 5% carbon dioxide, and 90% humidity). The plates were washed gently twice to remove nonadherent cells, and remaining adherent cells were quantified by counting 16 high-power fields under light microscopy.
Cell adhesion to immobilized proteins.
Plates containing 96 wells were coated with human fibrinogen, vitronectin, or mouse ICAM-1–Fc (20 μg/mL each) for 2 hours at 37°C and blocked with 1% (wt/vol) bovine serum albumin for 30 minutes at 25°C. Pretreated leukocytes (70 000 cells/well) or BAF3 cells were seeded in the absence or presence of blocking or control mAbs for 30 minutes. After removal of nonadherent cells by 2 washing steps, adhesion was quantified by a peroxidase reaction using p-nitrophenol as a substrate in an enzyme-linked immunosorbent assay (ELISA) reader (BioRad).
Flow cytometry
Blood samples were handled as described previously.20 Briefly, 1.5 mL blood was collected into a polypropylene syringe containing 0.5 mL sodium citrate, phosphate buffer, dextrose, and adenine (Greiner, Nürtingen, Germany). Immediately after blood sampling, staining with the primary mouse antihuman mAb was performed, followed by lysis of red blood cells, leukocyte fixation (lysing solution and fixing reagent from Coulter Electronics, Germany), and staining with the secondary phycoerythrin-conjugated antimouse mAb. Cells were washed twice, fixed with paraformaldehyde (1%), and analyzed on a FACScalibur flow cytometer (Becton Dickinson, Mountain View, CA). Monocytes were identified by forward- compared with side-scatter analysis. BAF3 cells were washed twice with HEPES-buffered saline before mAb staining and fixation with paraformaldehyde (1%). Nonspecific fluorescence was determined by using isotype-matched mouse IgG as the control antibody.
Reverse transcriptase–polymerase chain reaction (RT-PCR)
Total RNA was extracted from cells by using an RNeasy mini-kit (Qiagen, Cologne, Germany). Contaminating DNA was digested with DNase using a Message Clean kit (Gene Hunter, Brookline, MA), and 1.5 μg total RNA was transcribed to cDNA by using Omniscript RT (Qiagen) and random hexamers (Gibco BRL Life Technologies, Karlsruhe, Germany). Primer sequences for RT-PCR were as follows: uPAR (forward), 5′-GCCCTGGGACAGGACCTCTG-3′; uPAR (reverse), 5′-CATTGATTCATGGGGCCTCGGC-3′; cyclophillin (forward), 5′-CATCTGCACTG-CCAAGACTG-3′; and cyclophillin (reverse), 5′-CTGCAATCCAGCTAGGCATG-3′. PCR was performed with HotStarTaq DNA polymerase (Qiagen). Annealing temperature for uPAR and cyclophillin (internal standard) was 63°C. Real-time PCR analysis was done with a SYBR Green PCR reagents kit and an ABI Prism 7700 sequence detection system (Applied Biosystems, Foster City, CA). Each PCR amplification sample was performed with cDNA derived from 50 ng total RNA. For amplification of uPAR cDNA, the exon-spanning oligonucleotides were 5′-GCCCAATCCTGGAGCTTG A-3′ and 5′-TCCCCTTGCAGCTGTAACACT-3′. Amplification of 18S ribosomal RNA cDNA was used as the internal standard; the primers were 5′-CGGCTACCACATCCAGGAA-3′ and 5′-GCTGGATTACCGCGGCT-3′. Each sample was measured in triplicate, and a blank containing no template cDNA was used as the negative control.
ELISA
Soluble uPAR in plasma from patients was studied by using a modified ELISA described previously.21
Statistical analysis
Results with normally distributed continuous variables were reported as mean ± SD and were analyzed by unpaired ttest or analysis of variance followed by the Scheffe test, as appropriate. In general, P < .05 was regarded as representing significance.
Results
Up-regulation of uPAR and β2-integrin activation on monocytes in AMI
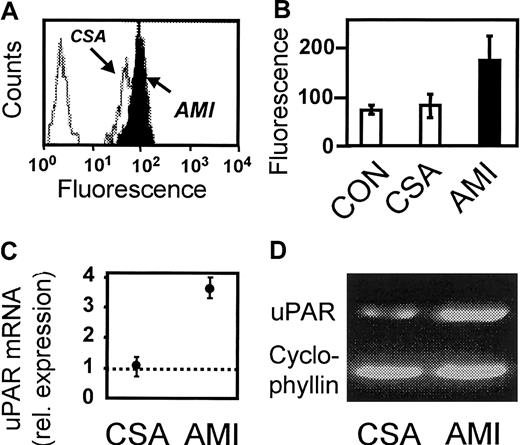
Monocyte expression of uPAR was studied by flow cytometry. Figure1 shows representative histograms from an analysis of one patient with AMI and one with CSA (Figure 1A) and the results of the statistical analysis of the 3 groups (Figure 1B). Surface expression of uPAR was significantly higher on monocytes from patients with AMI compared with patients with CSA (mean immunofluorescence, 179 ± 96 vs 80 ± 53; P < .005). Consistent with the results of the cell-surface analysis, quantitative RT-PCR showed a 3.5-fold increased expression of uPAR messenger RNA (mRNA; range, 3.2-3.9) in isolated MNCs from 3 patients with AMI compared with 3 patients with CSA (Figure 1C). No differences were found between patients with CSA and healthy donors. Nevertheless, enhanced uPAR expression was not associated with detectable changes in plasma levels of soluble uPAR or urokinase plasminogen activator (uPA), whereas levels of soluble plasminogen activator inhibitor (PAI-1) were found to be elevated (Table 2), as was observed previously.22 In addition, monocytic cells (MonoMac6) were incubated with pooled plasma from patients with AMI and CSA. Plasma from patients with AMI was found to up-regulate uPAR mRNA (Figure 1D) and uPAR surface expression (data not shown), suggesting that released soluble factors in the circulating blood of AMI patients may be involved in uPAR up-regulation.
Expression of uPAR on monocytes in AMI.
(A) Representative flow cytometric histograms showing uPAR expression (mAb R4) in one patient with AMI and one with CSA. The far left peak represents the nonspecific control mAb. (B) Flow cytometric analysis of healthy controls (CON) and patients with CSA or AMI (n = 20 in each group). Values shown are the mean immunofluorescence ± SD. (C) Relative uPAR mRNA expression in MNCs isolated from 3 patients with CSA or AMI. Values shown are the means; error bars indicate range in relation to healthy controls (dotted line). (D) Expression of uPAR mRNA in monocytic MonoMac6 cells incubated with pooled plasma from patients with CSA or AMI. One experiment representative of 3 is shown.
Expression of uPAR on monocytes in AMI.
(A) Representative flow cytometric histograms showing uPAR expression (mAb R4) in one patient with AMI and one with CSA. The far left peak represents the nonspecific control mAb. (B) Flow cytometric analysis of healthy controls (CON) and patients with CSA or AMI (n = 20 in each group). Values shown are the mean immunofluorescence ± SD. (C) Relative uPAR mRNA expression in MNCs isolated from 3 patients with CSA or AMI. Values shown are the means; error bars indicate range in relation to healthy controls (dotted line). (D) Expression of uPAR mRNA in monocytic MonoMac6 cells incubated with pooled plasma from patients with CSA or AMI. One experiment representative of 3 is shown.
Soluble hemostatic factors in patients with AMI and CSA
| Factor . | CSA group (ng/mL; n = 20) . | AMI group (ng/mL; n = 20) . |
|---|---|---|
| uPAR | 3.69 ± 1.3 | 3.6 ± 1.39 |
| uPA | 0.35 ± 0.09 | 0.38 ± 0.12 |
| PAI-I | 25.8 ± 11.7 | 41.3 ± 18.3* |
| Factor . | CSA group (ng/mL; n = 20) . | AMI group (ng/mL; n = 20) . |
|---|---|---|
| uPAR | 3.69 ± 1.3 | 3.6 ± 1.39 |
| uPA | 0.35 ± 0.09 | 0.38 ± 0.12 |
| PAI-I | 25.8 ± 11.7 | 41.3 ± 18.3* |
P < .05 for the difference between groups.
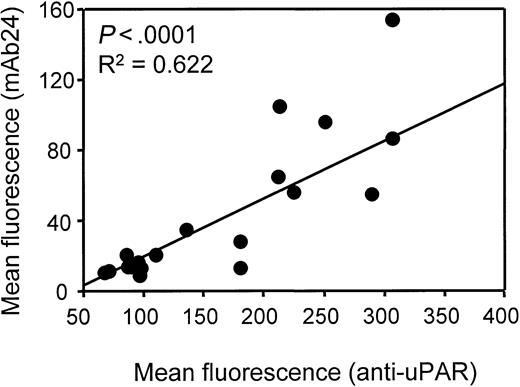
Next, β2-integrin activation and Mac-1 expression were studied (Figure 2). Compared with samples from patients with CSA, samples from patients with AMI had increased β2-integrin activation, as indicated by increased detection of epitopes CBRM1/5 (mean immunofluorescence, 39 ± 21 vs 13 ± 7; P < .01) and 24 (mean immunofluorescence, 35 ± 22 vs 13 ± 8; P < .05). Notably, the surface density of total Mac-1 (mAb Bear-1) was comparable in patients with AMI and those with CSA (mean immunofluorescence, 1448 ± 1212 vs 1585 ± 1630; P not significant). No significant differences were found between patients with CSA and healthy controls with regard to uPAR and Mac-1 surface expression or Mac-1 activation. Linear regression analysis revealed a significant correlation between uPAR expression and β2-integrin activation in AMI samples (Figure 3).
Activation of β2-integrin on monocytes in AMI.
(A) Representative flow cytometric histograms showing β2-integrin activation (mAbs CBRM1/5 and mAb 24) and total Mac-1 expression (mAb Bear1) in one patient with AMI and one with CSA. The broken line represents the nonspecific control mAb. (B) Flow cytometric analysis of healthy controls and patients with CSA or AMI (n = 20 in each group). Values shown are the mean immunofluorescence ± SD.
Activation of β2-integrin on monocytes in AMI.
(A) Representative flow cytometric histograms showing β2-integrin activation (mAbs CBRM1/5 and mAb 24) and total Mac-1 expression (mAb Bear1) in one patient with AMI and one with CSA. The broken line represents the nonspecific control mAb. (B) Flow cytometric analysis of healthy controls and patients with CSA or AMI (n = 20 in each group). Values shown are the mean immunofluorescence ± SD.
Linear regression analysis of uPAR expression and β2-integrin activation on monocytes in AMI.
Linear regression analysis of uPAR expression and β2-integrin activation on monocytes in AMI.
β2-integrin and uPAR-mediated adhesion of MNCs from patients with AMI
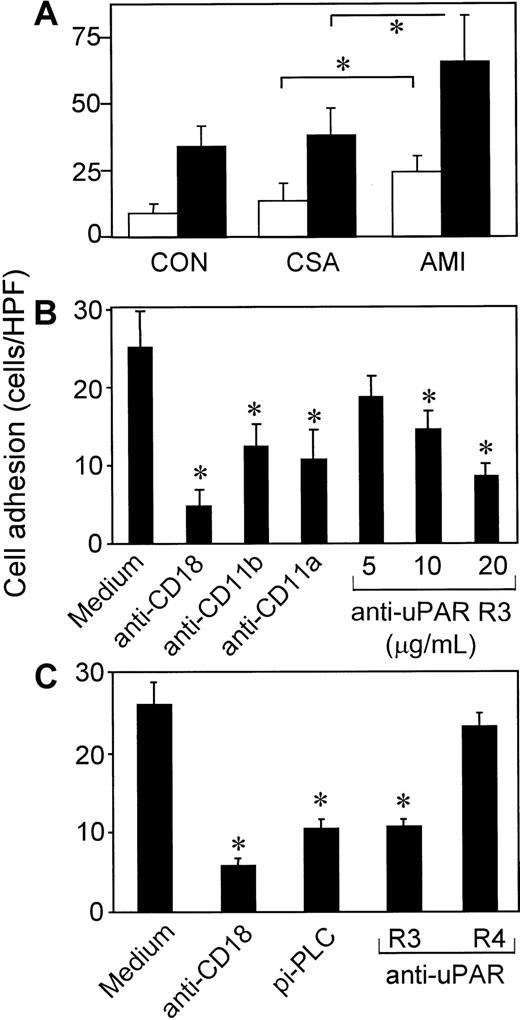
In the adhesion assay, 2% to 5% of unstimulated isolated MNCs (4-10 cells/high-power field) from healthy volunteers adhered to the endothelium, and these were identified as monocytes (> 95%) by flow cytometry. Under the experimental conditions used, lymphocytes did not discernibly adhere to the endothelium, even when stimulated with phorbol ester (PMA) or isolated from patients with AMI. Nonstimulated MNCs from patients with AMI showed significantly greater adhesion to HUVECs than cells from patients with CSA or healthy volunteers (Figure4A). Maximum cellular adhesiveness in response to ex vivo stimulation with phorbol ester (PMA) was also enhanced in cells from patients with AMI compared with cells from patients with CSA or healthy donors.
The uPAR-dependent cell adhesion to endothelial cells in AMI.
(A) MNCs from patients with AMI (n = 16) or CSA (n = 16) or from healthy volunteers (CON; n = 16) were pretreated with medium (white bars) or 10 ng/mL PMA (black bars) for 30 minutes at 37°C and washed, and adhesion to HUVEC monolayers was performed. Values shown are the means ± SEM from 16 independent experiments performed in triplicate. (B) Adhesion of MNCs from patients with AMI to HUVEC monolayers in the presence or absence of mAbs anti-CD18 (IB4; 20 μg/mL), anti-CD11b (CBRM1/5; 50 μg/mL), anti-CD11a (L15; 20 μg/mL), or different concentrations of anti-uPAR R3. Values shown are the means ± SEM from 3 experiments performed in triplicate. (C) MNCs from patients with AMI were pretreated with medium or pi-PLC (0.5 U/mL; 90 minutes) and washed. Adhesion to HUVEC monolayers was performed in the absence or presence of 20 μg/mL mAb anti-CD18 (IB4), anti-uPAR (R3), or anti-uPAR (R4). Values shown are the means ± SEM from 3 experiments performed in triplicate. The asterisks indicateP < .05.
The uPAR-dependent cell adhesion to endothelial cells in AMI.
(A) MNCs from patients with AMI (n = 16) or CSA (n = 16) or from healthy volunteers (CON; n = 16) were pretreated with medium (white bars) or 10 ng/mL PMA (black bars) for 30 minutes at 37°C and washed, and adhesion to HUVEC monolayers was performed. Values shown are the means ± SEM from 16 independent experiments performed in triplicate. (B) Adhesion of MNCs from patients with AMI to HUVEC monolayers in the presence or absence of mAbs anti-CD18 (IB4; 20 μg/mL), anti-CD11b (CBRM1/5; 50 μg/mL), anti-CD11a (L15; 20 μg/mL), or different concentrations of anti-uPAR R3. Values shown are the means ± SEM from 3 experiments performed in triplicate. (C) MNCs from patients with AMI were pretreated with medium or pi-PLC (0.5 U/mL; 90 minutes) and washed. Adhesion to HUVEC monolayers was performed in the absence or presence of 20 μg/mL mAb anti-CD18 (IB4), anti-uPAR (R3), or anti-uPAR (R4). Values shown are the means ± SEM from 3 experiments performed in triplicate. The asterisks indicateP < .05.
Adhesion of MNCs from patients with AMI to HUVECs was significantly inhibited by mAbs anti-CD18 (84%), anti-CD11a (51%), and anti-CD11b (57%), indicative of the predominant contribution of Mac-1 and LFA-1 to cell adhesion (Figure 4B). Additionally, excessive cell adhesion of AMI-MNCs to HUVECs was inhibited by the blocking anti-uPAR mAb R3 in a dose-dependent manner (Figure 4B and 4C) but not by the nonblocking mAb anti-uPAR R4 (Figure 4C), indicating that the total amount of functionally active uPAR on the cell surface may limit β2-integrin–dependent cell adhesion. Consistently, preincubation with pi-PLC, which removes glycolipid-anchored receptors such as uPAR from the cell surface, inhibited β2-integrin–mediated cell adhesion of AMI cells.
Consistent with the results described above, isolated MNCs from patients with AMI showed enhanced adhesion to the adhesive ligand of Mac-1, fibrinogen (Figure 5B). Enhanced cell adhesion to fibrinogen in AMI samples was abrogated by mAb anti-CD18 but also by anti-uPAR R3. Similarly, adhesion of MNCs to immobilized vitronectin (uPAR ligand) was enhanced in AMI samples and blocked in the presence of anti-uPAR R3 but was not affected by mAb anti-CD18 (Figure 5A). Notably, no significant differences in cell adhesiveness to fibrinogen or vitronectin were found between cells from patients with CSA and cells from healthy volunteers.
The uPAR-dependent cell adhesion to fibrinogen and vitronectin in AMI.
Adhesion of MNCs from patients with AMI or CSA or from healthy volunteers (CON; n = 6 in each group) to immobilized vitronectin (A) or fibrinogen (B). Adhesion of cells from patients with AMI was performed in the presence or absence of 20 μg/mL of blocking mAb anti-uPAR (R3) or anti-CD18 (IB4). Values shown are the means ± SEM from 6 independent experiments performed in triplicate using cells from 6 different patients or donors. The asterisk indicatesP < .05. Nonsignificant P values between data sets are indicated with NS.
The uPAR-dependent cell adhesion to fibrinogen and vitronectin in AMI.
Adhesion of MNCs from patients with AMI or CSA or from healthy volunteers (CON; n = 6 in each group) to immobilized vitronectin (A) or fibrinogen (B). Adhesion of cells from patients with AMI was performed in the presence or absence of 20 μg/mL of blocking mAb anti-uPAR (R3) or anti-CD18 (IB4). Values shown are the means ± SEM from 6 independent experiments performed in triplicate using cells from 6 different patients or donors. The asterisk indicatesP < .05. Nonsignificant P values between data sets are indicated with NS.
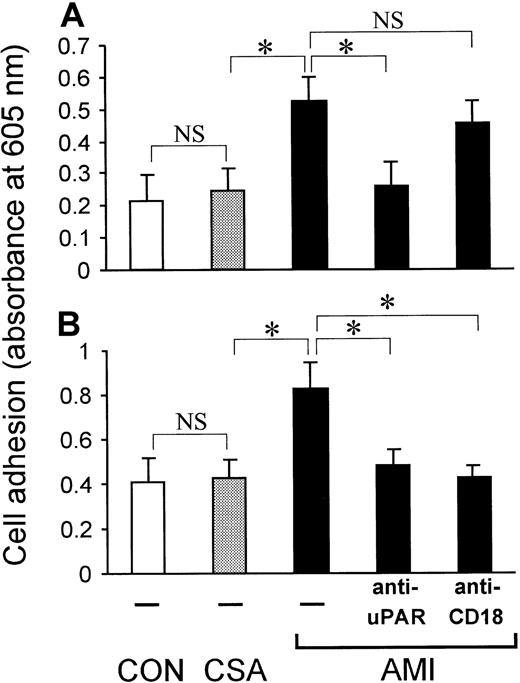
Plasma from patients with AMI induces monocytic cell adhesion by means of β2-integrins and uPAR
To study the effect of patient plasma on adhesion of healthy monocytic cells, MonoMac6 cells were incubated with plasma from either healthy controls, patients with CSA, or patients with AMI for 1 hour before the adhesion assay. Compared with plasma from healthy subjects, plasma from patients with CSA did not alter cell adhesion (Figure6). In contrast, plasma from patients with AMI increased cell adhesion to immobilized fibrinogen (Mac-1 ligand; Figure 6A), immobilized vitronectin (uPAR ligand; Figure 6B), and endothelial cell monolayers (Figure 6C). AMI plasma–induced cell adhesion to endothelial cells was inhibited by antibody blockade of the common β2-integrin chain CD18 or mAb anti-uPAR. Thus, apart from inducing uPAR synthesis (Figure 1D), soluble factors in AMI plasma seem to activate monocyte adhesion by means of β2-integrin and uPAR activation.
Plasma from patients with AMI induces adhesion of MonoMac6 cells.
MonoMac6 cells were incubated with plasma (plasma:medium, 1:1 vol/vol) from patients with AMI or CSA or from healthy volunteers (CON; n = 3 in each group) for 60 minutes, and adhesion assays were performed. (A) Adhesion to immobilized fibrinogen. (B) Adhesion to immobilized vitronectin. (C) Adhesion to endothelial cell monolayers in the presence or absence of 20 μg/mL of blocking mAb anti-uPAR (R3) or anti-CD18 (IB4). Values shown are means ± SEM from 3 independent experiments performed in triplicate using plasma from 3 different patients or donors. The asterisk indicates P < .05.
Plasma from patients with AMI induces adhesion of MonoMac6 cells.
MonoMac6 cells were incubated with plasma (plasma:medium, 1:1 vol/vol) from patients with AMI or CSA or from healthy volunteers (CON; n = 3 in each group) for 60 minutes, and adhesion assays were performed. (A) Adhesion to immobilized fibrinogen. (B) Adhesion to immobilized vitronectin. (C) Adhesion to endothelial cell monolayers in the presence or absence of 20 μg/mL of blocking mAb anti-uPAR (R3) or anti-CD18 (IB4). Values shown are means ± SEM from 3 independent experiments performed in triplicate using plasma from 3 different patients or donors. The asterisk indicates P < .05.
Changes in uPAR expression regulate BAF3 cell adhesion by means of β2-integrins
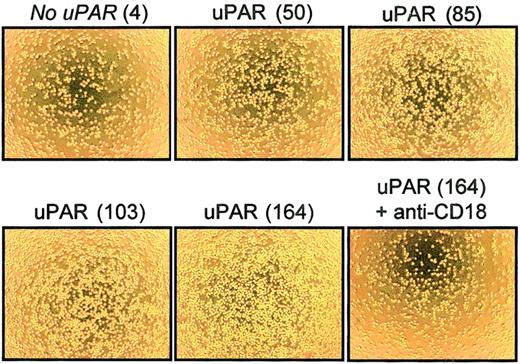
The relevance of changes in uPAR expression on cell adhesion was tested in an isolated in vitro system lacking the inflammatory environment of AMI. Mouse BAF3 cells (pre-B cells) that constitutively express LFA-1 but do not express uPAR were stably transfected with various amounts of uPAR cDNA, resulting in 5 cell populations expressing different levels of uPAR on the cell surface, as assessed by flow cytometry (mean fluorescence, 4 [not transfected], 50, 85, 103, and 164; Figure 7). Surface expression of αL-chain (CD11a) and β2-chain (CD18) was found to be virtually identical on all BAF3 cells (data not shown). Increasing density of uPAR expression was associated with enhanced cell adhesion to the immobilized β2-integrin ligand ICAM-1 (Figure 7) as well as to the immobilized uPAR ligand vitronectin (data not shown). In AMI, inflammatory mediators may be involved in integrin activation and uPAR up-regulation. These experiments show clearly that changes in uPAR expression alone can augment β2-integrin–mediated cell adhesion independent of an inflammatory cellular environment.
Integrin-dependent adhesion of uPAR-transfected BAF3 cells.
BAF3 cells were transfected with various amounts of uPAR cDNA and analyzed by flow cytometry using mAb anti-uPAR R4. Adhesion to immobilized mouse ICAM-1–Fc of 5 BAF3 cell lines expressing no uPAR (mean immunofluorescence, 4) or different levels of uPAR (mean immunofluorescence, 50, 85, 103, and 164) was studied. Photomicrographs show results from 1 experiment representative of 5 comparable independent experiments.
Integrin-dependent adhesion of uPAR-transfected BAF3 cells.
BAF3 cells were transfected with various amounts of uPAR cDNA and analyzed by flow cytometry using mAb anti-uPAR R4. Adhesion to immobilized mouse ICAM-1–Fc of 5 BAF3 cell lines expressing no uPAR (mean immunofluorescence, 4) or different levels of uPAR (mean immunofluorescence, 50, 85, 103, and 164) was studied. Photomicrographs show results from 1 experiment representative of 5 comparable independent experiments.
Discussion
This study demonstrated that uPAR expression is enhanced on monocytes in AMI and affects integrin-dependent and -independent cell adhesion. In particular, uPAR surface expression on human monocytes was up-regulated in the peripheral blood of patients with AMI. The relevance of these changes in uPAR expression for cell adhesion was shown with respect to the function of uPAR as both an adhesion receptor and a coreceptor for integrins. Enhanced uPAR expression on monocytes from patients with AMI was associated with increased cell adhesion to the uPAR ligand vitronectin and was related to enhanced cell adhesion to the Mac-1 ligand fibrinogen as well as to the endothelium. Cell adhesion to vitronectin was dependent mainly on functionally active uPAR (Figure 5A). Cell adhesion to the endothelium was almost completely abrogated by mAbs against the β2-integrins LFA-1 and Mac-1 (Figure 4B), which were additionally found to be in an activated, ligand-binding state on monocytes from patients with AMI (Figure 2). These data indicate that functionally active LFA-1 and Mac-1 primarily mediate enhanced MNC binding to the endothelium in AMI. The blocking mAb anti-uPAR R3 (but not the nonblocking mAb R4) inhibited integrin-mediated cell adhesion in a dose-dependent manner, indicating that uPAR has a regulatory role in integrin-mediated cell adhesion in AMI.
Data from previous studies using transfected Chinese hamster ovary cells indicated that uPAR could mediate cell-to-cell adhesion not only by cis interactions with integrins on the same cell surface but also by transinteraction with integrins on apposing cells.23 In our experiments, such transinteractions between uPAR and integrins may have contributed to cell adhesion on endothelium. However, the finding of cell adhesion to fibrinogen (Figure 5) excluded possible integrin–uPAR transinteractions and clearly indicated the existence of uPAR-dependent, integrin-mediated adhesion of MNCs from patients with AMI, since Mac-1 binding activity was attenuated by blockade of uPAR.
The functional relevance of changes in uPAR expression was confirmed in an isolated in vitro system lacking any additional inflammatory conditions associated with AMI. In uPAR-transfected BAF3 cells, there was a strong correlation between uPAR expression and cell adhesion to vitronectin (data not shown) or ICAM-1 (Figure 7). Notably, the changes in uPAR expression that resulted in enhanced adhesiveness of transfected BAF3 cells were comparable to the differences observed between cells from patients with AMI and cells from patients with CSA.
Expression of uPAR, β2-integrins, and monocyte adhesion
Expression of uPAR on the cell surface may have several pathophysiologic consequences, such as uPA binding, activation, and resultant initiation of a pericellular proteolytic activation cascade.24,25 We focused on the possible influence of uPAR on cell adhesiveness because of findings by us9 and others26 indicating that uPAR can mediate leukocyte migration by means of nonproteolytic adhesive mechanisms. The urokinase receptor is constitutively expressed on monocytes, and even though it is a coreceptor for integrins essential for their adequate function,9,24,27 it has been unclear whether quantitative changes in surface expression of uPAR can effect integrin function. Little is known about the availability of uPAR for complex formation with β2-integrins.28 Factors that limit this receptor crosstalk may include the activation status of uPAR or integrins, the occupancy of either partner by their various soluble ligands (uPA, uPA PAI-1, kininogen, and vitronectin),5,6,9or possible occupancy by various known as well as unknown coreceptors that may compete with β2-integrins for association with uPAR (eg, gp130, α2-macroglobulin receptor, and β1-integrins such as very late antigen 4 [VLA-4] or VLA-5).25 28 Hence, the ratio of the absolute number of uPAR and β2-integrins does not allow any conclusions to be drawn about the availability of uPAR for complex formations. However, the current study clearly shows that the quantity of expression of uPAR on resting monocytes limits β2-integrin activation, since uPAR up-regulation in AMI samples was involved in enhanced cellular adhesiveness to the endothelium. Moreover, MNCs from patients with AMI had an enhanced adhesive response to maximum stimulation with PMA, although we did not observe any significant changes in quantitative surface expression of Mac-1. Thus, our data suggest that enhanced availability of uPAR in AMI may prime monocytes for enhanced adhesive responsiveness to additional inflammatory stimuli.
Apart from its role as an integrin coreceptor, uPAR is the main receptor on monocytes that directly binds to the extracellular matrix protein vitronectin. As a consequence, enhanced binding of MNCs from patients with AMI to vitronectin was found. In previous in vitro studies, soluble vitronectin was shown to enhance leukocyte-endothelium interactions.29 Because vitronectin is an adhesive protein present in plasma as well as a major component of the vascular extracellular matrix,15 enhanced monocyte binding capacity for vitronectin might also have substantial consequences for the entire process of cell recruitment.
Limitations of the study
Peripheral blood from patients with AMI was obtained on admission before revascularization therapy (stent placement). Thus, revascularization therapy did not influence our results. Nevertheless, before blood samples were obtained, patients with AMI had received standard antithrombotic agents (500 mg aspirin and 5000 IU heparin) given intravenously by the referring physician or in our emergency room. In 5 patients who received prehospitalization treatment with aspirin and heparin and were subsequently found not to have an AMI, uPAR expression did not differ from that in the 2 control groups (data not shown). The use of standard therapy (β blockers, statins, and low-dose aspirin) in patients with AMI and those with CSA was not significantly different. Thus, different patient management measures and prehospitalization treatment in the 2 patient groups does not appear to have accounted for the observed differences in uPAR expression.
Pathophysiologic considerations
In AMI, the adhesive phenotype of monocytes appears to be the result of a complex activation process. Soluble factors (probably inflammatory cytokines) in plasma from patients with AMI can directly activate β2-integrin–mediated and uPAR-mediated adhesion. Induced adhesion to the uPAR ligand vitronectin, the β2-integrin ligand fibrinogen, and the endothelium depends on uPAR. Expression of uPAR on circulating monocytes is elevated in AMI and is increased on monocytic cells on incubation with AMI plasma ex vivo (Figure 1). The up-regulation of uPAR may result from AMI-related processes such as hypoxia or release of inflammatory cytokines, both of which have been shown to up-regulate uPAR in vitro.12 30 Enhanced availability of uPAR can augment cell adhesion by means of β2-integrins (Figure 7). The existence of such a relation is supported by the fact that maximal β2-integrin stimulation with PMA led to enhanced integrin-mediated adhesion of AMI cells, which expressed more uPAR than cells from patients with CSA but comparable total amounts of Mac-1 (Figure 4A).
Leukocyte recruitment is fundamental for the development of atherosclerosis, restenosis after percutaneous coronary intervention, and myocardial damage after ischemia and reperfusion.31 32The current study shows for the first time that uPAR has a central role in AMI as a regulator of monocyte adhesiveness. It extends the concept that uPAR plays a key role in β2-integrin regulation to a relevant clinical situation, that is, AMI. Therapeutic blockade of the uPAR system may be effective because it would combine antiadhesive and antiproteolytic strategies. Our findings suggest that it may be useful to develop specific antagonists against the uPAR system to prevent detrimental monocytic cell recruitment during cardiovascular inflammatory processes.
We thank M. Hölderle and T. Schmidt-Wöll for excellent technical assistance.
Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-03-0778.
Supported in part by grants from the Deutsche Forschungs-gemeinschaft (F.-J.N.), Wilhelm Sander-Stiftung (A.E.M.), and Novartis Foundation (K.T.P. and T.C.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andreas E. May, 1. Medizinische Klinik und Deutsches Herzzentrum München, Technische Universität München, Lazarettstr 36, 80636 Munich, Germany; e-mail:may@dhm.mhn.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal