Abstract
Low-toxicity conditioning regimens prior to bone marrow transplantation (BMT) are widely explored. We developed a new protocol using hematopoietic growth factors prior to low-dose total body irradiation (TBI) in recipients of autologous transplants to establish high levels of long-term donor cell engraftment. We hypothesized that treatment of recipient mice with growth factors would selectively deplete stem cells, resulting in successful long-term donor cell engraftment after transplantation. Recipient mice were treated for 1 or 7 days with growth factors (stem cell factor [SCF] plus interleukin 11 [IL-11], SCF plus Flt-3 ligand [FL], or granulocyte colony-stimulating factor [G-CSF]) prior to low-dose TBI (4 Gy). Donor cell chimerism was measured after transplantation of congenic bone marrow cells. High levels of donor cell engraftment were observed in recipients pretreated for 7 days with SCF plus IL-11 or SCF plus FL. Although 1-day pretreatments with these cytokines initially resulted in reduced donor cell engraftment, a continuous increase in time was observed, finally resulting in highly significantly increased levels of donor cell contribution. In contrast, G-CSF treatment showed no beneficial effects on long-term engraftment. In vitro stem cell assays demonstrated the effect of cytokine treatment on stem cell numbers. Donor cell engraftment and number of remaining recipient stem cells after TBI were strongly inversely correlated, except for groups treated for 1 day with SCF plus IL-11 or SCF plus FL. We conclude that long-term donor cell engraftment can be strongly augmented by treatment of recipient mice prior to low-dose TBI with hematopoietic growth factors that act on primitive cells.
Introduction
Low-toxicity conditioning regimens are increasingly applied in settings of allogeneic or autologous bone marrow transplantation (BMT) for an expanding variety of clinical applications.1-6 Especially for transplantation protocols in gene therapy, or treatment of autoimmune diseases, a nonmyeloablated bone marrow environment is desired for autologous stem cells to engraft effectively.7-10 Most clinical transplantation protocols still use high-dose conditioning regimens to prevent rejection of the donor graft and to create a microenvironment in which transplanted (stem) cells are able to migrate efficiently to the bone marrow and “home” to appropriate niches.11-14 No unequivocal vision on the mechanism of homing and engraftment exists and discussion continues on whether conditioning prior to BMT leads to creating actual “space” in the bone marrow microenvironment or whether competition is induced between remaining recipient stem cells and infused donor cells.15-19 A recent study of Ponomaryov et al20 suggests that the up-regulation of stromal derived factor-1 (SDF-1) in response to total body irradiation (TBI) is important in the homing process, underscoring the role of humoral factors produced by stromal cells. In addition, evidence has been obtained that the cell cycle status of the transplanted cells plays a significant role and can be manipulated with growth factor treatment.21-24
We hypothesize that a strong depletion of the recipients' hematopoietic system prior to BMT will lead to enhanced donor cell engraftment. Specifically, we questioned whether long-term donor cell engraftment can be affected by pretreatment of transplant recipients with various hematopoietic growth factors prior to low-dose TBI. This concept is supported by data showing that chemotherapy (5-fluorouracil) after growth factor treatment with stem cell factor (SCF) can result in severe stem cell depletion.25 26 Apparently, growth factor treatment induces stem cells to proliferate and increase their sensitivity toward myeloablative treatment. We administered growth factors affecting primitive stem cells (SCF plus interleukin-11 [IL-11] or SCF plus Flt-3 ligand [FL]) or progenitor cells (granulocyte colony-stimulating factor [G-CSF]). Our results demonstrate that long-term donor cell engraftment after low-dose TBI can be strongly augmented by pretreatment of recipients with hematopoietic growth factors acting on primitive cells.
Materials and methods
Mice
In all experiments female C57BL/6 mice (Harlan Nederland, Horst, The Netherlands) were used as recipients. Male C57BL/6.SJL.Ptprca congenic mice (kindly provided by Ms Helen Silvius, LUMC, Leiden, The Netherlands) were used as donor mice. Leukocytes from these mice express the CD45.1 antigen, whereas cells from recipients express CD45.2. Throughout this paper, we will name recipient mice CD45.2 and donors CD45.1.
Treatments
The following growth factors/growth factor combinations were administered for either 1 or 7 days: G-CSF, SCF plus IL-11, or SCF plus FL. Recombinant rat pegylated SCF and recombinant human G-CSF were donated by Amgen (Thousand Oaks, CA). Recombinant human IL-11 was a gift from Genetics Institute (Cambridge, MA); FL was provided by Immunex (Seattle, WA). Growth factors were appropriately diluted, mixed, and administered either for 1 day by 3 intraperitoneal injections at time points −24, −12, and −2 hours before TBI, or during 7 days by subcutaneously implanted osmotic minipumps (Alzet 1007D; Alza, Palo Alto, CA). SCF and G-CSF were administered at a dose of 2.5 μg per mouse per day, IL-11 at a dose of 2.0 μg per mouse per day, and FL at a dose of 5.0 μg per mouse per day.
Two hours after growth factor treatment, mice received a single dose of 4 Gy sublethal TBI at a dose rate of 0.89 Gy/min, using a137Cs γ-irradiation unit (IBL 637, CIS Biointernational, Gif-sur-Yvette Cedex, France).
Each group consisted of 9 to 11 mice; 3 were allocated for the cobblestone area–forming cell (CAFC) assay and the remaining animals were used as transplant recipients. Control mice, not treated with growth factors, were irradiated similarly.
CAFC assay
Growth factor administration in mice leads to migration of cells from bone marrow to spleen and liver.27-29 Because we wanted to assess the extent of stem cell depletion in recipients conditioned with TBI, we estimated the total number of stem cells before and after irradiation with CAFC assays on bone marrow, spleen, and liver cells, and calculated the percentage of surviving cells after irradiation. Bone marrow suspensions were obtained by flushing either 1 or 2 femurs of each mouse according to standard procedures. Spleen suspensions were acquired as described.29 Liver suspensions were obtained by dissecting and weighing the complete liver. A piece of about 10% was prepared and gently pressed through a stainless steel sieve after which liver cells were collected. Single cells were obtained by repeatedly flushing the liver cells through a 25-gauge needle. The remaining part of the liver was weighed again and the exact fraction of used liver material was calculated. Marrow, spleen, and liver cellularities were determined using a Coulter Counter (Coulter Electronics, Dunstable, United Kingdom) to calculate the appropriate cell numbers for the stem cell assays. Femur cellularity was assumed to represent 6% of total marrow cellularity.30 31 Each data point in the CAFC assay was obtained by pooling bone marrow, spleen, or liver suspensions of 3 mice. The total number of CAFCs per mouse was calculated by adding the CAFC number of bone marrow plus spleen plus liver.
Transplantation and determination of peripheral blood cell chimerism
Twenty-four hours after sublethal irradiation, female CD45.2 recipient mice received transplants of 3 × 106 freshly harvested unfractionated bone marrow cells from male CD45.1 donor mice by intravenous injection into the retro-orbital plexus. Peripheral blood cell chimerism in recipients was determined in 2 independent groups of mice (n = 3-4/group) by flow cytometry, at first every 2 weeks (up to week 12), and thereafter every 4 weeks until week 40. In short, 50 μL tail blood was drawn from each mouse and erythrocytes were eliminated by hypotonic lysis (0.16 M NH4Cl, 1.0 × 10−4 M EDTA, 0.017 M NaCl, 5 minutes at room temperature). Leukocytes were washed and stained with donor-specific anti-CD45.1 monoclonal antibody (mAb) conjugated with phycoerythrin (PE) and either host-specific anti-CD45.2 labeled with fluorescein isothiocyanate (FITC) or FITC-labeled anti–Gr-1, anti-B220, or anti-CD8a (all mAbs from Pharmingen, San Diego, CA). Stained cells were washed and resuspended in 200 μL phosphate-buffered saline plus 2% fetal calf serum. Two-color analysis (FACSCalibur; Becton Dickinson, Palo Alto, CA) was performed to determine the percentage of donor- and host-derived leukocytes or donor B and T lymphocytes and granulocytes to check for multilineage mixed chimerism.
Statistics
Unpaired Student t tests were performed to determine significant differences between engraftment levels of growth factor–treated mice and untreated controls. The 95% confidence limits (CLs) were calculated for each CAFC subset. Nonoverlapping 95% CLs were interpreted as P < .05.
Results
Stem cell numbers after growth factor administration before and after TBI
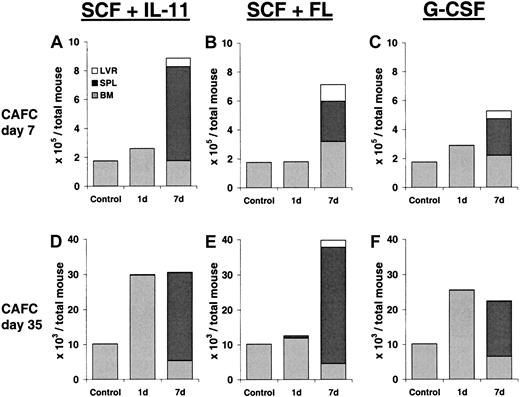
To determine the effect of growth factor treatment on stem cell pools, we performed CAFC assays on bone marrow, spleen, and liver cells of mice treated for 1 or 7 days with SCF plus IL-11, SCF plus FL, or G-CSF. Table 1 provides an overview of the relative CAFC frequencies that were measured in bone marrow, spleen, and liver cells. Figure 1demonstrates the corresponding total CAFC pool size in each organ and in the entire mouse. Figure 1A-C depicts the absolute numbers of CAFCs–day 7 (progenitor cells) per total mouse (ie, in bone marrow plus spleen plus liver). In 1-day growth factor–treated mice no significant expansion of progenitor cells was observed. Although the relative frequency of CAFCs-day 7 in mice treated with SCF plus IL-11 was higher than in control mice, the cellularity in the femur of these mice was lower (12 × 106/femur vs 22 × 106/femur). In contrast, after the 7-day growth factor treatments, progenitor cells expanded 3- to 4-fold and migrated from the bone marrow toward the liver and, in particular, the spleen. This effect was most notable with the combination of SCF plus IL-11.
Progenitor and stem cell frequency in bone marrow, spleen, and liver of growth factor–treated mice
| . | Control . | SCF + IL-11 . | SCF + FL . | G-CSF . | |||
|---|---|---|---|---|---|---|---|
| 1 d . | 7 d . | 1 d . | 7 d . | 1 d . | 7 d . | ||
| Bone marrow | |||||||
| CAFC-d 7 | 32-73 | 79-207 | 28-64 | 31-70 | 52-111 | 42-93 | 36-79 |
| CAFC-d 35 | 1.9-4.1 | 4.7-45 | 0.8-2.0 | 2.0-5.2 | 0.7-1.7 | 3.6-8.2 | 1.1-2.4 |
| Spleen | |||||||
| CAFC-d 7 | 0.4-0.9 | 0.2-0.5 | 73-370 | 0.5-1.2 | 51-109 | 0.7-1.7 | 57-121 |
| CAFC-d 35 | 0.1-0.2 | 0.1-0.3 | 6.7-14 | 0.1-0.2 | 6.1-13 | 0.1-0.2 | 6.2-13 |
| Liver | |||||||
| CAFC-d 7 | ND | ND | 46-99 | ND | 36-80 | ND | 23-48 |
| CAFC-d 35 | ND | ND | 0.1-0.3 | ND | 0.6-1.5 | ND | 0.1-0.2 |
| . | Control . | SCF + IL-11 . | SCF + FL . | G-CSF . | |||
|---|---|---|---|---|---|---|---|
| 1 d . | 7 d . | 1 d . | 7 d . | 1 d . | 7 d . | ||
| Bone marrow | |||||||
| CAFC-d 7 | 32-73 | 79-207 | 28-64 | 31-70 | 52-111 | 42-93 | 36-79 |
| CAFC-d 35 | 1.9-4.1 | 4.7-45 | 0.8-2.0 | 2.0-5.2 | 0.7-1.7 | 3.6-8.2 | 1.1-2.4 |
| Spleen | |||||||
| CAFC-d 7 | 0.4-0.9 | 0.2-0.5 | 73-370 | 0.5-1.2 | 51-109 | 0.7-1.7 | 57-121 |
| CAFC-d 35 | 0.1-0.2 | 0.1-0.3 | 6.7-14 | 0.1-0.2 | 6.1-13 | 0.1-0.2 | 6.2-13 |
| Liver | |||||||
| CAFC-d 7 | ND | ND | 46-99 | ND | 36-80 | ND | 23-48 |
| CAFC-d 35 | ND | ND | 0.1-0.3 | ND | 0.6-1.5 | ND | 0.1-0.2 |
Shown are the CAFC-day 7 and CAFC-day 35 frequency/105 nucleated cells.
Significant differences compared to control values (nonoverlapping 95% CLs) are depicted in bold.
ND indicates not detected.
Progenitor and stem cell number after growth factor treatment.
Total number of CAFCs–day 7 and CAFCs–day 35 per mouse (bone marrow plus spleen plus liver, n = 3) after treatment with SCF plus IL-11, SCF plus FL, or G-CSF for either 1 or 7 days. The numbers of CAFCs–day 7 are depicted in panels A to C, whereas panels D to F show the numbers of CAFCs– day 35.
Progenitor and stem cell number after growth factor treatment.
Total number of CAFCs–day 7 and CAFCs–day 35 per mouse (bone marrow plus spleen plus liver, n = 3) after treatment with SCF plus IL-11, SCF plus FL, or G-CSF for either 1 or 7 days. The numbers of CAFCs–day 7 are depicted in panels A to C, whereas panels D to F show the numbers of CAFCs– day 35.
Primitive stem cells (CAFCs–day 35) demonstrated a different pattern in response to growth factor treatment (Figure 1D-F). Treatment with SCF plus IL-11 or G-CSF for 1 day resulted in a 2.5- to 3-fold expansion of stem cells. Continuation of the growth factor treatment for an additional 6 days did not result in further expansion, but led to a significant shift of primitive cells toward the spleen (Table 1). In contrast, 1-day treatment with SCF plus FL did not result in an increased number of stem cells, but stem cells expanded about 4-fold after a 7-day treatment. Again, most cells resided in the spleen.
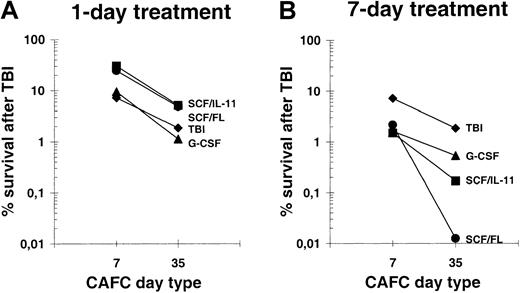
To establish the radiosensitivity of progenitors and primitive stem cells, we calculated the percentage of surviving cells 24 hours after irradiation per total mouse (Figure 2). Compared to TBI alone, 1 day of SCF plus IL-11 or SCF plus FL pretreatment resulted in a 4-fold reduced radiosensitivity of progenitor cells (CAFCs–day 7), whereas radiosensitivity of primitive stem cells (CAFCs–day 35) was reduced 2.7-fold (Figure 2A). A short G-CSF treatment did not significantly change the radiosensitivity of cells compared to controls. In contrast, after 7 days of growth factor administration, both CAFC subsets were more severely depleted than in mice conditioned with TBI alone (Figure 2B). Moreover, a 7-day growth factor treatment led to a much more pronounced difference in sensitivity between progenitors and stem cells. Whereas about 1% of progenitor cells survived in the 7-day growth factor-treated groups, almost complete depletion of primitive stem cells was achieved in SCF plus FL and to a lesser extent in mice treated with SCF plus IL-11. These findings indicate that a 7-day treatment with SCF in combination with either IL-11 or FL results in a considerably increased sensitivity of hematopoietic cells toward low-dose TBI.
Survival of CAFCs–day 7 and CAFCs–day 35 after low-dose TBI.
(A) Fraction of surviving cells 24 hours after 4 Gy irradiation in 1-day growth factor-treated mice compared to control values. (B) Fraction of surviving cells 24 hours after 4 Gy irradiation in 7-day growth factor–treated mice.
Survival of CAFCs–day 7 and CAFCs–day 35 after low-dose TBI.
(A) Fraction of surviving cells 24 hours after 4 Gy irradiation in 1-day growth factor-treated mice compared to control values. (B) Fraction of surviving cells 24 hours after 4 Gy irradiation in 7-day growth factor–treated mice.
Donor cell engraftment after BMT
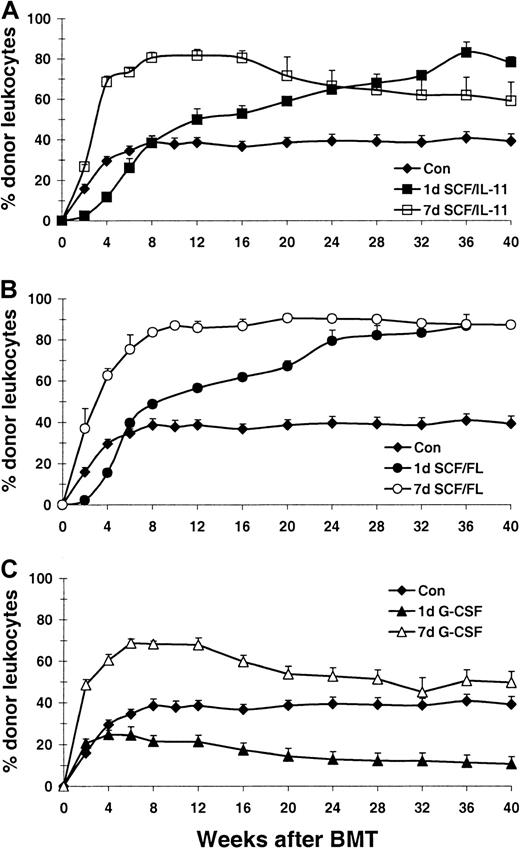
Recipient female mice received transplants of 3 × 106 unfractionated congenic bone marrow cells from male hosts after low-dose TBI. Subsequently, long-term donor cell engraftment was determined by fluorescence-activated cell-sorter (FACS) analysis of donor and recipient leukocyte contribution. Two independent experiments were performed and pooled data are shown in Figure3. The transplantation was considered successful when stable or increasing donor cell engraftment was maintained from week 12 onward (Figure 3). In 85% of control mice (17 of 20, Figure 3A-C) chimerism was achieved, reaching values of 40% ± 4.1% (SEM) donor leukocytes. Seven-day treatment with SCF plus IL-11 showed rapid donor cell reconstitution, resulting in significantly higher levels compared to control mice from week 4 onward (n = 6, P < 1 × 10−8; Figure3A). Although engraftment remained high throughout the study, donor cell contribution dropped very gradually over time. Interestingly, donor cell engraftment in 1-day SCF plus IL-11–treated mice showed a completely opposite pattern. The first month after transplantation, a significantly lower donor cell engraftment was observed (P < .02), which, however, was followed by a slow, but steady increase. Sixteen weeks after BMT, donor leukocyte contribution was significantly higher than control values (P < .05).
Donor cell engraftment in growth factor–pretreated recipients of transplants.
Percentage of donor-derived leukocytes in peripheral blood of sublethally (4 Gy) irradiated host mice after transplantation of 3.0 × 106 unfractionated donor bone marrow cells. Recipient mice were treated with growth factors for 1 day (closed symbols) or 7 days (open symbols) before irradiation. Mice treated with SCF plus IL-11, SCF plus FL, and G-CSF are depicted in panels A, B, and C, respectively. Each line represents the mean ± SEM of 2 independent experiments (n = 4 per experiment).
Donor cell engraftment in growth factor–pretreated recipients of transplants.
Percentage of donor-derived leukocytes in peripheral blood of sublethally (4 Gy) irradiated host mice after transplantation of 3.0 × 106 unfractionated donor bone marrow cells. Recipient mice were treated with growth factors for 1 day (closed symbols) or 7 days (open symbols) before irradiation. Mice treated with SCF plus IL-11, SCF plus FL, and G-CSF are depicted in panels A, B, and C, respectively. Each line represents the mean ± SEM of 2 independent experiments (n = 4 per experiment).
Figure 3B demonstrates donor cell engraftment in mice treated with SCF plus FL. Unlike 7-day, SCF-plus–IL-11–treated mice, chimerism in animals treated with SCF plus FL did not drop in the long-term. Almost full donor leukocyte chimerism (∼90%) was reached (n = 7,P < .00001). Although a significantly lower engraftment was found in the 1-day SCF-plus-FL–treated group compared to controls up to 4 weeks after transplantation (P < .01), from week 12 onward donor leukocyte contribution was significantly higher than in controls (n = 8, P < .01). Similar to the 1-day SCF-plus–IL-11 group, engraftment slowly but continuously kept increasing during the course of the study, reaching similar levels as the 7-day SCF plus FL group at week 32 (P < .0001).
It is apparent that 7-day G-CSF treatment prior to TBI had a different effect on donor cell engraftment after BMT than either SCF plus IL-11 or SCF plus FL (Figure 3C). Although increased donor cell levels (∼70%) were initially observed, (n = 8, P < .00001), subsequently, 16 weeks after BMT, engraftment gradually decreased toward control values at 24 weeks after transplantation. Mice treated for 1 day with G-CSF initially engrafted equally well as control animals, but all 8 mice eventually showed a gradual decrease in engraftment.
To ensure that a true multilineage mixed chimerism was established in all groups, we checked for donor B and T lymphocytes as well as granulocytes in all recipient mice 28 to 40 weeks after BMT, using anti-B220, anti-CD8a, and anti-Gr-1 antibodies. The contribution of total donor leukocytes, as shown in Figure 3, was proportionally reflected in all 3 lineages (data not shown).
Stem cell depletion and long-term donor cell engraftment
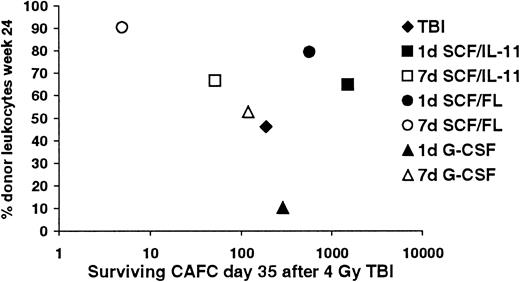
We hypothesized that for successful long-term donor cell engraftment after transplantation, depletion of recipient stem cells would be required. Thus, a relationship between the extent of depletion of host stem cells and levels of donor cell engraftment was predicted. In Figure 4 this relationship between residual recipient CAFCs–day 35 remaining after 4 Gy TBI and donor cell engraftment at 24 weeks after transplantation is shown. Evidently, in all 7-day growth factor-treated groups, the control group, and the 1-day G-CSF–treated group, a strong inverse correlation (R2 = 0.957) exists between long-term donor cell engraftment and surviving CAFCs-day 35 after TBI. Interestingly, although relatively many stem cells survived radiation after 1-day SCF plus IL-11 or 1-day SCF plus FL treatment, donor cell chimerism in these mice was very high.
Correlation between the extent of stem cell depletion and long-term donor cell engraftment.
Relationship between the absolute numbers of CAFCs–day 35 remaining in the recipient 24 hours after 4 Gy TBI and donor cell engraftment 24 weeks after transplantation.
Correlation between the extent of stem cell depletion and long-term donor cell engraftment.
Relationship between the absolute numbers of CAFCs–day 35 remaining in the recipient 24 hours after 4 Gy TBI and donor cell engraftment 24 weeks after transplantation.
Discussion
In this study, we show that growth factor treatment of recipient mice prior to low-dose TBI can modulate donor cell engraftment levels after syngeneic BMT. This modulation is dependent on the particular growth factor or growth factor combination used, but it is also highly dependent on the duration of the growth factor treatment.
Within 4 weeks after BMT donor cell engraftment in all mice pretreated for 7 days with either of the growth factor combinations was dramatically increased compared to engraftment in untreated mice. This high donor cell contribution remained stable for at least 4 months. Most pronouncedly, almost full donor chimerism (90%) was reached in the SCF plus FL group even after a 1-day treatment period. Also, after a short 1-day growth factor pretreatment with SCF plus IL-11, a highly significant increase in donor leukocyte contribution was seen, although this became apparent only several months after transplantation. These findings are in accordance with data that we have previously obtained by a 1-day treatment of recipient mice with SCF alone prior to low-dose TBI.34
Interestingly, a difference in donor cell engraftment was observed between mice treated with growth factors with an effect on primitive stem cells (SCF plus IL-11 or FL) and G-CSF, which primarily affects progenitors. Because it has been shown that G-CSF administered before chemotherapy enhanced stem cell damage,35,36 we expected that G-CSF would also sensitize the stem cell compartment toward TBI, resulting in higher donor leukocyte contribution after transplantation. However, although 1-day G-CSF–treated cells were equally sensitive toward low-dose TBI as controls, the absolute number of remaining CAFCs-day 35 after TBI was considerably higher than in controls. In agreement with our hypothesis on the correlation between percentage donor cell contribution and degree of stem cell depletion, donor cell engraftment was considerably lower in 1-day G-CSF–treated mice than in control mice and decreased gradually over time. Seven-day G-CSF treatment initially resulted in a high donor cell engraftment, but values dropped, as expected, toward controls several weeks after transplantation. Thus, the sensitizing effect of G-CSF was not observed. Mardiney and Malech37 showed that a 5-day G-CSF treatment prior to very low-dose TBI (1.6 Gy) and BMT led to significantly enhanced donor cell contribution in mice. However, they followed engraftment only up to 16 weeks after transplantation, whereas donor cell contribution in our experiment dropped toward control values only at 24 weeks after transplantation.
In the majority of our growth factor–treated groups we observed that the degree of stem cell depletion indeed is strongly correlated with the degree of donor cell engraftment, as we predicted. However, we observed an unexpectedly high donor cell chimerism in mice pretreated for 1 day with SCF plus IL-11 or SCF plus FL. This high level of engraftment cannot be explained by enhanced depletion of recipient stem cells, because many CAFCs-day 35 remained after TBI. We speculate that as yet unknown microenvironmental changes in the complex bone marrow stromal system caused by growth factor administration prior to TBI may influence engraftment.
Induction of mixed chimerism after allogeneic BMT in animals and in patients has been shown to cause increased immunocompetence, increased transplantation tolerance, reduced graft-versus-host disease, and reduced posttransplantation toxicity.2,38-40 Here, we were able to create almost full donor cell chimerism both short- and long-term after transplantation with male-into-female transplantations. It has been shown that engraftment can be slightly impaired after transplantation of male cells into mildly conditioned female recipients.41,42 In addition, it has been shown that the CD45.1 antigen, which is expressed by the congenic C57BL/6.SJL.Ptprca mice we used as donors, may be mildly immunogenic.43 Thus, our syngeneic, sex-mismatched transplantation model sets the stage for testing a similar approach in allogeneic transplantation settings. However, in these situations a growth factor pretreatment protocol may be combined with thymic irradiation or administration of antibodies causing a costimulatory blockade to obtain sufficient immune suppression.39,44 45
The authors would like to thank Geert Mesander for help with the FACS analyses and Dr Piet Wierenga for intravenous injection of bone marrow cells.
Supported by the Dutch Cancer Society, grant NKB1996-1204. G.d.H. is a fellow of the Royal Netherlands Academy of Arts and Sciences (KNAW).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gerald de Haan, Department of Stem Cell Biology, University of Groningen, A Deusinglaan 1, NL-9713 AV Groningen, The Netherlands; e-mail: g.de.haan@med.rug.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal