Abstract
Molecular events involved in specification of early hematopoietic system are not well known. In Xenopus, a paired-box homeodomain family (Mix.1-4) has been implicated in this process. Although Mix-like homeobox genes have been isolated from chicken (CMIX) and mice (Mml/MIXL1), isolation of a human Mix-like gene has remained elusive. We have recently isolated and characterized a novel human Mix-like homeobox gene with a predicted open reading frame of 232 amino acids designated the Mix.1 homeobox (Xenopus laevis)–like gene (MIXL). The overall identity of this novel protein to CMIX and Mml/MIXL1 is 41% and 69%, respectively. However, the identity in the homeodomain is 66% to that of Xenopus Mix.1, 79% to that of CMIX, and 94% to that of Mml/MIXL1. In normal hematopoiesis, MIXL expression appears to be restricted to immature B- and T-lymphoid cells. Several acute leukemic cell lines of B, T, and myeloid lineage express MIXL suggesting a survival/block in differentiation advantage. Furthermore, Xenopus animal cap assay revealed that MIXL could induce expression of the α-globin gene, suggesting a functional conservation of the homeodomain. Isolation of theMIXL gene is the first step toward understanding novel regulatory circuits in early hematopoietic differentiation and malignant transformation.
Introduction
Mechanisms governing specification, proliferation, and differentiation of hematopoietic progenitors into terminally differentiated circulating elements have begun to unfold in the past decade. Several transcription factors, uncovered by specific chromosomal translocations, are also regulators of normal hematopoietic differentiation as demonstrated by loss-of-function studies in murine models. More importantly, the striking evolutionary conservation of lineage-commitment mechanisms between fruit flies and mammals1 suggests that studies on mammalian orthologs of genes that regulate early hematopoiesis in invertebrates or amphibians may shed light on novel regulatory circuits.
Mix.1, a pairedlike homeobox gene in Xenopus, was initially identified as an inducer of ventral mesoderm and/or endoderm.2,3 Subsequently, several Mix.1-like genes were isolated and shown to be involved in the regulation of mesoderm and/or endoderm formation.4-7 At present, the Mix.1-like gene family includes the Mix family (Mix.1-4), Bix family (Bix1-4), and Mixer in Xenopus.3-7 However, in chicken (CMIX), mice (Mml) and humans, the Mix-like homeobox genes appear to be single copies.8-11 Although the proteins encoded by Mix-like genes vary in size, they are all modular with a highly conserved paired-type homeodomain and a conserved carboxy-terminal acidic domain.
The Xenopus Mix.1 gene is implicated in the process of patterning ventral mesoderm to hematopoietic fate induced by bone morphogenetic protein 4 (BMP-4), a member of the transforming growth factor β (TGF-β) family.12 In combination with mesoderm inducers fibroblast growth factor (FGF) or activin, BMP-4 induces abundant hematopoietic mesoderm in Xenopus animal caps.13,14 A dominant-negative mutant of Mix.1 blocks the BMP-4 response in this system.12 Consistent with theXenopus studies, BMP-4 expression in human embryos is polarized to the ventral wall of the dorsal aorta, which is associated with clusters of hematopoietic cells in the aorta-gonad-mesonephros (AGM) region.15 Strong BMP-4 expression is also detected in cells specifically associated with blood islands in human embryonic yolk sac.15 These observations in human embryos suggest a role for BMP-4 in the initiation of both yolk sac and AGM hematopoiesis.
The SMAD5 gene, a signal transducer of BMP-4, localizes to human chromosome 5q31.1, a region of loss in human acute myelogenous leukemia (AML).16 Our previous studies characterized a carboxy-terminally truncated alternate splice form SMAD5β that is preferentially expressed in hematopoietic stem cells and leukemia.17 Because of our interests in the functional consequences of persistent SMAD5β expression and possible haploinsufficiency for full-length SMAD5, we searched for a putative downstream target, the human Mix-like homeobox gene. Here, we report the isolation and characterization of the human MIXL(Mix.1 homeobox [Xenopus laevis]-like) gene, which mimics Xenopus Mix.1 in functional assays. Our findings raise the possibility of an essential role for MIXL in embryonic and adult hematopoiesis.
Materials and methods
Databases and bioinformatics
The databases Nucleotide (NR), High Throughput Genomic Sequences (HTGS), and Expressed Sequence Tags (dbEST) at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov), as well as the chromosome 1 sequences at the Sanger Center (http://www.sanger.ac.uk/HGP/Chr1/), were used to search for complementary DNAs (cDNAs), sequence tagged sites (STSs), and genomic sequences. High-resolution fluorescence in situ hybridization (FISH) of chromosome 1 markers was accessed at the websitehttp://cgap.nci.nih.gov/Chromosomes/BAC_Clone_Map?CHR = 1. The Mitelman Database of Chromosome Aberrations in Cancer culled from the literature by F. Mitelman, B. Johansson, and F. Mertens (editors) was accessed at the Cancer Genome Anatomy Project (CGAP) web sitehttp://cgap.nci.nih.gov/chromosomes/Mitelman.
Expressed sequence tags and bacterial artificial chromosome
Polymerase chain reaction
All the polymerase chain reactions (PCRs) were conducted under the following conditions: an initial denaturation for 4 minutes at 95°C, followed by 35 cycles of denaturation at 95°C for 30 seconds, annealing at 55°C to 60°C for 30 seconds, and extension at 72°C for 30 seconds, plus a final extension for 10 minutes at 72°C. The primers were used at the concentrations of 10 pmol in a total volume of 20 μL. The Taq polymerase was obtained from Gibco BRL (Rockville, MD).
The CITB Human BAC DNA Pool (CITB-HSP-C Library, Research Genetics) was screened with a pair of MIXL-specific primers:MIXL-EST1 forward primer 5′-CAA AGC TGG ACT CAT GGG AG-3′ and reverse primer 5′-AAC GAA TGC GGG AAC TCT GG-3′. TheMIXL intron was amplified from BAC 216I11 with the Expand High Fidelity PCR System (Roche, Indianapolis, IN). The primer pair used to amplify the putative intronic sequences was MIXL-HD forward primer 5′-TTT CAG CGC CGA ACA G-3′ and reverse primer 5′-ATC TCC GGC CTA GCC AAA GG-3′. The 3′ rapid amplification of cDNA ends (RACE) was performed with the primer MIX-3RACE 5′-GCG TGC CAA GTC TCG GCG TCA G-3′.
Radiation hybrid mapping
The G3 radiation hybrid (RH) panel (a panel of 83 whole genome human-hamster radiation hybrids) was used to generate the RH linkage data.19 Eighty-three PCR reactions were performed with a pair of MIXL primers: MIXL-EST2 forward primer 5′-TCT GGG AGA AAT CCG GAT AAG C-3′ and reverse primer 5′-TGT GAG AGG TGC TGT CAA AAC C-3′. The data were confirmed by PCR with a second pair of primers (MIXL-EST1). The combined RH linkage data set where 0 represents negative signals and 1 represents positive signals, was submitted to the Stanford Radiation Hybrid Mapping server (http://shgc.stanford.edu/RH/rhserverformnew.html) for statistical linkage analysis.
Northern blotting
The Human Immune System Multi-tissue Northern Blot II was purchased from Clontech (Palo Alto, CA). The blot was prehybridized for 5 hours with the prehybridization solution (5 times SSPE (150 mM sodium chloride, 10 mM sodium phosphate, 18 mM ethylenediaminetetraacetic acid [EDTA]), 10 times Denhardt solution, 2.0% sodium dodecyl sulfate (SDS), 50% deionized formamide, 100 μg/mL sheared salmon sperm DNA). The plasmid pCR2.1-TOPO-MIXL containing a 1 kilobase-pair (Kbp) MIXL insert (424 bp coding sequences including the Mix-like homeobox and 543 bp 3′ untranslated region [UTR] sequences) in the vector pCR2.1-TOPO (Invitrogen, Carlsbad, CA) was randomly primed with [α-32P]dCTP (Random Primed DNA Labeling Kit from Roche) and added to the hybridization solution at a final concentration of 1.3 × 107 cpm/mL. The blot was rinsed at room temperature 3 times with wash solution I (2 times SSC, 0.1% SDS) after 28 hours of hybridization. Final washes were done at 55°C with wash solution II (0.5 times SSC, 0.1% SDS) for 30 minutes before exposing the blot to x-ray films (X-OMT; Kodak, New Haven, CT) for 5 days. The blot was rehybridized with human β-actin cDNA (Clontech) to evaluate messenger RNA (mRNA) loading.
Cell sorting
Previously cryopreserved normal donor bone marrow (6 × 107 cells) or peripheral blood mononuclear cells (1 × 107 cells) were thawed, resuspended at 2 × 107 cells/mL in phosphate-buffered saline (PBS) and stained with appropriate combinations of monoclonal antibodies for 30 minutes at 4°C. The following monoclonal antibodies were used: CD5–fluorescein isothiocyanate (FITC), CD13-phycoerythrin (PE; Becton Dickinson, San Jose, CA); CD34-ECD (phycoerythrin-Texas Red-x; Beckman/Coulter, Hileah, FL); glycophorin A FITC, CD3-allophycocyanin (APC), CD45-APC-cyanine7 (Cy7), CD19-tri-color (R-PE-Cy5 tandem) (TC) (Caltag Labs, Burlingame, CA). After staining, cells were washed and resuspended for subsequent analysis and cell sorting on a MoFlo cell sorter (Cytomation, Fort Collins, CO). For bone marrow, live cells (CD45+) from bone marrow were gated for CD34 expression. Cells expressing CD34 (5.77% of CD45+ cells) were subgated for myeloid, T, and B lymphoid progenitors. Myeloid (CD34+CD45+CD13+), T (CD34+CD45+CD5+), or B (CD34+CD45+ CD19+) progenitors were sorted and deposited directly into tubes containing TriZol (Gibco). For peripheral blood, subgroups of cells were identified based on scatter characteristics and antibody staining: CD13+monocytes, glycophorin A+ erythrocytes, CD19+ B lymphocytes, or CD3+ T lymphocytes were sorted for total RNA extraction.
Reverse transcriptase-coupled PCR
Total RNAs from human cells, Xenopus embryos, or animal caps were extracted with TRIzol (Gibco) according to the manufacturer's instructions. The reverse transcription was performed with the oligo dT (Gibco) or the primer Qt.18The SuperScript II RNase H− M-MLV reverse transcriptase was purchased from Gibco. After reverse transcription (RT), the reactions were diluted 1:40, and 2 μL aliquots were used as templates for PCR reactions. MIXL expression in sorted cells was determined by RT-PCR with the MIXL-EXP primers 5′-GGTACCCCGACATCCACTTG-3′ and 5′-CTCCCATGAGTCCAGCTTTG-3′ and the β-actin primers 5′-AGAGCAAGAGAGGCATCCTC-3′ and 5′-ATAGCACAGCCTGGATAGCA-3′, followed by Southern blotting with aMIXL cDNA fragment and a β-actin cDNA fragment as described below. For each sample, 5 parallel reactions of RT-PCR were performed with different PCR cycles (18, 21, 24, 27, or 30 cycles).
The RT-PCR products were resolved on 1.5% agarose gels and then transferred to the Hybond N+ membranes (Amersham, Piscataway, NJ) with 0.4 M sodium hydroxide. The DNA on the blots was cross-linked by UV (Hoefer UV cross-linker, Amersham). The blots were prehybridized for 1 hour with the prehybridization solution (5 times SSPE, 5 times Denhardt solution, 0.5% SDS, 100 μg/mL sheared salmon sperm DNA). The 1 kbp MIXL and 286 bp β-actin cDNA fragments were randomly primed with [α-32P]dCTP (Random Primed DNA Labeling Kit from Roche) and added to the hybridization solution at a final concentration of 2.6 × 106 cpm/mL. After 14 hours of hybridization, the blot was rinsed at room temperature 3 times with wash solution I (2 times SSC, 0.1% SDS). Final washes were done at 65°C with wash solution II (0.05-0.1 times SSC, 0.1% SDS) for 30 minutes before exposing the blot to the Phosphor Imager screen (Molecular Dynamics/Amersham Biosciences, Sunnyvale, CA) for 1 hour.
Generation of antisera and immunoblotting
The amino-terminal antigen (TAESRALQFAEGAAF; single-letter amino acid codes) and carboxy-terminal antigen (GSKLDSWEEHIFSAF) from the predicted MIXL open reading frame (ORF) were synthesized in the synthetic antigen core facility at our institution. Rabbit polyclonal antibodies/antisera (anti-MIXL-N and anti-MIXL-C) were raised against both the peptides (Bethyl Lab, Montgomery, TX). The anti-MIXL-N antiserum was further affinity-purified (Bethyl Lab). Both antibodies were evaluated for specificity in nuclear extracts of transiently transfected COS-1 or 293T cells. Although both antibodies detected the same specific band, the affinity-purified amino-terminal antibody anti-MIXL-N with a higher titer was used in further studies.
Nuclear extracts were prepared by lysing 4 × 106 cells (rinsed twice with 1 times PBS) in 400 μL lysis buffer (10 mM HEPES [4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid], pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM ethyleneglycotetraacetic acid (EGTA), 1 mM dithiothreitol [DTT], 1 mM phenylmethylsylfonyl fluoride [PMSF], 2 μg/mL leupeptin, 2 μg/mL pepstatin A, 2 μg/mL aprotinin, 500 μg/mL benzamidine, 1 mM Na3VO4). After 15 minutes of lysis on ice, 12.5 μL of 10% Nonidet P-40 (NP-40) was added to the lysates and vortexed for 15 seconds. The pellets obtained after a quick spin at 14 000 rpm were resuspended in extraction buffer (20 mM HEPES, pH 7.9, 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF, 2 μg/mL leupeptin, 2 μg/mL pepstatin A, 2 μg/mL aprotinin, 500 μg/mL benzamidine, 1 mM Na3VO4). The extraction was continued on ice for 30 minutes with intermittent vortexing. The supernatant nuclear proteins obtained after centrifugation at 14 000 rpm for 5 minutes were resolved (50 μg proteins/lane) on precast 10% SDS-polyacrylamide gels (Invitrogen). After electrophoresis, the proteins were transferred to Hybond P nylon membrane (Amersham) at 30 V overnight. The protocol for immunoblotting was essentially as detailed elsewhere.20 The mouse monoclonal antibody against human histone H1 (Santa Cruz Biotechnology, Santa Cruz, CA) was used as loading control.
In vitro transcription, embryo injection, and animal cap assay
The predicted ORF of MIXL was cloned into the vector pBluescript RN3 (a kind gift from Nigel Garrett and Patrick Lemaire) and designated pRN3-MIXL. The mutated MIXL with an in-frame deletion of the entire homeodomain was also cloned into the same vector and designated pRN3-ΔMIXL. Capped mRNAs of MIXL and truncated MIXL were synthesized from the SfiI-linerized constructs pRN3-MIXL and pRN3-ΔMIXL by using T3 RNA polymerase (mMESSAGE mMACHINE; Ambion, Austin, TX), and purified through G50 Sephadex Column (Roche).
For animal cap assay, Xenopus embryos were in vitro fertilized, dejellied, and cultivated, and injected with synthetic mRNA at the one-cell stage in the animal pole. Then, animal caps were explanted at stage 8 and cultured to the sibling stage 36 in the presence of 200 ng/mL recombinant human basic FGF (bFGF). The expression of Xenopus αT4-globin gene was examined by RT-PCR with the primer pair 5′-TTG CTG TCT CAC ACC ATC CAG G-3′ and 5′-TCT GTA CTT GGA GGT GAG GAC G-3′. EF1α, an endoderm marker 5′-GGA AAG TCC ACA ACA ACT GG-3′ and 5′-GGA GCA TCA ATG ATA GTG AC-3′, was used as control. The multiplex PCR of 27 cycles was performed under conditions described above.
Results
Isolation of human MIXL
To molecularly clone the human Mix.1-like gene(s), we searched the human EST database against the homeodomain sequences ofXenopus Mix.1 with the tBLASTN software program (http://www.ncbi.nlm.nih.gov/BLAST) in the public domain. A single EST clone AA847809, containing a partial homeodomain with 66% identity and 72% similarity in the predicted ORF to that ofXenopus Mix.1, was identified from a CD20+germinal center pre-B cell library. Double-stranded sequencing of the 1.8 kbp insert of AA847809 allowed us to deduce a partial ORF of 155 amino acids followed by 3′ UTR of 1307 nucleotides, including 214 bp of an alu repeat and no polydenylation signal. Surprisingly, recursive searches with the AA847809 sequences identified only 3 other clones:AA911377, from a mammary carcinoma cDNA library, and AI654861 and BE552088, from germ cell tumors, in the entire human EST database of about 3.7 million entries from more than 30 different tissues. Thus the expression of the Mix.1-like homeobox gene may be tightly regulated.
Because all the 4 clones contain partial 3′ coding sequences of the presumptive gene, we attempted to isolate the 5′ and 3′ sequences by RACE of cDNA pools from fetal brain. Unfortunately, this approach did not yield readily discernible 5′ products due to the high GC content (> 75%) of the 5′ end, whereas the 3′-RACE yielded a 1.3-kbp product (data not shown).
An alternate approach was to isolate BAC clones, because these could be sequenced to deduce additional 5′ coding sequences. A pair of primers (MIXL-EST1) covering the unique 3′ region from the carboxy-terminal domain to the 3′ UTR yielded the predicted 271-bp fragment from normal human genomic DNA. This primer pair was used to screen the CITB-HSP-C human BAC library, and BAC clone 216I11 was isolated. By assembling sequences generated from the BAC clone with MIXL-specific primer with the sequences from EST clone AA847809, we predicted an ORF of 232 amino acids, similar to the previously reported murine Mix-like (Mml/MIXL1) gene. We designated the geneMIXL (Mix.1 homeobox [Xenopus laevis]-like gene, GenBank accession no. AF211891) in consultation with the Human Genome Nomenclature Committee.
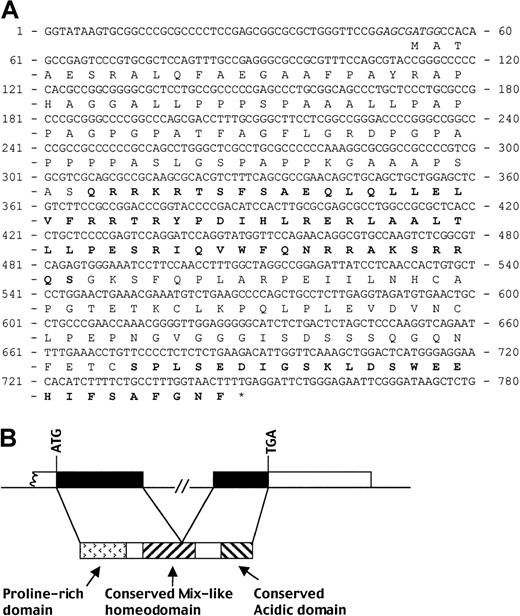
The deduced ORF has a putative ATG start codon with a good Kozak motif (G-3 and G+4[GGAGCGatgG])21 and contains a proline-rich amino-terminal region, a pairedlike homeodomain, and a carboxy-terminal acidic domain (Figure1A).
Human Mix.1-like homeobox gene
MIXL. (A) The predicted ORF of MIXL. Nucleotides 1-285 were derived from sequencing BAC CITB-HSP-C216I11. The rest was from cDNA clones AA847809, AA911377, and AI654861. The Kozak consensus around the initiation Met are in italics. The conserved Mix-like homeodomain and carboxy-terminal domain are in bold. (B) Genomic organization of MIXL. The 1.6-kbp genomic fragment containing the intron was amplified from BAC 216I11 with the primer pair MIXL-HD, subcloned, and sequenced. The exon-intron junctions with perfect splice donor and acceptor consensus thus identified between nucleotides 448 and 449 (panel A) were confirmed by direct sequencing of BAC 216I11. Exon 1 is predicted to be more than 393 bp and exon 2 to be about 1.3 kbp. The protein domains of the predicted MIXL ORF are indicated. The amino-terminal proline-rich domain, not found in Xenopus Mix.1, is unique to MIXL and Mml.
Human Mix.1-like homeobox gene
MIXL. (A) The predicted ORF of MIXL. Nucleotides 1-285 were derived from sequencing BAC CITB-HSP-C216I11. The rest was from cDNA clones AA847809, AA911377, and AI654861. The Kozak consensus around the initiation Met are in italics. The conserved Mix-like homeodomain and carboxy-terminal domain are in bold. (B) Genomic organization of MIXL. The 1.6-kbp genomic fragment containing the intron was amplified from BAC 216I11 with the primer pair MIXL-HD, subcloned, and sequenced. The exon-intron junctions with perfect splice donor and acceptor consensus thus identified between nucleotides 448 and 449 (panel A) were confirmed by direct sequencing of BAC 216I11. Exon 1 is predicted to be more than 393 bp and exon 2 to be about 1.3 kbp. The protein domains of the predicted MIXL ORF are indicated. The amino-terminal proline-rich domain, not found in Xenopus Mix.1, is unique to MIXL and Mml.
To characterize the genomic organization of MIXL, we designed several pairs of primers to probe the exon-intron junctions in BAC 216I11 by PCR. By using long-range PCR with the primer pair MIXL-HD that spans the codons 92-157, we amplified a 1.6-kbp genomic fragment. As a result, a 1.4-kbp intron was identified in the middle of the homeobox (Figure 1B). The possibility of one or more untranslated 5′ exons cannot be excluded at the present time.
Both Mix-like homeodomain and carboxy-terminal domain are evolutionarily conserved
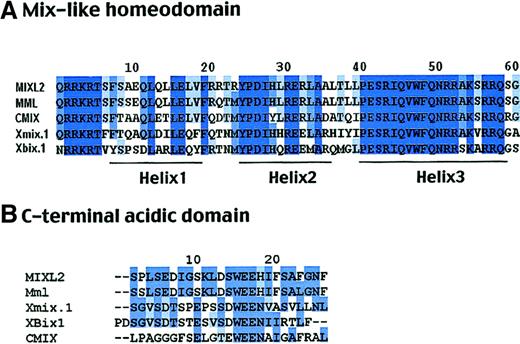
To determine the homology and phylogenetic distances between the human MIXL, other Mix-like genes (Mml andCMIX), and Xenopus Mix family members, the homeodomains of these genes were compared by the ClustalW program.22 A neighbor joining phylogenetic tree revealed the human MIXL gene to be more closely related to the mouseMml/MIXL1 gene than other genes (data not shown). The 2 proteins showed an overall identity of 69% with a 94% identity in the homeodomain (Figure 2A). Furthermore, the MIXL homeodomain is closer to that ofXenopus Mix.1 with 66% identity and 72% similarity than to that of Xenopus Bix.1 at 61% identity and 69% similarity (Figure 2A).
High degree of conservation in the homeo- and acidic carboxy-terminal domains in evolution.
(A) ClustalW alignment of the 60 amino acid homeodomain sequences. The MIXL homeodomain shows 66% identity to that of XenopusMix.1. Residues constituting the 3 helical motifs are denoted. Note the high identity in the amino-terminal arm and helix 3 responsible for DNA binding. (B) Homology in the carboxy-terminal acidic domain. The highly conserved SxxSD/E and WEE motifs are denoted.
High degree of conservation in the homeo- and acidic carboxy-terminal domains in evolution.
(A) ClustalW alignment of the 60 amino acid homeodomain sequences. The MIXL homeodomain shows 66% identity to that of XenopusMix.1. Residues constituting the 3 helical motifs are denoted. Note the high identity in the amino-terminal arm and helix 3 responsible for DNA binding. (B) Homology in the carboxy-terminal acidic domain. The highly conserved SxxSD/E and WEE motifs are denoted.
In the carboxy-terminal domain, MIXL also shows homology to Xenopus Mix.1 and other Mix-like proteins. As shown in Figure 2B, the 2 motifs SxxSD/E and WEE in the domain are conserved among most Mix-like proteins, suggesting that the carboxy-terminal domain is essential forMIXL function.
Localization of MIXL to chromosome 1q42.1
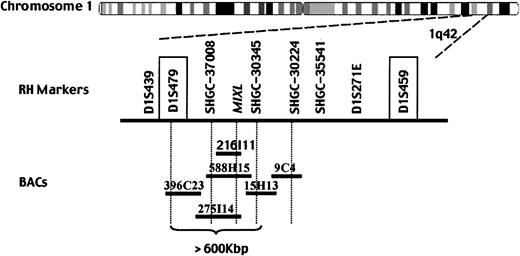
We localized MIXL by RH mapping to examine whether the gene maps to a chromosomal region implicated in malignancies. An RH data set generated by PCR with MIXL primers was submitted to the Stanford RH server for linkage mapping. The statistical analysis then demonstrated that the MIXL gene is linked to several markers (LOD > 6) located on chromosome 1q42, including SHGC-355541, SHGC-30345, SHGC-30224, and SHGC-37008 (Figure3). FISH studies with BAC 216I11 further confirmed the mapping to chromosome 1q42 (J. Ma, W.G., and L.N., unpublished results, January, 2000).
MIXL localizes to human chromosome 1q42.MIXL was localized to human chromosome 1q42 and linked to the markers in bold by radiation hybrid mapping. Tentative genomic sequences from BACs 216I11, RP11-588H15, and RP11-275I14 contain theMIXL gene. BACs 588H15 and 275I14 also span the marker SHGC-37008. BAC RP11-15H13, which overlaps with BAC 588H15, contains the marker SHGC-30345. BAC RP11-396C23 overlapping with BAC 275I14 contains the microsatellite marker D1S479. The microsatellite markers D1S479 and D1S459 (in text box) localize to band 1q42.12 and 1q42.2b by high-resolution FISH.
MIXL localizes to human chromosome 1q42.MIXL was localized to human chromosome 1q42 and linked to the markers in bold by radiation hybrid mapping. Tentative genomic sequences from BACs 216I11, RP11-588H15, and RP11-275I14 contain theMIXL gene. BACs 588H15 and 275I14 also span the marker SHGC-37008. BAC RP11-15H13, which overlaps with BAC 588H15, contains the marker SHGC-30345. BAC RP11-396C23 overlapping with BAC 275I14 contains the microsatellite marker D1S479. The microsatellite markers D1S479 and D1S459 (in text box) localize to band 1q42.12 and 1q42.2b by high-resolution FISH.
To further refine the mapping, a small BAC contig was built in silico by a search of the human genome database (Figure 3).MIXL sequences were detected in BACs RP11-257I14 (accession no. AL592045) and RP11-588H15 (accession no. AC021883). The RP11-588H15 sequences overlap with the RP11-15H13 (accession no. AC011651), which contains SHGC-30345, a marker identified by the RH mapping. Furthermore, BAC RP11-257I14 overlaps with BAC RP11-396C23 (accession no. AL512343) containing the polymorphic marker D1S479, which is physically localized to the human chromosome 1q42.12 by high-resolution FISH mapping in the public domain. Therefore, the MIXL gene is physically localized to human chromosome 1q42.1, a region with increased copy number (gain) as detected by comparative genomic hybridization in mammary cancer and lymphoid malignancies.23-25
MIXL expression is restricted to progenitor compartments
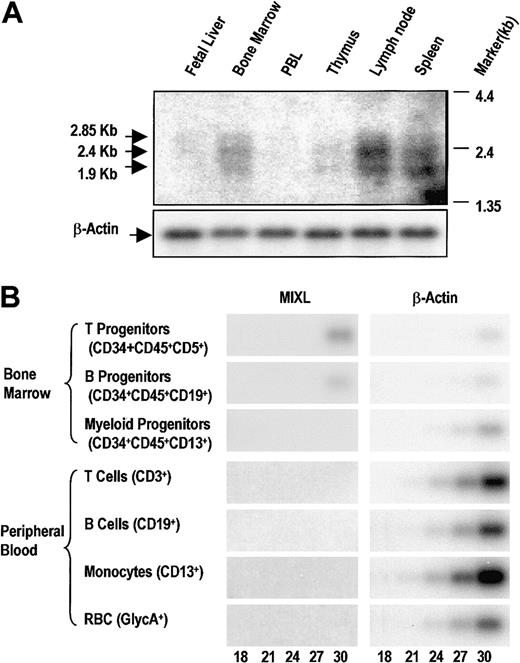
The MIXL transcript levels in hematopoietic tissues were evaluated by Northern blotting analysis. We detected 3 transcript forms: 2.85 kb, 2.4 kb, and 1.9 kb (Figure4A). It is likely that there are unidentified 5′ exons or different polyadenylation signals accounting for the 3 splice forms, as we could account for 1.7 kbp from theMIXL sequences and 3′-RACE results. All the 3 transcript forms were detected in lymph nodes, spleen, and bone marrow, suggesting that the MIXL expression is restricted to tissues with stem cell or progenitor compartments (Figure 4A). However, fetal liver did not reveal readily detectable expression, raising the possibility of a stage-specific low transcription during the 18- to 24-week stages of gestation. Future in situ hybridization experiments will address this important question.
MIXL expression is restricted in normal hematopoietic tissues.
(A) Human immune system multitissue Northern blot (Clontech Laboratories) was hybridized overnight with radiolabeledMIXL cDNA probe as described in “Materials and methods,” and the blot was exposed to x-ray film for 5 days. Arrows denote the 3 bands detected. The autoradiograph of the same blot rehybridized with β-actin is shown in the lower panel. PBL indicates peripheral blood leukocytes. (B) The T, B, or myeloid progenitors (1 × 103, 2.5 × 103, and 9 × 103 cells, respectively) from bone marrow or mature T cells, B cells, monocytes, and red blood cells (2 × 105, 1 × 105, 5 × 104, and 1 × 104 cells, respectively) were obtained for total RNA extraction. The RNA from each fraction was reverse-transcribed with an oligo dT primer. The cDNAs were approximately normalized based on a pilot β-actin amplification reaction. Aliquots of 2 μL of the direct or diluted cDNA pool were used in PCR of 18, 21, 24, 27, or 30 cycles with MIXL-EXP primers or β-actin primers as detailed in “Materials and methods.” The amplification products (341 bp for MIXL and 246 bp for β-actin) were resolved on a 1.5% agarose gel, transferred to Hybond N+ membrane, and probed with radiolabeled MIXL or β-actin cDNAs. The blots were exposed to the Phosphor Screen for 1 hour. The images were converted from the Phosphor Screen. The number of PCR cycles is denoted at the bottom.
MIXL expression is restricted in normal hematopoietic tissues.
(A) Human immune system multitissue Northern blot (Clontech Laboratories) was hybridized overnight with radiolabeledMIXL cDNA probe as described in “Materials and methods,” and the blot was exposed to x-ray film for 5 days. Arrows denote the 3 bands detected. The autoradiograph of the same blot rehybridized with β-actin is shown in the lower panel. PBL indicates peripheral blood leukocytes. (B) The T, B, or myeloid progenitors (1 × 103, 2.5 × 103, and 9 × 103 cells, respectively) from bone marrow or mature T cells, B cells, monocytes, and red blood cells (2 × 105, 1 × 105, 5 × 104, and 1 × 104 cells, respectively) were obtained for total RNA extraction. The RNA from each fraction was reverse-transcribed with an oligo dT primer. The cDNAs were approximately normalized based on a pilot β-actin amplification reaction. Aliquots of 2 μL of the direct or diluted cDNA pool were used in PCR of 18, 21, 24, 27, or 30 cycles with MIXL-EXP primers or β-actin primers as detailed in “Materials and methods.” The amplification products (341 bp for MIXL and 246 bp for β-actin) were resolved on a 1.5% agarose gel, transferred to Hybond N+ membrane, and probed with radiolabeled MIXL or β-actin cDNAs. The blots were exposed to the Phosphor Screen for 1 hour. The images were converted from the Phosphor Screen. The number of PCR cycles is denoted at the bottom.
To further assess MIXL expression during hematopoietic differentiation, we generated cDNA pools from fluorescence-activated cell sorter (FACS)–enriched progenitor populations of T, B, and myeloid lineages as well as mature B cells, T cells, monocytes and erythrocytes from normal peripheral blood. RT-PCR products of cDNA pools from these highly enriched fractions were detected by Southern hybridization with a radiolabeled MIXL probe. The products were screened at 18, 21, 24, 27, and 30 cycles to estimate the relative abundance of the target mRNA. The quality of the cDNA pools was first evaluated with a pair of β-actin primers. Under these conditions we could detect MIXL expression in both T- and B-cell progenitors from normal marrow (Figure 4B). In contrast, there were no detectable products from the RT-PCR of cDNA pools from mature T and B cells from peripheral blood (Figure 4B).
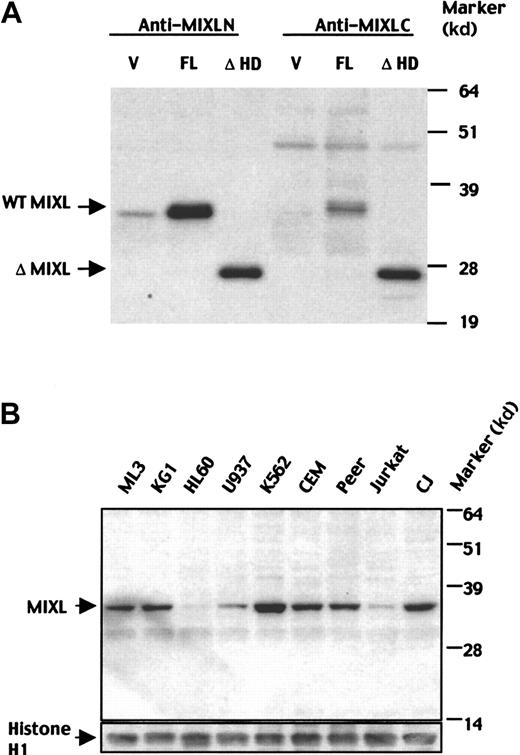
To further characterize MIXL protein expression, we developed polyclonal rabbit antisera against MIXL peptides. The specificities of the 2 antisera raised against 2 different epitopes were rigorously tested in transiently transfected 293T cells. Interestingly, the MIXL protein migrated at 36 kd, although the predicted molecular weight is 27 kd. Similarly, an expression construct lacking the homeodomain with a predicted molecular weight of 17 kd migrated at 26 kd (Figure5A). The aberrant mobility could be due to the high proline content (35%) of the amino-terminal segment between amino acids 31 and 83. Both the transfected and endogenous MIXL proteins showed similar mobilities (Figure 5B) confirming the predicted ORF.
Characterization of MIXL protein expression.
(A) Specificities of MIXL antibodies. 293T cells were transfected with wild-type (WT) MIXL and mutant MIXL without the homeobox (ΔMIXL) cloned in the vector pcDNA3.1-MycHis. Forty-eight hours after transfection, cytoplasmic lysates for homeodomain-less mutant MIXL and nuclear lysates for WT MIXL were resolved on a 12% SDS-PAGE gel and transferred to a Hybond P nylon membrane. The blot was probed with the affinity-purified antibody anti-MIXL-N at a dilution of 1:100 and antiserum anti-MIXL-C at a dilution of 1:1000. Both antibodies detected the same full-length MIXL protein at 36 kd and homeodomain-less mutant MIXL at 26 kd. V indicates vector alone; FL, full-length MIXL construct and ΔHD- homeodomain-less construct. (B) MIXL protein is expressed in hematologic malignancies. MIXL proteins were detected in leukemic cells expressing early myeloid markers (ML3, KG1), lymphoid markers (CEM, Peer, CJ) and erythroid markers (K562). The affinity purified antibody anti-MIXL-N was used at 1:100 dilution. The blot was reprobed with the mouse monoclonal antibodies against human histone H1 to quantify loading.
Characterization of MIXL protein expression.
(A) Specificities of MIXL antibodies. 293T cells were transfected with wild-type (WT) MIXL and mutant MIXL without the homeobox (ΔMIXL) cloned in the vector pcDNA3.1-MycHis. Forty-eight hours after transfection, cytoplasmic lysates for homeodomain-less mutant MIXL and nuclear lysates for WT MIXL were resolved on a 12% SDS-PAGE gel and transferred to a Hybond P nylon membrane. The blot was probed with the affinity-purified antibody anti-MIXL-N at a dilution of 1:100 and antiserum anti-MIXL-C at a dilution of 1:1000. Both antibodies detected the same full-length MIXL protein at 36 kd and homeodomain-less mutant MIXL at 26 kd. V indicates vector alone; FL, full-length MIXL construct and ΔHD- homeodomain-less construct. (B) MIXL protein is expressed in hematologic malignancies. MIXL proteins were detected in leukemic cells expressing early myeloid markers (ML3, KG1), lymphoid markers (CEM, Peer, CJ) and erythroid markers (K562). The affinity purified antibody anti-MIXL-N was used at 1:100 dilution. The blot was reprobed with the mouse monoclonal antibodies against human histone H1 to quantify loading.
We next examined MIXL protein levels in cell lines derived from hematopoietic malignancies (Figure 5B). ML3 and KG1, AML cell lines that express CD34+ antigen, expressed MIXL protein unlike the more committed myeloid leukemic cell lines HL60 and U937. The K562 cells derived from a patient with chronic myelogenous leukemia in erythroid blast crisis expressed significant levels. Among the cell lines derived from T-cell acute lymphocytic leukemia (ALL), the pre-T cell line CEM and the γ,δ+ cell line Peer had higher levels than the more committed Jurkat cells. CJ, a lymphoma cell line derived from a patient with diffuse large B-cell lymphoma, expressed high levels of MIXL. Thus, the expression of MIXLappears to correlate with malignancies of early progenitors of myeloid, erythroid, and B- and T-cell lymphoid lineages.
MIXL induces of α-globin expression in Xenopusanimal caps
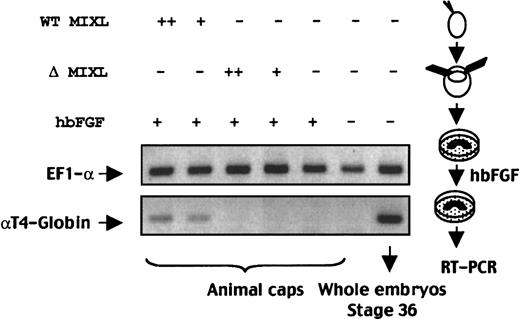
The structural conservation between human MIXL andXenopus Mix.1 led us to ask whether the humanMIXL is involved in embryonic hematopoiesis. One way to address the question is to examine whether ectopic expression ofMIXL would direct the mesoderm to a hematopoietic fate inXenopus embryos. Synthetic mRNA of wild-type MIXLor homeobox-truncated MIXL was injected into one-cellXenopus embryos. At stage 8, animal caps were collected and cultured with human recombinant bFGF, because FGF or other mesoderm inducers are required for induction of hematopoietic mesoderm by BMP-4 or GATA-1 in animal cap assay.13 At the equivalent of stage 36, animal caps were harvested for RNA extraction. As shown in Figure 6, bFGF alone could not induce αT4-globin expression in animal caps. With bFGF treatment, humanMIXL induced α-globin expression in animal caps, whereas homeobox-truncated MIXL failed to induce erythropoiesis. These findings demonstrated that human MIXL mimicsXenopus Mix.1 in the specification and development of the hematopoietic system in Xenopus embryos. Taken together,MIXL may be a novel regulatory factor involved in both embryonic and adult human hematopoiesis.
MIXL -induced expression of αT4-globin inXenopus animal caps.
Synthetic mRNAs (300 pg or 1 ng RNAs) were injected into Xenopus one-cell embryos in 2 separate sets. At the 8-cell stage, animal caps were excised and the explants were cultured in the presence of human recombinant bFGF (hbFGF). At the equivalent of stage 36, the animal caps from each set was pooled separately for RNA extraction and RT-PCR analysis. Twenty-seven cycles of multiplex PCR with the primer pairs for αT4-globin and EF1-α genes were performed. Note the αT4-globin expression in wild-type (WT) MIXL-injected animal caps but not in animal caps injected with homeobox-less MIXL(ΔMIXL) or FGF-treated embryos. +, 300 pg mRNA; ++, 1 ng mRNA.
MIXL -induced expression of αT4-globin inXenopus animal caps.
Synthetic mRNAs (300 pg or 1 ng RNAs) were injected into Xenopus one-cell embryos in 2 separate sets. At the 8-cell stage, animal caps were excised and the explants were cultured in the presence of human recombinant bFGF (hbFGF). At the equivalent of stage 36, the animal caps from each set was pooled separately for RNA extraction and RT-PCR analysis. Twenty-seven cycles of multiplex PCR with the primer pairs for αT4-globin and EF1-α genes were performed. Note the αT4-globin expression in wild-type (WT) MIXL-injected animal caps but not in animal caps injected with homeobox-less MIXL(ΔMIXL) or FGF-treated embryos. +, 300 pg mRNA; ++, 1 ng mRNA.
Discussion
MIXL is a XenopusMix.1-like gene
The Xenopus Mix.1 homeoboxlike genes have been found in several organisms, including zebrafish, chicken, mouse, and human. Although at least 9 Mix-like genes have been isolated inXenopus, only single-copy genes have been detected in each of the other vertebrates tested so far. How many Mix-like genes are there in higher organisms? Since Xenopus is tetraploid, there should be half the number of Mix-like genes in other organisms. However, our results strongly indicate that there is only one Mix-like gene in humans, because we could not identify any other Mix-like gene from the current genome database. Furthermore, Southern blotting studies with MIXL cDNA are consistent with MIXLbeing a single copy gene (H.L., W.G., and L.N., unpublished results, June, 1999).
A comparison between Mix-like genes indicated that the humanMIXL gene is more closely related to the mouseMml gene than other Mix-like genes. The homeodomain of humanMIXL has 94% identity to that of the mouse Mml, whereas the human MIXL protein shows an overall 69% identity to mouse Mml. Although the overall homology between human MIXL and mouse Mml is not very high, it is likely thatMIXL is the human ortholog of the mouse Mmlbecause both genes localize to evolutionarily conserved syntenic regions on chromosome 1.10
Structural and functional conservation between Mix.1-like genes
Mix-like genes constitute a distinct family of pairedlike homeobox regulators. Typically, they encode a conserved Mix-like homeodomain and a conserved carboxy-terminal “WEE” motif, except forXenopus Mixer that contains no “WEE” motif. However, there is a major difference in that Mix-like proteins in chicken, mouse, and human are much smaller in size (∼230 amino acids) than those in amphibians.8-11Xenopus Mix-like genes encode proteins of approximately 370 amino acids in size.3-7 There are 2 possible explanations that could account for the difference. The first is that only the conserved domains are evolutionarily critical for functions of Mix-like genes. Although homology between human MIXL and XenopusMix.1 is restricted in the conserved homeodomain and carboxy-terminal domain, ectopic expression of MIXL directedXenopus mesoderm to a hematopoietic fate in animal cap assay (Figure 6), as Xenopus Mix.1 did. In addition, microinjection of MIXL mRNA into Xenopus embryos led to the phenotypic consequences similar to those ofXenopus Mix.1 (A.P.C. et al, unpublished results, March, 2000). This possibility can also explain why the overall homology between human MIXL and mouse Mml is relatively low compared to other pairedlike homeobox gene families. An alternate possibility is that Mml,MIXL, and CMIX may belong to a novel subfamily of Mix-like homeodomain proteins, in case there are additional Mix-like genes with weak homology in higher organisms.
MIXL in human hematopoiesis and leukemogenesis
Our results strongly suggest MIXL to be involved in human hematopoietic differentiation. Ectopic expression ofMIXL could induce α-globin expression in animal caps (Figure 6), reflecting a possible role for human MIXL in embryonic hematopoiesis. Consistent with this observation was our detection of differential expression of MIXL in hematopoietic tissues and cell lineages (Figures 4 and 5). As shown in Figure 4A, the transcripts are readily detectable in lymph nodes and spleen. However, the apparent lack of expression in fetal liver is inconsistent with the expression in lymphoid tissues. The possibility of an oscillating expression pattern during hematopoietic maturation cannot be excluded. Clearly, the transcript levels are higher in the highly enriched B- and T-lymphoid progenitors from bone marrow than in the peripheral blood (Figure 4B). The high G+C content and the highly conserved homeodomain of MIXL are technical challenges in evaluating the expression pattern precisely. Future studies on how and when MIXL is induced in hematopoietic cells and whetherMIXL cooperates with other pairedlike homeobox genes and transcription factors to regulate downstream events in hematopoiesis will elucidate the significance of its highly restricted expression.
Another indication of the highly restricted expression comes from the representation of MIXL gene in the human EST database. Among the 3.9 million entries from more than 30 tissue types and 20 cell lines MIXL is found in only 4 tissues, namely CD20+ germinal center B cells, germ cell tumors, infiltrating ductal carcinoma, and fibrosarcoma.
BMP-4 signaling is hypothesized to be involved in initiation and specification of the mammalian hematopoietic system.26,27Although no direct evidence shows BMP-4 involvement in the mammalian hematopoietic system, Xenopus studies have implicated BMP-4 in hematopoiesis.13,14,28 The hypothesis is further supported by the polarized expression of BMP-4 in a region of densely packed cells underlying intra-aortic hematopoietic clusters located in the AGM region.15 Because Xenopus Mix.1 is a downstream target of the BMP-4 signaling pathway,12 it will be interesting to determine if the human gene MIXL is a direct target of BMP-4.
Mammalian hematopoiesis requires many factors, includingSCL, LMO2, and GATA2, that regulate the differentiation of uncommitted progenitors with high precision.29-34Xenopus SCL as well as GATA2 could induce the αT4-globin expression in animal caps.35,36 With bFGF treatment, human MIXLinduced αglobin expression in animal caps (Figure 6). Interestingly,Xenopus Mix.1 induces expression of XenopusSCL,36 suggesting that MIXL might be involved in SCL regulation. Considering the patterning of amphibian animal caps to hematopoietic fate by human MIXL (Figure 6) as well as Xenopus Mix.1,12 future studies will unveil whether MIXL is involved in the same cascade in mammalian hematopoiesis by regulating SCL. Reagents generated in the present study will be of value in elucidating this mechanism.
Activation of early transcription factors, including homeobox genes, by chromosomal translocation is a recurrent theme in human hematologic malignancies (for a review, see van Oostveen et al37). We detect high levels of MIXL expression in some less-committed myeloid and lymphoid malignancies (Figure 5B). One possible mechanism for these observations is that high levels of MIXLexpression may lead to activation and even overexpression of the oncogene SCL. Additionally, aberrant expression ofMIXL could directly confer a survival advantage or facilitate a block in differentiation.
Duplication or “jumping translocation” of 1q, resulting in increased gene dosage is considered a frequent secondary aberration in multiple myeloma.25 Gain of chromosome 1q42 is also a common anomaly in breast cancer.23-25 Thus, MIXLon chromosome 1q42 could be a novel candidate oncogene implicated in a subset of these anomalies.
We thank Dr Richard Ford for the CJ cell line, Elva Lopez for assistance with preparation of the manuscript, and members of our laboratory for critical reading of the manuscript. L.D.E. and L.N. are members of the Program in Genes and Development, Graduate School in Biomedical Sciences, University of Texas Health Science Center, Houston, TX.
Supported by the Department of Defense (DAMD 17-99-1-9267), National Institutes of Health (CA66982 and CA55164), funds from Katz Foundation (to L.N.), and core grant CA16672 to the University of Texas M. D. Anderson Cancer Center.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Lalitha Nagarajan, Department of Molecular Genetics, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Box 45, Houston, TX 77030; e-mail:lalitha@odin.mdacc.tmc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal