The engraftment of donor bone marrow (BM) cells in nonablated mice is inefficient. Niche availability has been thought to be the reason, and cytoablation with irradiation or cytotoxic agents is routinely used with the belief that this frees the preoccupied niches in recipients. In this study, donor cell redistribution and proliferation in ablated and nonablated mice were compared by implanting donor cells directly into the femur cavity of sedated mice. The redistribution of Lin− donor cells into BM was similar between ablated and nonablated mice. Poor engraftment in nonablated mice was shown to be the result of inefficient donor cell proliferation rather than because of a lack of space. Competitive repopulation assays demonstrated that the donor hematopoietic stem cells (HSCs) were present in nonirradiated recipients for at least 6 months after transplantation, but that they did not expand as did their counterparts in lethally irradiated mice. This study suggests that efficient bone marrow transplantation in nonablated recipients may be possible as a result of better understanding of HSC proliferative regulation and appropriate in vitro manipulation.
Introduction
Bone marrow transplantation (BMT) has widespread clinical application in the treatment of a variety of disorders. The classical BMT approach in both clinical patients and experimental animals is the injection of bone marrow (BM) cells or stem cells into the bloodstream, whereupon the blood-producing cells (progenitor cells or stem cells) find “niches” in the BM to “seed,” a process commonly referred to as homing. Little is known about the regulatory mechanisms that control this process. A niche is thought to be a space in the BM microenvironment composed of specific stromal cells and extracellular matrix elements that maintains and regulates the physiological activity of stem cells. Myeloablation is routinely used prior to BMT because it is thought that this opens stem cell niches for entry of donor cells. It is believed that successful engraftment of the transplanted hematopoietic stem cells (HSCs) depends on the ability of donor HSCs to home to the stem cell niches, which are occupied by host HSCs prior to myeloablation.1 This model has arisen from experiments in the murine model, which has shown that engraftment in nonablated mice is poor.2-4
Several groups have reported that with multiple large doses of BM cell infusions in the nonablated mouse, 10% to 42% of donor cell engraftment can be achieved.5-10 However, to reach this level of engraftment in the nonablated mouse, a total of 1 to 2 × 108 BM cells had to be infused, while only 2 × 105 BM cells were sufficient to repopulate an ablated (lethally irradiated) mouse. The different demand for donor cells was thought to result from the difference in niche availability. The dynamic distribution of BM cells is under physiological balance among the blood, BM, and other hematopoietic-related organs, such as the spleen. Homing is a process whereby progenitor cells and stem cells migrate from blood to BM niches, while mobilization refers to the process of progenitor cells and stem cells moving from the BM into the bloodstream; that is, redistribution. Changes in physiological conditions or levels of growth factors can influence this balance, but the mechanism is only partially understood. Introducing donor cells directly into the bloodstream makes it nearly impossible to study the dynamics of stem cell distribution and regulation among hematopoietic organs. Therefore, we developed a novel method by infusing the donor cells directly into the femur cavity of a sedated mouse and then monitoring stem cell redistribution and regulation. The purpose of this study is to understand the dynamics of stem cell distribution and regulation in the ablated versus the nonablated environment. Our results suggest that there is no significant difference in stem cell homing in ablated versus nonablated mice, but rather that the success of donor cell engraftment in ablated mice results from an up-regulation in stem cell proliferation, which does not occur in nonablated mice.
Materials and methods
Animals
C57BL/6J Ly5.2 and C57BL/6J Ly5.1 mice (8 to 12 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, ME). The first filial generation (F1) of Ly5.2 and Ly5.1 was bred at the animal facility of the University of Southern California, Los Angeles. Mice were housed in sterile microisolator cages and fed acidified water and sterilized lab chow. All procedures were approved by the Animal Care and Use Committee of the University of Southern California.
Antibodies
The antibodies used in the lineage cocktail were anti–Mac-1 (M1/70), anti–Gr-1 (RB6-8C5), anti-B220 (RA3-6B2), anti-CD3e (145-2C11), anti-CD4 (RM4-5), anti-CD5 (53-7.3), anti-CD8a (53-6.7), and antierythroid (Ter119). Other antibodies included anti-Ly5.1 (A20) conjugated with phycoerythrin (PE) and anti-Ly5.2 (104) conjugated with fluorescein isothiocyanate (FITC). All antibodies listed above were purified rat anti–mouse antibodies from Pharmingen (San Diego, CA). Goat anti–rat magnetic beads were from Miltenyi Biotech (Auburn, CA).
Lin− cell isolation
To isolate Lin− cells, BM cells were flushed from tibias, femurs, and humeri of male C57BL/6J Ly5.1 mice with Hanks buffer by means of a 25-gauge needle. After lysis of red blood cells with ammonium chloride Lysis Buffer (Ortho-mune Lysing Reagent; Ortho, Raritan, NJ), cells were labeled with the purified rat anti–mouse lineage marker antibody cocktail as described above. Lin+cells were depleted with goat anti–rat magnetic beads by using a CS column (Miltenyi Biotech, Bergisch Gladbach, Germany).
PKH26 labeling
PKH staining was performed according to the manufacturer's instructions (Sigma, St Louis, MO). In brief, Lin−cells were resuspended in PKH diluent and then were mixed with PKH26 dye (4 μM), followed by incubation at room temperature for 5 minutes with gentle agitation. After incubation, an equal volume of fetal calf serum was added to terminate the staining. Labeled cells were washed with alpha-minimal essential medium and resuspended in autologous mouse serum for femur implantation. More than 99% of the cells were labeled with PKH26. Up to 5 cell divisions can be traced with these labeling conditions as tested by an in vitro culture system (data not shown).
Femur implantation of Lin− cells
Male F1 mice, ablated or nonablated, were used as primary recipients. After being anesthetized with isoflurane (Abbott Lab, North Chicago, IL), the joint between the femur and tibia in one leg was surgically exposed. The PKH26-labeled Ly5.1 Lin− cells (5 × 105 in 2 μL) were injected into the femur medullary cavity near the medial epicondyle (preliminary experiments indicated that the femur cavity could accommodate 5 μL of trypan blue without leakage). The insertion hole was immediately sealed with bone wax, and the skin was closed by wound clips. This is designated the “implanted” or “injected” femur; the femur from the other leg served as the “nonimplanted” or “noninjected” femur. To confirm the location of implanted cells, 5-(and 6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE)–labeled Lin− cells were implanted into femurs for fluorescence microscopic analysis. As one of the controls, the same number of cells was infused via classic tail vein infusion into nonablated mice.
Analysis of transplanted cells
At 20 hours, 48 hours, 72 hours, 1 week, 16 weeks, and 12 months after femur implantation, primary recipient mice were killed. The peripheral blood was collected by retro-orbital bleeding for fluorescence-activated cell-sorter (FACS) analysis. Cells were harvested from the implanted and the nonimplanted femurs as well as from the spleen. After lysis of red blood cells (RBCs), all the nucleated cells were counted and analyzed by FACS. The percentage of PKH26-positive donor cells (Ly5.1) in BM and spleen was determined by PKH26 labeling and/or antibody staining to calculate the donor cell numbers. To determine the low frequency of donor cells, 1 million events were analyzed by FACS analysis. The background staining was determined to be less than 0.02%.
Long-term repopulation assay
At different times after implantation of cells in the femur (24 hours, 48 hours, 72 hours, and 1 week), cells from each primary recipient (F1) were collected from both femurs (implanted and nonimplanted). 1.5 × 106 cells were transplanted to lethally irradiated secondary recipients (Ly5.2) via tail vein as previously described.11 Peripheral blood was examined to determine the origin of the engrafting cells 6 and 18 weeks after secondary BMT. After lysis of RBCs, the nucleated cells were distributed into 2 aliquots. One was labeled with anti-Ly5.1 (PE or FITC), anti-Ly5.2 (FITC or PE), and antimyeloid (allophycocyanin-conjugated MAC-1 and Gr-1) antibodies; the other aliquot was labeled with anti-Ly5.1 (PE), anti-Ly5.2 (FITC), and antilymphoid marker antibodies (APC-conjugated CD4, CD8, and B220). FACS analysis was performed using a MoFlo flow cytometer with Summit analysis software (Cytomation, Fort Collins, CO) to determine the percentage of cells derived from the primary donor (Ly5.1), the primary recipients (F1), and the secondary recipients (Ly5.2). Ly5.1 and F1 cell numbers were determined by FACS analysis and cell counting prior to secondary transplantation. After secondary transplantation, the engraftment from Ly5.1 and F1 cells was determined by FACS analysis. Stem cell activity was measured by repopulation unit (RU) as previously described.12
Results
Implantation of donor cells
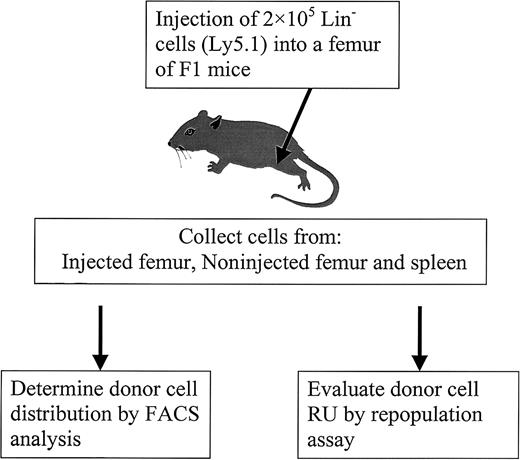
To study the distribution of BM cells, PKH26-labeled Ly5.1 (Lin−) cells were used as donor cells. Previous studies have demonstrated that PKH26 staining does not alter cell function,13-16 and our results support this conclusion given that PKH26-labeled cells had the same long-term repopulating activity as unstained cells (data not shown). After implantation, the donor cells were identified by PKH26 labeling and by antibody staining using FACS analysis. To better understand stem cell relocation and proliferation dynamics under different conditions (ablated versus nonablated), a novel transplantation method was developed by implanting 5 × 105 Lin− donor cells directly into a femur cavity (Figure 1).
Diagram of experimental design.
PKH26-labeled Lin− cells from C57BL/6J Ly5.1 mice were injected into the femur of primary recipients (F1 mice). At different times after injection, cells from different locations (injected femur, noninjected femur, and spleen) were harvested to determine donor cell distribution by FACS and to evaluate RU by competitive repopulation assay.
Diagram of experimental design.
PKH26-labeled Lin− cells from C57BL/6J Ly5.1 mice were injected into the femur of primary recipients (F1 mice). At different times after injection, cells from different locations (injected femur, noninjected femur, and spleen) were harvested to determine donor cell distribution by FACS and to evaluate RU by competitive repopulation assay.
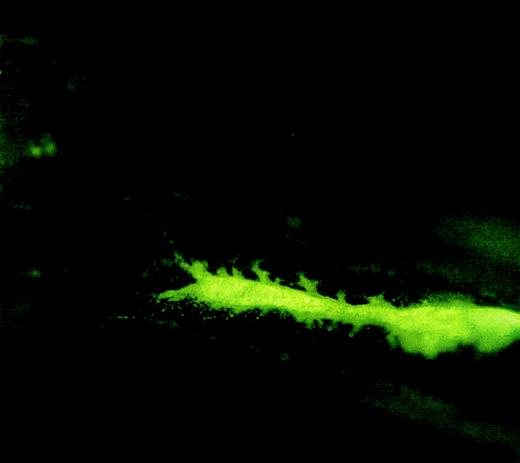
The following experiments were performed to ensure that the donor cells were not injected into the bloodstream. First, 5 minutes after implantation, the implanted femur was removed and examined under a fluorescence microscope. As shown in Figure2, donor cells (CFSE labeled) are concentrated within the femur cavity. Second, donor cell concentration in the bloodstream was analyzed and compared between the mice that received implantation and the mice that received traditional tail vein infusion.
Fluorescence microscopic picture of a femur longitudinal section showing the location of injected cells in the femur cavity.
Lin− cells were labeled with carboxyfluorescein diacetate, succinimidyl ester (CFDA) and injected into a femur cavity by a 26-gauge needle. The needle insertion hole was sealed with bone wax. The mouse was killed 5 minutes after injection, and the injected femur was removed for fluorescence microscopic analysis. Original magnification, 2 × 20.
Fluorescence microscopic picture of a femur longitudinal section showing the location of injected cells in the femur cavity.
Lin− cells were labeled with carboxyfluorescein diacetate, succinimidyl ester (CFDA) and injected into a femur cavity by a 26-gauge needle. The needle insertion hole was sealed with bone wax. The mouse was killed 5 minutes after injection, and the injected femur was removed for fluorescence microscopic analysis. Original magnification, 2 × 20.
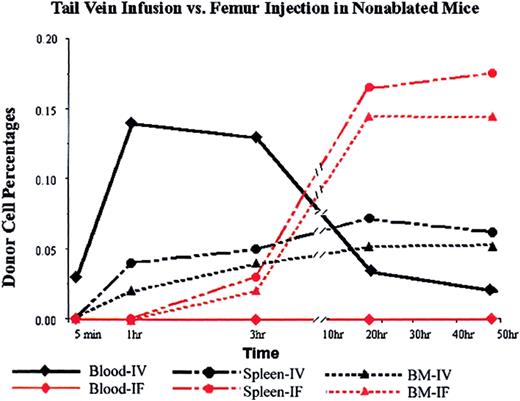
Donor cells appeared in the blood steam immediately after tail vein infusion and peaked at 1 hour after infusion at a concentration of 0.14%. In the mice that received the donor cells by femur implantation, the donor cell level in blood was undetectable at all time points checked: 5 minutes, 1 hour, 3 hours, 20 hours, 50 hours (Figure 3).
Donor cell distributions in nonablated mice after tail vein infusion or direct femur injection.
PKH26-labeled Lin− cells (5 × 105) were infused via tail vein or injected into the cavity of one of the femurs of untreated mice (3 mice per data point; SD was too small to be shown in the figure). At different times (5 minutes, 1 hour, 3 hours, 20 hours, and 50 hours) mice were killed, and the percentages of donor (PKH26-positive) cells in blood, BM, and spleen were determined by FACS analysis. Black lines represent data from intravenous (IV) injection, and red lines represent data from intrafemoral (IF) injection.
Donor cell distributions in nonablated mice after tail vein infusion or direct femur injection.
PKH26-labeled Lin− cells (5 × 105) were infused via tail vein or injected into the cavity of one of the femurs of untreated mice (3 mice per data point; SD was too small to be shown in the figure). At different times (5 minutes, 1 hour, 3 hours, 20 hours, and 50 hours) mice were killed, and the percentages of donor (PKH26-positive) cells in blood, BM, and spleen were determined by FACS analysis. Black lines represent data from intravenous (IV) injection, and red lines represent data from intrafemoral (IF) injection.
Surprisingly, however, implanted donor cells were detected in the nonimplanted femur and in the spleen 3 hours after implantation at concentrations of 0.02% and 0.03%, respectively. The concentration of donor cells in the nonimplanted femur continued to increase until 20 hours after implantation, and then reached a plateau. At the 20-hour time point, there were 3-fold more cells in the nonimplanted femur than there were in the BM of the femur in an animal receiving an equal number of donor cells via tail vein injection (Figure 3, triangles). Likewise, there were 3-fold more donor cells in the spleen (Figure 3, circles). These data suggest that the relocation of donor cells from implanted bone to other hematopoietic organs follows a different time course from the homing of donor cells from blood to hematopoietic organs.
Donor cell relocation in ablated and nonablated animals
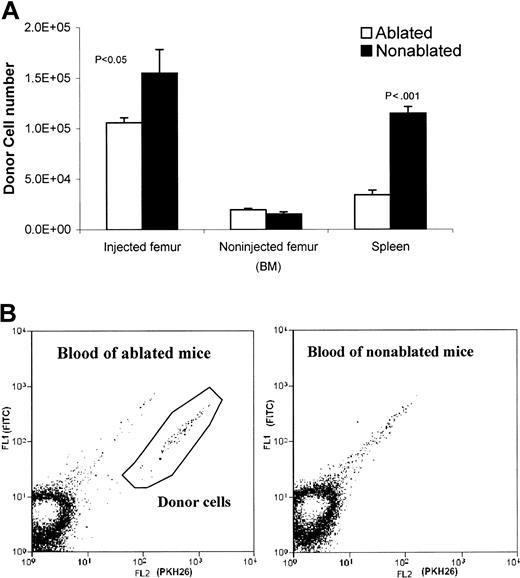
To compare the kinetics of donor cell distribution between ablated and nonablated mice, cells were collected at 20 hours after implantation from implanted femurs, nonimplanted femurs, and spleen. The donor cell (PKH26 labeled) percentages were determined by FACS analysis. After implantation (20 hours), the injected femur of the ablated mice contained somewhat fewer donor cells (1.06 ± 0.11 × 105) compared with nonablated mice (1.55 ± 0.05 × 105), indicating that implanted cells clearly egressed from the injected femur of ablated mice (Figure4A). In addition, donor cells were detected in ablated mice blood, but not in nonablated mice blood (Figure 4B). However, the number of donor cells detected in the noninjected femur was similar in ablated and nonablated mice (Figure4A). The movement of donor cells from the implanted femur to the nonimplanted femur represents homing of donor cells to whole body BM. These data suggest that lethal irradiation has little effect on the BM's ability to accept donor cells; that is, the redistribution capacity of irradiated and nonirradiated BM appears to be the same.
Comparison of donor cell distribution in ablated and nonablated mice 20 hours after injection.
(A) Donor cell numbers in injected ablated and nonablated mice (injected/noninjected femur and spleen). Both of the femurs (injected and noninjected) and the spleen were harvested 20 hours after injecting 5 × 105 PKH26-labeled Lin− cells into a femur cavity of untreated or lethally irradiated (950 rad) mice. Bone marrow cells were flushed out from the femurs. Spleens were dissected to wash out spleen cells. Cells from each tissue (injected femur, noninjected femur, and spleen) were counted and subjected to FACS analyses on a MoFlo flow cytometer. Donor cell numbers were calculated from the percentages of donor cells and the total cell count of each tissue. (B) FACS analysis of blood samples from ablated and nonablated mice 20 hours after injection of donor cells into the femur cavity. PKH26-labeled donor cells are present in the blood of ablated mice, but not in the blood of nonablated mice. The autofluorescent events were in the diagonal line and not counted. The positive events fall below the autofluorescent line.
Comparison of donor cell distribution in ablated and nonablated mice 20 hours after injection.
(A) Donor cell numbers in injected ablated and nonablated mice (injected/noninjected femur and spleen). Both of the femurs (injected and noninjected) and the spleen were harvested 20 hours after injecting 5 × 105 PKH26-labeled Lin− cells into a femur cavity of untreated or lethally irradiated (950 rad) mice. Bone marrow cells were flushed out from the femurs. Spleens were dissected to wash out spleen cells. Cells from each tissue (injected femur, noninjected femur, and spleen) were counted and subjected to FACS analyses on a MoFlo flow cytometer. Donor cell numbers were calculated from the percentages of donor cells and the total cell count of each tissue. (B) FACS analysis of blood samples from ablated and nonablated mice 20 hours after injection of donor cells into the femur cavity. PKH26-labeled donor cells are present in the blood of ablated mice, but not in the blood of nonablated mice. The autofluorescent events were in the diagonal line and not counted. The positive events fall below the autofluorescent line.
Donor cell proliferation kinetics in ablated and nonablated mice
At different times after implantation, donor cells (PKH26 labeled) or donor-derived cells (Ly5.1 antibody staining) were analyzed to study donor cell proliferation. Given that one femur contains 7.5% of the total BM,17 the donor cell numbers in total BM (excluding the implanted femur) could be calculated. Comparing the ablated and nonablated mice, the donor cell number in BM and spleen was not significantly different in the first 72 hours after implantation (Figure 5). However, in ablated mice, a rapid increase in the number of donor cells was observed from 72 hours to 1 week after implantation: 220-fold (implanted femur), 520-fold (nonimplanted femur), and 480-fold (spleen). From 1 week to 4 weeks, the rate of expansion declined: 2.8-fold for the implanted femur, 8.4-fold for the nonimplanted femur, and 14-fold for the spleen. This expansion of donor cells occurred only in ablated recipients, not in nonablated mice. The donor cell expansion in BM and spleen of ablated mice was also reflected in the donor cell–derived blood cells (Figure5). One month after implantation, the donor cells had repopulated the ablated BM to a normal cellularity (1.89 ± 0.18 × 107for implanted femur and 1.79 ± 0.45 × 107 for nonimplanted femur).
Proliferation profiles of donor cells in ablated and nonablated mice.
After injection of 5 × 105 Lin− Ly5.1 cells into the right femur, cells were collected from the injected femur, noninjected femur, spleen, and peripheral blood and were subjected to FACS analysis to determine the donor cell percentages. Two experiments were performed, with 5 to 8 mice per group. From 1 to 3 days, donor cells were identified by PKH26 labeling and antibody staining. After 3 days, donor cells were identified by PE-conjugated anti-Ly5.1 antibody. Donor cells rapidly proliferated in BM and spleen of ablated mice but did not proliferate in nonablated mice. The peripheral blood donor cell percentages also reflected this rapid proliferation of donor cells in ablated mice.
Proliferation profiles of donor cells in ablated and nonablated mice.
After injection of 5 × 105 Lin− Ly5.1 cells into the right femur, cells were collected from the injected femur, noninjected femur, spleen, and peripheral blood and were subjected to FACS analysis to determine the donor cell percentages. Two experiments were performed, with 5 to 8 mice per group. From 1 to 3 days, donor cells were identified by PKH26 labeling and antibody staining. After 3 days, donor cells were identified by PE-conjugated anti-Ly5.1 antibody. Donor cells rapidly proliferated in BM and spleen of ablated mice but did not proliferate in nonablated mice. The peripheral blood donor cell percentages also reflected this rapid proliferation of donor cells in ablated mice.
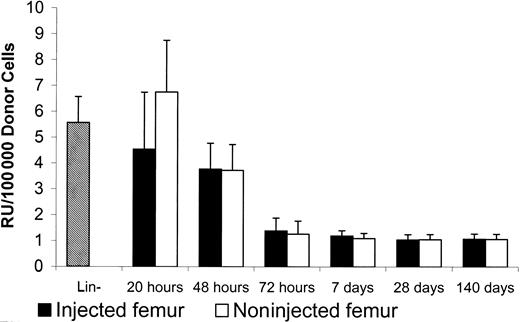
Distribution of long-term repopulating cells in nonablated mice
Our results indicate that although a similar number of donor cells were localized to the BM in both ablated and nonablated mice, rapid expansion of donor cells occurred only in ablated mice 72 hours after transplantation. However, the distribution of donor cells may not accurately represent the distribution of HSCs, because HSCs are only a small proportion of the Lin− cells. Therefore, the competitive repopulation assay was used to evaluate the distribution kinetics of the implanted long-term HSCs. To accurately compare the donor RU of HSCs (Ly5.1) in nonablated F1 mice, BM cells recovered from the primary recipient's implanted femur as well as the nonimplanted femur isolated at different time points were infused via tail vein into lethally irradiated Ly5.2 mice. To evaluate the RU in donor cells prior to implantation, Lin− cells from Ly5.1 mice and whole BM cells from F1 mice were mixed at a ratio of 1:50 in the competitive repopulation assay. The ratios, prior to and after repopulation in Ly5.2 recipients, were used to calculate RU.
In the nonablated mice, the donor RU between implanted (4.5 ± 2.2/105 donor cells) and nonimplanted femur (6.7 ± 2.0/105 donor cells) was not significantly different 20 hours after implantation (Figure6). The recovered donor RU at 20 hours after implantation was similar to the RU of donor cells prior to implantation (5.6 ± 1.0/105 donor cells). The donor stem cells were able to redistribute evenly into the BM in nonablated mice.
Repopulation units in injected and noninjected femur of nonablated mice.
After injection of 5 × 105 Lin− Ly5.1 cells into one of the femur cavities of F1 nonablated mice, 1.5 × 106 marrow cells were collected from each of the injected and noninjected femurs at different times. The collected cells, which contained Ly5.1 and F1 cells, then were infused into lethally irradiated Ly5.2 mice for the competitive repopulation assay. Lin− donor cells prior to femur injection were mixed with F1 BM (2% donor cells) for the competitive repopulation assay. Peripheral blood was collected from Ly5.2 recipients 6 months later for FACS analysis to determine the percentages of repopulation from Ly5.1 and F1 cells. Repopulation units per 100 000 donor cells were calculated based on the method described by Jordan et al.12
Repopulation units in injected and noninjected femur of nonablated mice.
After injection of 5 × 105 Lin− Ly5.1 cells into one of the femur cavities of F1 nonablated mice, 1.5 × 106 marrow cells were collected from each of the injected and noninjected femurs at different times. The collected cells, which contained Ly5.1 and F1 cells, then were infused into lethally irradiated Ly5.2 mice for the competitive repopulation assay. Lin− donor cells prior to femur injection were mixed with F1 BM (2% donor cells) for the competitive repopulation assay. Peripheral blood was collected from Ly5.2 recipients 6 months later for FACS analysis to determine the percentages of repopulation from Ly5.1 and F1 cells. Repopulation units per 100 000 donor cells were calculated based on the method described by Jordan et al.12
Our data, therefore, suggest that donor stem cells can home to their niches equally well in ablated and nonablated mice and that the increased efficiency of donor cell transplantation in ablated mice is due to stimulation of donor cell repopulation in ablated mice that does not occur in nonablated mice.
Discussion
To achieve a successful engraftment in BMT, 2 conditions have to be satisfied: the stem cells have to be able to home to their niches, and these stem cells have to be able to proliferate and differentiate. This study is an attempt to separate these 2 requirements. We asked the biologic question: is the lack of engraftment in nonablated mice the result of a lack of space for homing or a lack of proliferation of donor cells?
The classical BMT model in mice involves the infusion of donor cells intravenously. However, recovery of donor cells from BM and spleen is extremely low,18 and it is not possible to study stem cell relocation and the dynamic balance between homing and mobilization by infusing donor cells into the tail vein. Consequently, in this study, we implanted labeled donor cells directly into the femur BM cavity of ablated and nonablated mice. With this novel BMT approach, we were able to study the redistribution and proliferation profiles of 5 × 105 Lin− donor cells.
First, we demonstrated that donor cells did not go directly into the bloodstream in our bone implantation procedure. The following evidence supports this conclusion: (1) when implanted, donor cells were undetectable in the blood, while tail-vein injected donor cells were detected within 5 minutes; (2) implanted donor cells require 20 hours to reach equilibrium, while tail-vein injected donor cells were equilibrated in 3 hours; (3) donor cell concentration in BM from femur-implanted mice was higher (0.8%) than from tail-vein injected mice (0.43%); (4) although bone implanted donor cells were not detectable in the blood, more donor cells were detected in nonimplanted BM and spleen 20 hours after BMT than in animals who received donor cells via tail vein infusion. Although we cannot exclude the possibility of a very small number of donor cells leaking into the bloodstream because of the surgical wound, our data suggest that the primary process is a slow release of donor cells from the implanted BM and that this slow release minimizes the number of donor cells that are trapped in peripheral organs (such as lung and liver). We speculate that donor cell migration from the implanted femur to other locations might represent the normal BM cell traffic that occurs physiologically between BM, blood, and other organs.
Second, we studied donor cell relocation and proliferation in both ablated and nonablated mice. Donor cells were found in the blood of ablated mice, but not in the blood of nonablated mice after femur implantation. Vascular walls separate the extravascular compartment in the BM from the blood circulation.19-23 It has been reported that irradiation disrupts the BM/endothelial barrier.19,23 The donor cells detected in the bloodstream of irradiated mice are probably an indication of this disruption of BM integrity by irradiation. It also has been reported that irradiation can cause an increase in cytokines, including granulocyte-macrophage colony-stimulating factor (GM-CSF),24-28 which are known to be BM progenitor cell mobilization reagents. Although there was a detectable level of donor cells in the bloodstream of femur-implanted ablated mice, the level of donor cells detected in the nonimplanted femur was similar between ablated and nonablated animals. This observation suggests that homing is not simply donor cell diffusion from blood to the BM niches, but rather an apparent regulated process. We also realize the possibility that the intrafemoral injection may, by disturbing the natural anatomic structure, produce an artifact. From our relocation study, it appears that the majority of the intrafemoral injected cells follow different kinetics from tail vein–injected cells. Further work will determine if the intrafemoral approach is an appropriate model for progenitor cell homing and relocalization (mobilization).
Hendrikx et al15 compared the homing efficiency between nonablated and ablated mice. They found that the splenic homing was similar, however, the BM homing was 2.5 times higher in nonablated mice. Different experimental conditions may have resulted in the differences observed, since the experiments used different donor cells (sorted low-density cell, WGA+, 1.5.1.1−), a different radiation dose (875 rad), and different donor cell administration method (tail vein injection).
Given that donor cell recovery from the noninjected femur was the same as that obtained from the tibia and humeri (data not shown), the noninjected femur probably represents the whole BM. Comparing donor cell numbers in the nonimplanted femur, there is no significant difference between ablated and nonablated recipients (1.96 ± 0.25 × 104 vs 1.54 ± 0.47 × 10,4 Figure 4). Therefore, radio ablation does not increase the number of donor cells that home into BM. The homing of donor cells from circulating blood into the hematopoietic compartment of the BM involves specialized vascular sinuses whose walls consist of a complete endothelial layer and an incomplete adventitial layer.19 22 Our data suggest that radiation-induced alteration of sinus walls does not increase the number of donor cells that home to the BM, but rather increases the donor cell number in the circulating blood.
If homing efficiency is similar between ablated and nonablated mice, what is the cause for the lack of engraftment in nonablated mice? We studied the proliferation kinetics of donor cells in different locations in ablated versus nonablated mice. Donor cell proliferation could be observed only in ablated animals starting from day 3 after irradiation. The rate of proliferation in ablated mice continued for 3 to 4 weeks until it reached a plateau. In contrast, in nonablated mice, the donor cell–derived blood cells were hardly detectable (in 3 of 5 mice they were undetectable; in the other 2, the level was only 0.002%), and the donor cell number in BM and spleen remained at a low level at all times. Therefore, with the same homing efficiency, the lack of proliferation/amplification in nonablated mice resulted in the limited engraftment. With 2 × 108 donor BM cells, up to 42% donor-derived BM cells were detected by Stewart et al.8 Because the recipients contain 3 × 108BM cells,17 42% engraftment from 2 × 108donor cells suggests that there was no donor cell expansion but close to a dose-dependent replacement of host BM cells.
Bone marrow progenitor cell proliferation is controlled by complex, but still largely unknown, sets of regulatory mechanisms, including both systemic (growth factors, hormones, etc) and local environment variables (cell-to-cell interaction, extracellular matrix protein, autocrine and paracrine activities of cell cycle regulatory factors, etc). It has been reported that irradiated endothelial cells enhance the proliferation of CD34+ cells by means of increased production of cytokines such as GM-CSF, stem cell factor (SCF), interleukin 1α (IL-1α), IL-6, and tumor necrosis factor alpha (TNF-α).24,29 30 The rapid proliferation of stem cells in ablated mice appears to be a response of donor cells to the physiological changes after irradiation, including the augmentation of cytokine production and the decrease of BM cellularity. The donor cells in this study are lineage-negative cells, which include both stem and progenitor cells. The limited engraftment in nonablated mice might be the result of a lack of proliferation of the donor stem cells, or, alternatively, the donor cells that homed to the BM in the nonablated mice were not hematopoietic stem cells. To address this question, we did secondary BMT to test if the donor cells residing in the BM were, indeed, stem cells; that is, did they maintain the ability to repopulate ablated recipients. RU analysis was employed to examine the results.
In our system, the repopulating activity of lineage-negative cells was 5.6 times higher compared with whole BM nuclear cells. At different times after femur implantation, the RU of the retrieved donor cells varied. Although there were 10-fold more donor cells in the implanted femur than in the nonimplanted femur 20 hours after implantation, the donor cells' RU was similar in both femurs (implanted and nonimplanted). These results suggest that the cells with repopulating activity homed to BM preferentially. It also suggests that homing is a regulated process rather than a simple diffusion from bloodstream to BM. Donor cell RU dropped from 5.6 (20 hours after BMT) to 1 (72 hours after BMT) at 72 hours after implantation, and it stabilized at this level. Thus, the HSC-enriched lineage-negative cells (donor cells) developed into cells with the same repopulating activity as host BM cells. As shown in the peripheral blood, donor-derived cells differentiated into both myeloid and lymphoid cells. Our data suggest that donor stem cells could home to niches in nonablated mice, proliferate, differentiate, and be regulated, as host stem cells.
In conclusion, in nonablated mice, the donor cells home to bone marrow equally as efficiently as in the ablated mice, but the donor cells do not proliferate/expand as efficiently as they do in ablated mice. In the nonablated animal, the body has no need to activate stem cells into a rapid cell cycle to produce blood elements. In ablated mice, with the loss of host cells and the myelodepletion-induced production of stimulatory factors, donor HSCs take over hematopoiesis with little competition from host endogenous HSCs. Thus, the donor stem cell can play a major role in hematopoiesis if it has a selective advantage. Successful stem cell transplantation in the gene therapy trial in SCID-X1 patients without chemoablation supports this conclusion.31
In the treatment of various forms of cancer, conventional BMT is used following maximally tolerated doses of chemotherapy and/or radiation therapy. By understanding the regulatory mechanisms involved in cell cycle control in the bone marrow, it may be possible to pretreat donor cells before BMT in order to gain a proliferation advantage for the donor cell. If successful, then BMT could be carried out without ablation and the accompanying side effects. This may allow BMT for elderly or medically infirm patients who are not eligible for myeloablation.
We thank Dr Zhen Hai Chen for assistance with the femur injections, Jeffrey Louie for FACS analysis, and Dr Patrick Johnston for helpful comments on the manuscript.
Prepublished online as Blood First Edition Paper, July 12, 2002; DOI 10.1182/blood-2002-04-1256.
Supported by grants from the G. Harold and Leila Y. Mathers Charitable Foundation and from Farmal LLC.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Yi Zhao, Norris Cancer Center, Room 6316, University of South California, Keck School of Medicine, Los Angeles, CA 90033; e-mail: yizhao@hsc.usc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal