Abstract
The α6β4 integrin is the receptor for various laminin isoforms and is a component of the hemidesmosome. Increased expression levels of this integrin correlate with the aggressive phenotype of many epithelial tumors compared with surrounding normal tissue. Furthermore, the long cytoplasmic tail of the β4 integrin subunit has been implicated in several signal transduction pathways that are involved not only in invasion, but also in proliferation and apoptosis. Here we report that the exogenous expression of β4 integrin in 32D/v-abl–transformed cells reduces tumor aggressiveness in vivo and strongly inhibits cell proliferation in vitro by inducing monocytic differentiation. These effects are accompanied by growth arrest and p73 protein accumulation. The hypothesis that the inhibition of v-Abl oncogenic capacity could allow the activation of the endogenous c-Abl was tested in RKO cells. The results clearly demonstrated a strong increase of c-Abl phosphorylation that is accompanied by its association with p73 protein. Overall, the reported findings indicate that α6β4 integrin promotes growth arrest and differentiation by modulating Abl kinases and p73 protein pathway(s).
Introduction
The integrin α6β4 is expressed by epithelial and neuroepithelial cells. In epithelia, the distribution of this molecule is restricted to the basal layer of cells in contact with the basement membrane1 where it plays an essential role in the formation and stabilization of hemidesmosomes.2,3 The ligands for α6β4 integrin are various laminins isoforms.4,5 The α6β4 integrin was originally identified as a tumor-associated antigen (TSP-180).6 We and others have shown that the expression of the β4 subunit correlates with the metastatic phenotype of mouse tumors and increases in human invasive carcinomas relative to benign adenomas and normal tissues.7-10 This suggests a role for this integrin during tumor progression. It was recently observed that androgen receptor expression in prostate carcinoma cells suppresses α6β4 expression and the invasive phenotype,11 suggesting that the loss of androgen receptor expression, a common feature of aggressive prostate tumors, stimulates α6β4 expression and α6β4-mediated invasion. Further strong evidence implicating α6β4 in invasion was provided by the finding that the expression of the exogenous β4 subunit in β4-deficient colon and breast carcinoma cell lines increased their ability to invade in vitro.12,13 The β4 cytoplasmic domain is distinct both in size (approximately 1000 amino acids [aa]) and structure from any other integrin subunit.14 15 Although some progress has been made in identifying the specific regions and motifs within this domain that mediate α6β4-specific functions, the characterization is far from being conclusive.
Our previous work demonstrated that the stimulation of α6β4 integrin by a monoclonal antibody increases phosphorylation of the β4 subunit and promotes cell growth in vitro.16 We also found that the addition of insulin to intact carcinoma cells induces a 30-fold increase in the phosphorylation of the β4 subunit.17 This was the first evidence to implicate α6β4 in a signaling pathway. At present, it is well established that α6β4 participates in signaling events that regulate not only the invasion, but also cell proliferation, gene expression, and apoptosis.12,13,18-20 However, the molecular mechanisms underlining α6β4 activities remain to be elucidated. Recently, we found that α6β4 integrin associates in vitro and in vivo with the ErbB-2 tyrosine kinase receptor in human ovarian and mammary carcinoma cell lines.21 A significant increase in the proliferative and invasive capacity of ErbB-2–transformed NIH3T3 cells overexpressing α6β4 strongly suggested a role for this association during tumor progression. Using this model, we identified a specific region in the β4 cytoplasmic domain that is critical for the ability of α6β4 integrin to stimulate invasion. Interestingly, the extracellular domain of β4 is not necessary for α6β4 to stimulate invasion. We also observed a strong activation of PI3K only with those β4 deletion mutants that were able to stimulate invasion upon expression in the NIH3T3/ErbB-2 cell line.22 These data agree with the finding that α6β4 is implicated in carcinoma invasion through its ability to activate PI3K.13 22
In the present work, we investigated the possibility that α6β4 integrin cooperates with other oncogenes such as v-src and v-abl. For this purpose we used, as a model, 32D diploid murine hemopoietic cells and their transformed v-src or v-abl counterparts. The murine myeloid progenitor 32D cells do not express the β4 integrin subunit and have been extensively used to study transformation,23,24 cell spreading, and motility induced by different receptors and ligands.25 It has been previously reported that v-src and v-ablexpression in 32D cells induces transformation,23,24 and that 32D/v-Abl and 32D/v-src cells originate tumors in syngenic and nude mice.26,27 In these experimental conditions it was expected that de novo expression of the β4 integrin subunit might give additional information about its ability in modulating tumor phenotype.22 Surprisingly, we found that the β4 integrin subunit does not increase the malignant potential of transformed cells, but instead counteracts the transformed phenotype inducing differentiation. Indeed, we found that stable β4 integrin subunit expression significantly inhibits the proliferative and tumorigenic capacity of 32D/v-Abl cells. Moreover, morphologic analysis of 32D/v-Abl/β4 cells revealed that β4 expression induces monocytic differentiation that is accompanied by accumulation of p73 protein. Experiments undertaken to evaluate the role of p73 protein in 32D/v-Abl differentiation indicate that p73 overexpression induces monocytic differentiation of these transformed cells. The specificity of v-Abl and β4 interaction is demonstrated by the observation that β4 expression does not have any detectable effects in the proliferative and differerentiative capacity of 32D/v-src cells. These results provide for the first time evidence of a link between β4 integrin signaling and the p73 protein in the differentiation process.
Materials and methods
Cell lines and cDNAs
The murine myeloid progenitor cell line 32D, depending on interleukin 3 (IL-3) for growth and viability, was cultured in RPMI 1640 supplemented with 10% fetal calf serum (FCS), penicillin, streptomycin, and glutamine (Invitrogen, Milan, Italy), and 5% WEHI-3B–conditioned medium as a source of murine IL-3. The v-src– and v-abl–transformed counterparts are instead IL-3–independent either for survival or cell growth. The parental cell line, the 32D/v-Abl–transformed cell line,23,28 and the 32D/src–transformed cell line24 were transfected, by electroporation, either with the pRC/CMV expression vector alone or carrying the wild-type human β4 integrin subunit cDNA.5 The 32D/v-Abl cells were also transfected with pRC/CMV vector carrying the truncated cytoplasmic domain of the β4 integrin subunit (β4 L).29 Selection of neomycin-positive clones was carried out using 1 mg/mL G418 (Invitrogen). The human large-cell carcinoma cell line H1299 stable-transfected with an expression vector encoding p73 cDNA30 was maintained in RPMI 1640 supplemented with 10% FCS (Invitrogen) and used as positive control (H-p73α cells). 32D cells transfected with the expression vector encoding for a p53 Val 135, maintained in culture as described below, were also used as positive controls.28 32D/v-Abl cells were also transfected with the expression vector alone or encoding p73 cDNA which is also tagged with hemaglutinin (HA) epitope,30 and maintained as described below. After selection with G418, the population of 32D/v-Abl/neo and 32D/v-Abl/p73 cells was analyzed by morphology. Colon carcinoma cell line RKO20 was cultured in Dulbecco modified Eagle medium (DMEM) and transient transfected with the expression vector alone or encoding the β4 integrin subunit cDNA.5 After transfection, endogenous c-Abl phosphorylation level and β4 integrin expression were analyzed by immunoprecipitation and Western blot analyses.
Antibodies
The rat monoclonal antibodies (mAbs) 439-9B and 135-13C direct to the human β4 and α6 integrin subunits, respectively, were purified as previously described.6 The rat mAb 346-11A anti–mouse β4 integrin subunit was prepared and purified from ascitic fluid and used as a negative control.31 Rat anti–mouse CD11b (MAC-1 αM chain) (IgG2b) was from BD Pharmingen (San Diego, CA). The rat anti–mouse F4/80 (IgG2b) was kindly provided by Dr P. Giacomini (Regina Elena Cancer Institute, Italy). The rat anti–mouse CD4 (IgG2b) used as a negative control was from Southern Biotechnology Associates (Birminghan, AL). The antiphosphotyrosine (anti-P-tyr) mouse mAb 4G10 was from UBI (Lake Placid, NY). The rabbit polyclonal antibody against human and mouse c-Abl was from Santa Cruz (Santa Cruz, CA). The antiactive mitogen-activated protein kinase (MAPK) polyclonal antibody was from Promega (Madison, WI). The polyclonal antitotal MAPK was from Santa Cruz. The polyclonal antibody direct to p73 protein was kindly provided by Y. Shaul.32 The polyclonal antibodies direct to p73 protein were from Santa Cruz. To detect p53, a mixture of monoclonal antibodies D01 and 1801 was used.33 The anti-HA monoclonal antibody was from Roche Molecular Biochemicals (Monza, Italy). The secondary antibodies (F[ab′]2, fluorescein isothiocyanate [FITC]–conjugated or horseradish peroxidase [HRP]–conjugated) were from Cappel (Durham, NC).
Flow cytometry
The expression levels of cell surface receptors were analyzed by flow cytometric analysis (FACS) of stained cells. Cells were washed twice with cold phosphate buffered saline (PBS) containing 0.002% ethylenediaminetetraacetic acid (EDTA) and 10 mM NaN3 (washing buffer). Samples of 1 × 106control and transfected cells were incubated for 1 hour at 4°C with primary antibody diluted in PBS containing 0.5% bovine serum albumin (BSA). Cells were then washed 3 times with washing buffer (PBS containing 0.5% BSA) and incubated for 1 hour at 4°C with 50 μL FITC-conjugated secondary antibodies diluted 1:20 in PBS/BSA. After 3 washes, the cells were suspended in 1 mL washing buffer. Cell suspensions were analyzed by a flow cytometer (Epics XL analyzer; Coulter Corporation, Miami, FL) after addition of 5 μL propidium iodide (1 mg/mL stock solution) to exclude nonviable cells. The negative controls were treated as described below. At least 1 × 104 cells per sample were analyzed.
Total cell lysates and immunoprecipitation
In brief, as previously described,62 × 107 cells from each control and β4- transfected cells were labeled with 1 mCi (37 MBq) of 125I in the presence of 10 μL lactoperoxidase (2 mg/mL in 50% of glycerol) (Calbiochem, La Jolla, CA) and 5 μL of a 1:1000 dilution of H2O2 (30%). After labeling, cells were washed with PBS and solubilized in lysis buffer containing 5 mg/mL BSA, 1% NP-40, 1 mM NaN3, 1 mM phenylmethylsulfonylfluoride (Sigma, St Louis, MO), 5 μg/mL leupeptin, 10 μg/mL aprotinin (Sigma), and 10 mM EDTA. The lysates were clarified by centrifugation (30 000g) for 3 hours at 4°C and solubilized proteins (5 × 106 cpm) were immunoprecipitated. Direct immunoprecipitations were performed using primary mAb 439-9B collected with 50 μL protein G–agarose beads (Pierce, Rockford, IL) suspended in lysis buffer (50% vol/vol). The immunoprecipitates were washed at 4°C in lysis buffer, boiled, and analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). The autoradiography was performed with X-Omat RP film (Kodak, Rochester, NY).
Cell proliferation and viability curves
Exponentially growing cells were plated (3 × 104/mL) in RPMI 1640 with 10% FCS in the presence or absence of IL-3. Cell numbers were determined at daily intervals in triplicate with a Thoma hemocytometer (Marienfeld, Germany). Cells viability was determined by trypan blue exclusion.
In vivo studies
32D/v-Abl and 32D/v-Abl/β4 cells (2 × 107) were injected subcutaneously into 6-week-old female nu/nu mice. The mice were examined every day for the appearance of tumor masses. Tumor volume was measured every other day until day 37 from the first tumor appearance, when the first mouse died (Table1).
Tumorigenicity of 32D/v-Abl and 32D/v-Abl/β4 cell lines injected in nude mice
| Cell lines . | Tumor appearance, d . | Mean tumor volume, mm3 . |
|---|---|---|
| 32D/v-Abl | 10 ± 1 | 1849 ± 461 |
| 32D/v-Abl/β4 clone a | 25 ± 2 | 251 ± 50 |
| 32D/v-Abl/β4 clone b | 25 ± 2 | 320 ± 35 |
| Cell lines . | Tumor appearance, d . | Mean tumor volume, mm3 . |
|---|---|---|
| 32D/v-Abl | 10 ± 1 | 1849 ± 461 |
| 32D/v-Abl/β4 clone a | 25 ± 2 | 251 ± 50 |
| 32D/v-Abl/β4 clone b | 25 ± 2 | 320 ± 35 |
32D/v-Abl and 32D/v-Abl/β4 (clones a and b) cells (2 × 107) were injected subcutaneously into five 6-week-old female nu/nu mice. Tumor volume (mm3) was measured every other day. Mean tumor volume was calculated at day 37 when the first mouse died.
ApoTag staining
Cells (1 × 106) were plated for 48 hours on dishes coated with Poly-L-lysine, fibronectin (FN), vitronectin (VN), and laminin (LM) obtained from Invitrogen or on dishes coated with 10 μg/mL 439-9B and/or 135-13C mAbs in normal culture condition. Then the cells were harvested, washed in PBS, fixed in 1% paraformaldehyde in PBS, permeabilized in 70% ethanol, and stained with ApoTag reagent (Oncor, Gaithersburg, MD) and propidium iodide (PI) as described above. The stained cells were analyzed by FACS.
Cell cycle analysis
Analyses of DNA content of 32D control cells and β4 transfectans were done by FACS. In brief, the cells were fixed in cold methanol and acetone (1:4) for 30 minutes at 4°C and stained in PBS containing 50 μg/mL PI and 0.2 mg/mL RNAse A for 30 minutes at RT. DNA content was measured by the Epics XL analyzer.
Morphology
Approximately 2 × 104 cells were spun onto a glass slide in a cytocentrifuge. For morphologic analysis, cytospin preparations were fixed and stained with May-Grunwald-Giemsa stain (Sigma) and observed under a light microscope.
Western blot analyses
32D/v-Ab cells and/or β4-transduced cells were lysed in lysis buffer (50 mM Tris, pH 8, 100 mM NaCl, 10% glycerol, 1% Triton X-100, 1 mM EDTA, 1 mM MgCl2, 2 mM phenylmethylsulfonyl fluoride, and protease inhibitors mixture). The extracts were sonicated for 10 seconds and centrifuged at 14 000 rpm for 10 minutes to remove the debris. Protein concentration was determined by a coloritrimetic analysis assay (Bio-Rad, Milan, Italy). Total cell lysates (200 μg/lane) were resolved by SDS-PAGE. Protein gels were transferred to nitrocellulose membranes (Bio-Rad). The western blot analyses were resolved by the enhanced chemiluminescence (ECL) system (Amersham Pharmacia Biotech, Milan, Italy).
Reverse transcription–polymerase chain reaction analyses of mouse p73 mRNA
Expression of mouse p73 mRNA was analyzed by reverse transcription–polymerase chain reaction (RT-PCR) amplification. Total RNA was prepared from 32D/v-Abl/neo, 32D/v-Abl/β4 wild type, and 32D/v-Abl/β4 L cells using RNAzol B (Biotech, Rome, Italy) according to the manufacturer's procedure. Total RNA (7 μg) was reverse transcribed at 37°C for 1 hour in the presence of random hexamers and Moloney murine leukemia virus reverse transcriptase (Invitrogen). Mouse p73 mRNA quantitative analysiswas carried out by PCR amplification of a fragment of 298 base pair (bp) for a total of 36, 38, and 40 cycles using the follow specific primers: 5′GAGCACCTGTGGAGTTCTCTAGAG3′ and 5′GGTATTGGAAGGGATGACAGGCG-3′.
The housekeeping aldolase mRNA, used as an internal control, was amplified from the same cDNA reaction mixture for a total of 25, 30, and 35 cycles using the follow specific primers: 5′-TGGATCGGCTGTCTCAACGCTGT-3′, and 5′-TCACTGTCGTCCCCCGTGACA-3′ to amplify a fragment of 413 bp.
Amplified PCR products were electrophoresed on a 2% agarose gel containing ethidium bromide (0.5 μg/mL) and visualized under UV light.
Results
The cytoplasmic domain of the β4 integrin subunit influences the cell proliferation of 32D/v-Abl–transformed cells
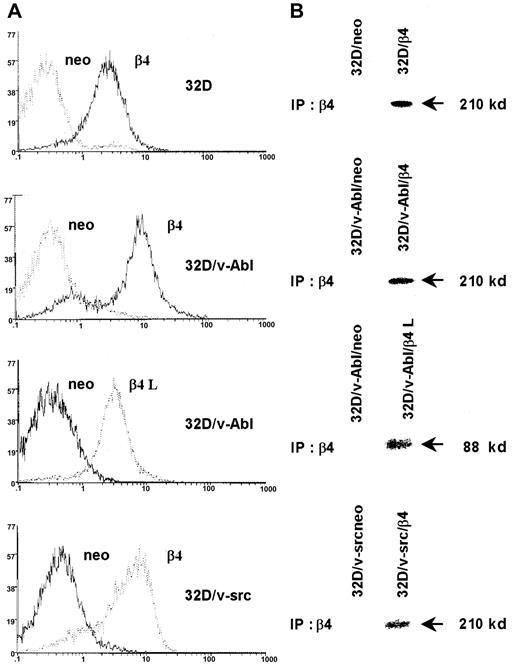
We stable transfected 32D cells, 32D/v-src–, and 32D/v-Abl–transformed cells with the expression vector pRC/CMV alone or carrying the β4 integrin subunit cDNA. The 32D/v-Abl cells were also transfected with the expression vector encoding the β4 integrin subunit devoid of its cytoplasmic domain (β4 L). Clones of transfected 32D, 32D/v-src, and 32D/v-Abl cells expressing a high level of either β4 wild type or β4 L molecules were isolated and analyzed by flow cytometry (Figure 1A) or by immunoprecipitation of surface-labeled proteins (Figure 1B). The expression of wild-type and truncated β4 proteins on the cell surface of selected clones is evident whereas the same were absent in clones transfected with the vector alone (neo) (Figure 1A-B).
Expression of the β4 integrin subunit in 32D cell lines.
32D cells and v-src– or v-abl–transformed counterparts were transduced with pRC/CMV expression vector alone and/or carrying wild-type β4 integrin subunit and cytoplasmic-deleted β4 molecule (β4 L) cDNAs. Neo cells and β4-transduced cells were analyzed by FACS for the expression of exogenous β4 molecules (A). Control and β4-transduced cells were exogenous-labeled and immunoprecipitated with the anti-β4 monoclonal antibody 439-9B (B).
Expression of the β4 integrin subunit in 32D cell lines.
32D cells and v-src– or v-abl–transformed counterparts were transduced with pRC/CMV expression vector alone and/or carrying wild-type β4 integrin subunit and cytoplasmic-deleted β4 molecule (β4 L) cDNAs. Neo cells and β4-transduced cells were analyzed by FACS for the expression of exogenous β4 molecules (A). Control and β4-transduced cells were exogenous-labeled and immunoprecipitated with the anti-β4 monoclonal antibody 439-9B (B).
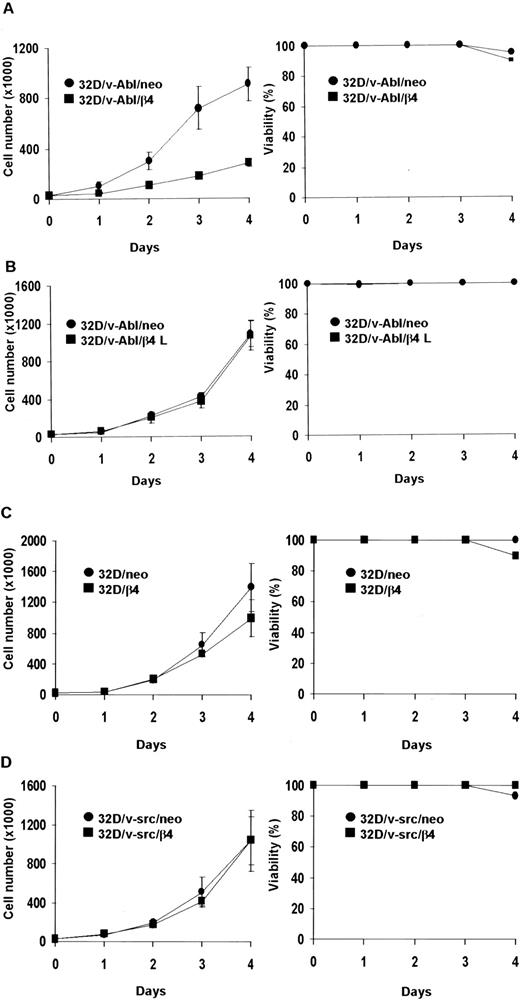
We asked whether the proliferative capacity of different 32D transfectans might be influenced by the expression of β4 protein. Proliferation rates were determined by daily counting the number of living cells. The data obtained showed that the expression of the wild-type β4 integrin subunit, but not the β4 L truncated protein, strongly inhibited the proliferation of 32D/v-Abl cells (Figure2A-B). Unlike 32D/v-Abl cells, the expression of the β4 subunit did not influence the proliferation capacity of nontransformed 32D and 32D/v-src–transformed cells (Figure 2C-D). Cell viability curves, obtained by trypan blue exclusion experiments, showed that all 32D β4-transfected cell lines survived as well as control cells (Figure 2, right panels). Similar results were also obtained when transformed v-src and v-abl control cells (neo) and β4-transduced cells were treated with IL-3 (data not shown). All analyses were performed with 2 clones for each β4 transfectant. The in vitro data were also confirmed by in vivo experiments (Table 1). The tumors were detectable at the site of injection 25 days after the injection of 32D/v-Abl/β4 cells, showing a delay of tumor appearance of 15 days compared with 32D/v-Abl cells. Furthermore, by evaluating tumor masses, we observed that 32D/v-Abl/β4 cells grow significantly less than parental 32D/v-Abl cells. The value of tumor masses indicates that 32D/v-Abl/β4–induced tumors grow 7-fold less than the 32D/v-Abl–induced ones. These data were interpreted hypothesizing that some 32D/v-Abl/β4 cells resistant to the selection but negative for β4 expression (Figure 1) grow in vivo, exhibiting a strong delay of tumor appearance.
Cell proliferation of 32D, 32D/v-src, 32D/v-Abl neo cells and/or transduced with wild-type and truncated β4 molecules.
Cells (3 × 104) were plated in triplicate and counted at daily intervals. The number of viable cells was determined by trypan blue exclusion. Panels show cell proliferation and viability of: ● 32D/v-Abl/neo and ■ 32D/v-Abl/β4 cells (A); ● 32D/v-Abl/neo and ■ 32D/v-Abl/β4 L (B); ● 32D/neo and ■ 32D/β4 (C); ● 32D/v-src/neo and ■ 32D/v-src/β4 (D).
Cell proliferation of 32D, 32D/v-src, 32D/v-Abl neo cells and/or transduced with wild-type and truncated β4 molecules.
Cells (3 × 104) were plated in triplicate and counted at daily intervals. The number of viable cells was determined by trypan blue exclusion. Panels show cell proliferation and viability of: ● 32D/v-Abl/neo and ■ 32D/v-Abl/β4 cells (A); ● 32D/v-Abl/neo and ■ 32D/v-Abl/β4 L (B); ● 32D/neo and ■ 32D/β4 (C); ● 32D/v-src/neo and ■ 32D/v-src/β4 (D).
The expression of the β4 integrin subunit induces growth arrest in 32D/v-abl–transformed cells
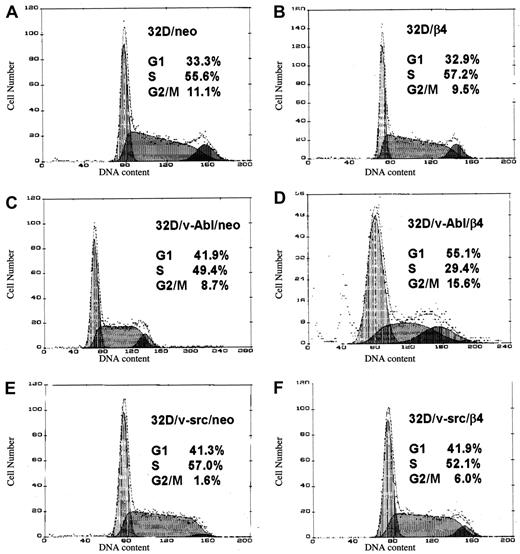
We analyzed the DNA content of control and β4-transfected cells. This analysis revealed that β4 expression in 32D/v-Abl cells causes an accumulation of the cells in G1 phase of the cell cycle (Figure3D). This effect was not evident in 32D/v-Abl cells transfected with either the β4 L molecule (data not shown) or the expression vector alone (Figure 3C). Moreover, the expression of β4 protein does not cause growth arrest in 32D as well as in 32D/v-src cells compared with the neo transfected ones (Figure 3A-B,E-F). Subsequently, we assessed whether β4 expression promotes apoptosis of 32D/v-Abl cells. The results showed that the level of apoptosis in 32D/v-Abl/neo cells was very low (approximately 3%) and that the expression of β4 did not increase this apoptotic feature (data not shown). Experiments were performed either in suspension or when the cells were allowed to adhere to various substrates or antibodies (“Materials and methods”), thus indicating that the reduced proliferative capacity induced by β4 expression was not due to an apoptotic event, in good agreement with the cell viability profile reported in Figure 2.
Effects of β4 integrin expression on the cell cycle of 32D, 32D/v-src, 32D/v-Abl neo cells and their counterparts transduced with wild-type β4 molecule.
(A) 32D/neo cells; (B) 32D/β4 cells; (C) 32D/v-Abl/neo cells; (D) 32D/v-Abl/β4 cells; (E) 32D/v-src/neo cells; (F) 32D/src/β4 cells. The DNA contents were measured by fluorimetric analysis of fixed and PI-stained cells.
Effects of β4 integrin expression on the cell cycle of 32D, 32D/v-src, 32D/v-Abl neo cells and their counterparts transduced with wild-type β4 molecule.
(A) 32D/neo cells; (B) 32D/β4 cells; (C) 32D/v-Abl/neo cells; (D) 32D/v-Abl/β4 cells; (E) 32D/v-src/neo cells; (F) 32D/src/β4 cells. The DNA contents were measured by fluorimetric analysis of fixed and PI-stained cells.
The expression of the wild-type β4 integrin subunit promotes the monocytic differentiation of 32D/v-Abl cells
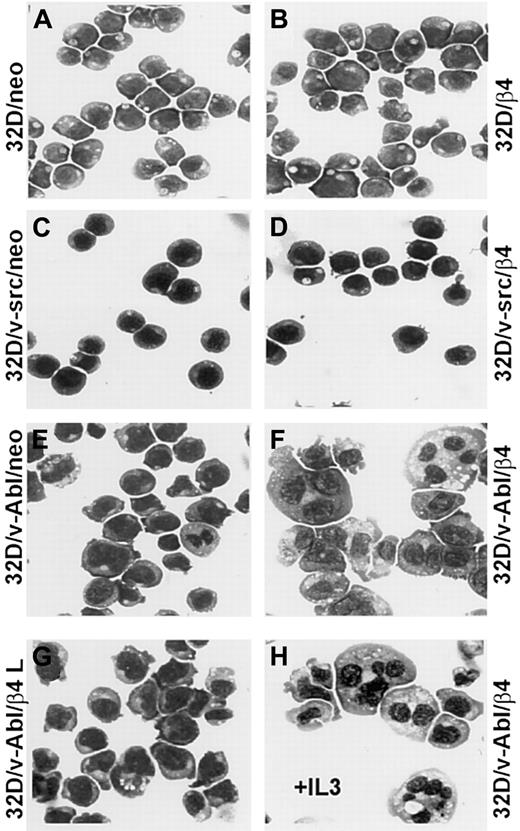
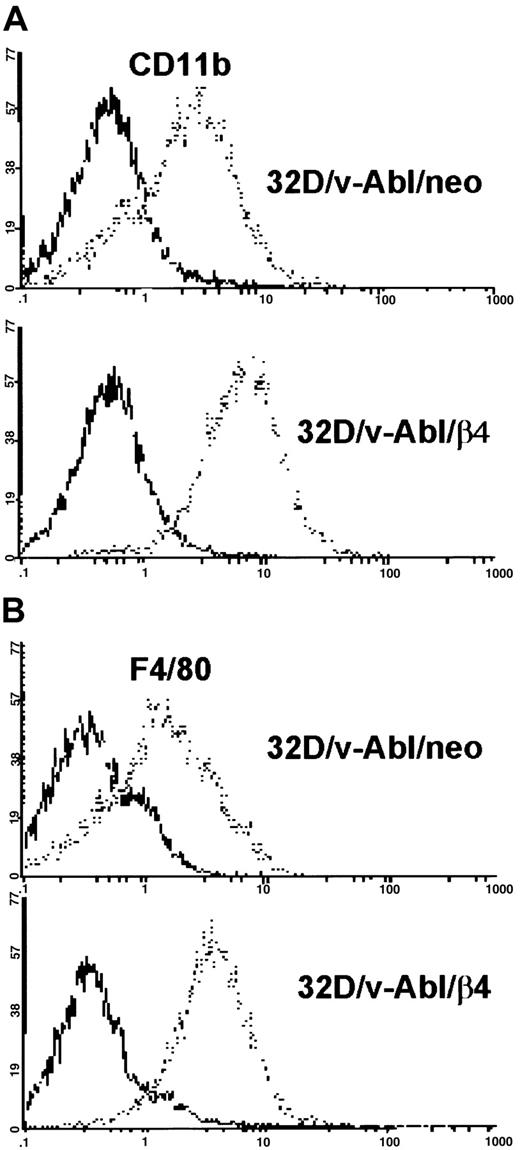
Since reduced proliferation capacity induced by β4 expression in 32D/v-Abl cells could not be attributed to a reduced cell viability, we verified whether these cells underwent differentiation. The morphologic analysis showed that 32D and 32D/v-src neo or β4-transduced cells did not differentiate (Figure 4A-D). In contrast, 32D/v-Abl cells expressing the β4 integrin subunit underwent monocytic differentiation independently whether the cells were grown in the absence or in the presence of IL-3 (Figure 4F,H). The latest result is in agreement with the fact that v-abloncogene expression in 32D cells abrogates their requirement for IL-3 and their capacity to differentiate in the presence of granulocyte–colony-stimulating factor (G-CSF).23Interestingly, since 32D/v-Abl cells transduced with the β4-truncated molecule (Figure 4G) did not differentiate, it is inferred that the cytoplasmic domain of the β4 subunit is directly involved in signaling events that generate monocytic differentiation of 32D/v-abl–transformed cells. To further confirm the monocytic differentiation observed in 32D/v-Abl/β4 cells, we tested, by FACS analysis, the expression levels of CD11b and F4/80 antigens, whose expression levels increase during monocytic differentiation. A strong up-regulation of CD11b (Figure 5A, lower panel) and F4/80 (Figure 5B, lower panel) antigens were found in 32D/v-Abl/β4 cells, confirming the differentiated status we observed in these cells by morphologic analysis. The results obtained from 2 different 32D/v-Abl/β4 clones used (A and B) were comparable. Conversely, the expression levels of CD11b and F4/80 antigens of the neo transfectans (Figure 5A-B, upper panels) were similar to their parental 32D/v-Abl counterparts (data not shown).
Representative samples of morphologic analyses of 32D, 32D/v-src, 32D/v-Abl control neo, and derivatives of β4-transduced cells.
(A) 32D/neo cells; (B) 32D/β4 cells; (C) 32D/v-src/neo cells; (D) 32D/src/β4 cells; (E) 32D/v-Abl/neo cells; (F) 32D/v-Abl/β4 cells; (G) 32D/v-Abl/β4 L cells; (H) 32D/vAbl cells maintained in IL-3 and transduced with wild-type β4 molecule. The morphology of the cytospin-fixed cells was analyzed by May-Grunwald-Giemsa stain. Original magnification × 630.
Representative samples of morphologic analyses of 32D, 32D/v-src, 32D/v-Abl control neo, and derivatives of β4-transduced cells.
(A) 32D/neo cells; (B) 32D/β4 cells; (C) 32D/v-src/neo cells; (D) 32D/src/β4 cells; (E) 32D/v-Abl/neo cells; (F) 32D/v-Abl/β4 cells; (G) 32D/v-Abl/β4 L cells; (H) 32D/vAbl cells maintained in IL-3 and transduced with wild-type β4 molecule. The morphology of the cytospin-fixed cells was analyzed by May-Grunwald-Giemsa stain. Original magnification × 630.
Cytofluorimetric analysis of CD11b and F4/80 antigens in 32D/v-Abl/neo and 32D/v-Abl/β4 cells.
32D/v-Abl/neo and 32D/v-Abl/β4 cells (clone a) were stained with anti-CD11b and/or F4/80 monoclonal antibodies and analyzed by flow cytometry. (A) Samples of 1 × 106 of control (neo) and β4-transduced cells were incubated with purified rat anti–mouse CD11b, and (B) with rat anti–mouse F4/80 primary antibodies. The cells were washed, incubated with FITC-conjugated secondary antibody, and analyzed by a flow cytometer after addition of 5 μL PI. (A,B) Negative controls were stained with rat anti–mouse CD4 monoclonal antibody and processed as described in “Materials and methods.”
Cytofluorimetric analysis of CD11b and F4/80 antigens in 32D/v-Abl/neo and 32D/v-Abl/β4 cells.
32D/v-Abl/neo and 32D/v-Abl/β4 cells (clone a) were stained with anti-CD11b and/or F4/80 monoclonal antibodies and analyzed by flow cytometry. (A) Samples of 1 × 106 of control (neo) and β4-transduced cells were incubated with purified rat anti–mouse CD11b, and (B) with rat anti–mouse F4/80 primary antibodies. The cells were washed, incubated with FITC-conjugated secondary antibody, and analyzed by a flow cytometer after addition of 5 μL PI. (A,B) Negative controls were stained with rat anti–mouse CD4 monoclonal antibody and processed as described in “Materials and methods.”
The expression of β4 in 32D/v-Abl cells promotes monocytic differentiation by p73 protein accumulation
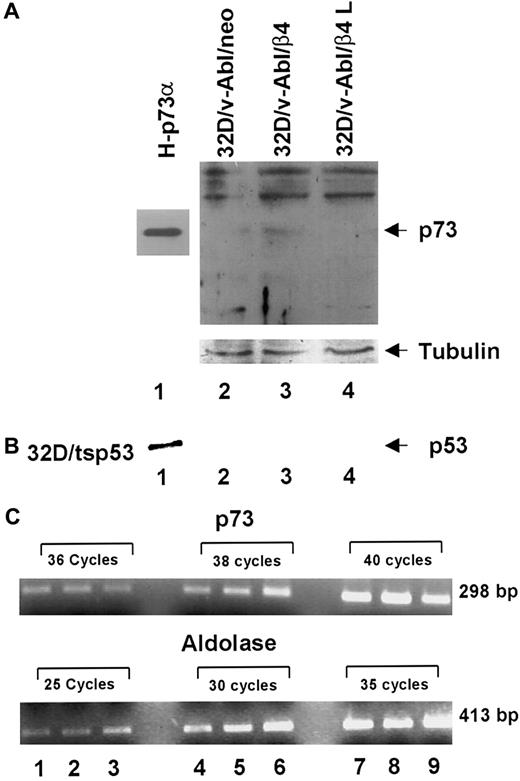
It has been previously reported that p53 family members induce cell cycle arrest, differentiation and apoptosis. Furthermore, the p73 protein, a new member of p53 family, is able to activate p53 target genes as well as to induce cell cycle arrest and apoptosis.30,33-36 It has recently been reported that (1) p73 protein accumulates during epithelial differentiation,37 (2) p73 protein accumulates during myeloid leukemia and neuroblastoma cells upon treatment with differentiating agents,38,39 and (3) p73-null mice exhibit severe defects in development.40 In accordance with these results, we asked whether monocytic differentiation induced by β4 expression in 32D/v-Abl cells involves p73 and/or p53 proteins. To answer this issue, we tested by Western blot the expression level of p73 proteins in different transfectans. The H1299 cell line stable-expressing p73α protein was used as a positive control (Figure6, lane 1).32 We found that the expression of β4 integrin in 32D/v-Abl cells promotes the accumulation of p73 protein (Figure 6A, lane 3). Conversely, p73 protein was not detectable in control cells (32D/v-Abl/neo) and in cells expressing the truncated β4 protein (32D/v-Abl/β4 L) (Figure 6A, lanes 2 and 4). The lower panel of Figure 6A shows that the amount of total protein loaded in each lane was identical. To assess whether the accumulation of p73 protein was paralleled by its induction at the transcriptional level, p73 mRNA was measured by semiquantitative RT-PCR. As an internal control, the housekeeping aldolase gene was used (Figure 6C, lower panel). The reported data showed that the expression of β4 does not modulate the level of p73 mRNA of 32D/v-Abl/β4 cells (Figure 6C, lanes 2, 5, and 8, upper panel) compared with 32D/v-Abl/neo (Figure 6C, lanes 1, 4, and 7) and 32D/v-Abl/β4 L cells (Figure 6C, lanes 3, 6, and 9). These results indicate that β4 expression induces p73 protein accumulation in 32D/v-Abl cells and, apparently, does not modulate its transcriptional activation. The level of p53 protein in 32D/v-Abl/β4 cells was also evaluated. The reported data show, as expected, a high level of p53 protein in 32D/tsp53 control cells (Figure 6B, lane 1). By contrast, p53 protein was not detectable either in 32D/v-Abl/neo or in β4-transduced 32D/v-Abl cells (Figure 6B, lanes 2, 3, and 4), indicating that p53 might not be involved in the monocytic differentiation of 32D/v-Abl cells. These data agree with the finding that the overexpression of p53 in 32D/v-Abl cells induces growth arrest but not differentiation.28
Analyses of p73 protein and mRNA in 32D/v-Abl/neo cells and 32D/v-Abl cells expressing either wild-type or truncated β4 molecules.
(A) Total cell lysates from 32D/v-Abl/neo (lane 2), 32D/v-Abl/β4 wild type (lane 3), and 32D/v-Abl/β4 L (lane 4) were analyzed by SDS-PAGE and probed with anti–p73 antibody. H-p73α, stably transduced with p73α cDNA, was used as a positive control (lane 1). (B) Total cell lysates from 32D/v-Abl/neo (lane 2), 32D/v-Abl/β4 wild type (lane 3), and 32D/v-Abl/β4 L (lane 4) were analyzed by SDS-PAGE and probed with anti–mouse p53 antibody. 32D transduced with the expression vector encoding for a p53 Val 135 was used as a positive control (lane 1). The nitrocellulose used to probe p73 and p53 proteins was stripped and probed again with the anti–tubulin antibody to normalize equal loading of proteins (lanes 2, 3, and 4). (C) Total mRNA was extracted from 32D/v-Abl/neo (lanes 1, 4, and 7), from 32D/v-Abl/β4 wild type (lanes 2, 5, and 8), and from 32D/v-Abl/β4 L (lanes 3, 6, and 9). RT-PCR analysis was performed using primers specific for mouse p73 and the housekeeping aldolase gene to amplify 2 fragments of 298 bp and 413 bp, respectively. The conditions are described in “Materials and methods.” Molecular sizes of the 2 fragments are indicated.
Analyses of p73 protein and mRNA in 32D/v-Abl/neo cells and 32D/v-Abl cells expressing either wild-type or truncated β4 molecules.
(A) Total cell lysates from 32D/v-Abl/neo (lane 2), 32D/v-Abl/β4 wild type (lane 3), and 32D/v-Abl/β4 L (lane 4) were analyzed by SDS-PAGE and probed with anti–p73 antibody. H-p73α, stably transduced with p73α cDNA, was used as a positive control (lane 1). (B) Total cell lysates from 32D/v-Abl/neo (lane 2), 32D/v-Abl/β4 wild type (lane 3), and 32D/v-Abl/β4 L (lane 4) were analyzed by SDS-PAGE and probed with anti–mouse p53 antibody. 32D transduced with the expression vector encoding for a p53 Val 135 was used as a positive control (lane 1). The nitrocellulose used to probe p73 and p53 proteins was stripped and probed again with the anti–tubulin antibody to normalize equal loading of proteins (lanes 2, 3, and 4). (C) Total mRNA was extracted from 32D/v-Abl/neo (lanes 1, 4, and 7), from 32D/v-Abl/β4 wild type (lanes 2, 5, and 8), and from 32D/v-Abl/β4 L (lanes 3, 6, and 9). RT-PCR analysis was performed using primers specific for mouse p73 and the housekeeping aldolase gene to amplify 2 fragments of 298 bp and 413 bp, respectively. The conditions are described in “Materials and methods.” Molecular sizes of the 2 fragments are indicated.
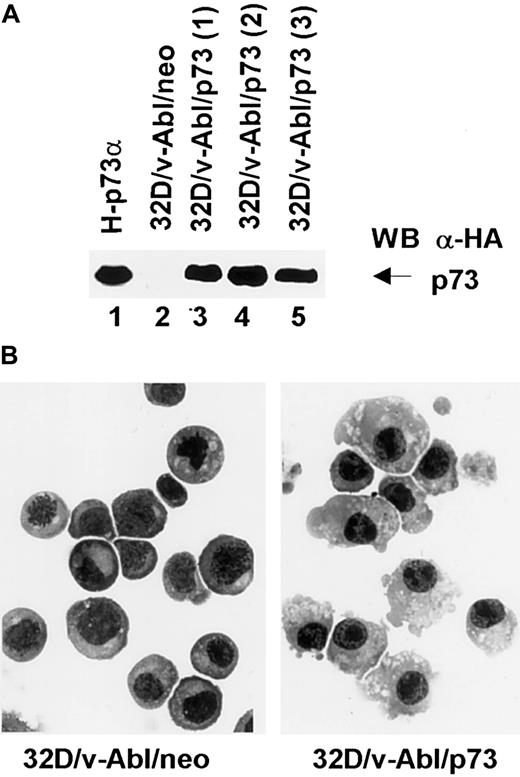
To further investigate the involvement of p73 in monocytic differentiation we transfected 32D/v-Abl cells with pcDNA3 expression vector carrying p73 cDNA. After selection, 3 different p73-positive populations (Figure 7, lanes 3, 4, and 5) were analyzed morphologically. The results showed that the 3 32D/v-Abl/p73 cell populations differentiated in monocytes at day 8 after selection. The percentage of differentiating cells was 55% (± 5% SD) compared with 32D/v-Abl/neo control cells (< 1%). After longer selection we found that almost 90% of the cells differentiated in monocytes as shown in Figure 7B (right panel). This result, indicating that p73 expression is sufficient to induce the monocytic differentiation of 32D/v-Abl cells, strongly suggests that p73 accumulation mediates β4-induced monocytic differentiation.
Expression and morphologic analysis of 32D/v-Abl/neo and 32D/v-Abl/p73 cells.
(A) 32D/v-Abl cells were transduced with an expression vector alone and/or encoding p73 cDNA. After selection, total cell lysates from 32D/v-Abl/neo (lane 2), 3 derived 32D/v-Abl/p73α populations (lanes 3, 4, and 5), and H-p73α stably transduced with p73α cDNA (lane 1) used as a positive control were analyzed by SDS-PAGE and probed with anti–HA monoclonal antibody. (B) The morphology of the cytospin-fixed 32D/v-Abl/neo (left panel) and 32D/v-Abl/p73 (right panel) cells was analyzed by May-Grunwald-Giemsa stain as described in “Materials and methods.” Original magnification × 630.
Expression and morphologic analysis of 32D/v-Abl/neo and 32D/v-Abl/p73 cells.
(A) 32D/v-Abl cells were transduced with an expression vector alone and/or encoding p73 cDNA. After selection, total cell lysates from 32D/v-Abl/neo (lane 2), 3 derived 32D/v-Abl/p73α populations (lanes 3, 4, and 5), and H-p73α stably transduced with p73α cDNA (lane 1) used as a positive control were analyzed by SDS-PAGE and probed with anti–HA monoclonal antibody. (B) The morphology of the cytospin-fixed 32D/v-Abl/neo (left panel) and 32D/v-Abl/p73 (right panel) cells was analyzed by May-Grunwald-Giemsa stain as described in “Materials and methods.” Original magnification × 630.
β4 expression affects Abl and MAPK phosphorylation
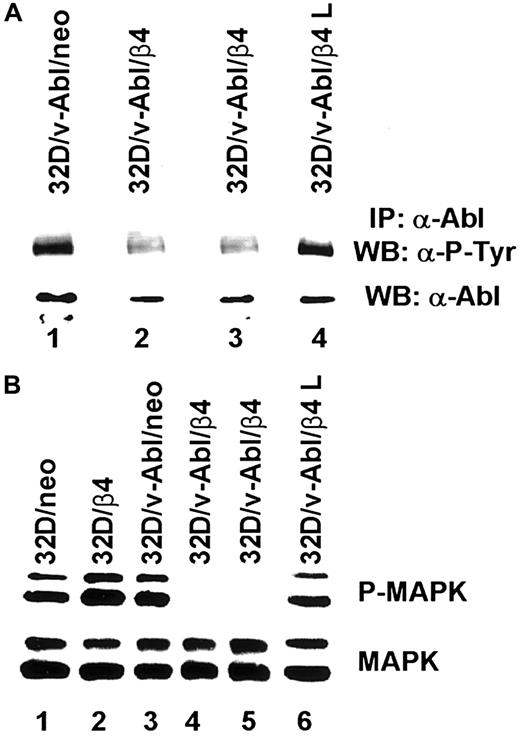
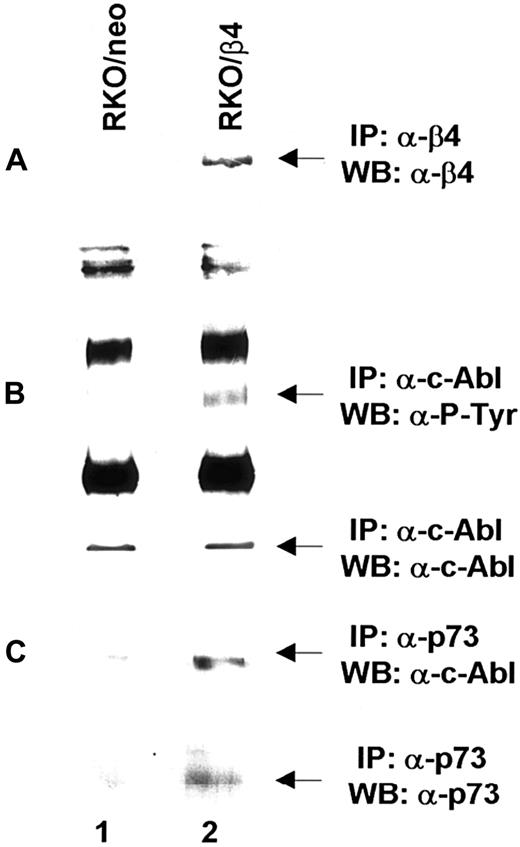
Based on the above reported findings that β4 integrin expression in 32D/v-Abl cells induces growth arrest and monocytic differentiation, we investigated whether β4 expression could affect v-Abl and MAPK phosphorylation. Both conditions are reported to be essential for transformation and cell proliferation.23,41 To this end, v-Abl protein was immunoprecipitated from 32D/v-Abl/neo cells, wild-type β4-transfected cells, and 32D/v-Abl/β4 L cells, and then, after SDS-PAGE, the phosphorylation level of the protein was evaluated by an antiphosphotyrosine antibody. As shown in Figure8A, the expression of the wild-type β4 molecule caused a clear decrease of v-Abl phosphorylation (lanes 2 and 3) compared with 32D/v-Abl/neo (lane 1), or 32D/v-Abl/β4 L (lane 4). The lower part of Figure 8A shows that the amount of v-Abl immunoprecipitated and loaded in each lane was identical. By using a specific antibody that recognizes the active isoforms of ERK1 and ERK2, we also found a complete inhibition of MAPK phosphorylation in 32D/v-Abl/β4 clones (Figure 8B, lanes 4 and 5). No modulation of MAPK phosphorylation was evident in control 32D/v-Abl/neo cells (lane 3), in cells expressing the truncated form of β4 (32D/v-Abl/β4 L) (lane 6), and in the 32D untransformed counterparts (32D/neo, and 32D/β4) (lanes 1 and 2). The lower part of Figure 8B shows that the amount of total protein used in each lane was identical. We conclude that β4 expression in 32D/v-Abl cellular context inhibits both v-Abl and MAPK phosphorylation and modulates these events through its cytoplasmic domain. We interpreted these results, hypothesising that the inhibition of v-Abl function that we reported, upon expression of the β4 integrin subunit, could allow the endogenous tyrosine kinase c-Abl activation to induce growth arrest and differentiation. Indeed, it has been found that the induction of G1 arrest and apoptosis, by different stimuli, requires the kinase activity of c-Abl.36,42,43Since we have previously demonstrated that the expression of the β4 integrin subunit in RKO colon carcinoma cells induces growth arrest and apoptosis and it is known that carcinoma cells express the tyrosine kinase c-Abl, we asked whether β4 expression in these epithelial cells could activate endogenous c-Abl.20 We analyzed the phosphorylation status of endogenous c-Abl in RKO colon carcinoma cells transfected with the vector alone (Figure9A, lane 1) or carrying β4 cDNA (Figure9A, lane 2).20 Cell lysates from RKO/neo and RKO/β4 were immunoprecipitated with c-Abl antibody and blotted with an antiphosphotyrosine antibody. As shown in Figure 9B, the expression of the β4 molecule caused a clear increase of c-Abl phosphorylation (upper panel B, lane 2) compared with RKO/neo (upper panel B, lane 1) where the phosphorylation level of this kinase was undetectable. The lower part of Figure 9B shows that the amount of c-Abl immunoprecipitated and loaded in each lane was identical. Since it has been shown that in epithelial cells activated c-Abl induces growth arrest and apoptosis by its interaction with p73,42,43 we asked whether we were able to observe similar interaction in RKO cells upon β4 transduction. To this end, we immunoprecipitated p73 protein from RKO/neo and RKO/β4 cells (Figure 9C) and blotted with an anti–c-Abl antibody. We found an increase of association between p73 and c-Abl in RKO cells expressing the β4 integrin subunit (upper panel C, lane 2) compared with untrasfected β4 cells (upper panel C, lane 1). We also found that the interaction of c-Abl and p73 correlates with the accumulation of p73 protein (lower panel C, lane 2). These results are in good agreement with a previous finding showing that c-Abl/p73 interaction occurs when different stimuli activate c-Abl to induce G1 arrest and apoptosis.36,42 43
Analyses of v-Abl and MAPK phosphorylation on 32D neo and β4 transfectans.
(A) Total cell lysates from 32D/v-Abl/neo cells (lane 1), 2 derived 32D/v-Abl/β4 clones (lanes 2 and 3), and 32D/v-Abl/β4 L clone (lane 4), were immunoprecipitated with anti–Abl monoclonal antibody. The immunoprecipitated Abl proteins were analyzed by SDS-PAGE and probed with antiphosphotyrosine monoclonal antibody (upper panel A). The nitrocellulose was stripped and probed again with the anti–Abl polyclonal antibody (lower panel A). (B) Total cell lysates from 32D/neo (lane 1), 32D/β4 (lane 2), 32D/v-Abl/neo (lane 3), 32D/v-Abl/β4 (lanes 4 and 5), and 32D/v-Abl/β4 L cells (lane 6) were analyzed by SDS-PAGE and probed with an antibody to the active doubly phosphorylated forms of the MAPK (ERK 1 and 2) (upper panel B). The nitrocellulose was stripped and probed again with an antibody specific to the native unmodified form of ERK/MAPK (lower panel B).
Analyses of v-Abl and MAPK phosphorylation on 32D neo and β4 transfectans.
(A) Total cell lysates from 32D/v-Abl/neo cells (lane 1), 2 derived 32D/v-Abl/β4 clones (lanes 2 and 3), and 32D/v-Abl/β4 L clone (lane 4), were immunoprecipitated with anti–Abl monoclonal antibody. The immunoprecipitated Abl proteins were analyzed by SDS-PAGE and probed with antiphosphotyrosine monoclonal antibody (upper panel A). The nitrocellulose was stripped and probed again with the anti–Abl polyclonal antibody (lower panel A). (B) Total cell lysates from 32D/neo (lane 1), 32D/β4 (lane 2), 32D/v-Abl/neo (lane 3), 32D/v-Abl/β4 (lanes 4 and 5), and 32D/v-Abl/β4 L cells (lane 6) were analyzed by SDS-PAGE and probed with an antibody to the active doubly phosphorylated forms of the MAPK (ERK 1 and 2) (upper panel B). The nitrocellulose was stripped and probed again with an antibody specific to the native unmodified form of ERK/MAPK (lower panel B).
Analyses of c-Abl activation on RKO/neo and β4-transfected cells.
(A) Total cell lysates (500 μg) from RKO/neo (lane 1) and RKO/β4 (lane 2) cells were immunoprecipitated with anti–β4 monoclonal antibody. The immunoprecipitated β4 proteins were analyzed by SDS-PAGE and probed with an antibody direct to the β4 integrin subunit. (B) Equal amounts of cell lysates from RKO/neo cells (lane 1) and RKO/β4 cells (lanes 2) were immunoprecipitated with anti–Abl monoclonal antibody. The immunoprecipitated Abl proteins were analyzed by SDS-PAGE and probed with antiphosphotyrosine monoclonal antibody (upper panel B). The nitrocellulose was stripped and probed again with the anti–Abl polyclonal antibody (lower panel B). (C) Equal amounts of cell lysates from RKO/neo cells (lane 1) and RKO/β4 cells (lanes 2) were immunoprecipitated with anti–p73 polyclonal antibody and probed with anti–cAbl antibody (upper panel C). The nitrocellulose was stripped and probed again with the anti–p73 antibody (lower panel C).
Analyses of c-Abl activation on RKO/neo and β4-transfected cells.
(A) Total cell lysates (500 μg) from RKO/neo (lane 1) and RKO/β4 (lane 2) cells were immunoprecipitated with anti–β4 monoclonal antibody. The immunoprecipitated β4 proteins were analyzed by SDS-PAGE and probed with an antibody direct to the β4 integrin subunit. (B) Equal amounts of cell lysates from RKO/neo cells (lane 1) and RKO/β4 cells (lanes 2) were immunoprecipitated with anti–Abl monoclonal antibody. The immunoprecipitated Abl proteins were analyzed by SDS-PAGE and probed with antiphosphotyrosine monoclonal antibody (upper panel B). The nitrocellulose was stripped and probed again with the anti–Abl polyclonal antibody (lower panel B). (C) Equal amounts of cell lysates from RKO/neo cells (lane 1) and RKO/β4 cells (lanes 2) were immunoprecipitated with anti–p73 polyclonal antibody and probed with anti–cAbl antibody (upper panel C). The nitrocellulose was stripped and probed again with the anti–p73 antibody (lower panel C).
Discussion
In addition to many studies indicating that integrins promote survival,44-47 we previously demonstrated that α6β4 can also inhibit the proliferation of p53 wild-type carcinoma cell lines and induce their apoptosis by activating p5320 and increasing the expression of the cell cycle inhibitor p21.48 49 For the first time, we report that expression of the β4 integrin subunit promotes monocytic differentiation of 32D/v-Abl cells by a mechanism that involves β4-mediated growth arrest and p73 protein accumulation.
The p73 protein, which is a member of the p53 family, is a transcription factor whose functions recapitulate those of p53.35,50 Indeed, it has been shown that p73 induces cell cycle arrest at G1 phase and apoptosis when it is overexpressed either in p53+/+ and p53−/−cells.30,33-36,42,50 Recently, it was demonstrated that p73 protein plays an important role in differentiation.37-40,51 Although it has been described that p53 is a regulator of cell differentiation, at least in part by its transcriptional activity,52-54 we did not find any accumulation of p53 protein in 32D/v-Abl/β4 cells. Additional support for the hypothesis that accumulation of p73 protein in 32D/v-Abl/β4 cells promotes monocytic differentiation is the finding that the overexpression of p73 protein in 32D/v-Abl cells actually induces monocytic differentiation. Moreover, our results are in agreement with previous data indicating that the induction of p73 protein occurs during differentiation of the myeloid leukemia cell line.38 Further support for the role of p73 in differentiation has also been provided by the phenotype of p73-deficient mice that exhibit neurologic and inflammatory defects.40 These studies suggest a direct involvement of p73 in the proper development of white lineage. The murine myeloid progenitor cell line 32D does not express the β4 integrin subunit that is instead expressed by adult, neonatal, and fetal mouse thymocytes.55 We used 32D cells and v-src– or v-abl–transformed 32D cells as a model to study a possible cooperation between β4 integrin and the above-mentioned oncogenes as we previously demonstrated with ErbB-2.17,22 Surprisingly, we found that β4 expression inhibits the transformed phenotype of 32D/v-Abl cells inducing growth arrest, differentiation, and p73 protein accumulation. To gain more insight into the mechanism by which β4 expression interferes with the oncogenic potential of v-abl, we observed strong reduction of v-Abl phosphorylation. Furthermore, the fact that the expression of a truncated β4 molecule in 32D/v-abl cells does not affect the level of either v-Abl or MAPK phosphorylation strongly indicates the involvement of the β4 cytoplasmic domain in these events. Recently, an immune T-cell inhibitory motif (ITIM) in the β4 cytoplasmic domain has been identified, which is a potential binding site for SHP-1/2 (src-homology 2 domains protein tyrosine phosphatases) protein tyrosine phosphatases.56 Accordingly, it might be that the binding of SHP-1/2 phosphatases to the β4 cytoplasmic domain could induce directly or indirectly a down-regulation of v-Abl phosphorylation. As a consequence, the strong reduction of v-Abl phosphorylation in 32D/v-Abl cells by β4 might cause the inhibition of cell cycle progression. Based on the fact that during cell proliferation MAPK proteins are highly active,41 the strong inhibition of MAPK phopshorylation we found on 32D/v-Abl/β4 cells is in agreement with v-Abl phosphorylation status and the growth arrest of these cells. The observation that β4 integrin expression does not inhibit cell proliferation either of 32D/v-src–transformed cells or parental cells indicates a specific interference of α6β4 receptor signaling on v-abl oncogenic pathways. These results suggested the possibility that the inhibition of v-abl oncogenic capacity upon expression of the β4 integrin subunit could allow the endogenous c-Abl activation and its cooperation with p73 to induce growth arrest and differentiation. This hypothesis was confirmed by the fact that the expression of the β4 integrin subunit in colon carcinoma RKO, where we previously demonstrated that the integrin induces growth arrest,20 causes a strong activation of endogenous c-Abl phosphorylation that is accompanied by its association with p73. This result is in agreement with other data where it has been shown that c-Abl and p73 associate and cooperate to induce growth arrest in several cell types.36,42 43 All together, these results—while confirming the interaction between c-Abl and p73 to induce growth arrest—indicate that β4 acts as stimuli to activate specific signaling on this pathway(s).
Finally, our results provide evidence that by modulating tumor phenotype the α6β4 receptor signaling promotes differentiation. This finding is important and relevant because α6β4 is highly expressed in most epithelial cells, which are growth arrested and differentiated.57 The possible involvement of the α6β4 integrin during differentiation is also supported by the fact that laminins, whose isoforms are α6β4 substrates, strongly induce epithelial differentiation.58 59 Our results should stimulate studies aimed to clarify the possible contribution of the α6β4 integrin to epithelial differentiation.
In summary, our data provide evidence for a role of the β4 integrin subunit in counteracting tumor phenotype by inducing monocytic differentiation through accumulation of p73 protein, and suggest that the α6β4 integrin receptor not only signals for invasion, proliferation, and apoptosis, but also for cell differentiation.
We are grateful to Laura Spinardi and Filippo Giancotti for providing us with β4 cDNAs. We are particularly grateful to Patrizio Giacomini for providing us with anti–F4/80 antibody, to Yosef Shaul for providing us with anti–p73 antibody, and to David Lane for providing us with anti–p53 antibody. We are also particularly grateful to Isabella Manni, Leslie Shaw, Silvia Soddu, and Sabrina Strano for helpful discussion.
Supported by Italia-USA; Associazione Italiana Ricerca sul Cancro (AIRC) and Progetto Strategico “Oncologia” CNR-MURST projects (R.F.); Teleton Grant N. 369/bi, and AIRC (G.B.).
A.M. and S.R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Rita Falcioni, Molecular Oncogenesis Laboratory, Regina Elena Cancer Institute, Via delle Messi d'Oro, 156-00158 Rome, Italy; e-mail: falcioni@ifo.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal