Recent experiments show that hematopoietic progenitor cell populations contain endothelial precursor cells. We have isolated a population of CD34+ cells that expresses fibroblast growth factor receptor-1 (FGFR-1) and that differentiates into endothelial cells in vitro. We find that 4.5% ± 2.1% of CD34+cells isolated from bone marrow, cord blood, and mobilized peripheral blood express FGFR-1 and that viable CD34+FGFR+cells are small, with little granularity, and express both primitive hematopoietic and endothelial markers on their surface. The primitive hematopoietic markers AC133, c-kit, and Thy-1 are coexpressed by 75%, 85%, and 64% of CD34+FGFR+ cells, respectively. Most of the CD34+FGFR+ cells also express antigens found on endothelial cells, such as CD31, vascular endothelial growth factor receptor-2, and the endothelial-specific cell surface marker, vascular endothelial cadherin (VE-cadherin), whereas 56% to 60% of the cells express Tie, Tek, and the endothelial-specific marker, P1H12. The CD34+FGFR+ population is enriched in cells expressing endothelial-specific antigens compared with the CD34+ population. Isolated CD34+FGFR+ cells grow slowly in culture, are stimulated by fibroblast growth factor-2 and vascular endothelial growth factor, and give rise to cells that express von Willebrand factor and VE-cadherin and that incorporate acetylated low-density lipoprotein. These experiments show that FGFR-1 is expressed by a subpopulation of CD34+ cells that give rise to endothelial cells in vitro, indicating that this population contains endothelial stem/progenitor cells.
Introduction
The CD34+ population contains hematopoietic stem cells as well as endothelial stem/progenitor cells that can differentiate into endothelial cells. A small number of CD34+ hematopoietic progenitor cells express receptors for fibroblast growth factors,1-5 and fibroblast growth factor-2 (FGF-2) has both synergistic and direct effects on progenitor cell proliferation.6-10 FGF-2 also promotes the self-renewal11 and proliferation12 of primitive hematopoietic cell lines, indicating its relevance in early hematopoiesis.
Hematopoietic and endothelial cells have a close association during ontogeny. A common embryonic precursor, the hemangioblast, that gives rise to both hematopoietic and endothelial cells, has been identified within the Tek+ fraction of the aorta-gonad-mesonephros (AGM) region of the embryo,13 and FGF-2 has been shown to stimulate the proliferation of hemangioblasts.14 In addition, FGF-2 acts as a potent angiogenic cytokine, stimulating endothelial proliferation15 and inducing angiogenesis.16-18 FGF-2 and its receptors are therefore relevant in hemangioblast, early hematopoietic, and endothelial cell biology.
Hematopoietic stem cells have been isolated from CD34+ cells obtained from bone marrow, cord blood, and peripheral blood.19-22 CD34+ cells have also been shown to contain endothelial stem/precursor cells.23-29 Human CD34+ cells isolated from peripheral blood are incorporated into the endothelium of ischemic blood vessels of recipient animals.23,24 They also promote neovascularization of ischemic myocardium, improve cardiac function,25 and accelerate the restoration of blood flow in diabetic mice undergoing neovascularization.26CD34+ cells purified from umbilical blood give rise to von Willebrand factor (VWF)–expressing endothelial cells in vitro,27 whereas bone marrow–derived CD34+cells are found lining the surfaces of vascular prostheses in sex-mismatched canine grafts.28 Furthermore, endothelial cells of donor origin have been cultured from the peripheral blood of human subjects who had previously received sex-mismatched allogeneic bone marrow transplants, thereby definitively identifying the existence of circulating progenitor endothelial cells.30 31
Because hematopoietic and endothelial cells share many cell surface markers, including CD34,21,32 it is important to establish whether circulating cells that give rise to endothelium are endothelial precursors or merely mature endothelial cells derived from vessel walls. The primitive hematopoietic cell surface marker, AC133,33-37 is not expressed on differentiated endothelial cells29,38 and therefore coexpression of AC133 with endothelial markers can be used to distinguish endothelial precursors from mature endothelial cells. Human AC133+ cells isolated from mobilized peripheral blood have been shown to differentiate into endothelial cells in vitro and to form new blood vessels in vivo when injected with tumor cells into immunodeficient mice.39
We have isolated a subpopulation of CD34+ cells from bone marrow, cord blood, and mobilized peripheral blood that expresses FGFR-1 and that coexpresses a number of antigens found on primitive hematopoietic cells, including AC133. This population is selectively enriched for the expression of antigens specific for endothelial cells (P1H1230,40 and vascular endothelial cadherin41-45 [VE-cadherin]). The isolated CD34+FGFR+ cells grow slowly in culture, are stimulated by FGF-2 and vascular endothelial growth factor (VEGF), and give rise to cells that express VWF and VE-cadherin and that incorporate acetylated low-density lipoprotein (ac-LDL). These data indicate that the CD34+FGFR+ population contains endothelial stem/progenitor cells.
Materials and methods
Cell preparation
Fresh or frozen cells from bone marrow, cord blood, or cytokine-mobilized peripheral blood were obtained from donors after informed consent. Bone marrow or cord blood samples were diluted with 4 volumes RPMI 1640 medium containing 10% fetal calf serum (FCS). Mononuclear cells were separated on a Histopaque-1077 density gradient (Sigma Diagnostics, St Louis, MO) and washed twice with phosphate-buffered saline (PBS)–citrate (PBS containing 13.6 mM sodium citrate, 1 mM adenosine, and 2 mM theophylline). Leukapheresis samples were not usually subjected to Histopaque-1077 density gradient centrifugation and were washed twice with PBS-citrate. Cells washed in PBS-citrate (from all sources) were filtered through 40-μm nylon cell strainers (Becton Dickinson, Franklin Lakes, NJ), resuspended in 2 mL PBS-citrate, overlaid on a 3-mL PBS-citrate/10% bovine serum albumin (BSA) cushion, and subsequently centrifuged for 10 minutes at 200g at room temperature to remove platelets.46If necessary, DNAse 1 (Sigma Diagnostics) was added to increase recovery of viable cells from frozen samples.
Samples were enriched for CD34+ cells by immunomagnetic separation, using either CD34 magnetically activated cell sorter (MACS) microbeads, magnetic columns, and the MiniMACS system (Miltenyi Biotec, Auburn, CA) or the Dynal CD34 Progenitor Cell Selection System (Dynal AS, Oslo, Norway). Separation of CD34+ cells was carried out according to the manufacturers' recommendations. In some experiments, lineage depletion was performed to obtain lineage-negative (Lin−) cells. This was done by using a cocktail of lineage-specific antibodies (CD2, CD3, CD14, CD16, CD19, CD24, CD41, CD56, CD66b, and Glycophorin A)47 obtained from StemCell Technologies (Vancouver, BC, Canada), goat antimouse magnetic beads (Miltenyi Biotech), or sheep antimouse magnetic beads (Dynal) and immunomagnetic separation.
Antibodies and reagents
The following antibodies (all antibodies are mouse antihuman monoclonal antibodies unless otherwise stated) and reagents were used for immunofluorescent staining: Thy-1–fluorescein isothiocyanate (FITC) and Thy-1–phycoerythrin (PE) (Immunotech, Marseille, France); goat–antimouse-FITC, goat–antirabbit-FITC, mouse immunoglobulin G (IgG), goat IgG, rabbit IgG, and DNAse 1 (Sigma Chemicals, St Louis, MO); CD31-FITC (Caltag Laboratories, Burlingame, CA); CD31-PE (Becton Dickinson, San Jose, CA); biotinylated goat-antihuman kinase domain receptor (KDR) (R&D Systems, Minneapolis, MN); goat antimouse IgG2a-PE (Southern Biotechnology Associates, Birmingham, AL); human IgG (Massachusetts Public Health Biologic Laboratories, Boston, MA); AC133-PE (Miltenyi Biotec). CD34-allophycocyanin (APC), mouse IgG-APC, and mouse IgG biotin were obtained either from Caltag or Becton Dickinson. The following antibodies were purchased from Dako (Glostrup, Denmark): CD34-FITC, CD34-PE, CD34-PE–CY5, c-kit–PE, CD38-FITC, mouse IgG-FITC, mouse IgG-PE, mouse IgG-PE–Cy5, mouse IgG2a, rabbit antihuman von Willebrand factor, streptavidin-PE, and goat antimouse-PE. Additional antibodies used were unconjugated VE-cadherin (Hec 1.2) and VE-cadherin–FITC (Dr W. A. Muller, Cornell University, New York, NY); Tie-FITC and biotinylated Tek (Dr T. Suda, Kumamoto University, Kumamoto, Japan); P1H12-FITC and biotinylated P1H12 (Dr R. P. Hebbel, University of Minnesota, Minneapolis); FGFR-1 antibody (Dr W. L. McKeehan, Texas A & M University, Houston, and QED Bioscience, San Diego, CA). Anti–FGF receptor (R)-1–FITC was either purchased from QED or prepared in our laboratory. Anti–FGFR-1 was conjugated to APC in our laboratory. Conjugation of the antibody to APC was performed by using a Phycolink conjugation kit purchased from Prozyme (San Leandro, CA). Recombinant human FGF-2 was obtained from Scios Nova (Mountain View, CA); VEGF and stem cell factor (SCF) were obtained from PeproTech (Rocky Hill, NJ). DiI-ac-LDL (1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate) and goat antirabbit IgG conjugated to Alexa Fluor 488 were purchased from Molecular Probes (Eugene, OR).
Cell staining and flow cytometry
MACS-selected or Dynal-selected CD34+ cells or Lin− cells were resuspended in fluorescence-activated cell sorting (FACS) buffer that comprised PBS supplemented with BSA (0.1%), sodium azide (0.01%), and aprotinin (20 μg/mL). Fc receptors and nonspecific binding of immunoglobulins to cell surfaces were blocked with human IgG and either mouse or goat IgG, where appropriate. In most experiments, staining of FGFR+ cells was performed by using directly labeled FGFR-1–APC, although FGFR-1–FITC was used in some cases. In a few experiments unconjugated FGFR-1 was used together with either goat antimouse-PE or goat antimouse IgG2a-PE as secondary antibodies. Cells were incubated with appropriate antibodies for 30 minutes on ice, using FACS buffer to wash the cells between staining steps. Cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson), equipped with an argon laser to excite FITC, PE, and RPE-CY5 fluorochromes and a helium-neon diode, with time delay adjusted according to manufacturer's recommendations, for excitation of APC. CD34+ or Lin− selected cells (30 000-150 000) were analyzed with the use of CellQuest software (Becton Dickinson). The dye 7-aminoactinomycin D (7-AAD), at a final concentration of 1 μg/mL, was added 5 minutes before flow cytometry to identify dead cells. This task was done to ensure analysis of viable CD34+FGFR+ cells.48
For the detection of VWF expression, cells were fixed in 2% paraformaldehyde for 10 minutes at 37°C and 10 minutes at 4°C, permeabilized with 9:1 methanol/PBS for 20 minutes at −20°C,30 and treated with antibodies to VWF (see below). Cells were also examined for their ability to incorporate ac-LDL labeled with the fluorescent probe DiI-ac-LDL. The cells were incubated with DiI-ac-LDL (10 μg/mL) for 4 hours at 37°C,49 washed twice with PBS to remove free DiI-ac-LDL, and treated with CD34-FITC and FGFR-1–APC prior to FACS analysis.
Culture of CD34+FGFR+ cells
CD34-enriched cells were incubated with antibodies to CD34 and FGFR-1 and sorted on an Epics Elite Cell Sorter (Beckman Coulter, Fullerton, CA) into CD34+FGFR+ and CD34+FGFR− populations. Sorted cells were seeded at 1000 cells/well in 96-well plates in long-term culture medium (Myelocult H5100; StemCell Technologies) containing 12.5% horse serum and 12.5% FCS, in the presence or absence of FGF-2 (10 ng/mL) or FGF-2 and VEGF (10 ng/mL). Cells were fed twice weekly by replacing half the medium with fresh medium. Growth was assessed by determining the number of viable cells per well present after various times in culture.
Purified CD34+FGFR+Lin−cells were examined for evidence of endothelial maturation after culture in endothelial growth medium (EGM-2 medium from Clonetics, San Diego, CA) supplemented with SCF (50 ng/mL). The cells were cultured in fibronectin-coated (50 μg/mL in 0.1% gelatin) wells (Lab-Tek chamber slides; Miles Scientific, Naperville, IL) and incubated in a humidified incubator in the presence of 5% CO2. Half the medium was removed and replaced with fresh medium once weekly. After 5 to 8 weeks the cells were fixed with 3% paraformaldehyde in PBS, permeabilized with ice-cold acetone/methanol (50/50), and examined for the expression of VWF, using rabbit antihuman VWF antibodies and either goat antirabbit-FITC or goat antirabbit IgG conjugated to Alexa Fluor 488. Control wells were incubated with rabbit IgG in place of rabbit antihuman VWF antibody. Human umbilical vein cells (HUVECs) and cells of the erythroleukemic cell line, K562, were used as positive and negative controls, respectively, for ascertaining the specificity of the reagents used to determine VWF expression.
Cultured CD34+FGFR+ cells were also examined for concomitant incorporation of DiI-ac-LDL and expression of VWF. Cells were incubated with DiI-ac-LDL (10 μg/mL) for 4 hours at 37°C,49 washed twice with PBS to remove free DiI-ac-LDL, and fixed with 3% paraformaldehyde in PBS. Cells were subsequently permeabilized with ice-cold acetone/methanol (50/50), treated with VWF antibodies (see above), and examined by fluorescent microscopy for VWF and evidence of DiI-ac-LDL uptake.
Sorted CD34+FGFR+ and CD34+FGFR− cells were also seeded on OP9 feeder layers13 (1000 viable cells/well on a 96-well dish) in long-term culture medium containing FGF-2 (10 ng/mL) and VEGF (10 ng/mL). Cells were fed twice weekly by replacing half the medium with fresh growth factor-containing medium. After 12 weeks cells were fixed with 1% paraformaldehyde and examined for expression of VE-cadherin by using antibodies to VE-cadherin and secondary goat antimouse-FITC antibodies.
Statistical analysis
Data are expressed as the mean ± SD. When comparing the data from 2 populations, paired 2-tailed Student t tests were used to determine levels of significance. A P value of < .05 was considered statistically significant.
Results
Phenotype of the CD34+FGFR+ population
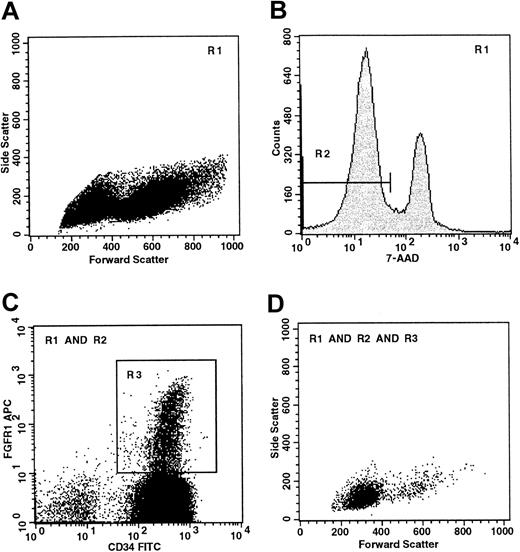
Purified CD34+ cells (Figure1A) were examined for evidence of FGFR-1 expression. CD34+ cells were incubated with 7-AAD to ensure that only viable cells (R2, Figure 1B) that excluded 7-AAD were analyzed for expression of FGFR-1 and other antigens. CD34+cells from bone marrow, umbilical cord blood, and mobilized peripheral blood contained a distinct population of FGFR-1–expressing cells (R3, Figure 1C). These cells comprised 4.5% ± 2.1% of the total CD34+ population (Table 1) and have low forward (FSC) and side scatter (SSC) properties (Figure1D), indicating that they are small cells with little granularity. It is important to ensure the viability of the CD34+FGFR+ cells, because substantial numbers of these small cells are found in a region of the FSC/SCC dotplot that is often excluded from FACS analysis, as this region may contain nonviable cells.
Flow cytometry of the CD34+FGFR+ population.
(A) Forward and side scatter analysis of CD34+-enriched cells isolated from mobilized peripheral blood. R1 defines a region that excludes debris with low FSC and cells with high SSC. (B) R2 defines viable (7-AAD−) cells. (C) R3 defines viable CD34+FGFR+ cells. (D) Boolean gating is used to illustrate the FSC/SSC characteristics of viable CD34+FGFR+ cells and shows that most of these cells are small (low FSC) and display little granularity (low SSC).
Flow cytometry of the CD34+FGFR+ population.
(A) Forward and side scatter analysis of CD34+-enriched cells isolated from mobilized peripheral blood. R1 defines a region that excludes debris with low FSC and cells with high SSC. (B) R2 defines viable (7-AAD−) cells. (C) R3 defines viable CD34+FGFR+ cells. (D) Boolean gating is used to illustrate the FSC/SSC characteristics of viable CD34+FGFR+ cells and shows that most of these cells are small (low FSC) and display little granularity (low SSC).
CD34+ cells express FGFR-1
| Source . | Selection method . | CD34+FGFR+ cells* (% of CD34+ cells) . |
|---|---|---|
| mPB | Dynal | 3.2 |
| mPB | Dynal | 4.7 |
| mPB | Dynal | 5.6 |
| mPB | Dynal | 5.3 |
| mPB | MACS | 7.9 |
| mPB | MACS | 3.6 |
| mPB | MACS | 3.3 |
| mPB | MACS | 6.5 |
| mPB | MACS | 3.6 |
| mPB | MACS | 8.2 |
| mPB | MACS | 3.5 |
| BM | Dynal | 4.9 |
| BM | MACS | 2.1 |
| CB | Dynal | 5.8 |
| CB | MACS | 1.3 |
| CB | MACS | 6.5 |
| CB | MACS | 1.1 |
| Source . | Selection method . | CD34+FGFR+ cells* (% of CD34+ cells) . |
|---|---|---|
| mPB | Dynal | 3.2 |
| mPB | Dynal | 4.7 |
| mPB | Dynal | 5.6 |
| mPB | Dynal | 5.3 |
| mPB | MACS | 7.9 |
| mPB | MACS | 3.6 |
| mPB | MACS | 3.3 |
| mPB | MACS | 6.5 |
| mPB | MACS | 3.6 |
| mPB | MACS | 8.2 |
| mPB | MACS | 3.5 |
| BM | Dynal | 4.9 |
| BM | MACS | 2.1 |
| CB | Dynal | 5.8 |
| CB | MACS | 1.3 |
| CB | MACS | 6.5 |
| CB | MACS | 1.1 |
mPB, mobilized peripheral blood; BM, bone marrow; CB, cord blood.
Mean = 4.5; SD = 2.1; n = 17.
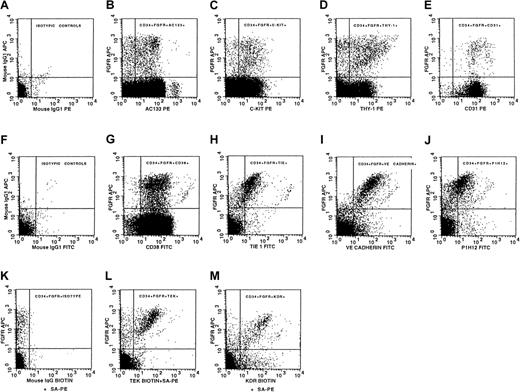
To determine the phenotypic nature of the CD34+FGFR+ population, we examined viable CD34+FGFR+ cells isolated from mobilized peripheral blood for the expression of the following 10 antigens: CD38, AC133 (CD133), Thy-1, c-kit, CD31, Tie, Tek, vascular endothelial growth factor receptor-2 (VEGFR-2/KDR), P1H12, and VE-cadherin (Table2, Figure2). This examination was done by using 4-color flow cytometry: 3 different fluorochromes were used for the staining of CD34, FGFR-1, and one of each of the 10 antigens, and the dye, 7-AAD, comprised the fourth color. The number of CD34+FGFR+ cells obtained in each experiment limited the number of antigens examined per sample. Most of the CD34+FGFR+ cells expressed cell surface antigens that are present on primitive hematopoietic cells, such as AC13333-37 (75%, Table 2, Figure 2B), c-kit50(85%, Table 2, Figure 2C), and Thy-151 (64%, Table 2, Figure 2D). In addition, substantial numbers of CD34+FGFR+ cells expressed the endothelial-specific markers VE-cadherin41-45 (88%, Table2, Figure 2I) and P1H1230,40 (58%, Table 2, Figure 2J). A number of antigens that are expressed by both hematopoietic and endothelial cells were also present on the CD34+FGFR+ population: CD3152,53(84%, Table 2, Figure 2E), Tie54,55 (56%, Table 2, Figure2H), Tek56,57 (60%, Table 2, Figure 2L), and KDR58 59 (76%, Table 2, Figure 2M). CD38 was found on 95% of CD34+FGFR+ cells (Table 2, Figure 2G). (Percentages provided here are rounded; for more precise data, refer to Table 2.) Isotypic control antibodies were used to indicate the specificity of the staining (Figure 2A,F,K). CD34+FGFR+ cells isolated from bone marrow and cord blood samples showed a pattern of expression of hematopoietic and endothelial markers similar to that found on cells isolated from mobilized peripheral blood (data not shown). These results demonstrate that substantial numbers of viable CD34+FGFR+ cells express antigens that are found on both early hematopoietic and endothelial cells.
Comparison of the expression of the indicated antigens by CD34+FGFR+ and CD34+ cells
| Antigen . | % CD34+FGFR+ cells expressing antigen (mean ± SD) . | % CD34+ cells expressing antigen (mean ± SD) . | No. of samples (n) . | Enrichment (fold) . | P . |
|---|---|---|---|---|---|
| CD38 | 94.9 ± 5.7 | 95.6 ± 2.9 | 4 | 0 | |
| AC133 | 75.3 ± 6.4 | 79.7 ± 6.4 | 6 | 0 | |
| c-kit | 84.5 ± 11.3 | 77.4 ± 18.3 | 6 | 0 | |
| CD31 | 84.0 ± 22.6 | 84.5 ± 18.3 | 6 | 0 | |
| Thy-1 | 63.9 ± 10.9 | 12.0 ± 8.9 | 8 | 5.3 | <.001 |
| Tie | 55.8 ± 15.1 | 4.4 ± 2.4 | 7 | 12.6 | <.001 |
| Tek | 60.0 ± 18.5 | 3.6 ± 2.6 | 6 | 16.6 | <.001 |
| P1H12 | 58.0 ± 17.1 | 4.2 ± 2.0 | 7 | 13.8 | <.001 |
| VE-cadherin | 87.8 ± 10.3 | 8.4 ± 4.0 | 4 | 10.4 | <.001 |
| KDR | 75.5 ± 16.0 | 5.2 ± 1.9 | 3 | 14.5 | =.014 |
| Antigen . | % CD34+FGFR+ cells expressing antigen (mean ± SD) . | % CD34+ cells expressing antigen (mean ± SD) . | No. of samples (n) . | Enrichment (fold) . | P . |
|---|---|---|---|---|---|
| CD38 | 94.9 ± 5.7 | 95.6 ± 2.9 | 4 | 0 | |
| AC133 | 75.3 ± 6.4 | 79.7 ± 6.4 | 6 | 0 | |
| c-kit | 84.5 ± 11.3 | 77.4 ± 18.3 | 6 | 0 | |
| CD31 | 84.0 ± 22.6 | 84.5 ± 18.3 | 6 | 0 | |
| Thy-1 | 63.9 ± 10.9 | 12.0 ± 8.9 | 8 | 5.3 | <.001 |
| Tie | 55.8 ± 15.1 | 4.4 ± 2.4 | 7 | 12.6 | <.001 |
| Tek | 60.0 ± 18.5 | 3.6 ± 2.6 | 6 | 16.6 | <.001 |
| P1H12 | 58.0 ± 17.1 | 4.2 ± 2.0 | 7 | 13.8 | <.001 |
| VE-cadherin | 87.8 ± 10.3 | 8.4 ± 4.0 | 4 | 10.4 | <.001 |
| KDR | 75.5 ± 16.0 | 5.2 ± 1.9 | 3 | 14.5 | =.014 |
Antigen expression on viable CD34+FGFR+ cells.
Isotypic control antibodies were used to indicate the specificity of staining of cells stained with (A) CD34-FITC, (F) CD34-PE, and (K) biotinylated antibody plus streptavidin-PE. CD34+FGFR+ cells express the following antigens: (B) AC133, (C) c-kit, (D) Thy-1, (E) CD31, (G) CD38, (H) Tie, (I) VE-cadherin, (J) P1H12, (L) Tek, and (M) KDR. All graphs except panel M were obtained from the analysis of cells from the same mobilized peripheral blood sample and are representative of at least 4 separate experiments. KDR results (M) are representative of 3 experiments.
Antigen expression on viable CD34+FGFR+ cells.
Isotypic control antibodies were used to indicate the specificity of staining of cells stained with (A) CD34-FITC, (F) CD34-PE, and (K) biotinylated antibody plus streptavidin-PE. CD34+FGFR+ cells express the following antigens: (B) AC133, (C) c-kit, (D) Thy-1, (E) CD31, (G) CD38, (H) Tie, (I) VE-cadherin, (J) P1H12, (L) Tek, and (M) KDR. All graphs except panel M were obtained from the analysis of cells from the same mobilized peripheral blood sample and are representative of at least 4 separate experiments. KDR results (M) are representative of 3 experiments.
To determine whether CD34+FGFR+ cells coexpressed both primitive and endothelial antigens on the same cells, we excluded the dye, 7-AAD, from analysis so that we could simultaneously examine the cells for the expression of AC133 and VE-cadherin. We found that 74% of CD34+FGFR+cells coexpress AC133 and VE-cadherin (Table3). We also determined whether CD34+FGFR+ cells demonstrated concomitant expression of Thy-1 with either Tek or VE-cadherin. We found that 65% of CD34+FGFR+ cells express both Thy-1 and VE-cadherin, whereas 57% express Thy-1 and Tek (Table 3). Because the majority (68%) of cells that expressed AC133 also expressed Thy-1 (Table 3), this finding indicates that substantial numbers of CD34+FGFR+ cells coexpress AC133, Tek, VE-cadherin, and Thy-1.
Coexpression of primitive hematopoietic and endothelial antigens by CD34+FGFR+ cells
| Phenotype . | % CD34+FGFR+ cells expressing the phenotype . |
|---|---|
| CD34+FGFR+AC133+VE-cadherin+ | 74 |
| CD34+FGFR+Thy-1+VE-cadherin+ | 65 |
| CD34+FGFR+Thy-1+Tek+ | 57 |
| CD34+FGFR+AC133+Thy-1+ | 68 |
| Phenotype . | % CD34+FGFR+ cells expressing the phenotype . |
|---|---|
| CD34+FGFR+AC133+VE-cadherin+ | 74 |
| CD34+FGFR+Thy-1+VE-cadherin+ | 65 |
| CD34+FGFR+Thy-1+Tek+ | 57 |
| CD34+FGFR+AC133+Thy-1+ | 68 |
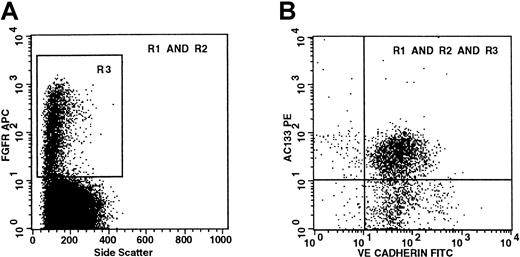
To confirm the coexpression of hematopoietic and endothelial markers on viable cells, we examined cells that had been depleted of lineage markers (ie, Lin− cells) for the simultaneous coexpression of FGFR-1, the primitive hematopoietic marker AC133, and the endothelial-specific marker VE-cadherin. Because we were limited to 4-color flow cytometry, the use of Lin− cells enabled us to examine a progenitor population for the coexpression of 2 surface antigens on viable 7-AAD−FGFR-1+ cells. This experiment is in contrast to others described earlier in which CD34+FGFR+ cells were analyzed for the coexpression of either one surface antigen on viable cells or 2 surface antigens on cells in which viability staining was omitted. The viable Lin−FGFR+ cells have a low SSC (Figure3A), and 68% of Lin−FGFR+ cells coexpress both AC133 and VE-cadherin (Figure 3B). Because the majority (> 99%) of AC133+ cells express CD34,34,35 the viable Lin−FGFR+AC133+VE-cadherin population (upper right quadrant in Figure 3B) must also be CD34+. These data confirm that viable CD34+FGFR+ cells simultaneously express both early hematopoietic and endothelial antigens on their surface. As AC133 is not found on mature or circulating endothelial cells,29 38 the expression of AC133 with VE-cadherin on CD34+FGFR+ cells indicates that these cells are candidates for endothelial stem/precursor cells.
Viable (7-AAD−) Lin−FGFR+ cells coexpress both primitive hematopoietic (AC133) and endothelial-specific (VE-cadherin) cell surface markers.
CD34+-enriched cells were isolated from lineage-depleted mobilized peripheral blood and gated to exclude debris with low FSC and cells with high SSC (R1) and nonviable (7-AAD+) cells (R2). (A) R3 defines viable Lin−FGFR+cells. Most of the viable Lin−FGFR+ cells are shown to have low SSC. (B) Boolean gating is used to show coexpression of both AC133 and VE-cadherin by viable Lin−FGFR+ cells. These plots are representative of 2 separate experiments.
Viable (7-AAD−) Lin−FGFR+ cells coexpress both primitive hematopoietic (AC133) and endothelial-specific (VE-cadherin) cell surface markers.
CD34+-enriched cells were isolated from lineage-depleted mobilized peripheral blood and gated to exclude debris with low FSC and cells with high SSC (R1) and nonviable (7-AAD+) cells (R2). (A) R3 defines viable Lin−FGFR+cells. Most of the viable Lin−FGFR+ cells are shown to have low SSC. (B) Boolean gating is used to show coexpression of both AC133 and VE-cadherin by viable Lin−FGFR+ cells. These plots are representative of 2 separate experiments.
We compared the levels of expression of endothelial and hematopoietic antigens on CD34+FGFR+ cells with that of the CD34+ population to determine if any of these antigens were preferentially expressed on CD34+FGFR+ cells. We found that CD38, AC133, c-kit, and CD31 are expressed to a similar extent by CD34+ and CD34+FGFR+cells (Table 2). In contrast, substantially increased numbers of CD34+FGFR+ cells expressed Thy-1, Tie, Tek, P1H12, VE-cadherin, and KDR compared with the CD34+population (Table 2). Thy-1 is expressed by 64% of CD34+FGFR+ cells compared with 12% of the CD34+ cells (Table 2). This finding indicates that there is a 5.3-fold enrichment of Thy-1+ cells in the CD34+FGFR+ subset as compared with the whole CD34+ population (P < .001, Table2). The differences in expression of the endothelial-specific markers, VE-cadherin and P1H12, between these 2 populations are even more striking, with a 10- to 14-fold increase in expression by the CD34+FGFR+ population compared with the CD34+ population (P < .001, Table2). Similar significant increases in expression (12- to 16-fold) of Tie (P < .001), Tek (P < .001), and KDR (P = .013) were noted by the CD34+FGFR+ cells compared with the CD34+ cells (Table 2). The increased numbers of cells expressing the endothelial-specific markers, P1H12 and VE-cadherin, as well as the antigens Tie, Tek, and KDR, found within the CD34+FGFR+ population compared with that of the CD34+ population, indicates that the CD34+FGFR+ subset is enriched for cells that express endothelial antigens.
These results show that approximately 4.5% of CD34+ cells from bone marrow, cord blood, and mobilized peripheral blood express FGFR-1 and that they simultaneously express antigens found on both primitive hematopoietic and endothelial cells. The expression of a number of antigens present on endothelial cells (Tie, Tek, KDR, P1H12, VE-cadherin) is enriched in the CD34+FGFR+population as compared with the CD34+ population. As 2 of these antigens (VE-cadherin, P1H12) are endothelial cell specific, the FGFR-1+ cells are likely to contain endothelial stem/progenitor cells.
Growth characteristics of CD34+FGFR+cells
To determine whether CD34+FGFR+ cells respond to endothelial growth factors and give rise to endothelial cells, we isolated and cultured them under conditions that promote endothelial cell growth. CD34+FGFR+ and CD34+FGFR− cells isolated on the FACS were cultured in the absence and presence of FGF-2 alone or FGF-2 and VEGF together. In the absence of growth factors both populations had minimal growth (Table 4). An increase in the numbers of CD34+FGFR+ cells (2.5- to 6.9-fold) was noted when these cells were cultured with FGF-2 or with FGF-2 and VEGF, whereas substantially less growth was found when CD34+FGFR− cells were cultured under similar conditions (Table 4). These data demonstrate that CD34+FGFR+ cell growth is stimulated by factors known to promote endothelial cell proliferation.
FGF-2 and VEGF promote growth of CD34+FGFR+ cells
| Experiment4-150 . | Incubation time (wk) . | Population . | Control4-151 . | FGF-2 (10 ng/mL)4-151 . | FGF-2 + VEGF (both at 10 ng/mL)4-151 . |
|---|---|---|---|---|---|
| 1 | 19 | CD34+FGFR+ | 1734 | 11 978 | ND |
| CD34+FGFR− | 1244 | 2 500 | ND | ||
| 2 | 5 | CD34+ | 1617 | 1 457 | 2 311 |
| CD34+FGFR+ | 3227 | 8 057 | 17 140 | ||
| CD34+FGFR− | 1493 | 2 098 | 3 093 |
| Experiment4-150 . | Incubation time (wk) . | Population . | Control4-151 . | FGF-2 (10 ng/mL)4-151 . | FGF-2 + VEGF (both at 10 ng/mL)4-151 . |
|---|---|---|---|---|---|
| 1 | 19 | CD34+FGFR+ | 1734 | 11 978 | ND |
| CD34+FGFR− | 1244 | 2 500 | ND | ||
| 2 | 5 | CD34+ | 1617 | 1 457 | 2 311 |
| CD34+FGFR+ | 3227 | 8 057 | 17 140 | ||
| CD34+FGFR− | 1493 | 2 098 | 3 093 |
ND, not determined.
Wells were seeded with 1000 cells.
Cells/well.
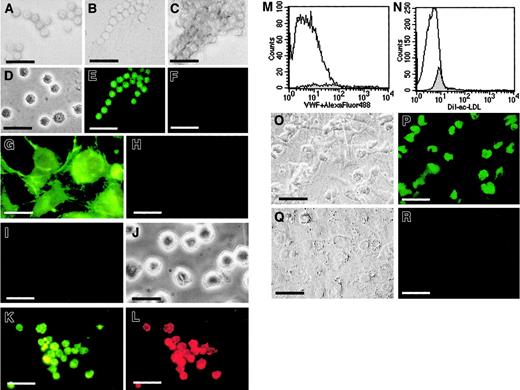
We next determined whether CD34+FGFR+cells could differentiate into endothelial cells as evidenced by the expression of the endothelial-specific markers VWF and VE-cadherin and the incorporation of ac-LDL. CD34+FGFR+ cells were cultured in fibronectin-coated wells in the presence of endothelial growth factors and examined for evidence of VWF expression. CD34+FGFR+ cells cultured under these conditions grew in small groups (Figure4A), beadlike strings (Figure 4B), or clusters (Figure 4C) of small round cells (∼6 μm in diameter), similar in size to freshly isolated cells (Figure 4D). Cultured cells displayed VWF expression (Figure 4E), whereas no staining was found in wells that received control antibodies (Figure4F). HUVECs (Figure 4G,H) and cells of the erythroleukemic cell line, K562 (Figure 4I,J), were used as positive (Figure 4G) and negative (Figure 4I) controls, respectively, to ensure the specificity of the VWF staining. These data indicate that CD34+FGFR+ cells give rise to cells that express VWF.
CD34+FGFR+ cells give rise to endothelial cells in culture.
CD34+FGFR+ cells grow in small groups (A), beadlike strings (B), or clusters (C) of small round cells, similar in size to freshly isolated cells (D). Cultured cells treated with rabbit antihuman VWF IgG and secondary goat antirabbit FITC IgG show expression of VWF (E), whereas those treated with control IgG in place of antibodies to VWF do not stain (F). To ensure the specificity of VWF staining, HUVECs and K562 cells were used as positive and negative controls, respectively. HUVECs display positive staining with VWF IgG (G) and no staining with control IgG (H), whereas no expression of VWF (I) is observed in K562 cells (J). Cultured CD34+FGFR+ cells that express VWF (K) also incorporate DiI-ac-LDL (L). Freshly isolated CD34+FGFR+ cells neither express VWF (M, shaded histogram) nor incorporate ac-LDL (N, shaded histogram). Open histograms represent control histograms, ie, cells incubated in the absence of antibodies to VWF (M) or in the absence of DiI-ac-LDL (N). CD34+FGFR+ (O,P) and CD34+FGFR− (Q,R) cells were cultured on OP9 feeder layers in the presence of FGF-2 and VEGF. Wells seeded with CD34+FGFR+ cells contained substantial numbers of adherent VE-cadherin–expressing cells (P), whereas cells in wells containing CD34+FGFR− cells lacked expression of VE-cadherin (R). Phase contrast microscopy of the same fields is shown in O and Q. Scale bars = 20 μm for panels A-L and O-R.
CD34+FGFR+ cells give rise to endothelial cells in culture.
CD34+FGFR+ cells grow in small groups (A), beadlike strings (B), or clusters (C) of small round cells, similar in size to freshly isolated cells (D). Cultured cells treated with rabbit antihuman VWF IgG and secondary goat antirabbit FITC IgG show expression of VWF (E), whereas those treated with control IgG in place of antibodies to VWF do not stain (F). To ensure the specificity of VWF staining, HUVECs and K562 cells were used as positive and negative controls, respectively. HUVECs display positive staining with VWF IgG (G) and no staining with control IgG (H), whereas no expression of VWF (I) is observed in K562 cells (J). Cultured CD34+FGFR+ cells that express VWF (K) also incorporate DiI-ac-LDL (L). Freshly isolated CD34+FGFR+ cells neither express VWF (M, shaded histogram) nor incorporate ac-LDL (N, shaded histogram). Open histograms represent control histograms, ie, cells incubated in the absence of antibodies to VWF (M) or in the absence of DiI-ac-LDL (N). CD34+FGFR+ (O,P) and CD34+FGFR− (Q,R) cells were cultured on OP9 feeder layers in the presence of FGF-2 and VEGF. Wells seeded with CD34+FGFR+ cells contained substantial numbers of adherent VE-cadherin–expressing cells (P), whereas cells in wells containing CD34+FGFR− cells lacked expression of VE-cadherin (R). Phase contrast microscopy of the same fields is shown in O and Q. Scale bars = 20 μm for panels A-L and O-R.
To confirm the endothelial nature of the cultured CD34+FGFR+ cells, they were also examined for their ability to incorporate DiI-ac-LDL. Because VWF is expressed only by megakaryocytic cells and endothelial cells60 and because ac-LDL is only taken up by endothelial cells and macrophages,49 we examined cultured CD34+FGFR+ cells for the presence of these characteristics. Dual fluorescence (Alexa Fluor 488 = green fluorescence, DiI = red fluorescence), indicating simultaneous VWF expression (Figure 4K) and ac-LDL incorporation (Figure 4L), confirmed the endothelial nature of the cells. To ensure the absence of endothelial cells in the original cell population, freshly isolated CD34+FGFR+ cells were examined by FACS analysis for the expression of VWF and their ability to incorporate DiI-ac-LDL. We show that freshly isolated CD34+FGFR+ cells do not express VWF (Figure 4M) or incorporate DiI-ac-LDL (Figure 4N). These data confirm that CD34+FGFR+ cells are able to give rise to endothelial cells.
Because OP9 cells have been shown to promote the differentiation of progenitor cells to mature endothelial cells,13 we also cultured CD34+FGFR+ cells on OP9 feeder layers. Phase contrast microscopy shows that sorted CD34+FGFR+ cells adhered to the OP9 feeder layer in the presence of FGF-2 and VEGF (Figure 4O). The expression of VE-cadherin in wells seeded with CD34+FGFR+ and CD34+FGFR− cells was examined by using a mouse monoclonal antibody to VE-cadherin and a goat antimouse FITC-conjugated antibody. Wells seeded with CD34+FGFR+ cells contained substantial numbers of VE-cadherin–expressing cells (Figure4P), whereas cells in wells containing CD34+FGFR− cells (Figure 4Q) lacked expression of VE-cadherin (Figure 4R). These results indicate that the CD34+FGFR+ cells maintained their expression of the endothelial-specific antigen, VE-cadherin, when cultured under conditions that promote endothelial growth.
Our results show that the CD34+FGFR+ population grows slowly in culture in an FGF-2– and VEGF-dependent manner, indicating that these cells are stimulated by angiogenic factors. The cultured CD34+FGFR+ cells give rise to cells that incorporate ac-LDL and express VWF, whereas freshly isolated cells do not. This finding indicates that the CD34+FGFR+ population contains cells that differentiate into endothelial cells.
Discussion
We show that endothelial precursor cells reside within a population of FGFR+ progenitor cells. FGFR-1 is expressed on a small population of CD34+ cells (Table 1, Figure 1) that coexpresses primitive hematopoietic and endothelial cell surface antigens (Tables 2 and 3, Figures 2 and 3) and gives rise to endothelial cells in culture (Figure 4). The CD34+FGFR+ population is considerably enriched (10- to 16-fold) with respect to the expression of many antigens that are found on endothelial cells (VE-cadherin, P1H12, Tie, Tek, KDR) compared with the CD34+ population (Table 2). The CD34+FGFR+ population also contains 5-fold more cells expressing Thy-1, a primitive hematopoietic marker,51 than the CD34+ population, indicating that Thy-1 is also expressed on most endothelial progenitor cells. Most CD34+FGFR+ cells express AC133, a primitive hematopoietic antigen33 that is not expressed on mature endothelial cells.29 38 Because VE-cadherin and P1H12 are expressed exclusively on endothelial cells and because Thy-1 and AC133 are expressed on early hematopoietic cells, the CD34+FGFR+ population that coexpresses these markers is likely to represent an endothelial stem/progenitor cell population. Cultured CD34+FGFR+ cells respond to angiogenic cytokines (Table 4), maintain their expression of VE cadherin (Figure 4P), and give rise to cells that express VWF (Figure4K) and incorporate ac-LDL (Figure 4L). The freshly isolated CD34+FGFR+ population does not express VWF (Figure 4M) or incorporate ac-LDL (Figure 4N), indicating that the CD34+FGFR+ population contains cells that differentiate into endothelial cells.
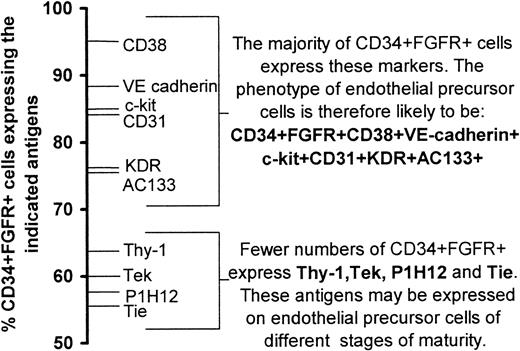
We have combined the results from 4-color fluorescence staining (Table 3, Figure 3B) with those of the incidence of expression of antigens on the CD34+FGFR+ cells (Table 2) to obtain the following phenotype to characterize endothelial progenitor cells: CD34+FGFR+CD38+VE-cadherin+c-kit+CD31+KDR+AC133+(Figure 5). As fewer cells express Thy-1, Tie, Tek, and P1H12, it is possible that these antigens may be expressed on endothelial progenitor cells of different stages of maturity in a manner analogous to the expression of lineage-specific markers on hematopoietic cells as they mature along different lineages.
Phenotype of endothelial stem/precursor cells.
The incidence of cell surface antigen expression was used to construct a probable phenotype of endothelial stem/precursor cells.
Phenotype of endothelial stem/precursor cells.
The incidence of cell surface antigen expression was used to construct a probable phenotype of endothelial stem/precursor cells.
Cells that have the capacity to develop into endothelial cells have recently been isolated from the circulation.23,27-31,39,61,62 Previous studies indicating the presence of endothelial precursor cells in cord blood or peripheral blood have used either unfractionated mononuclear cells or samples enriched for CD34+,23,27,29,61,63AC133+,39 P1H12+,30or CD14+ cells.62 In these studies the phenotype of the circulating endothelial precursors was determined by either single-color flow cytometry alone or together with immunocytochemisty, or alternatively by 2-color flow cytometry. Our data indicate that endothelial precursor cells can be isolated and identified by the expression of FGFR-1 and provides the first detailed description of the phenotype of these cells by using FACS isolation and 4-color flow cytometry.
The identification of progenitor cells that express FGFR-1 as well as primitive hematopoietic and endothelial cell antigens and that give rise to endothelial cells in vitro is not surprising, as hematopoietic and endothelial systems have a close association during ontogeny. A common embryonic precursor, the hemangioblast, that gives rise to both hematopoietic and endothelial cells has been identified,13and experiments using murine embryonic stem cell lines carrying mutations for FGFR-1 show that FGF-2–mediated signaling is essential for hemangioblast proliferation.14 FGFRs are expressed on pluripotent human embryonic stem cells and embryoid bodies64 as well as on CD34+ hematopoietic cells1-5 and on leukemic cell lines, such as K562, U937, Hel, Daudi, MO7E, HL60, Jurkat, Molt 3, and TF-1.65-67FGF-2 has been shown to promote the self-renewal of a multipotent hematopoietic cell line11 and the growth of a primitive erythroid cell line.12 FGF-2 also stimulates the growth of early hematopoietic progenitors in synergy with other cytokines.7-10 In addition to its effects on embryonic and hematopoietic cells, FGF-2 also acts as a potent angiogenic cytokine. It stimulates endothelial cell proliferation15 and is a potent inducer of angiogenesis.16-18 FGF-2 is able to induce uncommitted mesoderm to differentiate into endothelial precursors (angioblasts) in the quail embryo68,69 and is required for the organization of vascular endothelial cells into functional networks in the murine embryo.70 FGF-2 has also been shown to induce the development of blood islands and endothelial cells from dissociated quail epiblasts,71 and FGF-2 stimulates the growth of both endothelial and hematopoietic cells from embryonic avian mesoderm.72 Furthermore, experiments using 3-dimensional spheroid models of endothelial cell differentiation have shown that FGF-2 is a survival factor for immature endothelial cells, inhibiting the apoptosis of these cells.73 There is therefore considerable evidence that indicates that FGF-2 is a relevant cytokine that affects endothelial cells as well as hemangioblasts and early hematopoietic cells.
Although regarded as an endothelial-specific marker of mature endothelial cells, VE-cadherin is also expressed by embryonic cells that have hemangioblast potential.74,75 Because FGFR-1 and FGF-2 are required for hemangioblast proliferation,14 it is possible that CD34+FGFR+ cells that express VE-cadherin may have hemangioblast potential. It is currently unknown whether hemangioblasts exist in postnatal life.
In summary, we show that the FGFR+ progenitor population contains endothelial precursor cells. These cells may be of significant clinical benefit in treating a number of disorders such as cardiovascular disease, diabetes, and cancer. Endothelial stem cells have been used to repair sites of vascular injury,76 to improve cardiac function,25 and to enhance the blood flow in diabetic mice after hindlimb ischemia.26CD34+FGFR+ cells from syngeneic individuals may also be useful for delivering angiogenic or antitumor agents to the rapidly expanding vascular bed associated with tumors.
This paper is dedicated to the memory of Eugene B. Dowdle, a mentor, friend, and collaborator of P.E.B., S.C., and E.L.W.
Supported by the Cancer Association of South Africa (CANSA), the University of Cape Town Staff Research Fund, and the National Institutes of Health DK48728.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Patricia E. Burger, Department of Immunology, H53-22 Old Main Bldg, Groote Schuur Hospital, Observatory 7925, Cape Town, South Africa; e-mail:pburger@uctgsh1.uct.ac.za.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal