Abstract
To understand the molecular mechanism by which interleukin-6 (IL-6) regulates myeloid differentiation primary response (MyD) genes at the onset of M1 myeloid differentiation, we used JunB as a representative MyD gene to isolate and characterize IL-6 responsive elements. An IL-6 responsive element was localized between −65 and −52 of the JunB promoter (−65/−52 IL-6RE). By using antibody and oligonucleotide competition assays in electrophoretic mobility shift assay experiments, we have shown that the heterotrimeric transcription nuclear factor Y (NF-Y) complex binds to this element. A dominant-negative form of NF-YA, ectopically expressed in M1 cells, blocked NF-Y binding to the −65/−52 IL-6RE and reduced induction of JunB by IL-6. Furthermore, inhibition of NF-Y binding also reduced MyD gene induction by IL-6 and dampened the IL-6–induced M1 differentiation program. These findings are consistent with the observation that most MyD genes contain intact NF-Y binding motifs in their promoter regions. In contrast to M1 cells, during myeloid differentiation of bone marrow (BM), there was induction of NF-Y binding to the −65/−52 IL-6RE. This induced binding can be attributed to the observed induction of NF-YA protein expression and may reflect the molecular mechanism that couples proliferation to terminal differentiation of normal myeloblasts. Similar to M1 cells, blocking NF-Y binding in BM resulted in a reduction in mature macrophages. It can be concluded that NF-Y plays a role in the transcriptional regulation of MyD genes and is required for optimum myeloid differentiation.
Introduction
To understand the molecular mechanism by which interleukin-6 (IL-6) regulates myeloid differentiation primary response (MyD) genes at the onset of M1 myeloid differentiation, we used JunB as a representative MyD gene to isolate and characterize IL-6 responsive elements. An IL-6 response element (IL-6RE), localized between −65 and −52 of the JunB promoter (−65/−52 IL-6RE) relative to its transcription start site, was shown to be necessary and sufficient for IL-6 inducibility.1 This element is regulated in a cell type–dependent manner and has no effect on the IL-6 inducibility of JunB in the human HepG2 hepatoma cell line.1-3 The same element was shown to be leukemia inhibitory factor–inducible in M1 cells but not in HepG2 cells.1 Sequence analysis of the −65/−52 IL-6RE revealed both a CCAAT box and an inverted repeat (IR) region4; the latter does not seem to play a role in the IL-6 inducibility of JunB in M1 cells.1 Therefore, it is likely that the CCAAT box would play a crucial part in the IL-6–mediated MyD gene response.
The CCAAT box is a widespread regulatory sequence that has been identified in many eukaryotic promoters and is usually located between −60 and −100 base pair (bp) upstream of the transcription start site.5-7 The orientation and position of CCAAT boxes are highly conserved within the same gene across different species.8 Functional analyses have indicated the importance of the CCAAT box in constitutively expressed,5,9,10 fully differentiated,7,11,12or cell cycle–regulated genes,13-19 and mutations within the CCAAT box cause a decrease in transcriptional activity.5 To date, several transcription factors have been shown to interact with the CCAAT box, including CCAAT enhancer binding protein (C/EBP), CCAAT displacement protein (CDP), nuclear factor Y (NF-Y), and NF-1. Among these transcription factors, only NF-Y requires an intact and complete CCAAT motif for its DNA sequence specificity.
NF-Y is a ubiquitous factor that is evolutionarily conserved. It was originally identified as the component that interacts with the Ea Y box of the major histocompatibility complex class II promoter.9 It consists of 3 subunits, NF-YA, NF-YB, and NF-YC, all of which are required for DNA interaction.20NF-YB and NF-YC belong to the histone H2A-H2B subfamily; they have conserved domains of 90 and 84 amino acids, containing histonelike fold motifs important for subunit interaction,21 allowing for NF-YB–NF-YC dimerization, which is a prerequisite for NF-YA interaction. NF-Y plays a crucial role in transcriptional activity at several levels. It increases the DNA affinity of other transcription factors that bind to neighboring enhancer elements, participates in the correct positioning of other transcription factors, and interacts with the TBP complex and proteins that have intrinsic histone acetyltransferase activity.22-25
In this study, we identified NF-Y as the major protein that binds to the −65/−52 IL-6RE. Mutagenesis within the CCAAT motif caused inhibition of both NF-Y binding and induction of JunB by IL-6. Expression of a dominant-negative (DN) form of NF-YA, which inhibits NF-Y binding to DNA, was able to significantly reduce the MyD response following IL-6 stimulation and also reduced the IL-6–mediated differentiation of M1 cells. In contrast to M1 cells, during myeloid differentiation of bone marrow (BM) cells there is induction of NF-Y binding to the −65/−52 IL-6RE and induction of NF-YA expression. Similar to the effect in M1 cells, blocking NF-Y binding in BM leads to a reduction in mature macrophages. This is the first incidence in which NF-Y has been shown to be a target of IL-6, to play a role in the transcriptional regulation of MyD genes, and to be required for optimum myeloid differentiation.
Materials and methods
Cells and cytokines
The murine M1 myeloid leukemic cell line, M1Neo, and M1NF-YA DN clones were grown in Dulbecco modified Eagle medium (DMEM; GIBCO-BRL, Gaithersburg, MD) supplemented with 10% heat-inactivated horse serum (GIBCO-BRL) and 1% penicillin and streptomycin (P/S) (GIBCO-BRL). M1Neo and M1NF-YA DN clones were maintained in the presence of 400 μg/mL G418 (Geneticin; GIBCO-BRL). PA317, a retrovirus packaging cell line, was cultured in DMEM supplemented with 10% fetal bovine serum (GIBCO-BRL) plus 1% P/S. PA317 cells were periodically selected in hypoxanthine, aminopterin, and thymidine medium (Sigma, St Louis, MO) to maintain their packaging function. WEHI-3B–conditioned medium, a source for IL-3, and L929 fibroblasts (L-cell)–conditioned medium, a source for macrophage colony-stimulating factor (MCSF), were prepared in our laboratory as described previously.26 Bosc23 cells, a high-titer retroviral packaging cell line, were used for BM infections. These cells were grown in DMEM supplemented with 10% fetal bovine serum and 1% P/S with 5% CO2 at 37°C. Recombinant human IL-6 (100 ng/mL) and rat stem cell factor (200 ng/mL) were a generous gift from Amgen (Thousand Oaks, CA).
Infection of BM cells and colony formation assays
BM cells were obtained from femurs of 4- to 6-week-old female Balb/C mice (Charles River Laboratories, Wilmington, MA). Erythrolysis of BM, retroviral infections, colony formation assays, and assays for differentiation-associated properties were previously described.26 The retroviral expression plasmid murine stem cell virus (pMSCV) EB-Neo and pMSCV EB-Neo/NF-YA DN (10 μg) was used. Colonies were raised in the presence of IL-3 (10% WEHI-3B–conditioned medium) or MCSF (10% L-cell–conditioned medium) plus 100 ng/mL IL-6.
Assays for differentiation-associated properties
Morphologic differentiation was determined by counting at least 300 cells on May-Grünwald-Giemsa–stained cytospin smears and scoring the proportion of blast cells, mature granulocytes, and macrophages as previously described.26,27 Immature blast cells are characterized by scant cytoplasm and round or oval nuclei; cells at intermediate monocyte stages of differentiation are flattened, with a larger cytoplasm-to-nucleus ratio, irregularly shaped nuclei, and few interspersed or no vacuoles; granulocyte intermediates are characterized by dented but not lobulated nuclei; mature macrophagelike cells are flattened and spread out cells interspersed with numerous vacuoles in a greatly enlarged cytoplasm; mature granulocytelike cells are characterized by enlarged cytoplasm and lobulated nuclei. Analysis of the expression of the specific cell surface marker of macrophages was done by fluorescence-activated cell-sorting (FACS) analysis by using fluorescein isothiocyanate (FITC)–conjugated F4/80 (Caltag Laboratories, Burlingame, CA) as previously described.26Cells (2 × 106) were harvested by centrifugation at 200 rpm and washed twice with 1 × phosphate-buffered saline (PBS). To eliminate background, cells were resuspended in 1% bovine serum albumin and allowed to incubate at room temperature for 30 minutes. Cells were further incubated with FITC-conjugated F4/80, washed 2 times, resuspended in 1 × PBS, and analyzed by using the Coulter Epic Elite system (Coulter, Miami, FL) for variation in signal.
General recombinant DNA techniques and probes
The use of the XhoP2GD construct was described in detail.1 The DNA probes for the murine MyD genes have been previously described.28-30 Probes were purified and labeled as described.1 The retroviral expression plasmid, MSCV EB-neo, used in this study was a generous gift from Dr Robert G. Hawley (University of Toronto, Toronto, Ontario, Canada). The DN NF-YA29 (NF-YA DN) was a gift from Dr Peter Edwards (UCLA, Los Angeles, CA).31 The EcoRI/BglII 1.0-kb NF-YA29 insert was subcloned into the EcoRI andBglII restrictions sites of MSCV EB-neo by directional ligation.
Gross deletions
The −102GD was obtained by digesting XhoP2GD withHpaI and NcoI, and the resulting 1.3-kilobase (kb) fragment was isolated. This fragment was further digested withBfaI and XcmI, and the 355-bp fragment that was obtained was cloned into the XbaI site of pCAT-Basic.
Smaller gross deletions were obtained by using the polymerase chain reaction (PCR). For all PCR amplifications, the same 3′ primer, 5′-GATCCTCTAGAGGGCTCGCTGCGG-3′, was used, which changed aSmaI restriction site into XbaI. The −102GD and −80GD were obtained by using the 5′ primers, 5′-CGCGGGGGCCGTCGACTGGCTCCCGCGTC-3′ and 5′-TGGCTCCCGCGTCGACGTCGGCCAATCGGA-3′, respectively. The PCR amplification was carried out by using 25 ng XhoP2GD plasmid DNA as template, 0.5 mg/mL bovine serum albumin, 1 mM dNTP, 5 mM primers, 1 × vent buffer (NEB, Beverly, MA) and 2.5 U Taq DNA polymerase (NEB), heated at 94°C for 3 minutes, annealed at 68°C for 1 minute, and subjected to 3 cycles using a Perkin-Elmer DNA Thermal Cycler (Perkin-Elmer, Exton, PA). Samples were then further subjected to 30 cycles of PCR by using 1 minute of denaturation at 94°C, 2 minutes of annealing at 76°C, and finally 5 minutes of polymerization at 72°C. The −70GD and the −31GD were constructed by using 5′ primers, 5′-ACTTCCGCAGGTCGACCTGACAAATTCAGTA-3′ and 5′-TGACAAATTCGTCGACAGTATAAAAGCTTGG-3′, respectively, with denaturation at 94°C for 1 minute, annealing at 68°C for 2 minutes, and subjected to 40 cycles. The −51GD and −41GD were obtained by using 5′ primers 5′-TCGGGTCGACAGTGCACTTCCGCAG-3′ and 5′-GTGCAGTCGACCTTCCGCAGCTGAC-3′, respectively. Amplifications were done similar to −70GD and −31GD, except 2 mM MgCl2 was added in the PCR reactions. The primers used were selected with the aid of the program PCRPLAN of PCGENE (Intelligenetics, Mountain View, CA). The 5′ primers all contained a SalI restriction recognition site and the 3′ primer an XbaI site. PCR products were purified by using Qiaquick Gel Extraction kit and digested with SalI and XbaI and subcloned into the SalI andXbaI sites of pCAT-Basic vector by directional ligation. All primers were prepared by the Chemistry Department at the University of Pennsylvania (UPENN; Philadelphia, PA) (unless otherwise indicated). All constructs were verified by sequencing using T7Sequenase v2.0 kit (United States Biochemical Corporation, Cleveland, OH) with 3′ primer (PE1JunB), 5′-CTGCTTTCTCGGCGTCGCTTC-3′ (Fels Institute, Temple University, Philadelphia, PA).
Northern blot analysis
RNA extraction, loading of the gel, and hybridization conditions were all described in detail elsewhere.1 β-Actin and glyceraldehyde-3-phosphate dehydrogenase 3 (GADPH3) probes were commercially obtained from Clonetech (Palo Alto, CA). Relative messenger RNA (mRNA) levels were determined with the aid of a Fuji BAS 2000 phosphoimage analyser.
Electrophoretic mobility shift assays
All nuclear extracts and electrophoretic mobility shift assay (EMSA) reactions were performed as mentioned before.1 NF-Y antibodies (1 μg) were incubated with extracts for 2 hours on ice prior to the addition of labeled probe. NF-YA and NF-YB antibodies obtained from Rockland (Gilbertsville, PA) were used in Western analysis, and antibodies obtained from Santa Cruz (Santa Cruz, CA) were used in EMSA experiments. All remaining antibodies were obtained from Santa Cruz. Most antibodies (2-5 μg), unless otherwise indicated, were incubated with extracts for 30 minutes at room temperature prior to the addition of labeled probe.
Synthetic oligonucleotides and minimal promoter CAT gene constructs
The synthesized oligonucleotide (UPENN), names, and sequence were as follows (only coding strands are indicated): JunBWT, 5′-GATCCCCCGCGTCGGCCAATCGGAGTGCACTTCCGCAGCTGA-3′; JunBMut, 5′-GATCCCCCGCGTCGGCCTGCAGGTCGACCCTTCCGCAGCTGA-3′; JunB Mut (−67/−65), 5′-GATCCCCCGCGTCCTGCAATCGGAGTGCACTTCCGCAGCTGA-3′; JunB Mut (−63/−62), 5′-GATCCCCCGCGTCGGCCTGTCGGAGTGCACTTCCGCAGCTGA-3′; JunB Mut (−61/−60), 5′-GATCCCCCGCGTCGGCCAAAGGGAGTGCACTTCCGCAGCTGA-3′; JunB Mut (−54/−52), 5′-GATCCCCCGCGTCGGCCAATCGGAGTACCCTTCCGCAGCTGA-3′; NF-Y, 5′-GATCCTTTTTCTGATTGGTTAAAAGA-3′; NF-Y Mut, 5′-GATCCTTTTTCTGCGGTTTTAAAAGA-3′; NF-1, 5′-TTTTGGATTGAAGCCAATATGATAA-3′; Egr-1, 5′-GATCCATCCCGGCGCGGGGGCGAGGGCGTA-3′; Sp-1, 5′-ATTCGATCGGGGCGGGGCGAGC-3′; and C/EBP consensus binding sequence, 5′-TGCAGATTGCGCAATCTGCA-3′. For protein interaction to the longer probe the following oligonucleotides were used: GC-CAAT, 5′-GATCCTCTAGGAGGGGGCCGCGGGGGCCTGGCCTCCCGCGTCGGCCAATCGGAGTGCACTTCCGCAGCTGA-3′; GCmut-CAAT, 5′-GATCCTCTAGGAGGTTTCCGCGTTTTCCTGGCCTCCCGCGTCGGCCAATCGGAGTGCACTTCCGCAGCTGA-3′; GC-CAATmut, 5′-GATCCTCTAGGAGGGGGCCGCGGGGGCCTGGCCTCCCGCGTCGGCCTGCAGGTCGACCCTTCCGCAGCTGA-3′; and GCmut-CAATmut, 5′-GATCCTCTAGGAGGTTTCCGCGTTTTCCTGGCCTCCCGCGTCGGCCTGCAGGTCGACCCTTCCGCAGCTGA-3′. Mutations are indicated by underlined sequences. The Sp-1, NF-1, and C/EBP oligonucleotides were obtained from Santa Cruz Biotechnology. Complementary oligonucleotides were made so that there was aBamHI restriction site at the 5′ end and a BglII site at the 3′ end, respectively. DNA probes (1 μg) were made by annealing complementary oligonucleotides by heating to 75°C for 10 minutes and then slowly cooling to room temperature.
Methyl interference assay
For methyl interference experiments either coding or noncoding strands of the JunBWT probe were 5′ end labeled by using 5′ DNA Terminus Labeling System with [∼−32P]ATP (NEN). The complementary strands were annealed at 75°C for 10 minutes and allowed to slowly cool to room temperature, ensuring that the probe was labeled only at one 5′ end. Probes were gel purified and isolated as mentioned before. Limited methylation on labeled probe (107 cpm) was carried out with dimethylsulfate (Sigma) in 200 μL dimethylsulfate buffer (Sigma) for 2.5 minutes at 30°C. The methylated probe (50 000 cpm) was used in gel shift binding reactions with 20 μg nuclear extract and 5 pg poly (dl-dC) and electrophoresed on a 5% nondenaturing polyacrylamide gel. Following autoradiography, bound and free probes were excised and eluted with extraction buffer (10 mM Tris [pH 8.0], 10 mM EDTA, and 200 mM NaCl) overnight at room temperature, passed through Dextran-sulfate (Sigma) capillary columns, washed with washing buffer (10 mM Tris [pH 8.0], 10 mM EDTA, and 300 mM NaCl), and eluted with elution buffer (10 mM Tris [pH 8.0], 10 mM EDTA, and 600 mM NaCl). The recovered DNA was cleaved with 1 M piperidine (Sigma) at 90°C for 30 minutes, lyophilized and resuspended in 10 μL formamide sample loading buffer (80% formamide, 10 mM NaOH, 1 mM EDTA, 0.1% xylene cyanole, and 0.1% bromophenol blue). An equal number of counts were loaded on a 12% polyacrylamide DNA sequencing gel.
Western blot analysis
Whole extracts were prepared from 3 × 107 cells, which were washed twice with 5 mL cold PBS and lysed in RIPA lysis buffer (150 mM NaCl, 1% NP-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 50 mM Tris [pH 8.0], 2 μg/mL Aprotinin, 2 μg/mL Benzamidine, 2 μg/mL Pepstatin A, 2 μg/mL Leupeptin, and 100 μg/mL 4-(2-Aminoethyl)benzenesulfonyl fluoride hydrochloride [AEBSF]). Cells were allowed to sit on ice for 30 minutes, and extracts were collected as supernatants after centrifugation. Protein concentrations were determined according to the Bradford method, using a Bio-Rad protein assay kit.
Unless otherwise indicated, 50 μg of each protein extract was electrophoresed on 7.5% to 12.5% SDS-polyacrylamide gels (SDS-PAGE), and transferred to polyvinylidene diflouride (Millipore, Bedford, MA) membranes using 3-(cyclohexylamino)-l-propanesulfonic acid (CAPS) (Sigma) transfer buffer for 30 minutes at 65 V.
For Western blot analysis, membranes were blocked with Blotto A solution (5% nonfat dry milk in 1 × PBS and 0.05% Tween-20) for 1 hour at room temperature, probed with primary antibodies overnight at 4°C, and finally incubated with secondary antibody antirabbit or antigoat immunoglobulin horseradish peroxidase-linked whole antibody (1:5000 dilution; Amersham Life Science, Cleveland, OH) for 2 hours at room temperature. The antibodies were detected by using enhanced chemiluminescence reagents (Amersham). Rabbit polyclonal NF-YA and NF-YB antibodies were obtained from Rockland (Gilbertsville, PA) and used at 1:1000 and 1:500 dilution, respectively. Goat polyclonal NF-YC antibody, obtained from Santa Cruz, was used at a 1:1000 dilution.
Results
CCAAT box is the critical component of the −65/−52 IL-6RE
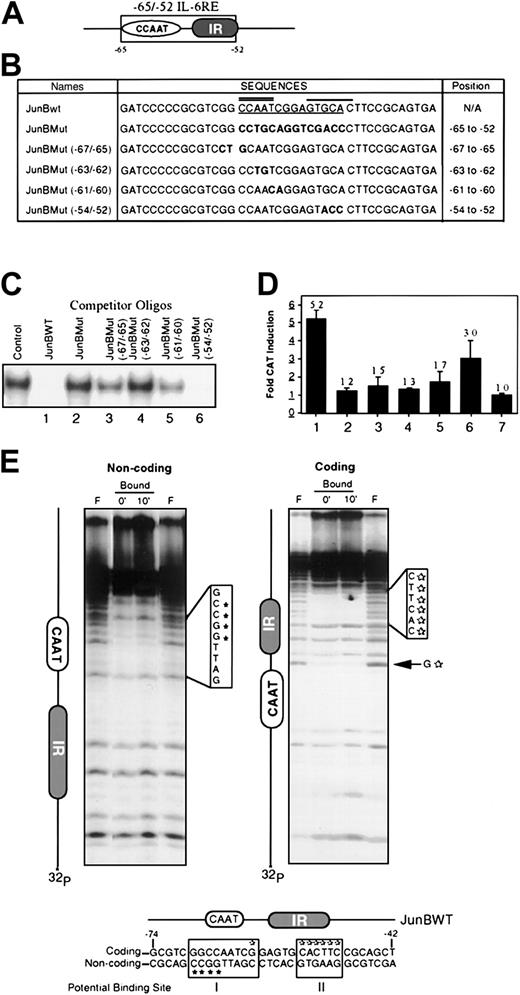
Using JunB as a representative MyD gene induced by IL-6, we identified and characterized the −65/−52 IL-6RE in its promoter region.1 This site contains both a CCAAT box and an IR repeat region (Figure 1A). Previously, we have shown that a specific protein complex is bound to the −65/−52 IL-6RE.1 To more precisely define the binding site of the −65/−52 IL-6RE to the nuclear protein complex, mutant oligonucleotides were used in gel retardation experiments, each having 2 or 3 bases of the wild-type −65/−52 IL-6RE (JunBWT) sequence mutated (Figure 1B).
The CCAAT box is the critical component of the −65/−52 IL-6RE of the JunB promoter.
(A) The IL-6 responsive element of the JunB promoter was localized between −65 and −52 and termed the −65/−52 IL-6RE.1This element contains 2 motifs, a CCAAT box and an IR repeat. (B) Sequences of wild type and mutant oligonucleotides that were used in EMSA experiments and in the minimal promoter constructs. Sequences of mutant oligos were derived from the JunBMut fragment. Only the coding strand is indicated. Underlined sequence indicates the −65/−52 IL-6RE. Bold sequences indicate the mutated sequences. Double line indicates the CCAAT box and the thick solid line shows the IR region. Indicated position of the mutated sequences is relative to the transcription start site. (C) Binding analysis by EMSA of mutant oligonucleotides. Cold competition assays using nuclear extract from IL-6–stimulated (15 minutes) M1 cells and JunBWT as probe, with excess unlabeled competitor oligos (100-fold) added 5 minutes prior to the addition of probe. Control lane contains no competitor oligos. (D) In vivo CAT induction by IL-6 when mutant oligonucleotides are cloned into promoter region of JunB minimal promoter expression vector. Mutant oligonucleotides were cloned upstream from the −31GD minimal JunB promoter and constructs were transiently transfected into M1 cells, either untreated or treated with IL-6 (100 ng/mL). CAT activity was ascertained, as previously described.1 The sample numbers in D correspond to the sample numbers in C, with regard to the oligos used. Lane 7 indicates transfection with pCAT-Basic vector. Values are averages of 3 independent experiments, and SDs are indicated. (E) Methyl interference assay on the −65/−52 IL-6 response element to identify protein binding sites. Noncoding and coding strands were 5′ end labeled and the probe was methylated. Binding reactions were done with 50 000 cpm methylated probe and 20 μg nuclear extracts from cells treated with IL-6 for the indicated times. Resulting protein-DNA complexes were resolved on 5% nondenaturing polyacrylamide gel. After autoradiography, free and bound probes were excised and eluted from the gel. Eluted DNA was digested with piperidine for 30 minutes at 90°C and resolved on 12% DNA sequencing PAGE. F, Free Probe. Two potential binding sites were detected (indicated by open boxes). Area I covers the GGCCAATCG sequence and area II the CACTTC sequence. Schematic diagram of the JunBWT probe that was used is indicated. *Indicates G residues that are protected from cleavage based on labeling of the noncoding strand, and open stars, of the coding strand.
The CCAAT box is the critical component of the −65/−52 IL-6RE of the JunB promoter.
(A) The IL-6 responsive element of the JunB promoter was localized between −65 and −52 and termed the −65/−52 IL-6RE.1This element contains 2 motifs, a CCAAT box and an IR repeat. (B) Sequences of wild type and mutant oligonucleotides that were used in EMSA experiments and in the minimal promoter constructs. Sequences of mutant oligos were derived from the JunBMut fragment. Only the coding strand is indicated. Underlined sequence indicates the −65/−52 IL-6RE. Bold sequences indicate the mutated sequences. Double line indicates the CCAAT box and the thick solid line shows the IR region. Indicated position of the mutated sequences is relative to the transcription start site. (C) Binding analysis by EMSA of mutant oligonucleotides. Cold competition assays using nuclear extract from IL-6–stimulated (15 minutes) M1 cells and JunBWT as probe, with excess unlabeled competitor oligos (100-fold) added 5 minutes prior to the addition of probe. Control lane contains no competitor oligos. (D) In vivo CAT induction by IL-6 when mutant oligonucleotides are cloned into promoter region of JunB minimal promoter expression vector. Mutant oligonucleotides were cloned upstream from the −31GD minimal JunB promoter and constructs were transiently transfected into M1 cells, either untreated or treated with IL-6 (100 ng/mL). CAT activity was ascertained, as previously described.1 The sample numbers in D correspond to the sample numbers in C, with regard to the oligos used. Lane 7 indicates transfection with pCAT-Basic vector. Values are averages of 3 independent experiments, and SDs are indicated. (E) Methyl interference assay on the −65/−52 IL-6 response element to identify protein binding sites. Noncoding and coding strands were 5′ end labeled and the probe was methylated. Binding reactions were done with 50 000 cpm methylated probe and 20 μg nuclear extracts from cells treated with IL-6 for the indicated times. Resulting protein-DNA complexes were resolved on 5% nondenaturing polyacrylamide gel. After autoradiography, free and bound probes were excised and eluted from the gel. Eluted DNA was digested with piperidine for 30 minutes at 90°C and resolved on 12% DNA sequencing PAGE. F, Free Probe. Two potential binding sites were detected (indicated by open boxes). Area I covers the GGCCAATCG sequence and area II the CACTTC sequence. Schematic diagram of the JunBWT probe that was used is indicated. *Indicates G residues that are protected from cleavage based on labeling of the noncoding strand, and open stars, of the coding strand.
JunBMut (−67/−65), JunBMut (−63/−62), and JunBMut (−61/−60) mutant sequences, located between −67 and −60, overlap the CCAAT box. The JunBMut (−54/−52) mutant sequence, between −54 to −52, is within the IR site (Figure 1B), and the JunBMut mutant sequence spans the entire −65/−52 element. These mutant oligonucleotides were used in cold competition EMSA experiments, in which labeled JunBWT oligonucleotide was the probe (Figure 1C). As expected, excess unlabeled JunBWT probe completely competed away the binding (Figure 1C, lane 1), whereas excess unlabeled JunBMut probe did not (Figure 1C, lane 2). Excess unlabeled JunBMut (−67/−65) and JunBMut (−61/−60) oligonucleotides competed for more than 50% of the bound protein (Figure 1C, lanes 3 and 5), whereas excess unlabeled JunBMut (−63/−62), where the AA nucleotides of the CCAAT sequence were mutated, did not disrupt the complex at all (Figure 1C, lane 4). In contrast, excess unlabeled JunBMut (−54/−52) competed for most of the bound protein (Figure 1C, lane 6). Oligonucleotides with mutated sequences between −59 and −54 also were used, and similar results were obtained as with JunBMut (−54/−52) oligonucleotides (data not shown). These data are consistent with an intact CCAAT box being necessary for efficient protein binding to the −65/−52 IL-6RE; mutating the AA within the CCAAT box eliminates any detectable protein binding.
Further evidence that the CCAAT box, and not the IR site, is important for binding of the protein complex to the −65/−52 IL-6RE, comes from methyl interference assays. By using labeled JunBWT probe in methyl interference assays, the site protected from cleavage encompassed the CCAAT box and sequences flanking this region but not the IR site (Figure 1E). Furthermore, we have previously shown that mutating the IR site has no effect on IL-6 inducibility.1
It was also of interest to examine whether the sequences that are important for protein binding in vitro are also important for the IL-6–mediated induction of JunB in vivo. To do this, the mutant oligonucleotides were subcloned upstream from the JunB minimal promoter in the JunB minimal promoter expression vector, which is unresponsive to IL-6.1 These constructs were transiently transfected into M1 cells and assayed for IL-6 responsiveness (Figure 1D). JunBMut (−67/−65), JunBMut (−63/−62), and JunBMut (−61/−60) exhibited only a marginal increase in CAT activity (Figure 1D, lanes 3-5), whereas JunBMut (−54/−52) was induced 3-fold by IL-6 (Figure 1D, lane 6). Although JunBMut (−54/−52) was able to compete away the complex as effectively as JunBWT (Figure 1C, compare lanes 1 with 6), only 60% of IL-6 inducibility was recovered, suggesting that sequences flanking the CCAAT box may be important for stabilization of the protein-DNA complex. Because the in vivo CAT activity studies correlate with the in vitro binding experiments, it can be concluded that the CCAAT box is important not only for the binding of the protein complex but also for IL-6 inducibility of JunB.
NF-Y is the protein complex bound to the CCAAT box of the −65/−52 IL-6RE
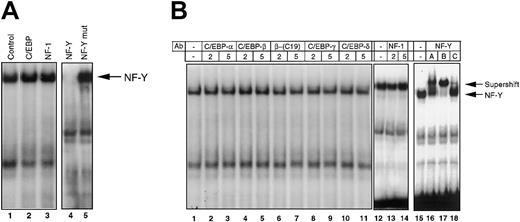
There are several transcription factors that interact with CCAAT-related sequences, including C/EBP, nuclear factor-1 (NF-1/CCAAT box transcription factor [CTF]), and NF-Y. To identify the protein that binds to the CCAAT box in the −65/−52 IL-6RE, competition experiments were performed by using JunBWT-labeled probe and cold competitor oligonucleotides containing cognate sequences specific for binding to C/EBP family proteins, NF-1/CTF, and NF-Y (Figure 2A). Excess unlabeled C/EBP and NF-1/CTF oligonucleotides did not compete with the probe for complex formation (Figure 2A, lanes 2 and 3). However, the NF-Y consensus sequence successfully competed with the labeled probe for protein binding, whereas a NF-Y mutant oligonucleotide did not (Figure 2A, lanes 4 and 5). This result indicates that NF-Y binds to the −65/−52 IL-6RE.
Identification of NF-Y as the transcription factor that binds to the −65/−52 IL-6RE of the JunB promoter.
M1 cells stimulated with IL-6 (100 ng/mL) for 15 minutes were used to prepare nuclear extracts. Protein-DNA complexes were analyzed by EMSA, as described in “Materials and methods.” (A) Oligonucleotide competition. The 100-fold excess unlabeled oligonucleotides were added to nuclear extracts 5 minutes before the addition of labeled JunBWT probe. Control sample had no oligonucleotide competitor. Arrow indicates the NF-Y-DNA complex. (B) Antibody competition. Antibodies (2-5 μg), unless otherwise indicated, were incubated with nuclear extract at RT for 30 minutes before addition of labeled JunBWT probe. Two different antibodies for C/EBP-b were used (lanes 4-7). NF-YA, NF-YB, and NF-YC antibodies (1 μg) were incubated with extract on ice for 2 hours before the addition of labeled probe. All antibodies were obtained from Santa Cruz.
Identification of NF-Y as the transcription factor that binds to the −65/−52 IL-6RE of the JunB promoter.
M1 cells stimulated with IL-6 (100 ng/mL) for 15 minutes were used to prepare nuclear extracts. Protein-DNA complexes were analyzed by EMSA, as described in “Materials and methods.” (A) Oligonucleotide competition. The 100-fold excess unlabeled oligonucleotides were added to nuclear extracts 5 minutes before the addition of labeled JunBWT probe. Control sample had no oligonucleotide competitor. Arrow indicates the NF-Y-DNA complex. (B) Antibody competition. Antibodies (2-5 μg), unless otherwise indicated, were incubated with nuclear extract at RT for 30 minutes before addition of labeled JunBWT probe. Two different antibodies for C/EBP-b were used (lanes 4-7). NF-YA, NF-YB, and NF-YC antibodies (1 μg) were incubated with extract on ice for 2 hours before the addition of labeled probe. All antibodies were obtained from Santa Cruz.
Direct evidence that NF-Y is bound to the CCAAT box comes from antibody competition experiments, in which antibodies specific against known CCAAT box binding proteins were used. NF-Y is a heterotrimeric factor composed of 3 subunits, NF-YA (40-43 kd), NF-YB (32 kd), and NF-YC (30 kd). As shown in Figure 2B, antibodies specific against each of these subunits caused a super shift of the complex (lanes 16-18), whereas antibodies against C/EBP family proteins and NF-1 had no effect (lanes 2-14). This finding clearly indicates that NF-Y is bound to the CCAAT box within the −65/−52 IL-6RE of the JunB promoter. These data are consistent with our previously reported observation that a 100-kDa protein complex is bound to the −65/−52 IL-6RE.1
Because an intact CCAAT box is necessary for both protein binding to the −65/−52 IL-6RE of the JunB promoter and IL-6 inducibility of JunB and the protein that binds to the −65/−52 IL-6RE is NF-Y, it is highly probable that NF-Y participates in IL-6 signaling that results in induction of JunB transcription.
NF-Y proteins are constitutively expressed in M1 cells
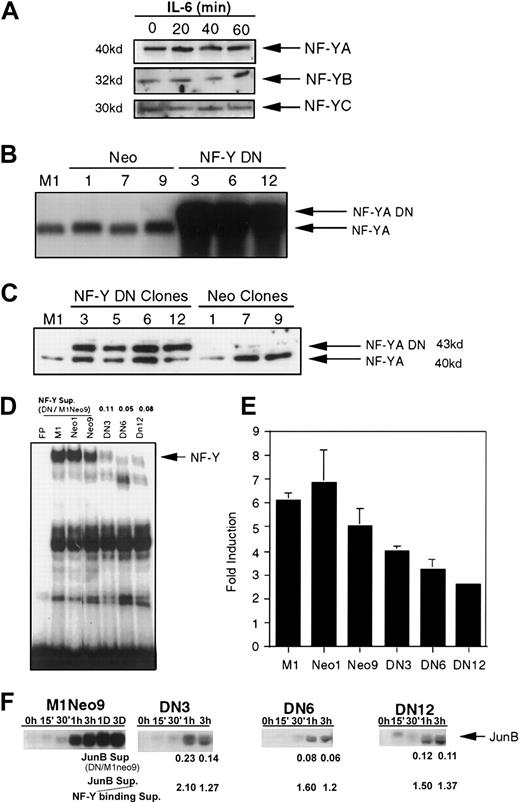
Previously, we have shown that there is no IL-6–inducible binding to the −65/−52 IL-6RE.1 Because it has been demonstrated that NF-Y is a component of the −65/−52 IL-6RE protein-DNA complex, Western analysis was performed to examine NF-Y expression during M1 myeloid differentiation (Figure3A). NF-YA mRNA is differentially spliced to produce 2 isoforms, a 40-kDa and 43-kDa protein. Only the 40-kDa isoform was detected, both prior to and following IL-6–induced M1 differentiation. In addition, both NF-YB and NF-YC expression, like NF-YA, is not regulated by IL-6. Therefore, NF-YA, NF-YB, and NF-YC proteins are constitutively expressed in M1 cells. Possibilities for transcriptional activation of the −65/−52 IL-6RE/NF-Y complex include posttranslational activation of NF-Y by IL-6 and/or interaction with and modification of other proteins.
NF-Y participates in induction of JunB following IL-6 stimulation.
(A) Protein expression of NF-Y subunits. Extracts were derived from IL-6–treated M1 cells for the indicated times. Protein extracts (50 μg) were resolved on SDS-PAGE, using 10% acrylamide for NF-YA and 12.5% acrylamide for NF-YB and NF-YC expression. Western blots were prepared as described in “Materials and methods” and probed with antibodies. Antibodies against both NF-YA (1:1000) and NF-YB (1:500) were rabbit polyclonal and obtained from Rockland. NF-YC antibodies (1:1000) were goat polyclonal and obtained from Santa Cruz. (B,C) Establishment of M1 NF-YA DN cell lines. (B) RNA was extracted from several neo-resistance clones and analyzed by Northern blots, probing with NF-YA DN cDNA fragment. (C) Expression of NF-YA DN protein. TheNF-YA DN transgene encodes for the 43-kDa isoform. Westerns were performed as described earlier, probing with NF-YA antibodies (1:1000). (D) Expression of NF-YA DN inhibits NF-Y binding to −65/−52 IL-6RE DNA. EMSA experiments were performed with labeled JunBWT probe (10 000 cpm) and nuclear extracts (5 μg) from untreated cells. The cells used for the extracts are indicated above each lane. NF-Y binding was quantitated by using a Fuji BAS 2000 phosphoimage analyzer and software and expressed as a ratio in DN versus M1Neo9 cells (DN/M1Neo9). (E,F) NF-YA DN inhibits IL-6 induction of JunB. (E) XhoP2GD was transiently transfected into M1, M1Neo, and M1NF-YA DN cell lines, treated with or without IL-6 (100 ng/mL), CAT activity measured, and IL-6 induction determined. The SD of DN12 is too small to be seen. (F) JunB expression in M1Neo control and M1NF-YA DN IL-6–treated cells. RNA was extracted from the cell lines, untreated or treated with IL-6 (100 ng/mL) for the indicated time points, and Northern blots were probed with the appropriate labeled complementary DNA (cDNA) fragments. As in D, above, the level of expression was quantitated and expressed as a ratio to assess if NF-YA-DN suppresses JunB similarly to DNA binding. The suppression ratios of each were compared (JunB sup/NF-Y binding sup).
NF-Y participates in induction of JunB following IL-6 stimulation.
(A) Protein expression of NF-Y subunits. Extracts were derived from IL-6–treated M1 cells for the indicated times. Protein extracts (50 μg) were resolved on SDS-PAGE, using 10% acrylamide for NF-YA and 12.5% acrylamide for NF-YB and NF-YC expression. Western blots were prepared as described in “Materials and methods” and probed with antibodies. Antibodies against both NF-YA (1:1000) and NF-YB (1:500) were rabbit polyclonal and obtained from Rockland. NF-YC antibodies (1:1000) were goat polyclonal and obtained from Santa Cruz. (B,C) Establishment of M1 NF-YA DN cell lines. (B) RNA was extracted from several neo-resistance clones and analyzed by Northern blots, probing with NF-YA DN cDNA fragment. (C) Expression of NF-YA DN protein. TheNF-YA DN transgene encodes for the 43-kDa isoform. Westerns were performed as described earlier, probing with NF-YA antibodies (1:1000). (D) Expression of NF-YA DN inhibits NF-Y binding to −65/−52 IL-6RE DNA. EMSA experiments were performed with labeled JunBWT probe (10 000 cpm) and nuclear extracts (5 μg) from untreated cells. The cells used for the extracts are indicated above each lane. NF-Y binding was quantitated by using a Fuji BAS 2000 phosphoimage analyzer and software and expressed as a ratio in DN versus M1Neo9 cells (DN/M1Neo9). (E,F) NF-YA DN inhibits IL-6 induction of JunB. (E) XhoP2GD was transiently transfected into M1, M1Neo, and M1NF-YA DN cell lines, treated with or without IL-6 (100 ng/mL), CAT activity measured, and IL-6 induction determined. The SD of DN12 is too small to be seen. (F) JunB expression in M1Neo control and M1NF-YA DN IL-6–treated cells. RNA was extracted from the cell lines, untreated or treated with IL-6 (100 ng/mL) for the indicated time points, and Northern blots were probed with the appropriate labeled complementary DNA (cDNA) fragments. As in D, above, the level of expression was quantitated and expressed as a ratio to assess if NF-YA-DN suppresses JunB similarly to DNA binding. The suppression ratios of each were compared (JunB sup/NF-Y binding sup).
Effect of the NF-YA DN mutant protein on IL-6–mediated induction of JunB
Because NF-Y can bind to the CCAAT box in the −65/−52 IL-6RE and the integrity of the CCAAT box is necessary for IL-6 induction of JunB, it is important to know the functional significance of NF-Y in the IL-6–mediated induction of JunB. Toward this end, we used a NF-YA DN mutant, which allows heterotrimirization with NF-YB and NF-YC but prevents binding to DNA.31 By using a MSCV retroviral expression vector, M1 cell lines were established that express NF-YA DN. Several neo-resistant clones were isolated and analyzed for NF-YA DN expression and binding to the −65/−52 IL-6RE. As shown in Figure3B, the M1NF-YA DN clones expressed exogenous NF-YA DN transcripts, whereas the parental M1 cells or M1 cells infected with the MSCV EB-neo empty vector did not. The NF-YA DN transgene encodes for the 43-kDa splice variant of NF-YA, and, when expressed in M1 cells, both the larger exogenous NF-YA DN protein band and the endogenous 40-kDa NF-YA protein band were detected (Figure 3C). The difference in intensity of the protein bands was due to unequal loading of the gel.
To confirm that the NF-YA DN protein could inhibit NF-Y binding, EMSA experiments were performed with labeled JunBWT probe and extract from M1 cells expressing the NF-YA DN transgene (Figure3D). It can be seen that NF-Y binding was suppressed 10- to 20-fold in the M1NF-YA DN cell lines, indicating that NF-YA DN could inhibit NF-Y transcriptional activity.
To examine the effect of NF-YA DN protein on the IL-6 inducibility of JunB, the XhoP2GD CAT construct, which is IL-6 responsive, was transiently transfected into the different established cell lines and assayed for IL-6 induction of CAT expression (Figure 3E). By using this approach, IL-6 inducibility was inhibited by about 50% in the M1NF-YA DN cell lines, compared with the parental M1 or M1Neo control cells. Given the complexity of the transcriptional machinery, it is not surprising that there was not a linear relationship in the effect of NF-YA DN on suppression in NF-Y DNA binding and suppression of JunB induction that is driven by a limited promoter region of an exogenously transfected expression vector. Thus, to further establish the role NF-Y plays in JunB inducibility by IL6, endogenous JunB expression was examined in 3 independently isolated M1 NF-YA DN cell lines following IL-6 stimulation. As shown in Figure 3F, JunB mRNA was significantly suppressed in M1 NF-YA DN cells compared with M1Neo control cells on IL-6 stimulation. Furthermore, determining the ratio of JunB suppression to suppression of NF-Y DNA binding (Figure 3D) has indicated that suppression of JunB inducibility by NF-YA DN correlates well with suppression of NF-Y DNA binding (Figure 3F). These data are consistent with NF-Y–mediating induction of JunB gene expression by IL-6, indicating that NF-Y participates in the regulation of JunB induction by IL-6 in M1 myeloid cells.
NF-Y plays a role in the induction of MyD genes by IL-6
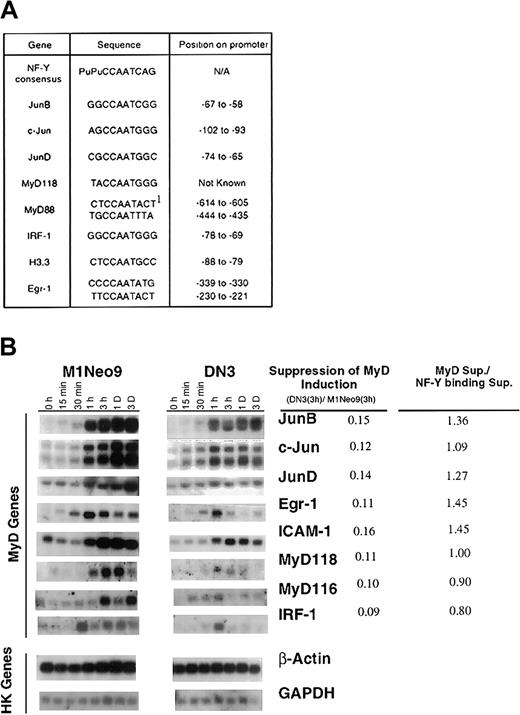
So far we provided evidence that the immediate early activation of JunB, a representative MyD gene, is regulated by a novel IL-6 response element, which binds the NF-Y transcription factor. If NF-Y plays a role in regulating the coordinate activation of MyD genes at the onset of myeloid differentiation, NF-Y binding elements should be present in the promoter regions of these genes. By using the GAP aligning program of the GCG package (Madison, WI), NF-Y binding motifs, with the essential CCAAT motif intact, were found in the promoter regions of most MyD genes (Figure 4A).
NF-YA DN reduces MyD gene induction by IL-6.
(A) NF-Y binding motifs in MyD gene promoter regions. Underlined bases indicate a match with the NF-Y consensus sequence. No match was found for MyD116 and H10. Position on the promoter is relative to the transcription start site, which has not been determined for MyD118. Pu, Purine residues. 1Indicates the inverse sequence. (B) MyD expression in M1 control and M1NF-YA DN IL-6–treated cells. RNA was extracted from the cell lines, untreated or treated with IL-6 (100 ng/mL) for the indicated time points, and Northern blots were probed with the appropriate labeled cDNA fragments. Genes were categorized into MyD genes and housekeeping (HK) genes. Similar levels of MyD gene expression were observed for 2 additional M1NF-YA DN clones. As detailed in Figure 3D, the level of MyD gene expression in M1NF-YA DN and M1Neo cells was quantitated and expressed as a ratio. To assess if NF-YA DN suppresses MyD similarly to DNA binding, the suppression ratios of each were compared (MyD sup/NF-Y binding sup).
NF-YA DN reduces MyD gene induction by IL-6.
(A) NF-Y binding motifs in MyD gene promoter regions. Underlined bases indicate a match with the NF-Y consensus sequence. No match was found for MyD116 and H10. Position on the promoter is relative to the transcription start site, which has not been determined for MyD118. Pu, Purine residues. 1Indicates the inverse sequence. (B) MyD expression in M1 control and M1NF-YA DN IL-6–treated cells. RNA was extracted from the cell lines, untreated or treated with IL-6 (100 ng/mL) for the indicated time points, and Northern blots were probed with the appropriate labeled cDNA fragments. Genes were categorized into MyD genes and housekeeping (HK) genes. Similar levels of MyD gene expression were observed for 2 additional M1NF-YA DN clones. As detailed in Figure 3D, the level of MyD gene expression in M1NF-YA DN and M1Neo cells was quantitated and expressed as a ratio. To assess if NF-YA DN suppresses MyD similarly to DNA binding, the suppression ratios of each were compared (MyD sup/NF-Y binding sup).
To ascertain if NF-Y does play a role in the coordinate transcriptional activation of MyD genes by IL-6, MyD gene expression was examined in the M1 NF-YA DN cells following IL-6 stimulation. As shown in Figure4B, all MyD genes were induced at lower levels in M1 NY-YA DN cells compared with the M1Neo control cells on IL-6 stimulation. Furthermore, it can be seen that suppression of MyD gene induction correlated with suppression of NF-Y DNA binding. These data are consistent with NF-Y–mediating induction of MyD gene expression by IL-6.
Effect of NF-YA DN on M1 myeloid differentiation
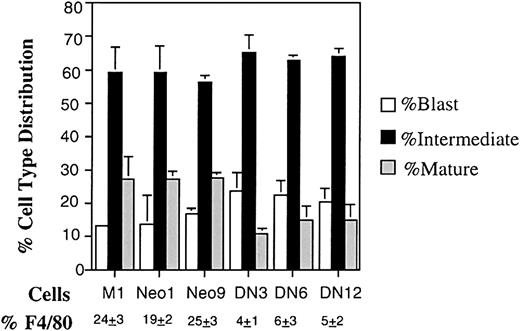
Because the MyD gene response was inhibited following IL-6 treatment of M1 NF-YA DN cells, we wanted to examine what effect inhibiting NF-Y function would have on terminal myeloid differentiation of M1 cells. NF-YA DN cells were observed to be less sensitive to IL-6 treatment at low concentrations compared with control cells, which was expected because of the reduced MyD response in these cells (data not shown). Analysis of cell morphology showed that at low concentration of IL-6 (10 ng/mL) M1NF-YA DN cells had a lower percentage of mature macrophages compared with the parental M1 and M1Neo controls (Figure5, 10%-15% compared with 27%-28%). This results in a higher percentage of cells with blastlike and intermediate-stage morphology for M1NF-YA DN cells compared with M1 and M1Neo cells. To further corroborate the results of the cytologic examination, M1Neo and M1 M1NF-YA DN cells untreated and following treatment with IL-6 (10 ng/mL) were analyzed for the expression of the macrophage-specific cell surface differentiation marker F4/80. As indicated in Figure 5, a lower percentage of M1NF-YA DN cells treated with IL-6–expressed F4/80 compared with the parental M1 and M1Neo controls. Collectively, these data indicate that NF-Y plays a role in the progression of M1 myeloid differentiation following IL-6 stimulation.
Differentiation characteristics of M1, M1Neo, and M1NF-YA DN cell in mass culture.
Cells were collected 3 days following IL-6 stimulation (10 ng/mL), and morphologic differentiation was determined by counting at least 300 cells on May-Grünwald Giemsa–stained cytospin smears and scoring the proportion of immature blast cells, cells at the intermediate monocytic differentiation, and mature macrophages as detailed in “Materials and methods.” Percentage of cells expressing the macrophages specific cell surface marker F4/80 was determined by FACS analysis as detailed in “Materials and methods.” Values are averages of 3 independent experiments, and SDs are indicated. The SD of M1 blast cells is too small to be seen.
Differentiation characteristics of M1, M1Neo, and M1NF-YA DN cell in mass culture.
Cells were collected 3 days following IL-6 stimulation (10 ng/mL), and morphologic differentiation was determined by counting at least 300 cells on May-Grünwald Giemsa–stained cytospin smears and scoring the proportion of immature blast cells, cells at the intermediate monocytic differentiation, and mature macrophages as detailed in “Materials and methods.” Percentage of cells expressing the macrophages specific cell surface marker F4/80 was determined by FACS analysis as detailed in “Materials and methods.” Values are averages of 3 independent experiments, and SDs are indicated. The SD of M1 blast cells is too small to be seen.
NF-Y expression during myeloid differentiation of normal cells from BM
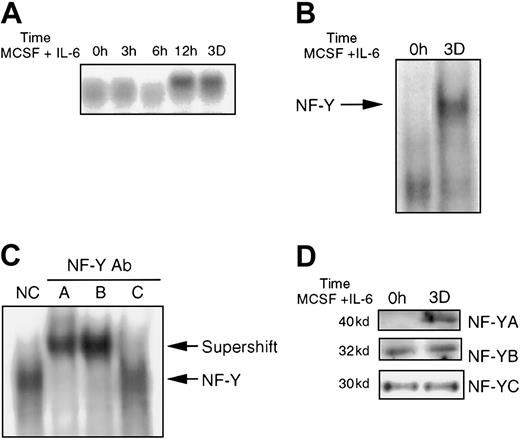
Because all the previously described data were obtained using the M1 myeloid leukemia cell line, it was necessary to ascertain if the same holds true in normal BM cells induced for macrophage differentiation. BM cells were treated with MCSF and IL-6, which results in terminal macrophage differentiation (data not shown). RNA was extracted from these cells and analyzed for JunB expression (Figure6A). JunB induction was detected by 12 hours following MCSF/IL-6 treatment and continued to be expressed up to 3 days. This induction is different from IL-6–induced M1 differentiation in which JunB expression reaches peak levels after only 1 hour.28 29 EMSA analysis, using labeled JunBWT probe with extracts from BM, either untreated or treated with MSCF/IL-6, showed induction of the protein-DNA complex (Figure 6B). Similar results were obtained when the BM cells were treated with MCSF alone (data not shown). To verify that NF-Y is a component of the protein-DNA complex, antibody competition experiments were performed, using extracts obtained from BM treated with MCSF/IL-6 for 3 days. As shown in Figure 6C, antibodies specific against all 3 NF-Y subunits disrupted the complex, indicating that NF-Y is indeed part of the induced protein-DNA complex. To determine if NF-Y protein expression is regulated, protein expression was analyzed using Western blots. NF-YA was induced following MCSF/IL-6 treatment, whereas NF-YB and NF-YC expression remained unchanged (Figure 6D). The regulation of NF-YA expression may account for the observed MCSF/IL-6–induced NF-Y binding to −65/−53 IL-6RE during BM differentiation; however, it is possible that posttranslational modifications are also required for the induced binding.
NF-Y expression in BM cells treated with MCSF plus IL-6.
(A) JunB expression in BM following stimulation with MCSF (10%) plus IL-6 (100 ng/mL), for indicated times. Preparation of RNA and Northern blot analysis was performed as mentioned earlier. (B) Nuclear extracts (5 μg) from untreated and treated (MCSF plus IL-6 for 3 days) BM were incubated with JunBWT probe (10 000 cpm). EMSA was performed as indicated in “Materials and methods.” (C) EMSA analysis was done as in B, except extracts from treated cells were incubated with antibody, as indicated for each sample, prior to addition of probe, as in Figure2. (D) NF-Y protein expression in BM. Western blot analysis and probing with antibodies were performed as previously described.
NF-Y expression in BM cells treated with MCSF plus IL-6.
(A) JunB expression in BM following stimulation with MCSF (10%) plus IL-6 (100 ng/mL), for indicated times. Preparation of RNA and Northern blot analysis was performed as mentioned earlier. (B) Nuclear extracts (5 μg) from untreated and treated (MCSF plus IL-6 for 3 days) BM were incubated with JunBWT probe (10 000 cpm). EMSA was performed as indicated in “Materials and methods.” (C) EMSA analysis was done as in B, except extracts from treated cells were incubated with antibody, as indicated for each sample, prior to addition of probe, as in Figure2. (D) NF-Y protein expression in BM. Western blot analysis and probing with antibodies were performed as previously described.
Effect of NF-YA DN expression on myeloid differentiation of BM cells
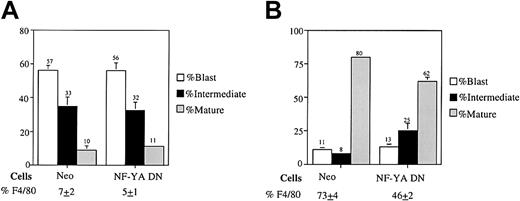
Because NF-Y is a positive modulator of M1 myeloid cell differentiation and is induced during myeloid differentiation of BM, it was asked if NF-Y plays a role in normal hematopoietic cell development. To do this, we used high-efficiency retroviral transduction to infect normal BM cells with a retroviral expression vector expressing the NF-YA DN transgene. Retroviral particles were generated by transfecting the pMSCV retroviral vectors (MSCV-neo and MSCV-NF-YA29) into the high-efficiency Bosc23 packaging cell line. Normal BM cells were then infected with the resulting retroviral particles, seeded in methylcellulose supplemented with G418, and treated with either IL-3 or MCSF plus IL-6. After 8 days, the neo-resistant colonies were counted. As shown in Table1, no difference in colony numbers was observed for Neo-infected and NF-YA DN–infected BM cells when the cells were treated with IL-3 alone, which promotes extensive proliferation as well as differentiation. However, when the cells were induced for macrophage differentiation with MCSF plus IL-6, more colonies were observed in the NF-YA DN–infected BM than the Neo-infected controls (Table 1). Inhibiting NF-Y function reduces terminal differentiation; the inhibition of differentiation would account for the higher colony number observed for MCSF plus IL-6–treated BM expressing the NF-YA DN transgene (Table 1). To further corroborate this notion, analysis of cell morphology was carried out in which the neo-resistant colonies were removed by pipet from the methylcellulose and were used to prepare cytospin smears. As shown in Figure 7A, there was no difference in cell morphology for Neo–infected BM and NF-YA DN–infected BM, when the cells were treated with IL-3 alone. However, when treated with MCSF plus IL-6, NF-YA DN–infected BM had fewer mature cells, with a concomitant increase in intermediate differentiated cells compared with Neo–infected BM (Figure 7B). Similar results were obtained when the cells were treated with MCSF alone (data not shown). Analysis for expression of the macrophage specific cell surface marker F4/80 correlated with the data obtained by the morphologic analysis (Figure 7A,B) Taken together, this data indicate that, like in M1 cells, NF-Y plays a role in modulating normal myeloid differentiation.
Colony formation efficiency
| BM infection . | IL-3 . | MCSF + IL-6 . |
|---|---|---|
| Neo | 143.3 ± 8.9 | 35.0 ± 2.9 |
| NF-YA DN | 142.7 ± 9.1 | 52.7 ± 4.4 |
| BM infection . | IL-3 . | MCSF + IL-6 . |
|---|---|---|
| Neo | 143.3 ± 8.9 | 35.0 ± 2.9 |
| NF-YA DN | 142.7 ± 9.1 | 52.7 ± 4.4 |
Bone marrow (BM) cells were infected with Neo or dominant negative nuclear factor YA (NF-YA DN) retroviral particles, seeded in 1 mL methylcellulose supplemented with G418 (650 μg/mL), and treated with either interleukin 3 (IL-3; 10%) alone or macrophage colony-stimulating factor (MCSF) plus IL-6 (100 ng/mL). After 8 days G418-resistant colonies were counted. Data are the average of 3 independent experiments, with SE indicated. According to the Studentt test, the observed difference in the means of Neo–infected BM and NF-YA DN–infected BM treated with MCSF plus IL-6 is significant with more than 95% confidence.
Cell type distribution of Neo and NF-YA DN–infected BM cells treated with (A) IL-3 alone or (B) MCSF plus IL-6 (100 ng/mL).
Neo-resistant colonies were removed by pipet from the methylcellulose and used for cytospin smears. Morphologic differentiation was determined by counting at least 300 cells on May-Grünwald Giemsa–stained cytospin smears and scoring the proportion of immature blast cells, intermediate monocytes (A,B), intermediate granulocytes (A), mature granulocytes (A), and mature macrophages (A,B) determined as detailed in “Materials and methods.” Percentage of cells expressing the macrophages specific cell surface marker F4/80 was determined by FACS analysis as described in “Materials and methods.” Values are averages of 3 independent experiments, and SDs are indicated. The SDs of NF-YA DN–infected BM treated with IL-3 for mature cells, and Neo–infected BM treated with MCSF plus IL-6 for both mature and intermediate cells are too small to be seen.
Cell type distribution of Neo and NF-YA DN–infected BM cells treated with (A) IL-3 alone or (B) MCSF plus IL-6 (100 ng/mL).
Neo-resistant colonies were removed by pipet from the methylcellulose and used for cytospin smears. Morphologic differentiation was determined by counting at least 300 cells on May-Grünwald Giemsa–stained cytospin smears and scoring the proportion of immature blast cells, intermediate monocytes (A,B), intermediate granulocytes (A), mature granulocytes (A), and mature macrophages (A,B) determined as detailed in “Materials and methods.” Percentage of cells expressing the macrophages specific cell surface marker F4/80 was determined by FACS analysis as described in “Materials and methods.” Values are averages of 3 independent experiments, and SDs are indicated. The SDs of NF-YA DN–infected BM treated with IL-3 for mature cells, and Neo–infected BM treated with MCSF plus IL-6 for both mature and intermediate cells are too small to be seen.
Discussion
At the onset of M1 differentiation, a set of MyD primary response genes is induced. JunB was chosen as a paradigm to dissect the molecular mechanisms of the IL-6–mediated induction of MyD response genes. We have identified a novel IL-6 responsive element on the JunB promoter, termed −65/−52 IL-6RE, which was demonstrated to be important for IL-6 responsiveness in M1 myeloid cells but not in HepG2 liver cells,1 suggesting that the −65/−52 IL-6RE of the JunB promoter may be regulated in a tissue-specific manner by IL-6. The −65/−52 IL-6RE contains 2 sequence motifs, a CCAAT box located between sequences −65 and −61 and an IR element. The IR element was originally identified as an element that could confer phorbol ester (TPA) and protein kinase A (PKA) responsiveness in mouse hepatoma BW1 cells. It is unlikely that the IR element plays a role in the IL-6 inducibility of JunB in M1 cells because (1) in M1 cells the IL-6 signaling cascade is distinct from TPA and PKA signaling pathways, and no JunB induction was observed following TPA and PKA treatment of M1 cells4; (2) mutagenesis analysis of oligonucleotides used in in vitro and in vivo assays showed that mutating these sequences had no effect on protein binding to the −65/−52 IL-6RE (Figure 1C), and 60% of IL-6 response was maintained (Figure 1D); and (3) methyl interference assays showed that only the CCAAT box was protected from cleavage (Figure 1E). Collectively, these data indicate that the CCAAT box is important for protein-DNA interaction and for induction of JunB by IL-6. Interestingly, recently, while this work was in progress, evidence has been obtained that JunB induction in keratinocytes by okadaic acid also involves NF-Y.32
By using antibodies against known CCAAT box binding factors in protein-DNA binding experiments, it was shown that NF-Y binds specifically to the −65/−52 IL-6RE. This observation, taken together with the demonstrated importance of an intact CCAAT box for both protein-DNA binding and induction of JunB by IL-6, is the first incidence in which NF-Y was identified as a target for IL-6 signaling. Although it has been reported that NF-Y and Sp-1 interactions are needed for transcriptional activation,16 33-37 we have observed that Sp-1 (and Egr-1) is not part of the NF-Y complex that is required for IL-6 induction of JunB (data not shown). However, it cannot be ruled out that Sp-1 does play a role in IL-6 inducibility in the context of the full JunB promoter. Moreover, our results seem to indicate that Sp-1 may regulate JunB basal expression, rather than IL-6–mediated induction of JunB (data not shown).
Direct evidence for NF-Y involvement in JunB induction comes from experiments using the NF-YA DN transgene, which inhibits NF-Y interaction with DNA. It has been shown that inhibition of NF-Y leads to a decrease in JunB induction. Furthermore, the observation that NF-Y binding motifs are present in the promoter regions of other MyD genes and that their induction is also regulated by NF-Y suggests that NF-Y plays a role in the regulation of coordinate induction of MyD genes by IL-6. NF-Y has been shown to be involved in regulating the expression of other myeloid genes, including myeloperoxidase in response to granulocyte colony-stimulating factor,38 and the ferritin heavy chain39 and gpc91-phox40 on spontaneous differentiation of peripheral blood monocytes to macrophages in vitro. Blocking the IL-6–mediated MyD response by expression of the NF-YA DN transgene leads to a decrease in mature macrophages, verifying the role of NF-Y in terminal myeloid differentiation of M1 cells.
Unlike in M1 cells, inducible NF-Y binding was detected in BM cells on myeloid differentiation. When BM cells are stimulated with MCSF plus IL-6, the cells first proliferate before undergoing terminal differentiation. In contrast, M1 cells proliferate autonomously and, following stimulation with IL-6 M1 cells, undergo rapid growth arrest and terminal differentiation. The induced expression of NF-YA and induced interaction of NF-Y with the −65/−53 IL-6RE in stimulated BM cells may reflect a molecular mechanism that couples proliferation to differentiation of normal myeloblasts. Induction of NF-YA expression has also been shown on spontaneous differentiation of peripheral blood monocytes to macrophages in vitro.7 11
BM cells expressing the NF-YA DN transgene are less sensitive to macrophage differentiation compared with control cells following stimulation with MCSF plus IL-6 (Table 1 and Figure 7B), or MCSF alone (data not shown). This finding is shown by both a higher colony formation efficiency in methylcellulose and a decrease in mature macrophages. These observations suggest that NF-Y plays a role in myeloid differentiation in normal myeloid cells as well as in M1 cells.
This is the first incidence in which NF-Y has been shown to be a target of IL-6, to play a role in the transcriptional regulation of MyD genes, and to be required for optimum myeloid differentiation. Furthermore, Western analysis (Figure 3A) revealed no detectable difference in expression of NF-Y subunits on IL-6–induced differentiation of M1 cells. Because JunB is an immediate early response gene in IL-6–stimulated M1 cells, it must be induced in the absence of de novo protein synthesis28,30; therefore, its transcription must be regulated through a posttranslational mechanism, possibly phosphorylation events. It is likely that the NF-Y–protein complex bound to the −65/−52 IL-6RE in untreated M1 cells is in an inactive state, and, following IL-6 stimulation, this complex is posttranslationally modified to turn on JunB expression. It has been reported that NF-Y can interact with Sp-1, proteins that have intrinsic histone acetyltransferase activity, and C/EBP.39 Here, we have shown that in the context of the −65/−52 IL-6RE, Sp-1 plays no role in the response to IL-6. The exact role for NF-Y in MyD gene induction, including its posttranslational modifications, association with other factors, and influence on MyD gene expression within the context of the full promoter are open questions. How NF-Y expression and activation are regulated during normal myeloid differentiation and if alterations in its expression and activity can be exploited for therapeutic or diagnostic purposes are also open for further experimentation.
Supported by core program on carcinogenesis (5P30CA12227) and partially supported with funds from Amgen (Thousand Oaks, CA) to B.H. and D.A.L.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Dan A. Liebermann or Barbara Hoffman, Fels Institute for Cancer Research and Molecular Biology, Temple University School of Medicine, 3307 N Broad St, Philadelphia, PA 19140; e-mail:lieberma@unix.temple.edu;hoffman@unix.temple.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal