Abstract
The glutathione S-transferase (GST) genes are involved in the metabolism of environmental carcinogens and of some classes of chemotherapy drugs. GSTM1 and GSTT1 genotypes are polymorphic in humans, and the phenotypic absence of enzyme activity is caused by a homozygous inherited deletion of the gene. Previous, smaller studies of childhood acute lymphoblastic leukemia (ALL) provided contrasting data on the role of the GST genotype in susceptibility and treatment outcomes. We analyzed GST genotypes in 710 children with ALL treated by the Children's Cancer Group. Frequencies were compared with those of normal controls, and outcomes were analyzed according to genotype. Comparisons of gene frequencies in ALL case and control patients showed similar frequencies (54% vs 53% GSTM1 null in whites,P = .9; 40% versus 32% in blacks,P = .45; 16% versus 15% GSTT1 null in whites,P = .8; 17% versus 28% in blacks,P = .3). ALL was not associated with the GSTM1-null genotype or the double-null genotype in blacks or whites, in contrast to previous reports. Stratification of cases by age at diagnosis, sex, white blood cell count at diagnosis, B or T lineage, or cytogenetics revealed no differences in genotype frequencies. Analysis of treatment outcomes showed no differences in outcome according to GST genotype; in particular, there were no differences in frequencies of relapse at any site. These data, representing a larger series than any reported previously, suggest that GST genotype does not affect etiology or outcome of childhood ALL.
Introduction
Conjugation of electrophilic compounds to glutathione, mediated by the family of glutathioneS-transferase (GST) enzymes, is an important detoxifying pathway for mutagens, such as organophosphates (including pesticides), alkylating agents, epoxides, and polycyclic aromatic hydrocarbons.1,2 The GSTM1 and the GSTT1 genotypes are polymorphic in humans, and the phenotypic absence of enzyme activity is caused by a homozygous inherited deletion of the gene.3,4Previous studies that examined the relationship between GST genotype and etiology and outcome of therapy for childhood acute lymphoblastic leukemia (ALL) produced somewhat contradictory results.5-7 In our study of 710 children with ALL, our goal was to provide additional and more definitive data addressing this topic by analyzing a large group of patients treated according to uniform protocols. The data do not support an important effect of GST genotype on susceptibility to childhood ALL or treatment outcome.
Patients and methods
Case and control patients
The study population included patients with 710 incident cases of childhood ALL treated on Children's Cancer Group (CCG) therapeutic studies between 1988 and 1994. Eligible patients were also participating in an epidemiologic study of ALL (CCG E15). Patients were treated on risk-adapted chemotherapy regimens (CCG protocols 1881, 1882, 1883, 1891, and 1901).8-10 In general, risk was defined by initial white blood cell (WBC) count, age, French-American-British morphology, and lymphomatous disease.11,12 Clinical data, including age, sex, immunophenotype, WBC count at diagnosis, presence of central nervous system (CNS) disease, and presence of Down syndrome, were collected prospectively. Cytogenetic data were available for 315 cases (44%). Given that the frequency of GST-null genotype varies by ethnicity and that previous reports described increases in GST double–null genotype only in black children, comparisons of genotype frequencies in case and control patients were restricted to 616 white (non-Hispanic) case patients and 35 black case patients.6 Case patients were drawn from the geographic areas served by CCG.13 Controls were 532 white (non-Hispanic) and 201 black individuals derived from normal blood donors and infant heel-stick cards. Proportions of the null genotype in the control populations were comparable to reported frequencies.14
GST genotyping
DNA for ALL cases was extracted from archival bone marrow slides or from cryopreserved marrow samples as reported previously.15 Polymerase chain reaction amplification and analysis were performed as described previously.16 Reactions were performed in duplicate. A negative control containing no DNA template was included in each experiment and showed no amplified products. DNA was extracted from control samples by means of standard techniques, and genotyping was performed as above, except for a small number of controls that were analyzed by means of the automated TaqMan allelic discrimination assay (PE Biosystems 7700; PE Applied Biosystems, Foster City, CA).17
Statistical analysis
Genotype frequencies in cases and controls were assessed with Pearson χ2 statistic or Fisher exact test. Survival estimates were based on the Kaplan-Meier method.18Differences in overall survival, disease-free survival, and event-free survival (EFS) were evaluated by means of the log-rank statistic.19 Disease-free survival was defined as the time from remission to relapse or death. EFS was defined as time from study entry to an event. Events included induction failure, leukemic relapse at any site, death in remission, and second malignant neoplasm. Patients who did not have events were censored at the time of last contact. Analyses were stratified by age (younger than 1 year, 1 to 2 years, 2 to 10 years, and older than 10 years) and by WBC count (below 10 × 109/L [<10 000/μL], 10 × 109/L to 20 × 109/L [10 000 to 20 000/μL], 20 × 109/L to 50 × 109/L [20 000 to 50 000/μL], 50 × 109/L to 100 × 109/L [50 000 to 100 000/μL], and more than 100 × 109/L [>100 000/μL]).
Results
Clinical characteristics of the study population
Clinical characteristics of the study population are shown in Table 1. Distribution of GST genotypes was examined according to sex, age at diagnosis, WBC count at diagnosis, presence of CNS disease at diagnosis, presence of testicular disease at diagnosis, B- or T-lineage disease, and cytogenetics. No differences were found in the various subgroups. However, numbers in each cytogenetic category were small. The 710 patients included in this study are a subset of the 4087 cases enrolled on CCG studies 1881, 1882, 1883, 1891, and 1891. Univariate analysis of survival in cases included and not included in the present study showed improved outcomes in cases studied (89% versus 82% surviving at 5 years). Multivariate analysis to adjust for known prognostic variables, such as age at diagnosis, WBC count at presentation, and sex, revealed that mortality was decreased for the cases included in the current cohort when compared with the rest of the cohort (relative risk = 0.8; P = .01). This indicates that these cases may be representative of a more favorable subset of cases than the group as a whole.
Demographics of patients and distribution of glutathioneS-transferase genotypes
| Characteristic . | Total patients, n = 710 (%) . | GSTM1-null patients, n = 381 (%) . | P . | GSTT1-null patients, n = 115 (%) . | P . |
|---|---|---|---|---|---|
| Male | 389 (55) | 213 (56) | 60 (52) | ||
| Female | 321 (45) | 168 (44) | .6 | 55 (48) | .6 |
| Age at diagnosis | |||||
| < 1 y | 15 (2) | 11 (3) | 4 (4) | ||
| 1-2 y | 67 (9) | 40 (11) | 14 (12) | ||
| 2-10 y | 527 (74) | 284 (75) | 84 (73) | ||
| > 10 y | 101 (14) | 46 (12) | .1 | 13 (11) | .4 |
| WBC count at diagnosis, × 109/L | |||||
| < 10 | 333 (47) | 178 (47) | 55 (48) | ||
| 10-20 | 117 (16) | 67 (18) | 23 (20) | ||
| 20-50 | 109 (15) | 57 (15) | 19 (17) | ||
| 50-100 | 64 (9) | 34 (9) | 7 (6) | ||
| > 100 | 87 (12) | 45 (12) | .9 | 11 (10) | .5 |
| Race | |||||
| White | 616 (87) | 331 (87) | 96 (84) | ||
| Black | 35 (5) | 14 (4) | 6 (5) | ||
| Hispanic | 35 (5) | 19 (5) | 9 (8) | ||
| Asian | 6 (1) | 5 (1) | 1 (1) | ||
| Other/mixed | 18 (2.5) | 12 (3) | .2 | 3 (3) | .6 |
| Lineage | |||||
| B-lineage | 463 (65) | 242 (64) | 73 (63) | ||
| T-lineage | 95 (13) | 55 (14) | 15 (13) | ||
| Null | 1 (0.3) | 1 (0.3) | 1 (1) | ||
| Mixed lineage | 64 (9) | 37 (9.7) | 10 (9) | ||
| Undefined/missing | 87 (12) | 46 (12) | .7 | 16 (14) | .6 |
| CNS disease at diagnosis | |||||
| Yes | 25 (3) | 12 (3) | 2 (2) | ||
| No | 679 (96) | 368 (96.5) | 112 (97) | ||
| Missing | 6 (1) | 1 (0.5) | .2 | 1 (1) | .5 |
| Testicular disease at diagnosis (males) | |||||
| Yes | 14 (3.5) | 8 (3.5) | 5 (8) | ||
| No | 373 (96) | 204 (96) | 55 (92) | ||
| Missing | 2 (0.5) | 1 (0.5) | .8 | 0 | .1 |
| Cytogenetics (numerical) | |||||
| Normal | 104 (14) | 48 (13) | 16 (14) | ||
| Hyperdiploidy, no. of chromosomes (47-50) | 37 (5) | 16 (4) | 5 (4) | ||
| Hyperdiploidy, no. of chromosomes (> 50) | 84 (12) | 49 (13) | 10 (9) | ||
| Pseudodiploidy | 79 (11) | 48 (13) | 11 (10) | ||
| Hypodiploidy | 11 (1.5) | 7 (2) | 0 | ||
| Missing | 395 (56) | 213 (56) | .2 | 73 (63) | .7 |
| Cytogenetics (structural) | |||||
| Normal | 104 (15) | 48 (29) | 16 (38) | ||
| Multiple abnormalities | 186 (26) | 104 (62) | 21 (50) | ||
| t(1;19) | 12 (1.5) | 6 (4) | 2 (5) | ||
| t(4;11) | 8 (1) | 6 (4) | 2 (5) | ||
| t(9;22) | 4 (0.5) | 3 (2) | 1 (2.5) | ||
| t(8;14) | 1 (0.5) | 1 (1) | .3 | 0 (0) | .7 |
| Down syndrome | |||||
| Yes | 12 (2) | 6 (2) | 3 (3) | ||
| No | 698 (98) | 375 (98) | .9 | 112 (97) | .3 |
| Characteristic . | Total patients, n = 710 (%) . | GSTM1-null patients, n = 381 (%) . | P . | GSTT1-null patients, n = 115 (%) . | P . |
|---|---|---|---|---|---|
| Male | 389 (55) | 213 (56) | 60 (52) | ||
| Female | 321 (45) | 168 (44) | .6 | 55 (48) | .6 |
| Age at diagnosis | |||||
| < 1 y | 15 (2) | 11 (3) | 4 (4) | ||
| 1-2 y | 67 (9) | 40 (11) | 14 (12) | ||
| 2-10 y | 527 (74) | 284 (75) | 84 (73) | ||
| > 10 y | 101 (14) | 46 (12) | .1 | 13 (11) | .4 |
| WBC count at diagnosis, × 109/L | |||||
| < 10 | 333 (47) | 178 (47) | 55 (48) | ||
| 10-20 | 117 (16) | 67 (18) | 23 (20) | ||
| 20-50 | 109 (15) | 57 (15) | 19 (17) | ||
| 50-100 | 64 (9) | 34 (9) | 7 (6) | ||
| > 100 | 87 (12) | 45 (12) | .9 | 11 (10) | .5 |
| Race | |||||
| White | 616 (87) | 331 (87) | 96 (84) | ||
| Black | 35 (5) | 14 (4) | 6 (5) | ||
| Hispanic | 35 (5) | 19 (5) | 9 (8) | ||
| Asian | 6 (1) | 5 (1) | 1 (1) | ||
| Other/mixed | 18 (2.5) | 12 (3) | .2 | 3 (3) | .6 |
| Lineage | |||||
| B-lineage | 463 (65) | 242 (64) | 73 (63) | ||
| T-lineage | 95 (13) | 55 (14) | 15 (13) | ||
| Null | 1 (0.3) | 1 (0.3) | 1 (1) | ||
| Mixed lineage | 64 (9) | 37 (9.7) | 10 (9) | ||
| Undefined/missing | 87 (12) | 46 (12) | .7 | 16 (14) | .6 |
| CNS disease at diagnosis | |||||
| Yes | 25 (3) | 12 (3) | 2 (2) | ||
| No | 679 (96) | 368 (96.5) | 112 (97) | ||
| Missing | 6 (1) | 1 (0.5) | .2 | 1 (1) | .5 |
| Testicular disease at diagnosis (males) | |||||
| Yes | 14 (3.5) | 8 (3.5) | 5 (8) | ||
| No | 373 (96) | 204 (96) | 55 (92) | ||
| Missing | 2 (0.5) | 1 (0.5) | .8 | 0 | .1 |
| Cytogenetics (numerical) | |||||
| Normal | 104 (14) | 48 (13) | 16 (14) | ||
| Hyperdiploidy, no. of chromosomes (47-50) | 37 (5) | 16 (4) | 5 (4) | ||
| Hyperdiploidy, no. of chromosomes (> 50) | 84 (12) | 49 (13) | 10 (9) | ||
| Pseudodiploidy | 79 (11) | 48 (13) | 11 (10) | ||
| Hypodiploidy | 11 (1.5) | 7 (2) | 0 | ||
| Missing | 395 (56) | 213 (56) | .2 | 73 (63) | .7 |
| Cytogenetics (structural) | |||||
| Normal | 104 (15) | 48 (29) | 16 (38) | ||
| Multiple abnormalities | 186 (26) | 104 (62) | 21 (50) | ||
| t(1;19) | 12 (1.5) | 6 (4) | 2 (5) | ||
| t(4;11) | 8 (1) | 6 (4) | 2 (5) | ||
| t(9;22) | 4 (0.5) | 3 (2) | 1 (2.5) | ||
| t(8;14) | 1 (0.5) | 1 (1) | .3 | 0 (0) | .7 |
| Down syndrome | |||||
| Yes | 12 (2) | 6 (2) | 3 (3) | ||
| No | 698 (98) | 375 (98) | .9 | 112 (97) | .3 |
GSTM1 indicates glutathione S-transferase μ1; GSTT1, glutathione S-transferase θ1; WBC, white blood cell; CNS, central nervous system.
GST genotypes and genetic susceptibility to ALL
Analysis of GST genotypes showed that 16% of 710 ALL patients were GSTT1 null and 54% were GSTM1 null. GST gene frequencies are known to differ in healthy black populations, so comparison with frequencies in healthy controls was performed separately for black and white case patients (Table 2). There were no differences between cases and controls in GSTM1 or GSTT1 gene frequencies. In addition, in contrast to previous reports, there were no differences between cases and controls in frequencies of the double-null genotype in blacks or whites. There was no indication in cases or controls that the double-null genotype occurred more frequently than would expected if the genotypes were transmitted independently.
Glutathione S-transferase genotypes in white and black case and control patients
| Genotype . | Whites (%) . | P . | Blacks (%) . | P . |
|---|---|---|---|---|
| GSTM1 null | 1.0 | .45 | ||
| Control | 286/532 (54%) | 64/201 (32%) | ||
| ALL | 331/616 (54%) | 14/35 (40%) | ||
| GSTT1 null | .8 | .3 | ||
| Control | 87/532 (16%) | 56/201 (28%) | ||
| ALL | 96/616 (16%) | 6/35 (17%) | ||
| GSTM1 null and GSTT1 null | 1.0 | 1.0 | ||
| Control | 53/532 (10%) | 20/201 (10%) | ||
| ALL | 60/616 (9.7%) | 3/35 (8.6%) |
| Genotype . | Whites (%) . | P . | Blacks (%) . | P . |
|---|---|---|---|---|
| GSTM1 null | 1.0 | .45 | ||
| Control | 286/532 (54%) | 64/201 (32%) | ||
| ALL | 331/616 (54%) | 14/35 (40%) | ||
| GSTT1 null | .8 | .3 | ||
| Control | 87/532 (16%) | 56/201 (28%) | ||
| ALL | 96/616 (16%) | 6/35 (17%) | ||
| GSTM1 null and GSTT1 null | 1.0 | 1.0 | ||
| Control | 53/532 (10%) | 20/201 (10%) | ||
| ALL | 60/616 (9.7%) | 3/35 (8.6%) |
ALL indicates acute lymphoblastic leukemia; see Table 1 for other abbreviations.
GST genotypes and outcome of therapy for ALL
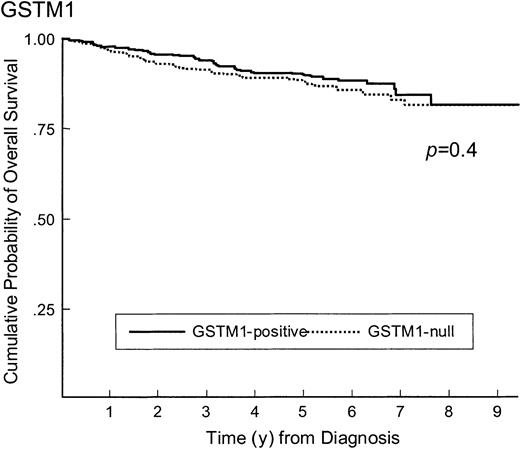
The impact of GST genotypes on the outcome of therapy was examined for the group overall, and no effect on survival, EFS, or disease-free survival was found for GSTM1, GSTT1, or the double-null genotype (Figures 1 and2 and Table3). Similar analyses, stratified by race, age at diagnosis, or WBC count at diagnosis, also showed no differences in outcomes (data not shown). There were 25 patients who had CNS disease relapses, and GSTM1 and GSTT1 genotype frequencies in those patients were similar to those with no relapses (54% versus 46% GSTM1 positive in patients with and without CNS disease, respectively,P = .6; 79% versus 84% GSTT1 positive in those with and without CNS disease relapse, respectively, P = .7). Marrow relapse occurred in 107 cases, and GSTM1 and GSTT1 genotype frequencies were not different from those with no relapses (48% versus 46% GSTM1 positive in those with and without marrow relapse, respectively,P = .6; 85% versus 84% GSTT1 positive in those with and without CNS disease relapse, respectively, P = .7).
Survival of ALL patients according to GSTM1 genotype.
Survival of ALL patients according to GSTM1-positive (n = 329) and GSTM1-null (n = 381) genotype (90% versus 88% survive at 5 years, P = .4).
Survival of ALL patients according to GSTM1 genotype.
Survival of ALL patients according to GSTM1-positive (n = 329) and GSTM1-null (n = 381) genotype (90% versus 88% survive at 5 years, P = .4).
Survival of ALL patients according to GSTT1 genotype.
Survival of ALL patients according to GSTT1-positive (n = 595 and GSTT1-null (n = 115) genotype (89% versus 91% survive at 5 years,P = .8).
Survival of ALL patients according to GSTT1 genotype.
Survival of ALL patients according to GSTT1-positive (n = 595 and GSTT1-null (n = 115) genotype (89% versus 91% survive at 5 years,P = .8).
Outcomes of treatment for acute lymphoblastic leukemia according to glutathione S-transferase genotype
| Genotype . | Overall survival, RR (P) . | Event-free survival, RR (P) . | Disease-free survival, RR (P) . |
|---|---|---|---|
| GSTT1+ | 1.0 | 1.0 | 1.0 |
| GSTT1− | 0.9 (.8) | 0.9 (.7) | 0.9 (.8) |
| GSTM1+ | 1.0 | 1.0 | 1.0 |
| GSTM1− | 1.2 (.3) | 1.0 (.9) | 0.9 (.7) |
| GSTM1+/GSTT1+ | 1.0 | 1.0 | 1.0 |
| GSTM1+/GSTT1− | 0.9 | 0.9 | 0.8 |
| GSTM1−/GSTT1+ | 1.2 | 1.0 | 0.9 |
| GSTM1−/GSTT1− | 1.1 (.8) | 0.9 (.9) | 0.9 (.9) |
| Genotype . | Overall survival, RR (P) . | Event-free survival, RR (P) . | Disease-free survival, RR (P) . |
|---|---|---|---|
| GSTT1+ | 1.0 | 1.0 | 1.0 |
| GSTT1− | 0.9 (.8) | 0.9 (.7) | 0.9 (.8) |
| GSTM1+ | 1.0 | 1.0 | 1.0 |
| GSTM1− | 1.2 (.3) | 1.0 (.9) | 0.9 (.7) |
| GSTM1+/GSTT1+ | 1.0 | 1.0 | 1.0 |
| GSTM1+/GSTT1− | 0.9 | 0.9 | 0.8 |
| GSTM1−/GSTT1+ | 1.2 | 1.0 | 0.9 |
| GSTM1−/GSTT1− | 1.1 (.8) | 0.9 (.9) | 0.9 (.9) |
RR indicates relative risk; see Table 1 for other abbreviations.
Discussion
Previous studies of GST genotype and genetic susceptibility to childhood ALL have provided somewhat contradictory results. A study of 197 children with ALL treated at St Jude Children's Research Hospital (Memphis, TN) showed similar GSTM1 and GSTT1 gene frequencies in case patients and healthy controls, in agreement with the present study.6 However, in contrast to the present study, subset analysis showed increased frequency of the double-null genotype in black children with ALL compared with normal controls (23% versus 3.9%, P = .004). The frequency of the double-null genotype was 8.6% in the current study, and was not different from the frequency in the control population (10%). Overall frequencies of the GSTM1- and GSTT1-null genotypes in the 2 studies were comparable (32% black controls were GSTM1 null in this study versus 28% GSTM1 null in the St Jude study; 28% black controls were GSTT1 null in this study versus 24% GSTT1 null in the St Jude study). However the frequency of the double-null genotype was notably lower in the black controls in the St Jude study (10% in the current study versus 3.9% in the St Jude study). In both studies, the number of black children studied was small (35 cases in the present study, 34 in the St Jude study). It is possible that a spurious result might have been generated as a result of the small sample size and/or multiple analyses. The low numbers of black children studied reflects the known reduced incidence of childhood ALL in this population, and further studies in this area will require a targeted effort to recruit adequate numbers of black case patients for study.
A Canadian study of 177 white children with ALL compared GST gene frequencies with 304 white controls.7 That study showed an increased frequency of the GSTM1-null genotype in children with ALL (odds ratio [OR], 1.8; 95% confidence interval [CI], 1.2-2.6;P = .004) and reported control frequencies similar to those in other studies. Neither our study nor the St Jude study showed a difference in frequency of the GSTM1-null genotype in children with ALL. The differences between the studies could indicate differences between populations in the influence of the GSTM1-null genotype on genetic susceptibility to leukemia or in exposures involved in leukemogenesis. However, the similar genotype frequencies reported in the 2 studies do not support major differences between the groups. It is also possible that the relatively small size of the Canadian study might have led to a chance statistical finding. A separate case-control study of 71 adult ALL cases showed no association with GSTM1 genotype, although there was an association with the GSTT1-null genotype (OR, 3.28; 95% CI, 1.31-8.26).20However, different etiologic mechanisms probably influence leukemogenesis in adults.21 Together, these data suggest that it is unlikely there is a major effect of GST genotype on susceptibility to childhood ALL.
We showed no effect of GST genotype on treatment outcome for leukemia. This is similar to the conclusion of the St Jude study of outcome in 161 cases, except for the observation of a trend toward reduction in CNS disease relapses in GSTM1-null cases in the St Jude study. In our study of 710 ALL cases, 24 CNS disease relapses occurred; the number of CNS disease relapses in the St Jude study is not reported, but is likely to be small, indicating the success of current treatment regimens in preventing relapse at this site. It is likely that neither study has sufficient power to detect or exclude a modest effect on relapse in the CNS disease.
A case-control study of 64 German children treated on the ALL–Berlin-Frankfurt-Münster (BFM)-86 and ALL–BFM-90 trials showed a significant reduction in risk of relapse in association with GSTM1- and GSTT1-null genotypes.8 Subjects were matched with control ALL patients who had not relapsed according to sex, age at diagnosis, WBC count at diagnosis, immunophenotype, trial, risk group, and treatment arm within the risk group. The use of such an extensive matching strategy led to the inclusion of a small number of cases and controls from trials that enrolled 998 and 2178 cases, respectively. Cases included in the analysis were therefore a particular subgroup of B-lineage standard- and intermediate-risk cases, and results are not likely to be generalizable to the overall ALL population. It is also possible that the influence of GST genotype is different in different therapeutic regimens. However, the CCG studies included in this analysis were designed as a modification of BFM studies, making major differences unlikely.
In summary, this study represents the largest available study of GST genotypes in children with ALL. In contrast to earlier, smaller studies, the data do not support an important role for GST genotypes in genetic susceptibility to ALL or in outcome of therapy.
The following lists the principal investigators from the Children's Cancer Group, their institutions, and the grants that supported them in this work: W. Archie Bleyer, Anita Khayat, Harland Sather, Mark Krailo, Jonathan Buckley, Daniel Stram, and Richard Sposto, Group Operations Center, grant CA13539; Raymond Hutchinson, University of Michigan Medical Center, Ann Arbor, CA02971; Katherine Mattay, University of California Medical Center, San Francisco, CA17829; Paul Gaynon, University of Wisconsin Hospital, Madison, CA05436; Ronald Chard, Children's Hospital and Medical Center, Seattle, WA, CA10382; Susan Shurin, Rainbow Babies and Children's Hospital, Cleveland, OH, CA20320; Gregory Reaman, Children's National Medical Center, Washington, DC, CA03888; Edward Baum, Children's Memorial Hospital, Chicago, IL, CA07431; Jorge Ortega, Children's Hospital of Los Angeles, CA, CA02649; Frederick Ruymann, Children's Hospital of Columbus, OH, CA03750; Sergio Piomelli, Columbia Presbyterian College of Physicians and Surgeons, New York, NY, CA03526; Joseph Mirro, Children's Hospital of Pittsburgh, PA, CA36015; John Lukens, Vanderbilt University School of Medicine, Nashville, TN, CA26270; Lawrence Wolff, Doernbecher Memorial Hospital for Children, Portland, OR, CA26044; William Woods, University of Minnesota Health Sciences Center, Minneapolis, CA07306; Thomas Williams, University of Texas Health Sciences Center, San Antonio, CA36004; Anna Meadows, Children's Hospital of Philadelphia, PA, CA11796; Peter Steinherz, Memorial Sloan-Kettering Cancer Center, New York, NY, CA42764; Philip Breitfeld, James Whitcomb Riley Hospital for Children, Indianapolis, IN, CA13809; Mark Greenberg, Hospital for Sick Children, Toronto, Ont, Canada; Richard O'Brien, University of Utah Medical Center, Salt Lake City, CA10198; Harvey Cohen, Strong Memorial Hospital, Rochester, NY, CA11174; Cristopher Fryer, University of British Columbia, Vancouver, Canada, CA29013; Robert Wells, Children's Hospital Medical Center, Cincinnati, OH, CA26126; Jerry Finklestein, Harbor/UCLA and Miller Children's Medical Center, Torrance/Long Beach, CA, CA14560; Stephen Feig, University of California Medical Center, Los Angeles, CA27678; Raymond Tannous, University of Iowa Hospitals and Clinics, Iowa City, CA29314; Lorrie Odom, Children's Hospital of Denver, CO, CA28851; Gerald Gilchrist, Mayo Clinic and Foundation, Rochester, MN, CA28882; Dorothy Barnard, Izaak Walton Killam Hospital for Children, Halifax, Nova Scotia, Canada; Joseph Wiley, University of North Carolina, Chapel Hill; Milton Donaldson, University of Medicine and Dentistry of New Jersey, Camden; Maxine Hetherington, Children's Mercy Hospital Kansas City, MO; Peter Coccia, University of Nebraska Medical Center, Omaha; Donald Norris, Cleveland Clinic Foundation, OH; F. Leonard Johnson, Wyler Children's Hospital, Chicago, IL; R. Beverly Raney, MD Anderson Cancer Center, Houston, TX; David Baker, Princess Margaret Hospital, Perth, Western Australia; Jean Sanders, Fred Hutchinson Cancer Research Center, Seattle, WA; Aaron Rausen, New York University Medical Center, NY; Mitchell Cairo, Children's Hospital of Orange County, CA.
Supported by the Division of Cancer Treatment, National Cancer Institute, National Institutes of Health, Supplement to NIH 5U10-CA 13539, US Department of Health and Human Services. Contributing Children's Cancer Group investigators, institutions, and grant numbers are provided in the .
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
S. M. Davies, Children's Oncology Group, PO Box 60012, Arcadia, CA 91066-6012; e-mail: davie008@umn.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal