Abstract
Tyr phosphorylation of the multifunctional transmembrane protein band 3 has been implicated in several erythrocyte functions and disorders. We previously demonstrated that pervanadate treatment of human erythrocytes induces band-3 Tyr phosphorylation, which is catalyzed by the sequential action of tyrosine kinase Syk and tyrosine kinase(s) belonging to the Src family. In this study, we show that Tyr phosphorylation of band 3, elicited by pervanadate, N-ethylmaleimide, or diamide, greatly increases band-3 interaction with the tyrosine phosphatase SHP-2 in parallel with the translocation of SHP-2 to erythrocyte membranes. These events seem to be mediated by Src-like catalyzed phosphorylation of band 3 because both SHP-2 translocation to cellular membranes and its interaction with Tyr-phosphorylated protein are greatly counteracted by PP2, a specific inhibitor of Src kinases. Binding-competition experiments demonstrate that SHP-2 recruitment to band 3 occurs via its SH2 domain(s). In particular, our data support the view that SHP-2 docks specifically with P-Y359 of band 3. Experiments performed with intact erythrocytes in the presence of the SHP-2 inhibitor calpeptin suggest that, once recruited to Tyr-phosphorylated band 3, the tyrosine phosphatase dephosphorylates the protein. P-Y8, 21, and 904 are the residues affected by SHP-2, as judged by 32P-peptide mapping of band 3 digested with trypsin. These results indicate that in treated erythrocytes, recruitment of cytosolic SHP-2 to band 3 is a prerequisite for the subsequent dephosphorylation of the transmembrane protein.
Introduction
Phosphorylation/dephosphorylation of protein tyrosine residues (Tyr) has been implicated in the regulation of several erythrocyte functions, including metabolic pathways,1-6 membrane transport,7,8 and cellular volume and shape.8-10 The Tyr-phosphorylated state of cellular proteins reflects the balance between the competing activities of protein tyrosine kinases and protein tyrosine phosphatases (PTPs). Because the activity of PTPs is much higher than that of protein tyrosine kinases, basal levels of protein phosphotyrosine (P-Y) in cells are very low.11-13Treatment of human erythrocytes with pervanadate, diamide, orN-ethylmaleimide (NEM), which act as both oxidizing agents and PTP inhibitors, induces an increase in protein Tyr phosphorylation. Under these conditions, the multifunctional transmembrane band 3 has been demonstrated to represent the main Tyr-phosphorylated protein.14-16 We have recently found that pervanadate treatment of human red cells induces a sequential phosphorylation of band 3 catalyzed by the concerted action of tyrosine kinase Syk and a tyrosine kinase belonging to the Src family (most probably Lyn, even if other members of the family cannot be excluded). Tyr8 and Tyr21 of band 3, primarily phosphorylated by Syk, act as docking sites for the Src homology domain 2 (SH2) domain of the Src-like kinase, which, once recruited to band 3, phosphorylates Tyr359 and Tyr904 of the protein.16 It has been shown that a PTP activity, which seems to be related to PTP1B, is associated with band 3 of human erythrocytes,17 but the enzyme or enzymes catalyzing protein dephosphorylation are presently unknown. Because our preliminary experiments showed that a PTP-containing fraction, partially purified from human erythrocyte cytosol, contained SHP-2 tyrosine phosphatase and displayed dephosphorylating activity toward32P-Y band 3 (L.B., G.C., A.M.B., unpublished data, February 1999), we aimed to test whether band 3 of intact human erythrocytes is a physiologic target of SHP-2.
SHP-2 is a ubiquitous non-transmembrane PTP containing 2 SH2 domains.18 It is involved in the signaling pathway of a variety of growth factors and cytokines and plays an important role in relaying signals from the cell surface to the nucleus.19-21 SHP-2 interacts with many proteins by recognizing specific Tyr-phosphorylated motifs [(V/L)XpYXX(V/L)] through its amino-terminal SH2 domain.22 This protein–protein interaction enhances SHP-2 activity by relieving the inhibitory intramolecular interactions between the amino-terminal SH2 domain and the catalytic phosphatase domain.23 Thus, the SH2 domains play a crucial role both in the recruitment of cellular substrates and in the modulation of the enzyme catalytic activity.
We show here that SHP-2 tyrosine phosphatase is expressed in human erythrocytes, where it is mostly present in the cytosol, and that it translocates to the cell membranes following increased Tyr phosphorylation of transmembrane protein band 3 induced by the PTP inhibitors pervanadate, diamide, and NEM. The present data support the view that band 3 represents not only an anchor protein for SHP-2, but also a target substrate for it.
Materials and methods
Materials
Anti–P-Y and anti–band 3 monoclonal antibodies were purchased from ICN Biotechnology (Irvine, CA) and Sigma (Dorset, United Kingdom), respectively. Rabbit anti-SHPTP2 (C-18) polyclonal antibody, mapping at the carboxyl-terminus of SHP-2, was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Synthetic phosphopeptides of band 3 (351-364 and 896-909) were provided by Advanced Biotech Italia (Seveso, Italy). PP2 inhibitor, calpeptin, and protease inhibitor cocktail were obtained from Calbiochem (Darmstadt, Germany). γ[32P] adenosine 5′-triphosphate (ATP) was purchased from Amersham Pharmacia Biotech (Little Chalfont, United Kingdom).
Enzymes
Isolation of human erythrocytes
Human erythrocytes were prepared from fresh blood collected from healthy donors, as previously described.26
Treatment of erythrocytes
Packed cells, prepared as described above, were resuspended (at 20% hematocrit) in buffer A (20 mmol/L Tris-HCl, pH 7.5; 125 mmol/L NaCl, 10 mmol/L KCl, 1 mmol/L MgCl2, 100 μg/mL streptomycin, 25 μg/mL chloramphenicol, 50 mmol/L glucose, and 1 mmol/L adenosine). Resuspended cells (250 μL for each sample) were incubated for 30 minutes at 35°C either alone or in the presence of either 1 mmol/L pervanadate (prepared by mixing 3 mmol/L hydrogen peroxide with 2 mmol/L sodium orthovanadate16) or the indicated amounts of diamide or NEM. When indicated, 5 μmol/L PP2 inhibitor was added to the incubation mixture together with pervanadate, diamide, or NEM. After incubation, each sample was centrifuged, and the packed cells were subjected to hemolysis in 1.5 mL of a hypotonic buffer containing 5 mmol/L sodium phosphate, pH 8; 0.02% NaN3, 30 μM phenylmethylsulfonyl fluoride (PMSF), 1 mmol/L sodium orthovanadate, and protease inhibitor cocktail.
Membranes were separated from the cytosol by centrifugation (20 000g for 20 minutes) and washed once in hypotonic buffer. Aliquots of membranes (15 μg) or diluted supernatant (15 μL) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; 10% gels), transferred to nitrocellulose membranes, and immunostained with the appropriate antibody.
Immunostaining
Proteins transferred to nitrocellulose membranes were incubated with the indicated antibody followed by the appropriate biotinylated second antibody and developed using an enhanced chemiluminescence detection system (ECL; Amersham Pharmacia Biotech).
Anti–SHP-2, anti-Syk, and anti-Lyn immunoprecipitations
Immunoprecipitates (IPs) from erythrocyte lysates.
For each sample, 3 mL erythrocytes (20% hematocrit) were washed with buffer A, centrifuged, and then treated for 1 hour at 4°C with buffer B (20 mmol/L Tris-HCl, pH 7.5; 10% glycerol, 1% Nonidet P-40, 1 mmol/L EDTA, 150 mmol/L NaCl, 1 mmol/L sodium orthovanadate, and protease inhibitor cocktail).
IPs from erythrocyte membranes.
For each sample, packed membranes prepared from 3 mL erythrocytes (20% hematocrit) were treated as described above and then extracted for 1 hour at 4°C with buffer B. After centrifugation of either cell lysates or membrane extracts, supernatants were diluted 1:1 in 20 mmol/L Tris HCl, pH 7.5; 10 mmol/L glycerol, 5 mmol/L NaCl, 50 mmol/L PMSF, and 10 mmol/L β-mercaptoethanol; precleared with protein A–Sepharose; and then incubated for 5 hours at 4°C with anti–SHP-2, anti-Syk, or anti-Lyn antibodies bound to protein A–Sepharose. The immunocomplexes were washed 3 times in 50 mmol/L Tris-HCl, pH 7.5, containing protease inhibitor cocktail and 1 mmol/L sodium orthovanadate, and then resuspended in the same buffer. When SHP-2 activity was tested in the immunocomplexes, SHP-2 IPs were resuspended in buffer B (25 mmol/L imidazole, pH 7; 1 mmol/L EDTA, 0.02% NaN3, 10% glycerol, 10 mmol/L β-mercaptoethanol, 10 mg/mL leupeptin, and 50 μmol/L PMSF).
SHP-2 IPs from cytosol.
Cytosol obtained from 10 mL packed erythrocytes was treated with ammonium sulfate to reach 60% saturation,27 dialyzed in buffer B, precleared with protein A–Sepharose, immunoprecipitated for 5 hours at 4°C with anti–SHP-2 antibody, washed with buffer B, and recovered in a final volume of 150 μL of the same buffer.
Syk and Lyn IP kinase assays
Tyrosine kinase assays of immunocomplexes were performed in 30 μL incubation mixture containing 50 mmol/L Tris-HCl, pH 7.5; 10 mmol/L MnCl2, 50 μmol/L γ[32P]ATP (specific activity 1000 cpm/pmol), 100 μmol/L sodium orthovanadate (basal medium), and either 300 ng of the cytoplasmic domain of band 316 or 200 μM cdc2 (6-20) peptide,16 which served as substrates for Syk and Lyn, respectively. Following incubation for 10 minutes at 30°C, samples were subjected to SDS-PAGE (15% gels), and substrate phosphorylation was evaluated by an Instant Imager (Packard Instrument Company, Meriden, CT).
Dephosphorylation of band 3 by coimmunoprecipitated SHP-2
Membranes obtained from erythrocytes (500 μL for each sample) treated with 2 mM diamide were immunoprecipitated with anti–SHP-2 antibody. Immunocomplexes were then washed, as described above, in the absence of 1 mmol/L sodium orthovanadate. Tyrosine phosphatase activity of SHP-2 IP was measured by incubation for 30 minutes at 30°C in 20 μL buffer B containing 4 mM dithiothreitol to reverse diamide inhibition. Proteins were then subjected to SDS-PAGE (10% gels) and immunostained with either anti–P-Y or anti–band 3 antibodies.
32P-peptide mapping of band 3
Erythrocyte membranes (30 μg), isolated from red cells treated by hemolysis as described elsewhere,16 were phosphorylated for 10 minutes at 30°C in 30 μL of the above-described basal medium containing 150 nmol/L Syk.16 Syk-phosphorylated membranes were separated by Syk and other reagents by centrifugation and washed twice, as described elsewhere.26 Syk-phosphorylated membranes were then 32P-phosphorylated by 300 nmol/L Lyn in the same basal medium for 10 minutes. 32P-membranes were microfuged for 20 minutes, washed twice in buffer B, resuspended in the same buffer, and incubated at 30°C for 20 minutes in the absence or presence of 50 μL cytosolic SHP-2 IP. Solubilized proteins were subjected to SDS-PAGE (10% gels) and transferred onto nitrocellulose membranes. 32P-phosphorylated band 3, localized by autoradiography, was excised and digested with trypsin.26The resulting peptides were separated in 2 dimensions on thin-layer cellulose plates by electrophoresis in 1% ammonium bicarbonate (pH 8; 1 hour, 1000 V), followed by ascending chromatography in a buffer containing 25% pyridine, 7.5% acetic acid, and 37.5% butanol.26
Results
Detection of SHP-2 tyrosine phosphatase in human erythrocytes
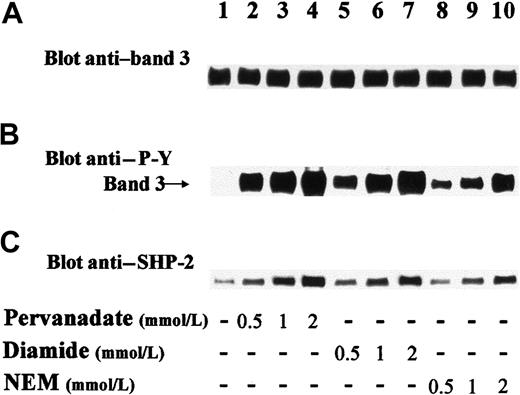
Western blot analysis of lysates from human erythrocytes indicated that the PTP SHP-2 was substantially expressed in these cells. More than 95% of the cellular SHP-2 was found in the cytosolic fraction, as revealed by densitometric analysis (data not shown). However, a small amount of tyrosine phosphatase remained bound to erythrocyte membranes even after several washes (Figure 1C; lane 1). We have previously detected PTP activities in both cytosolic and membrane fractions of human erythrocytes28,29 and found that erythrocyte cytosol, subjected to diethylaminoethyl-cellulose chromatography followed by Ultragel AcA44 gel filtration (Amersham Pharmacia Biotech, Little Chalfont, United Kingdom), gave rise to 2 elution peaks displaying PTP activity.28Preliminary experiments in the present study demonstrated that the first peak of the AcA44 column both immunoreacted with anti–SHP-2 antibody and displayed phosphatase activity toward in vitro32P-Y–phosphorylated band 3 (data not shown). These results prompted us to assess whether SHP-2 plays a physiologic role in the transmembrane protein dephosphorylation. For this purpose, we treated intact human erythrocytes with PTP inhibitors (ie, either oxidizing agents pervanadate and diamide or thiol alkylating agent NEM). Figure 1B shows that treatment of red cells with pervanadate (lanes 2-4), diamide (lanes 5-7), or NEM (lanes 8-10) highly stimulated Tyr phosphorylation of band 3, in contrast to the total absence of Tyr phosphorylation in controls (lane 1). Because it has been previously demonstrated that, upon external stimuli, SHP-2 is recruited to its target substrates and directed to the correct intracellular location,20-22 we assayed for the presence of SHP-2 in membranes isolated from control and differently treated erythrocytes. Figure 1C shows that the amount of tyrosine phosphatase detected by anti–SHP-2 immunostaining was very small in membranes from control cells (lane 1) and greatly increased in membranes from treated erythrocytes (lanes 2-10). It is noteworthy that incubation of red cells with increasing concentrations of the 3 compounds induced parallel dose-dependent enhancement of both band-3 Tyr phosphorylation and the amount of SHP-2 recruited to the erythrocyte membranes (compare Figure 1B and C), indicating that SHP-2 translocation to membranes is associated with band-3 Tyr phosphorylation.
Treatment of human erythrocytes induces both Tyr phosphorylation of band 3 and translocation of cytosolic SHP-2 to membranes.
Red blood cells were incubated in the absence (lane 1) or presence of indicated amounts of pervanadate (lanes 2-4), diamide (lanes 5-7), and NEM (lanes 8-10). Cell membranes were isolated, solubilized, and submitted to SDS-PAGE followed by transfer to nitrocellulose, as described in “Materials and methods.” The upper part of blots containing band 3 (101.8 kd) was immunostained with anti–P-Y antibody (B), stripped, and reprobed with anti–band 3 antibody (A). The lower part of blots containing SHP-2 (68 kd) was immunostained with anti–SHP-2 antibody (C). Panels are representative of at least 7 separate experiments.
Treatment of human erythrocytes induces both Tyr phosphorylation of band 3 and translocation of cytosolic SHP-2 to membranes.
Red blood cells were incubated in the absence (lane 1) or presence of indicated amounts of pervanadate (lanes 2-4), diamide (lanes 5-7), and NEM (lanes 8-10). Cell membranes were isolated, solubilized, and submitted to SDS-PAGE followed by transfer to nitrocellulose, as described in “Materials and methods.” The upper part of blots containing band 3 (101.8 kd) was immunostained with anti–P-Y antibody (B), stripped, and reprobed with anti–band 3 antibody (A). The lower part of blots containing SHP-2 (68 kd) was immunostained with anti–SHP-2 antibody (C). Panels are representative of at least 7 separate experiments.
Diamide and NEM trigger the phosphorylation of band 3 by Syk and Src-like tyrosine kinase(s)
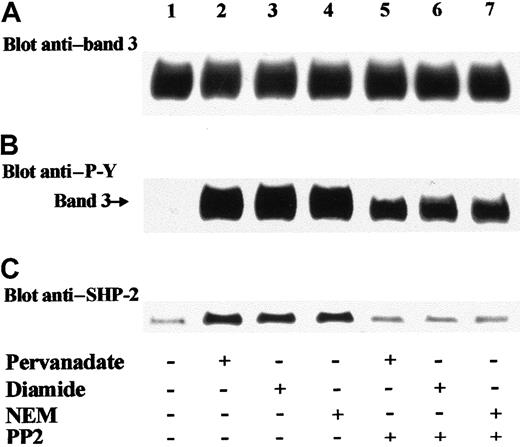
We previously demonstrated that band 3 is phosphorylated according to a sequential model.16 Treatment of erythrocytes with pervanadate induced the activation of tyrosine kinase Syk, which triggered the phosphorylation of band 3 at Tyr8 and Tyr21. This primary phosphorylation generates the docking sites for the SH2 domain of a Src kinase, most probably Lyn, which in turn catalyzes a secondary protein phosphorylation at Tyr359 and Tyr904.16 In agreement with this model, Figure 2B shows that the addition of PP2, which is a specific inhibitor of Src kinases,30 together with diamide or NEM to red cells caused a decrease in band-3 Tyr phosphorylation (compare lanes 3,4 and 6,7) similar to that observed in pervanadate-treated cells (Figure 2B; lanes 2 and 5).16 Therefore, it may be presumed that exposure of erythrocytes to either diamide or NEM triggers band-3 Tyr phosphorylation according to a sequential model like that evoked by pervanadate, which involves activation of both PP2-resistant (Syk) and PP2-sensitive (Src-like) tyrosine kinases. To confirm this hypothesis, we performed anti-Syk and anti-Lyn immunoprecipitation experiments using extracts of membranes isolated from control and treated cells and tested the in vitro activity of the IPs. Table1 shows that pervanadate, diamide, and NEM stimulated the activity of both Syk and Lyn tyrosine kinases and that, as expected, PP2 inhibited only Lyn and not Syk activity. These data confirm that diamide and NEM promote the same sequential mechanism of phosphorylation previously described in pervanadate-stimulated red cells.16 Moreover, it may be assumed that in membranes isolated from erythrocytes stimulated in the presence of PP2, band-3 Tyr phosphorylation is ascribable only to Syk (Figure 2B, lanes 5-7; Table 1). It is interesting to note that under these conditions (ie, when band 3 is only phosphorylated by Syk), SHP-2 does not translocate to the membranes, as shown by the detected amount of membrane-bound tyrosine phosphatase (Figure 2C; lanes 5-7), which was similar to that found in untreated cells (Figure 2C; lane 1). This finding suggests that secondary phosphorylation of band 3, catalyzed by a Src-like kinase, is involved in the recruitment of SHP-2 to the membranes of treated erythrocytes. Because we always found similar data by treating erythrocytes with pervanadate, diamide, or NEM, henceforward we present only results obtained upon diamide treatment, which induces reversible PTP inhibition.15
SHP-2 translocation to erythrocyte membrane is mediated by PP2-sensitive phosphorylation of band 3.
Red blood cells were incubated in the absence (lane 1) or presence of 1 mmol/L pervanadate (lanes 2, 5), 2 mmol/L diamide (lanes 3, 6), or 1 mmol/L NEM (lanes 4, 7). PP2 (5 μM) was added to the incubation mixture of assays of lanes 5-7. Erythrocytes were then subjected to hemolysis, and isolated membranes were treated as described in the legend of Figure 1 (B-C). Panels are representative of 4 separate experiments.
SHP-2 translocation to erythrocyte membrane is mediated by PP2-sensitive phosphorylation of band 3.
Red blood cells were incubated in the absence (lane 1) or presence of 1 mmol/L pervanadate (lanes 2, 5), 2 mmol/L diamide (lanes 3, 6), or 1 mmol/L NEM (lanes 4, 7). PP2 (5 μM) was added to the incubation mixture of assays of lanes 5-7. Erythrocytes were then subjected to hemolysis, and isolated membranes were treated as described in the legend of Figure 1 (B-C). Panels are representative of 4 separate experiments.
Activation of both Syk and Lyn tyrosine kinases by treatment of intact erythrocytes
| Treatment . | Tyrosine kinase activity, cpm . | |
|---|---|---|
| Anti-Syk IP . | Anti-Lyn IP . | |
| None | 2 730 | 1 010 |
| Pervanadate | 5 948 | 10 566 |
| Pervanadate + PP2 | 5 725 | 910 |
| Diamide | 6 215 | 10 416 |
| Diamide + PP2 | 5 838 | 1 120 |
| NEM | 6 063 | 10 275 |
| NEM + PP2 | 5 790 | 930 |
| Treatment . | Tyrosine kinase activity, cpm . | |
|---|---|---|
| Anti-Syk IP . | Anti-Lyn IP . | |
| None | 2 730 | 1 010 |
| Pervanadate | 5 948 | 10 566 |
| Pervanadate + PP2 | 5 725 | 910 |
| Diamide | 6 215 | 10 416 |
| Diamide + PP2 | 5 838 | 1 120 |
| NEM | 6 063 | 10 275 |
| NEM + PP2 | 5 790 | 930 |
Human erythrocytes were incubated without or with 1 mmol/L pervanadate, 2 mmol/L diamide, and 1 mmol/L NEM in the absence or presence of 5 μM PP2. Isolated erythrocyte membranes were treated with extraction buffer, and extracted proteins were immunoprecipitated with anti-Syk or anti-Lyn antibodies. Tyrosine kinase activities of immunocomplexes were tested in vitro, as described in “Materials and methods.” Reported values represent the means for 4 separate experiments. SEMs were always less than 20%. IP indicates immunoprecipitate; NEM, N-ethylmaleimide.
Recruitment of SHP-2 to Tyr-phosphorylated band 3
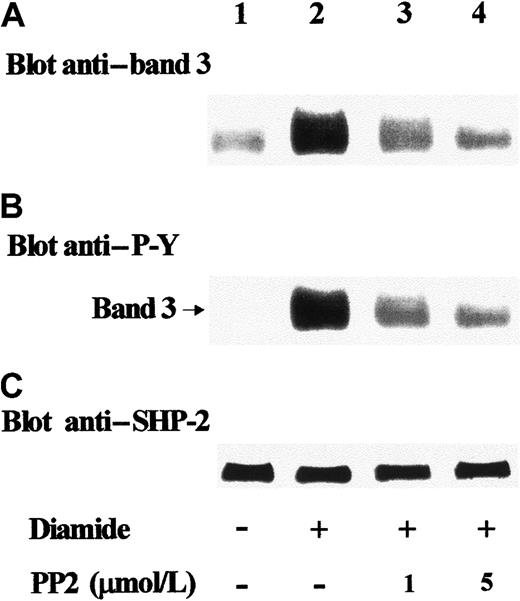
To assess the actual occurrence of interactions between SHP-2 and band 3 in intact erythrocytes, we assayed for the presence of band 3 in anti–SHP-2 IPs obtained from cellular lysates. Figure3A shows that in untreated cells, a small amount of transmembrane protein coimmunoprecipitated with SHP-2 (lane 1). However, because of the great amount of band 3 present in red cells, contamination cannot be excluded. Diamide stimulation of erythrocytes promoted remarkable coimmunoprecipitation of band 3 with tyrosine phosphatase (lane 2), which was greatly reduced in the presence of PP2 (lanes 3, 4). These results demonstrate that PP2 counteracts diamide-induced recruitment of SHP-2 by Tyr-phosphorylated band 3.
Detection of Tyr-phosphorylated band 3 in anti–SHP-2 IPs.
Red blood cells were treated in the absence (lane 1) or presence of 2 mmol/L diamide (lanes 2-4). Indicated concentrations of PP2 were added to the incubation mixture of assays of lanes 3 and 4. Erythrocyte lysates were immunoprecipitated with anti–SHP-2 antibody; immunocomplexes were subjected to SDS-PAGE and immunostained with anti–band 3 antibody (A). Blots were then stripped and reprobed with anti–P-Y antibody (B). In parallel experiments, blots were first immunostained with anti–band 3 antibody and then stripped and reprobed with anti–SHP-2 antibody (C). Panels are representative of at least 4 separate experiments.
Detection of Tyr-phosphorylated band 3 in anti–SHP-2 IPs.
Red blood cells were treated in the absence (lane 1) or presence of 2 mmol/L diamide (lanes 2-4). Indicated concentrations of PP2 were added to the incubation mixture of assays of lanes 3 and 4. Erythrocyte lysates were immunoprecipitated with anti–SHP-2 antibody; immunocomplexes were subjected to SDS-PAGE and immunostained with anti–band 3 antibody (A). Blots were then stripped and reprobed with anti–P-Y antibody (B). In parallel experiments, blots were first immunostained with anti–band 3 antibody and then stripped and reprobed with anti–SHP-2 antibody (C). Panels are representative of at least 4 separate experiments.
Subsequent analysis of the same Western blot with anti–P-Y antibody showed that in treated cells, SHP-2 interacted with Tyr-phosphorylated band 3 (Figure 3B; lane 2). In the presence of PP2, Tyr phosphorylation was greatly reduced, indicating that both Src-like phosphorylation of the protein and its binding to SHP-2 were inhibited (Figure 3A,B; lanes 3, 4).
SHP-2 recruitment to Tyr-phosphorylated band 3 is mediated by phosphatase SH2 domain(s)
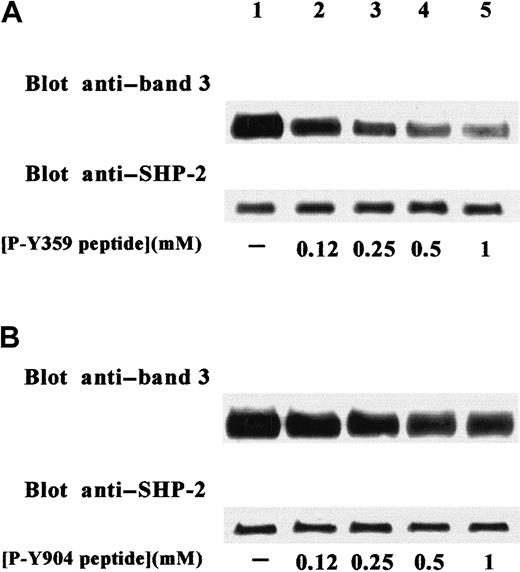
The evident importance of secondary Tyr phosphorylation of band 3 in the recruitment of SHP-2 suggests the involvement of SHP-2 SH2 domain(s) in interactions between phosphatase and transmembrane protein. This hypothesis is also supported by the sequences containing the tyrosyl residues phosphorylated by Src-like kinase, which present a hydrophobic residue at +3 position, a motif required for the specific binding to SHP-2 SH2 domains.31,32 To confirm our hypothesis, we used the synthetic phosphopeptides PAKPDSSFpY359KGLDL and DEEEGRDEpY904DEVAM, which reproduce the sequences of band 3 containing the tyrosines secondarily phosphorylated by the Src-like kinase in stimulated erythrocytes.16 The phosphopeptides were tested as potential competitors with the endogenous band-3 docking site(s) for the binding to SHP-2 SH2 domain(s). For this purpose, increasing concentrations of the 2 phosphopeptides were added during anti–SHP-2 immunoprecipitation of membrane extracts obtained from diamide-stimulated red cells. Figure 4A shows that P-Y359–containing peptide counteracted the diamide-induced interaction of phospho–band 3 with SHP-2, causing the displacement of protein from immunoprecipitating SHP-2. In contrast, P-Y904–containing peptide was a less effective competitive inhibitor of the interaction between band 3 and tyrosine phosphatase (Figure 4B).
Specific inhibition of band-3 coimmunoprecipitation in SHP-2 IPs by P-Y359–containing phosphopeptide.
Erythrocytes were stimulated with 2 mM diamide. Extracts from cell membranes were immunoprecipitated with anti–SHP-2 antibody in the absence (lane 1) or presence (lanes 2-5) of increasing concentrations of synthetic phosphopeptides reproducing band-3 sequences containing either P-Y359 (A) or P-Y904 (B). SHP-2 IPs were submitted to SDS-PAGE, transferred to nitrocellulose, and immunostained with anti–band 3 antibody. Blots were then stripped and reprobed with anti–SHP-2 antibody. Figure is representative of at least 3 separate experiments.
Specific inhibition of band-3 coimmunoprecipitation in SHP-2 IPs by P-Y359–containing phosphopeptide.
Erythrocytes were stimulated with 2 mM diamide. Extracts from cell membranes were immunoprecipitated with anti–SHP-2 antibody in the absence (lane 1) or presence (lanes 2-5) of increasing concentrations of synthetic phosphopeptides reproducing band-3 sequences containing either P-Y359 (A) or P-Y904 (B). SHP-2 IPs were submitted to SDS-PAGE, transferred to nitrocellulose, and immunostained with anti–band 3 antibody. Blots were then stripped and reprobed with anti–SHP-2 antibody. Figure is representative of at least 3 separate experiments.
SHP-2 dephosphorylates band 3 both in vitro and in intact erythrocytes
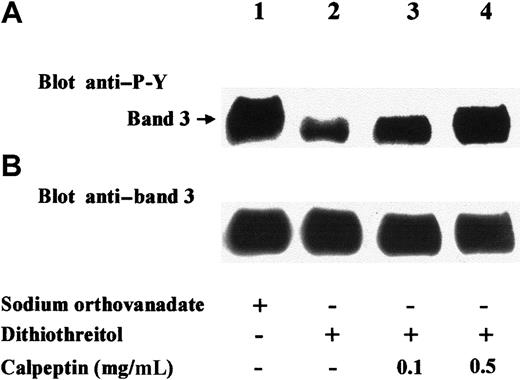
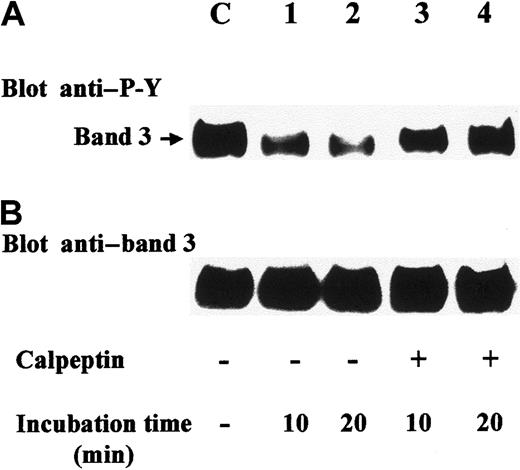
Having shown that diamide stimulation of erythrocytes induces translocation of SHP-2 to Tyr-phosphorylated band 3, we aimed to determine whether the recruited phosphatase was active and whether band 3 itself was a target substrate. Anti–SHP-2 immunocomplexes from diamide-treated cell membranes were therefore incubated in phosphatase assay buffer in the presence of either the phosphatase inhibitor sodium orthovanadate (Figure 5; lane 1) or dithiothreitol, which counteracts the inhibitory effect of diamide15 (Figure 5; lanes 2-4). Anti–P-Y immunostaining of the later Western blot showed that the phosphatase greatly reduced Tyr phosphorylation of coimmunoprecipitated band 3 (Figure 5A; lane 2). Band-3 dephosphorylation was almost completely abolished when the phosphatase assay was performed in the presence of calpeptin (0.5 mg/mL), a selective inhibitor of SHP-233 (Figure 5A; lane 3). To assess whether SHP-2 is involved in band-3 dephosphorylation also in intact erythrocytes, we treated red cells with diamide in the presence of calpeptin, washed them, and incubated them in basal buffer lacking both diamide and calpeptin. Figure6 shows that under these conditions, band 3 was greatly dephosphorylated (lanes 1, 2). On the contrary, the presence of calpeptin in washings and following incubations highly counteracted protein dephosphorylation (lanes 3, 4), indicating that band 3 is a target of SHP-2.
SHP-2 dephosphorylates coimmunoprecipitated band 3.
Erythrocytes were treated with diamide, and extracts from cell membranes were immunoprecipitated with anti–SHP-2 antibody. Immunocomplexes were washed twice in the absence of sodium orthovanadate and then incubated for 30 minutes at 30°C in phosphatase assay buffer containing 1 mM sodium orthovanadate (lane 1) or 4 mM dithiothreitol (lanes 2-4). The indicated amount of calpeptin was present in the phosphatase assay buffer of experiments of lanes 3 and 4. After incubation, samples were submitted to SDS-PAGE, transferred to nitrocellulose, and immunostained with anti–P-Y (A) or anti–band 3 (B) antibodies. Figure represents at least 3 separate experiments.
SHP-2 dephosphorylates coimmunoprecipitated band 3.
Erythrocytes were treated with diamide, and extracts from cell membranes were immunoprecipitated with anti–SHP-2 antibody. Immunocomplexes were washed twice in the absence of sodium orthovanadate and then incubated for 30 minutes at 30°C in phosphatase assay buffer containing 1 mM sodium orthovanadate (lane 1) or 4 mM dithiothreitol (lanes 2-4). The indicated amount of calpeptin was present in the phosphatase assay buffer of experiments of lanes 3 and 4. After incubation, samples were submitted to SDS-PAGE, transferred to nitrocellulose, and immunostained with anti–P-Y (A) or anti–band 3 (B) antibodies. Figure represents at least 3 separate experiments.
Dephosphorylation of band 3 is inhibited by calpeptin in treated erythrocytes.
Erythrocytes were stimulated with 2 mM diamide in basal buffer containing calpeptin (2 mg/mL) for 20 minutes. In experiments of lanes 1-4, red cells were washed 3 times, resuspended, and incubated in basal buffer either in the absence (lanes 1, 2) or presence of calpeptin (lanes 3, 4) for 10 (lanes 1, 3) or 20 minutes (lanes 2, 4). Lane C refers to control erythrocytes treated with diamide and calpeptin without the following washes and incubation. Cell membranes were then isolated and solubilized, and an aliquot (15 μg) was submitted to SDS-PAGE, followed by transfer to nitrocellulose. Blots were immunostained with anti–P-Y antibody (A), stripped, and reprobed with anti–band 3 antibody (B). Panels are representative of 3 separate experiments.
Dephosphorylation of band 3 is inhibited by calpeptin in treated erythrocytes.
Erythrocytes were stimulated with 2 mM diamide in basal buffer containing calpeptin (2 mg/mL) for 20 minutes. In experiments of lanes 1-4, red cells were washed 3 times, resuspended, and incubated in basal buffer either in the absence (lanes 1, 2) or presence of calpeptin (lanes 3, 4) for 10 (lanes 1, 3) or 20 minutes (lanes 2, 4). Lane C refers to control erythrocytes treated with diamide and calpeptin without the following washes and incubation. Cell membranes were then isolated and solubilized, and an aliquot (15 μg) was submitted to SDS-PAGE, followed by transfer to nitrocellulose. Blots were immunostained with anti–P-Y antibody (A), stripped, and reprobed with anti–band 3 antibody (B). Panels are representative of 3 separate experiments.
Identification of band-3 sites dephosphorylated by SHP-2
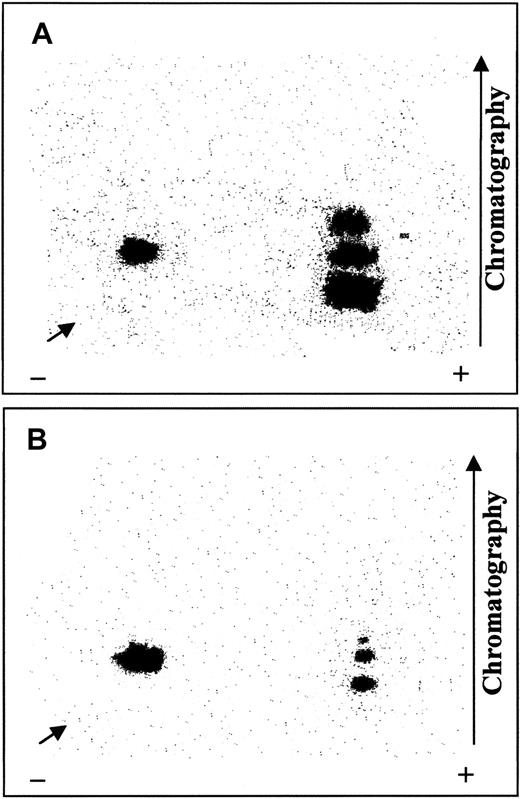
We previously demonstrated16 that Syk and Lyn phosphorylate in vitro the same band-3 residues that are phosphorylated in pervanadate-stimulated red cells.16 Therefore, to shed light on the site specificity of SHP-2, we treated isolated erythrocyte membranes by 32P-phosphorylation in vitro by the sequential addition of Syk and Lyn tyrosine kinases.32P-phosphorylated membranes were then dephosphorylated by SHP-2 immunoprecipitated from the cytosol of untreated erythrocytes. Control and SHP-2–dephosphorylated membranes were subjected to SDS-PAGE and electrotransferred to nitrocellulose.32P-phosphorylated band 3 was then excised and digested with trypsin, and the radiolabeled tryptic peptides were separated in 2 dimensions on thin-layer cellulose plates. Figure7 shows that SHP-2 greatly affected 3 radioactive fragments, which displayed high mobility toward the anode and thus included several acidic amino acids. As previously demonstrated,16 the phosphorylated residues contained in these sequences correspond to Tyr8, Tyr21, and Tyr904 of band 3. In contrast, P-Y359, which is located in a less mobile fragment, was not dephosphorylated by SHP-2. Indeed, complete band-3 dephosphorylation was never achieved, even using higher phosphatase concentrations or longer incubation times than those described in “Materials and methods.”
Mapping of tryptic fragments obtained by32P-phosphorylated band 3, untreated or treated with SHP-2.
Isolated erythrocyte membranes were 32P-phosphorylated by Syk and Lyn and incubated in the absence (A) or presence (B) of cytosolic SHP-2 IP, as described. Samples were subjected to SDS-PAGE, and radiolabeled band 3 was excised and digested with trypsin. Resulting 32P-peptides were separated in 2 dimensions on thin-layer cellulose plates and autoradiographed. Arrows indicate sample starting point. Panels represent 3 separate experiments.
Mapping of tryptic fragments obtained by32P-phosphorylated band 3, untreated or treated with SHP-2.
Isolated erythrocyte membranes were 32P-phosphorylated by Syk and Lyn and incubated in the absence (A) or presence (B) of cytosolic SHP-2 IP, as described. Samples were subjected to SDS-PAGE, and radiolabeled band 3 was excised and digested with trypsin. Resulting 32P-peptides were separated in 2 dimensions on thin-layer cellulose plates and autoradiographed. Arrows indicate sample starting point. Panels represent 3 separate experiments.
Discussion
This study demonstrates that the tandem SH2 domain–containing PTP SHP-2 is present in human erythrocytes. As in other hematopoietic cells,21 22 the tyrosine phosphatase is mainly located in the cytosol. Under conditions that stimulate Tyr phosphorylation, cytosolic SHP-2 is recruited to the erythrocyte membranes and catalyzes band-3 dephosphorylation.
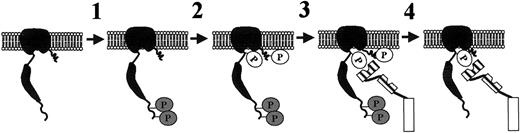
Figure 8 depicts the steps underlying the mechanism of phosphorylation/dephosphorylation of the transmembrane protein band 3, as suggested by our experimental data.
Sequential steps underlying the mechanism of band-3 phosphorylation and dephosphorylation in treated human erythrocytes.
Step 1: Tyrosine kinase Syk phosphorylates Tyr8 and Tyr21 of band 3; step 2: tyrosine kinase(s) of Src family phosphorylates Tyr359 and Tyr904 of band 3; step 3: cytosolic SHP-2 is recruited by P-Y359 of band 3; step 4: SHP-2 dephosphorylates Tyr8, Tyr21, and Tyr904 of band 3. For simplicity, band 3 is represented as a monomer.
Sequential steps underlying the mechanism of band-3 phosphorylation and dephosphorylation in treated human erythrocytes.
Step 1: Tyrosine kinase Syk phosphorylates Tyr8 and Tyr21 of band 3; step 2: tyrosine kinase(s) of Src family phosphorylates Tyr359 and Tyr904 of band 3; step 3: cytosolic SHP-2 is recruited by P-Y359 of band 3; step 4: SHP-2 dephosphorylates Tyr8, Tyr21, and Tyr904 of band 3. For simplicity, band 3 is represented as a monomer.
Step 1: Treatment of red cells with pervanadate, diamide, or NEM stimulates Syk and Lyn tyrosine kinases (Table 1).16 Syk acts as primary kinase and phosphorylates Tyr8 and Tyr21 of band 3.
Step 2: Syk phosphorylation of band 3 generates the docking sites for the SH2 domain of the secondary kinase, namely Lyn or another member of the Src family, which affects Tyr359 and Tyr904 of the protein.16
Step 3: Once Src-like phosphorylation is accomplished, part of cytosolic SHP-2 is recruited to the Tyr-phosphorylated protein via the phosphatase SH2 domain(s). This hypothesis is based on the following evidence: (1) Anti–SHP-2 immunostaining of membrane proteins, isolated from stimulated erythrocytes, reveals a dose-dependent increase of band-3 Tyr phosphorylation in parallel with SHP-2 translocation to the cell membranes (Figure 1); (2) coimmunoprecipitation of Tyr-phosphorylated band 3 in SHP-2 IPs demonstrates that the tyrosine phosphatase interacts with the protein (Figure 3); (3) the drastically reduced translocation of SHP-2 in the presence of the Src-specific inhibitor PP2 indicates that the secondary phosphorylation of band 3 generates the docking site(s) for SHP-2 recruitment (Figures 2, 3); and (4) competition experiments point out the involvement of SHP-2 SH2 domain(s) in the recruitment of the phosphatase to secondarily Tyr-phosphorylated band 3. In particular, P-Y359 seems to be the dominant site in binding to SHP-2 because the corresponding phosphorylated peptide strongly counteracts coimmunoprecipitation of tyrosine phosphatase with phospho–band 3 (Figure 4A). The finding that the phosphopeptide containing P-Y904 is a poor competitor, although its sequence contains, similarly to P-Y359 peptide, a determinant for binding to SHP-2 SH2 domains, is most probably due to other residues of the sequence that play a negative role in the interaction with the phosphatase (Figure 4B).
Step 4: Once recruited to Tyr-phosphorylated band 3, SHP-2 dephosphorylates the protein. It has been demonstrated that, in the unbound state, SHP-2 adopts a “closed” conformation, in which the amino-terminal SH2 domain interacts extensively with the phosphatase domain, blocking the active site.23 Upon binding of the N-terminal SH2 domain to a specific P-Y target, the enzyme adopts an “open” active conformation. Hence, selective interaction of P-Y359 with the N-terminal SH2 domain of SHP-2 is probably critical for band 3–mediated stimulation of tyrosine phosphatase activity. By using the phosphatase inhibitor calpeptin, we demonstrated that SHP-2 dephosphorylates band 3 both in coimmunoprecipitates (Figure 5) and in intact erythrocytes (Figure 6).
Band-3 residues affected by SHP-2 are P-Y8, P-Y21, and P-Y904 (Figure7). It has been proposed that the members of the PTP family achieve selectivity through different combinations of both specific targeting strategies and intrinsic catalytic-domain specificity.12,20,31,34,35 Because sites dephosphorylated by SHP-2 are located in highly acidic sequences, our data suggest that the presence of several acidic residues surrounding the target amino acid is a positive determinant for SHP-2 site recognition. The finding that P-Y359, the only band-3 site unaffected by SHP-2, is not located in a highly acidic sequence supports our hypothesis. However, because we have previously demonstrated in vitro that binding between a recombinant SH2 domain and its cognate Tyr-phosphorylated sequence inhibits dephosphorylation regardless of either the nature or the amount of the tyrosine phosphatase used,36 it cannot be excluded that the inability of SHP-2 to dephosphorylate P-Y359 may also depend on the inaccessibility of tyrosine phosphatase to this phosphorylated residue. If this is the case, in vivo dephosphorylation of band-3 P-Y359 by its specific PTP must be preceded by disruption of the interaction between P-Y and the SHP-2 SH2 domain.
The hypothesis that SHP-2 dephosphorylation of band 3 represents a general mechanism implicated in erythrocyte functions mediated by protein Tyr phosphorylation has been supported by a set of preliminary experiments performed with erythrocytes exposed to osmotic stress. A correlation between SHP-2 translocation and band-3 Tyr phosphorylation/dephosphorylation similar to that found upon oxidative stress has been also observed by exposing erythrocytes to hypertonic followed by isotonic conditions (not shown).
SHP-2 phosphatase has been demonstrated to be involved in various signaling cascades. Critical to the function of this cytosolic phosphatase is its recruitment to membrane receptors bearing the phospho-ITIM (immunoreceptor tyrosine-based inhibition motif).22 Our results demonstrate that, in stimulated human erythrocytes, band 3 is the anchor protein bearing docking sites for SHP-2, which catalyzes its dephosphorylation.
Band 3 is a multifunctional protein that plays a crucial role in the flexibility and shape of erythrocyte membranes37-39 and in the regulation of several cellular functions, including lifespan.2,40-42 Its Tyr phosphorylation has been reported to be involved in the modulation of a variety of erythrocyte functions, such as glycolysis, cell shape, cytoskeleton movements, and anion transport,1-10,43 and is associated with erythrocyte disorders.44 45 Therefore, elucidation of the mechanisms of Tyr phosphorylation and dephosphorylation of band 3 will be useful for better understanding of their implications in the regulation of erythrocyte functions.
Grateful thanks are due to Miss Carla Munari for technical assistance and to Mr Dario Spinello for supplying fresh blood from volunteers.
Supported by the Centro di Studio delle Biomembrane del CNR, the Armenise-Harvard Foundation, Associazione Italiana per la Ricerca sul Cancro (AIRC), the Italian Ministry of Health (Project AIDS), Italian Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica (MURST) (Co-Finanziamento, 2000), and CNR (n. 98.03280.ST74 and Target Project on Biotechnology).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Arianna Donella-Deana, Dipartimento di Chimica Biologica, Viale G. Colombo, 3, 35121 Padova, Italy; e-mail:arianna.donella@unipd.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal