Abstract
Clonality studies of mature cells suggest that the primary transformation event in myelodysplastic syndrome (MDS) most frequently occurs in a myeloid-restricted progenitor, a hypothesis supported by recent studies of purified CD34+Thy1+hematopoietic stem cells (HSCs) in cases with trisomy 8 (+8). In contrast, we recently demonstrated that a lymphomyeloid HSC is the target for transformation in MDS cases with del(5q), potentially reflecting heterogeneity within MDS. However, since +8 is known to frequently be a late event in the MDS transformation process, it remained a possibility that CD34+CD38−Thy1+ HSC disomic for chromosome 8 might be part of the MDS clone. In the present studies, although a variable fraction of CD34+CD38−Thy1+ cells were disomic for chromosome 8, they did not possess normal HSC activity in long-term cultures and nonobese diabetic–severe combined immunodeficiency (NOD-SCID) mice. Mixing experiments with normal CD34+CD38− cells suggested that this HSC deficiency was intrinsic and not mediated by indirect mechanisms. Furthermore, investigation of 4 MDS cases with combined del(5q) and +8 demonstrated that the +8 aberration was always secondary to del(5q). Whereas del(5q) invariably occurs in CD34+CD38−Thy-1+ HSCs, the secondary +8 event might frequently arise in progeny of MDS HSCs. Thus, CD34+CD38−Thy1+ HSCs are invariably part of the MDS clone also in +8 patients, and little HSC activity can be recovered from the CD34+ CD38−Thy1+ HSC. Finally, in advanced cases of MDS, the MDS reconstituting activity is exclusively derived from the minor CD34+CD38−HSC population, demonstrating that MDS stem cells have a similar phenotype as normal HSCs, potentially complicating the development of autologous transplantation for MDS.
Introduction
Myelodysplastic syndromes (MDSs) are a heterogeneous group of clonal disorders, characterized by ineffective hematopoiesis and frequent progression to acute myeloid leukemia (AML).1 The dysplastic changes and the resulting cytopenias involve all mature myeloid but not lymphoid blood lineages, and at variance with the hematopoietic stem cell (HSC) disease chronic myelogenous leukemia,2 transformation to acute lymphoblastic leukemia is extremely rare, suggesting that the primary transformation event in MDS in most cases might occur at a multipotent but myeloid-restricted progenitor cell level.3 This notion has been supported by most clonality studies, since mature myeloid cells are invariably part of the MDS clone, whereas B and T cells only rarely are derived from the MDS clone.4-14 However, the scarcity of evidence for mature lymphocytes being derived from the MDS clone does not exclude the possibility that the primary transformation event in MDS can occur at the HSC level. For example, the transformation events in MDS may be incompatible with further lymphoid differentiation and if so, the lymphocytes in patients with MDS may be derived from residual normal HSCs or long-lived lymphoid progenitor cells.14 15
Recently, major technological developments have allowed for prospective identification and purification of human HSCs and progenitor cells based on fluorescence activated cell sorting (FACS).16-18In addition, their functional characterization has become possible by improved in vitro and in vivo surrogate human HSC assays.19-23 This progress will facilitate a new line of studies in which the HSC and progenitor cell pools of patients with various hematologic malignancies can be prospectively identified and purified for further cytogenetic and functional studies. Unfortunately, the low frequency of phenotypically defined HSCs in healthy subjects as well as in patients with MDS makes it a challenging task to obtain sufficient cell numbers for functional studies. However, in a few studies it has been possible to perform fluorescence in situ hybridization (FISH) on FACS-purified HSC and progenitor cell populations from patients with MDS to address whether these carry known cytogenetic aberrations. Using this approach, we recently demonstrated that a multipotent (lymphomyeloid) HSC is the likely primary target for transformation in most MDS cases with 5q deletions.12 This was based on the observation that more than 95% of the cells in the CD34+CD38− HSC compartment of all investigated patients carried the del(5q), as did CD34+CD19+pro–B-cell progenitors in most patients in which this population could be purified.12 In addition, we demonstrated that the HSC pool in all these patients was severely compromised in its ability to reconstitute hematopoiesis in vitro and in vivo.
Saitoh et al24 investigated MDS cases with trisomy 8, and demonstrated in striking contrast to what we observed in cases with del(5q) that whereas myeloid committed progenitor cells were invariably part of the +8 clone, there was no evidence for involvement of the phenotypically (CD34+Thy-1+) defined HSC pool. Importantly, this lead to the conclusion that in MDS cases with trisomy 8 the transformation may predominantly originate in a committed myeloid progenitor, and as a consequence it was proposed that this made autologous HSC transplantation a meaningful approach in this patient group.13 24
Based on these and other studies it is now commonly viewed that MDS, being a heterogeneous disease, most often originates in a multipotent but myeloid-restricted progenitor cell population, although the primary transformation in some patients may occur at the lymphomyeloid HSC level. Hence, the potential involvement of HSCs in MDS remains a matter of controversy.3,14,15 However, the absence of trisomy 8 in the CD34+Thy1+ HSC pool of trisomy 8 patients does not exclude that this phenotyically defined HSC population may be part of the MDS disease because the possibility exists that the +8 aberration could be a secondary or late event in the MDS transformation as previously proposed,25 potentially occurring outside of the HSC pool. A functional evaluation of the HSC population in trisomy 8 patients would have contributed to a better understanding of whether the phenotypically defined HSCs were part of the disease in these patients. However, such studies were not performed by Saitoh et al,24 probably due to the limited number of cells obtained. In fact, sufficient CD34+Thy-1+cells for FISH analyses were only obtained in 3 patients. In the present study, we have investigated the clonal involvement and functional activity of the CD34+CD38− (and CD34+CD38−Thy1+) HSCs in MDS cases with trisomy 8. Using a dual FISH approach combined with a detailed functional evaluation of the HSC pool, we here demonstrate that the primary transformation also in this patient group might frequently occur in the phenotypically defined HSC pool. Thus, the absence of trisomy 8 in the HSC population of some of these patients rather reflects that this aberration usually is a late event in the MDS transformation process, and as a consequence might occur outside of the HSC pool.
Patients, materials, and methods
Patients
Bone marrow (BM) samples from 13 patients with MDS and 11 healthy subjects were collected at the hematology departments at Huddinge University Hospital, Aarhus Hospital, Rigshospitalet in Copenhagen, Danderyd Hospital, and University Hospital of Lund after obtaining informed consent and with the approval of the ethics committees at the medical faculties of the respective hospitals. Patient characteristics are provided in Table1. All patients had a confirmed diagnosis of MDS according to the French-American-British (FAB) classification and cytogenetic aberrations, including trisomy 8. Because all patients were selected for the presence of +8 there were no patients with low risk according to the International Prognostic Scoring System (IPSS) risk groups.26 For comparative purposes, 2 other patients with MDS, an 83-year-old female with refractory anemia (RA), less than 5% BM blasts, and del(5q) (Figure1) and an 81-year-old male with chronic myelomonocytic leukemia (CMML), 6% BM blasts, and del(20q) (Table 4), were included in the studies.
Clinical, hematologic, and cytogenetic characteristics of MDS patients with trisomy 8
| Patient no. . | Sex/ age, y . | FAB . | BM blasts, % . | Hemoglobin level, g/L . | WBC count, 109/L . | Platelet count, 109/L . | Karyotype . | Treatment . | |
|---|---|---|---|---|---|---|---|---|---|
| Ongoing . | Previous . | ||||||||
| 1 | M/62 | RA | < 5 | 133 | 6,1 | 8 | 47,XY,+8[8]/46,XY[17] | — | — |
| 2 | M/84 | RA | < 5 | 59 | 11,0 | 206 | 47,XY,+8[6]/47,idem,del(17)(p?)[4] | — | — |
| 3 | M/80 | RA | < 5 | 89 | 3,0 | 206 | 47,XY,del(5)(q?),+8[1]/46,XY[12] | — | — |
| 4 | M/74 | CMML | < 5 | 83 | 145,5 | 77 | 46-47,XY,+8[8]/47,idem,del(17)(p?)[4] | — | — |
| 5 | F/66 | RARS | < 5 | 80 | 3,0 | 85 | 45-48,XX,−4,del(5)(q?),del(7)(q?),+8,−16,−17, del(19)+1-6mar[33]/46,XX[2] | — | — |
| 6 | F/78 | RAEB | 10 | 100 | 3,0 | 100 | 47,XX,+8[27] | — | CT* |
| 7 | F/62 | RAEB | 10 | 65 | 7,7 | 211 | 47,XX,+8[5]/46,XX[5] | — | — |
| 8 | F/59 | RAEB | 10 | 75 | 4,4 | 20 | 50,XX,+8,+9,+14,+21[26] | IT† | — |
| 9 | F/73 | CMML | 12 | 48 | 80,8 | 105 | 47,XX,+8[4]/47,idem,del(17)(p?)[5] | — | Pred |
| 10 | F/74 | RAEB | 17 | 91 | 8,9 | 128 | 42-46,XX,del(5)(q22)[24]/47,XX,del(5)(q22),+8[1] | Pred | — |
| 11 | M/73 | RAEBt | 20 | 48 | 7,1 | 341 | 47,XY,+8[4]/46,XY[2] | — | — |
| 12 | M/51 | RAEBt | 25 | 51 | 4,3 | 71 | 45-47,XY,−5,del(7)(q22),+8,del(20)(q11.2),+20[7] | — | — |
| 13 | F/79 | MDS-AML‡ | 35 | 87 | 19,6 | 21 | 44,XX,−3,del(5)(q?),+8,−12,−13,−14,−15,−16[42] | — | — |
| Patient no. . | Sex/ age, y . | FAB . | BM blasts, % . | Hemoglobin level, g/L . | WBC count, 109/L . | Platelet count, 109/L . | Karyotype . | Treatment . | |
|---|---|---|---|---|---|---|---|---|---|
| Ongoing . | Previous . | ||||||||
| 1 | M/62 | RA | < 5 | 133 | 6,1 | 8 | 47,XY,+8[8]/46,XY[17] | — | — |
| 2 | M/84 | RA | < 5 | 59 | 11,0 | 206 | 47,XY,+8[6]/47,idem,del(17)(p?)[4] | — | — |
| 3 | M/80 | RA | < 5 | 89 | 3,0 | 206 | 47,XY,del(5)(q?),+8[1]/46,XY[12] | — | — |
| 4 | M/74 | CMML | < 5 | 83 | 145,5 | 77 | 46-47,XY,+8[8]/47,idem,del(17)(p?)[4] | — | — |
| 5 | F/66 | RARS | < 5 | 80 | 3,0 | 85 | 45-48,XX,−4,del(5)(q?),del(7)(q?),+8,−16,−17, del(19)+1-6mar[33]/46,XX[2] | — | — |
| 6 | F/78 | RAEB | 10 | 100 | 3,0 | 100 | 47,XX,+8[27] | — | CT* |
| 7 | F/62 | RAEB | 10 | 65 | 7,7 | 211 | 47,XX,+8[5]/46,XX[5] | — | — |
| 8 | F/59 | RAEB | 10 | 75 | 4,4 | 20 | 50,XX,+8,+9,+14,+21[26] | IT† | — |
| 9 | F/73 | CMML | 12 | 48 | 80,8 | 105 | 47,XX,+8[4]/47,idem,del(17)(p?)[5] | — | Pred |
| 10 | F/74 | RAEB | 17 | 91 | 8,9 | 128 | 42-46,XX,del(5)(q22)[24]/47,XX,del(5)(q22),+8[1] | Pred | — |
| 11 | M/73 | RAEBt | 20 | 48 | 7,1 | 341 | 47,XY,+8[4]/46,XY[2] | — | — |
| 12 | M/51 | RAEBt | 25 | 51 | 4,3 | 71 | 45-47,XY,−5,del(7)(q22),+8,del(20)(q11.2),+20[7] | — | — |
| 13 | F/79 | MDS-AML‡ | 35 | 87 | 19,6 | 21 | 44,XX,−3,del(5)(q?),+8,−12,−13,−14,−15,−16[42] | — | — |
BM morphology and blood values of the patients were investigated in connection with BM aspirations for the present studies. Karyotypes were established on whole BM by metaphase analysis.
MDS indicates myelodysplastic syndromes; FAB, French-American-British classification; BM, bone marrow; RA, refractory anemia; RARS, refractory anemia with ringsideroblasts; RAEB, refractory anemia with excess of blasts; RAEBt, refractory anemia with excess of blasts in transformation; CMML, chronic myelo-monocytic leukemia; CT, chemotherapy; IT, immune therapy; and Pred, prednisolone.
Mitoxantrone + etoposide + arabinosylcytosine, 2 years prior to this study.
Antithymocyte-globuline + cyclosporin A + prednisolone.
Tri-lineage dysplasia.
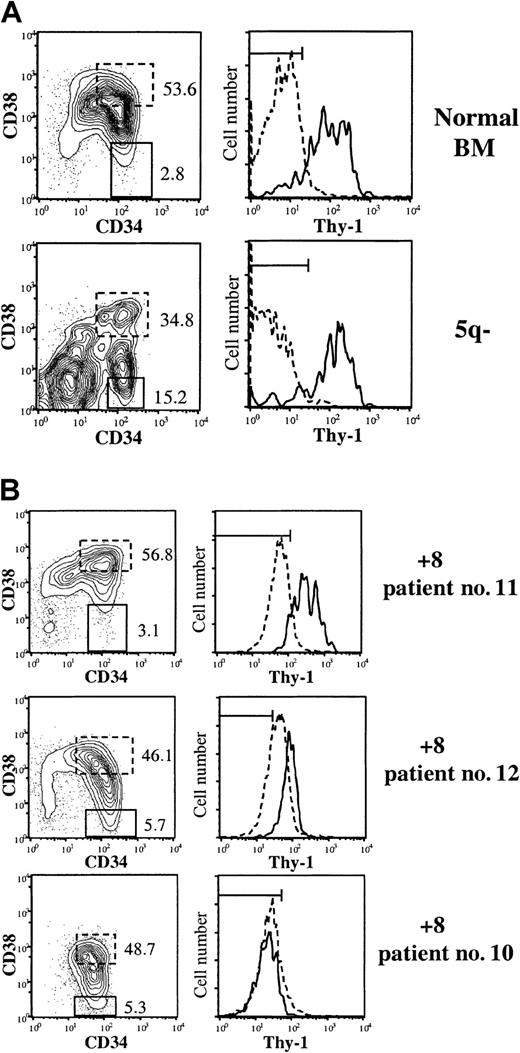
Thy-1 (CD90) expression within CD34+CD38− HSC compartment in MDS patients.
BM CD34-enriched cells (not MNC) were stained with MoAbs against CD34, CD38, and Thy-1 or irrelevant isotype control Abs. (A) Expression profiles of CD34+ cells from healthy subject and MDS case with del(5q). (B) Expression profiles of 3 MDS cases with trisomy 8. Left panels show coexpression of CD34 and CD38, with numbers indicating percentages of CD34+CD38+ and CD34+CD38− cells (of total CD34+cells) further investigated for Thy-1 expression in right panels. Dotted and solid lines represent Thy-1 expression on CD34+CD38+ and CD34+CD38− cells, respectively. Horizontal bars in the Thy-1 diagrams indicate gates for negative isotype control Ab, including at least 99% of CD34+CD38−cells.
Thy-1 (CD90) expression within CD34+CD38− HSC compartment in MDS patients.
BM CD34-enriched cells (not MNC) were stained with MoAbs against CD34, CD38, and Thy-1 or irrelevant isotype control Abs. (A) Expression profiles of CD34+ cells from healthy subject and MDS case with del(5q). (B) Expression profiles of 3 MDS cases with trisomy 8. Left panels show coexpression of CD34 and CD38, with numbers indicating percentages of CD34+CD38+ and CD34+CD38− cells (of total CD34+cells) further investigated for Thy-1 expression in right panels. Dotted and solid lines represent Thy-1 expression on CD34+CD38+ and CD34+CD38− cells, respectively. Horizontal bars in the Thy-1 diagrams indicate gates for negative isotype control Ab, including at least 99% of CD34+CD38−cells.
Purification of BM populations
BM cells were obtained from the posterior iliac crest and BM smears were used for May-Grünwald-Giemsa (MGG)–FISH for some patients. For the other patients, BM mononuclear cells (MNCs) were isolated by Ficoll-Hypaque (Nycomed, Oslo, Norway) gradient centrifugation and either processed directly or cryopreserved for later use. Positive selection of BM CD34+ cells was performed using a magnetically activated cell sorting (MACS) CD34 isolation kit (Miltenyi Biotec, Bergish Gladbach, Germany), as described previously.12,27 Mean purity of CD34+ cells was 72% (range 17%-99%). CD34-enriched cells were incubated with anti-CD38–phycoerythrin (PE) and CD34–fluorescein isothiocyanate (FITC) monoclonal antibodies (all from Becton Dickinson [BD], San Jose, CA). The 40% to 50% CD34+ cells expressing the highest levels of CD38 (CD34+CD38+) and CD34+CD38− cells were sorted on a FACS Vantage cell sorter (BD). A conservative approach was taken so that only the CD34+ cells with the “lowest” CD38 expression (approximately 3%) were sorted.27 For some patients a simultaneous staining with CD34 FITC, CD38 allophycocyanin (APC), and Thy-1 (CD90) PE was performed to evaluate the coexpression pattern of CD38 and Thy-1 on CD34+ cells. Sorted CD34+CD38− and CD34+CD38+ cells reproducibly had a purity of more than 90%.
FISH of FACS-purified BM populations
Cytospin preparations of various cell populations were pretreated as described previously.28 Briefly, slides were fixed in methanol/acetic acid (3:1) for 15 minutes, dehydrated in a graded (70% to 100%) series of ethanol, and air dried. The slides were then fixed in 1% neutral buffered formalin for 5 minutes, followed by microwave heat treatment in sodium citrate buffer, pH 6.0, 2 times for 5 minutes; 0.01% proteinase K (type VIII; Sigma, St Louis, MO) digestion for 5 minutes; and postfixation in neutral buffered formalin for 5 minutes before being dehydrated and air dried. The CEP 8 Spectrum Green centromeric probe was used either alone or together with the LSI EGR Spectrum Orange probe (both probes from Vysis, Downers Grove, MO) for 5q31. The probes were used according to the manufacturer′s instructions. After hybridization, the slides were washed in 1× standard saline citrate (SSC) for 5 minutes at 72°C, followed by washes in 0.05% TBS-Tween for 3 minutes at room temperature and counterstained with DAPI (4,6-diamino-2-phenyl-inol, 1 μg/mL; Sigma). In the nuclei of normal cells, the probe hybridizing to chromosome 8 appears as 2 green signals and cells with trisomy 8 appear as 3 green signals. Normal cells investigated with the probes hybridizing to 5q31 and chromosome 8 simultaneously, appear as 4 distinct signals, 2 orange and 2 green (2O+2G). Cells with del(5q) alone typically show 1 orange and 2 green (1O+2G), cells with trisomy 8 alone typically show 2 orange and 3 green (2O+3G), whereas cells with both del(5q) and trisomy 8 typically show 1 orange and 3 green (1O+3G). Occasionally, the probes may appear as other combinations of green and orange signals. Such combinations were excluded when calculating the frequency of 5q-deleted and/or trisomic 8 and normal cells in a specific cell population in the present study. Unless otherwise stated, 100 to 500 cells were evaluated for each cell population. For all cell populations investigated, cells from healthy subjects were treated the same way and used to determine the cut-off value for the FISH method; they always showed less than 0.3% of cells with 3 green signals when using the probe for chromosome 8 alone and less than 2.6% of 1O+2G, 0.1% of 2O+3G, and 0.04% of 1O+3G when using the probes for chromosome 8 and 5q31 simultaneously. When validating the specificity of FISH results on FACS-purified cell populations from patients with MDS, one also has to consider the likelihood of cells showing an abnormal signal pattern, originating from impurities. On the basis of the purity of sorted populations (> 90%) and with the cut-off value of the FISH methods, we generally considered findings of less than 15% cells with an abnormal signal pattern (depending on cell population) to be negative or inconclusive.
FISH on MGG-stained cells
BM smears were stained with MGG and individual cells were morphologically identified (erythroid = normoblasts, granulocytic = promyelocytes + myelocytes + metamyelocytes + bands + segmented + eosinophils) and by unique coordinates they were reidentified by dual FISH analysis for del(5q) and +8 simultaneously, as previously described.28When performing dual FISH on MGG-stained BM smears, the cut-off value for significant +8 findings was 2% to 3%.
Hematopoietic growth factors
Recombinant human (rh) megakaryocyte growth and development factor (MGDF), rh granulocyte–colony-stimulating factor (G-CSF), rh stem cell factor (SCF), rh interleukin 3 (IL-3), and rh granulocyte macrophage (GM)–CSF were generously provided by Amgen (Thousand Oaks, CA). Rh erythropoietin (Epo) was supplied by Boehringer Mannheim (Mannheim, Germany), and rh flt3 ligand (FL) was supplied by Immunex (Seattle, WA).
Single-cell clonogenic assay
As described previously,12,29CD34+CD38+ and CD34+CD38− cells were seeded in Terasaki plates (Nunc, Kamstrup, Denmark) at a density of one cell per well in 20 uL serum-depleted medium (X-vivo 15; BioWhittaker) supplemented with 1% bovine serum albumin (Stem Cell Technologies, Vancouver, Canada), 10−4 M 2-mercaptoethanol, and cytokines. Wells were scored for cell growth after 11 to 13 days of incubation, as previously described.27 A total of 120 wells were seeded per group, but since the statistical chance (based on Poisson probability distribution) of a well not receiving a cell is 37% by this method, the maximum expected clones was 76.
Long-term culture-initiating cell (LTC-IC) assay
Long-term cultures were either established with normal allogeneic BM MNCs according to previously described methods12,27 or with murine stromal feeders engineered to produce human growth factors (M2-10B4 and SI/SI mixed 1:1; kindly provided by D. E. Hogge, Vancouver, Canada).30 31 The murine stroma was established in 96-well collagen-coated microtiter plates with 5000 cells/well of each cell line, after irradiation with 8000 cGy and cultured in long-term culture medium (MyeloCult H5100; Stem Cell Technologies) with hydrocortisone 21-hemi-succinate 10−6M. CD34+CD38+ (250-25 000) and CD34+CD38− (75-11 000) cells from patients with MDS and healthy adults were added to the stroma layers and cocultures were maintained at 37°C in high humidity and with 50% medium exchange every week. After 6 weeks, nonadherent and adherent cells were plated in methylcellulose cultures, supplemented with SCF, GM-CSF, G-CSF, FL (all at 10 ng/mL), IL-3 (5 ng/mL), and Epo (5 U/mL). Colony-forming cells (CFCs; readout of LTC-IC assay) were scored after an additional 10 to 14 days in culture. Individual colonies were picked and transferred to slides for subsequent FISH analysis.
Nonobese diabetic–severe combined immunodeficiency (NOD-SCID) repopulating assay
NOD/LtSz-SCID or NOD/LtSz-SCID β2-microglobulin–deficient (B2mnull) mice (kindly provided by Dr L. D. Shultz, The Jackson Laboratory, Bar Harbor, ME) were bred and housed under sterile conditions in microisolator cages and given autoclaved food and acidified drinking water (and for the NOD/LtSz-SCID B2mnull mice also prophylactic trimethoprim-sulfadoxine [80/400 μg/mL]), 3 times a week during both breeding and experiments. Mice were irradiated with 350 to 375 cGy from a 137cesium source at 8 to 12 weeks of age and thereafter given prophylactic ciprofloxacin (100 μg/mL) in the drinking water until analysis (6-8 weeks after transplantation). Tail-vein transplantation/injection of hematopoietic cells suspended in 0.5 to 1.0 mL of medium was performed within 4 hours of irradiation. When less than 1 × 105 cells were transplanted, 1 × 106 irradiated (1500 rad) accessory cells (human MNCs or CD34-depleted cells) were coinjected. Femora and tibiae were collected after asphyxiation with CO2, and engraftment was investigated as described previously.12 31Briefly, cells were stained with anti-human CD45–FITC and CD71-FITC antibodies as well as anti-mouse CD45.1 (Ly 5.1)–PE. Mice that did not receive transplants (negative controls) and mixtures of 0.5% human cells in murine BM (positive controls) were always included. If engraftment was detected by CD45/71 analysis (detection level < 0.05%), staining with anti-CD34–FITC, CD19-PE, CD33-PE/CD66b-FITC, and CD15-PE was performed, including antihuman CD45-APC. 7-amino actinomycin D (7-AAD; Sigma) was always included to exclude dead cells. A minimum of 50 000 BM cells was examined for each sample. Only mice positive for both myeloid and lymphoid human engraftment (defined as > 10 positive events each per 50 000 viable BM cells) were evaluated as positive. Gates were set so that samples incubated with irrelevant isotype-matched control antibodies had a maximum of one positive event per 50 000 BM cells analyzed. If no engraftment was detected by flow cytometry or if the myeloid engraftment was low as defined by flow cytometric analysis, BM cells (5 × 104to 1 × 105) from NOD-SCID mice were plated in methylcellulose with human-specific cytokines (50 ng/mL GM-CSF, 25 ng/mL IL-3, and 25 ng/mL SCF) and 5 U/mL Epo, resulting in no colony formation of BM from NOD-SCID mice that did not receive transplants (L.N. and S.E.W.J., unpublished data, April 1999). Granulocyte macrophage–colony forming units (CFU-GMs) and erythroid–burst-forming units (BFU-Es) were scored after 10 to 14 days and transferred to slides for FISH analysis.
Results
Involvement of the CD34+CD38−Thy-1+ stem cell compartment in MDS trisomy 8 clones
Previous studies have demonstrated that most if not all HSC reconstituting activity in normal BM is derived from CD34+CD38− cells, although representing less than 0.1% of total BM cells.16,21,22 The CD34+CD38− fraction also appears to harbor the HSCs in patients with AML.32 Here we investigated the coexpression of CD34 and CD38 in 10 MDS cases with +8 (Table2). As expected, the mean frequency of CD34+ cells in BM MNCs was slightly higher than in healthy subjects, but as previously demonstrated for healthy individuals,16 the majority of the CD34+ cells in all investigated MDS cases with +8 coexpressed CD38 and only a minority of the cells were found to be CD34+CD38−, in agreement with what we recently observed in MDS cases with del(5q).12
Frequencies of CD34+CD38+ and CD34+CD38− progenitor/stem cells in patients with trisomy 8 and involvement in the trisomy 8 clone
| Patient no. . | %CD34+ . | %CD34+CD38+ . | %CD34+CD38− . | % cells with trisomy 8 . | ||
|---|---|---|---|---|---|---|
| CD34− . | CD34+CD38+ . | CD34+CD38− . | ||||
| 1 | 6.2 | 6.13 | 0.07 | 28 | 25 | 66 |
| 2 | 2.5 | 2.48 | 0.01 | nd | 80 | 67 (49)* |
| 4 | 0.8 | 0.60 | 0.20 | nd | 79 | 56 (32)* |
| 6 | 10 | 8.96 | 1.19 | 42 | 5 | 0 (56)* |
| 7 | 0.7 | 0.63 | 0.07 | nd | 67 | 33 |
| 8 | 8.8 | 8.27 | 0.53 | 60 | 78 | 71 |
| 9 | 14 | 11.2 | 2.78 | nd | 78 | 68 |
| 10 | 8.0 | 6.91 | 1.08 | nd | 20 | 66 |
| 11 | 0.7 | 0.68 | 0.02 | 14 | 44† | 52/63‡ |
| 12 | 0.7 | 0.53 | 0.17 | 26 | 69† | 73/79‡ |
| Mean (SEM) | 5.2 (1.5) | 4.64 (1.3) | 0.61 (0.3) | 34 (8.8) | 55 (9.5) | 56 (7.7) |
| Normal BM | 2.6 (0.2) | 2.4 (0.2) | 0.14 (0.03) | 0 | 0.2 | 0.3 |
| Patient no. . | %CD34+ . | %CD34+CD38+ . | %CD34+CD38− . | % cells with trisomy 8 . | ||
|---|---|---|---|---|---|---|
| CD34− . | CD34+CD38+ . | CD34+CD38− . | ||||
| 1 | 6.2 | 6.13 | 0.07 | 28 | 25 | 66 |
| 2 | 2.5 | 2.48 | 0.01 | nd | 80 | 67 (49)* |
| 4 | 0.8 | 0.60 | 0.20 | nd | 79 | 56 (32)* |
| 6 | 10 | 8.96 | 1.19 | 42 | 5 | 0 (56)* |
| 7 | 0.7 | 0.63 | 0.07 | nd | 67 | 33 |
| 8 | 8.8 | 8.27 | 0.53 | 60 | 78 | 71 |
| 9 | 14 | 11.2 | 2.78 | nd | 78 | 68 |
| 10 | 8.0 | 6.91 | 1.08 | nd | 20 | 66 |
| 11 | 0.7 | 0.68 | 0.02 | 14 | 44† | 52/63‡ |
| 12 | 0.7 | 0.53 | 0.17 | 26 | 69† | 73/79‡ |
| Mean (SEM) | 5.2 (1.5) | 4.64 (1.3) | 0.61 (0.3) | 34 (8.8) | 55 (9.5) | 56 (7.7) |
| Normal BM | 2.6 (0.2) | 2.4 (0.2) | 0.14 (0.03) | 0 | 0.2 | 0.3 |
The relative frequencies of CD34+, CD34+CD38+ and CD34+CD38− cells in bone marrow (BM) mononuclear cells (MNCs) were determined at the time of BM aspirations for the present studies. The values for normal controls (n = 10) represent the mean (SEM), analyzed in parallel.
CD34−, CD34+CD38+, and CD34+CD38− (and when indicated CD34+CD38−Thy-1+ and CD34+CD38−Thy-1−) cells were purified as described in “Patients, materials, and methods” and subsequently transferred to slides by cytospin centrifugation before being analyzed by fluorescence in situ hybridization (FISH).
Normal BM CD34+CD38+ and CD34+CD38− cells were sorted and analyzed by FISH in an identical manner and the percentage of cells with 3 signals was established.
nd indicates not determined.
Fewer than 100 cells counted (counted cells are shown in parentheses).
CD34-enriched cells.
Values for CD34+CD38−Thy-1+ and CD34+CD38−Thy-1− cells, respectively.
CD34+CD38+ and CD34+CD38− cells purified by FACS were next investigated by FISH for the presence of +8 (Table 2). In contrast to del(5q) MDS cases where (in all cases) 94% to 99% of the CD34+CD38− HSC population carried the 5q deletion,12 cases with +8 showed a tendency toward fewer cells being part of the +8 clone and much more variability, in that 0% to 71% of the CD34+CD38− cells carried the trisomy 8 (Table 2). Comparable results were observed for the CD34+CD38+ progenitor population (Table 2).
Whereas Thy-1 is known to be expressed on 5% to 25% of normal CD34+ cells, and CD34+Thy-1+ cells are highly enriched in HSC activity,17,33 its pattern of expression in hematologic malignancies is less clear. In AML, Thy-1 is usually not expressed or only at very low levels on CD34+ cells.34-36 Thus, it was of interest to also investigate Thy-1 expression in patients with MDS. In agreement with its usefulness as an HSC marker, the vast majority of CD34+CD38− cells from healthy subjects were Thy-1+, whereas CD34+CD38+ (high CD38-expressing) cells were Thy-1−, and strikingly, 2 investigated cases with del(5q) showed an identical (normal) pattern (Figure 1A). The pattern for the MDS cases with trisomy 8 was more heterogeneous in that some +8 cases showed the highest levels of Thy-1 expression within CD34+CD38− HSC compartment (2 patients) whereas in others (2 patients) no Thy-1 expression was observed (Figure1B). In 2 of the MDS cases with +8 (no. 11 and no. 12) we were able to sort sufficient CD34+CD38−Thy1+and CD34+CD38−Thy1− cells for FISH analysis, and found that both populations contained a high frequency of +8 cells but also cells disomic for chromosome 8 (Table2).
Functional evaluation of the HSC compartment in patients with MDS trisomy 8
The lack of CD34+Thy1+ cells carrying +8 in the study by Saitoh et al24 suggested that the HSC compartment in these patients with MDS may be normal. Although our FISH data indicated that a variable part of the CD34+CD38− HSC pool belonged to the +8 clone, there was always a sizeable fraction that did not carry this aberration. Therefore, as in the study by Saitoh et al,24these may potentially represent a residual and sizeable normal HSC pool, thereby providing a potential strategy for utilizing high-dose chemotherapy followed by autologous transplantation in these patients.13,24 However, the FISH data did not exclude the possibility that CD34+CD38− cells disomic for chromosome 8 are still part of the MDS clone (ie, the cells with trisomy 8 may represent a subclone). Although phenotypic (and gene expression) profiling is important for identifying HSCs, the presence of normal HSCs can only be demonstrated through their unique functional capacities.20 Normal HSCs show a characteristic response pattern to nonredundant HSC active cytokines,29,37 and reconstitute long-term cultures in vitro and immune-deficient mice in vivo.19,38 39 Thus, we here functionally characterized the CD34+CD38− (as well as the CD34+CD38+) HSC compartment of MDS cases with trisomy 8.
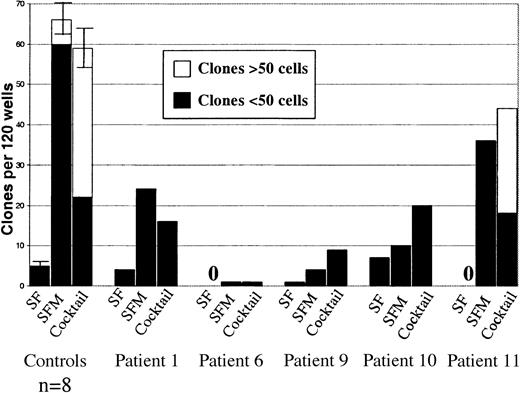
First, we tested the responsiveness of single CD34+CD38− cells to the cytokines SCF, FL, and MGDF that have been shown to be particularly effective at recruiting normal CD34+CD38− candidate HSCs into proliferation.29 As demonstrated before,29the combination of SCF and FL (in the absence of MGDF) showed little or no ability to recruit normal as well as MDS CD34+CD38− cells (Figure2). The addition of MGDF to SCF and FL resulted in efficient recruitment of normal CD34+CD38− cells into proliferation and also enhanced recruitment of CD34+CD38− cells in most patients with MDS (Figure 2). Finally, as for normal CD34+CD38− cells, the use of additional cytokines had little or no effect on recruitment of CD34+CD38− cells in most of the patients with MDS. Although purified CD34+CD38− cells from MDS cases with trisomy 8 showed a typical cytokine response pattern compatible with representing HSCs, they displayed a suboptimal growth potential compared with normal CD34+CD38−cells, as reflected in the reduced number and size of clones (Figure2).
Responsiveness of CD34+CD38−MDS cells to early-acting cytokines.
Single CD34+CD38− cells from 5 MDS cases with trisomy 8 were plated in 3 different cytokine combinations (SF = SCF + FL, SFM = SCF + FL + MGDF and Cocktail = SCF + FL + MGDF + IL-3 + G-CSF + GM-CSF + EPO) as described in “Patients, materials, and methods.” Also shown (to the left) are results from identical experiments with CD34+CD38− cells from 8 healthy subjects (error bars show SEM). Wells were scored for total clones per 120 wells and for size (3-50 cells or more than 50 cells) after 11-13 days of incubation. No clones were observed for patients #6 and #11 when stimulated only with SCF + FL. In general, clones from all MDS patients were too small to be analyzed by FISH.
Responsiveness of CD34+CD38−MDS cells to early-acting cytokines.
Single CD34+CD38− cells from 5 MDS cases with trisomy 8 were plated in 3 different cytokine combinations (SF = SCF + FL, SFM = SCF + FL + MGDF and Cocktail = SCF + FL + MGDF + IL-3 + G-CSF + GM-CSF + EPO) as described in “Patients, materials, and methods.” Also shown (to the left) are results from identical experiments with CD34+CD38− cells from 8 healthy subjects (error bars show SEM). Wells were scored for total clones per 120 wells and for size (3-50 cells or more than 50 cells) after 11-13 days of incubation. No clones were observed for patients #6 and #11 when stimulated only with SCF + FL. In general, clones from all MDS patients were too small to be analyzed by FISH.
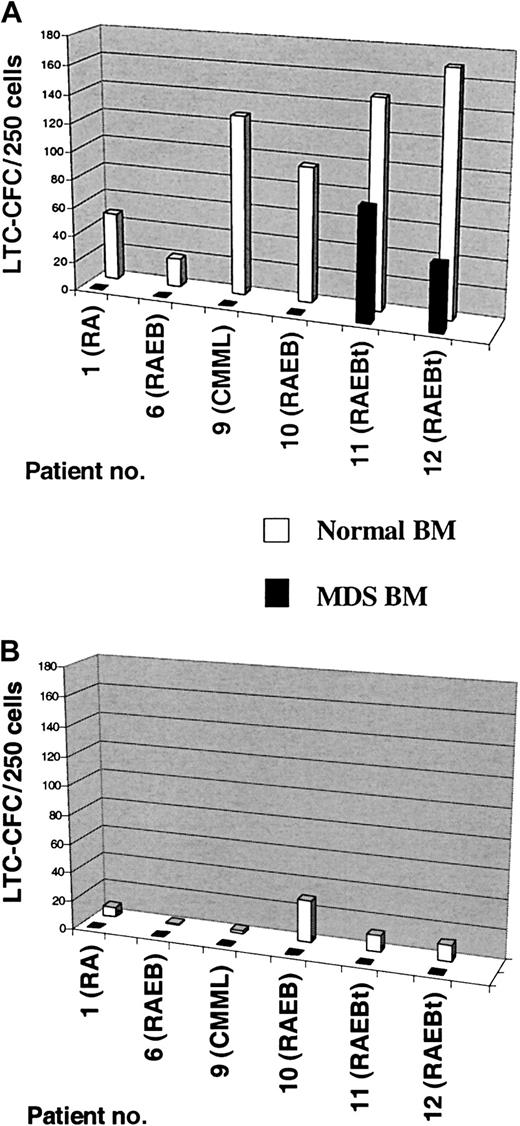
As previously demonstrated,19 normal CD34+CD38− cells were in each experiment (n = 6) found to be highly enriched in LTC-IC activity (Figure3A). Strikingly, in 4 patients with MDS (no. 1, no. 6, no. 9, and no. 10; all with < 20% BM blasts), absolutely no LTC-IC activity could be detected in the CD34+CD38− population, despite the fact that this subset always contained a sizeable fraction of cells without +8. In fact, even when as much as 100-fold more CD34+CD38− cells were used than in the normal controls, no LTC-IC activity was observed. In contrast, LTC-IC activity was observed in the CD34+CD38− cells purified from the 2 patients with MDS with most advanced disease (RAEBt; ≥ 20% blasts). However, in these 2 patients the LTC-CFC activity was derived from the +8 clone (L.N. and S.E.W.J., unpublished data, November 2001), and thus no evidence for normal HSC activity was observed in the CD34+CD38− population of any of the investigated MDS cases with trisomy 8. As expected, normal CD34+CD38+ cells contained much less LTC-IC activity than CD34+CD38− cells, and no LTC-CFC activity was observed from CD34+CD38+ cells in any of the 6 investigated MDS cases with trisomy 8 (Figure 3B).
LTC-IC activity of CD34+CD38−and CD34+CD38+ cells in MDS cases with +8.
Six-week LTC-CFC activity of MDS BM CD34+CD38−(A) and CD34+CD38+ (B) cells was evaluated as described in “Patients, materials, and methods.” CD34+CD38− and CD34+CD38+ cells from healthy subjects were included as controls in all experiments. After 6 weeks of culture, the number of CFCs produced was evaluated in methylcellulose. Results are the mean of 3-6 replicate wells from each cell population and patient. For patients #1 and #6 human allogeneic stroma was used while murine stromal feeders was used for patients #9, #10, #11, and #12. Note that the number of colonies found for the normal controls evaluated on human allogeneic stromas (patients #1 and #6) are significantly lower than those evaluated on murine stromal feeders (patients #9, #10, #11, and #12), reflecting the higher efficiency of the murine stromal feeders. Note also that no LTC-CFC activity was observed in the CD34+CD38+ populations of any MDS patient or in the CD34+CD38− population from patients #1, #6, #9, and #10 and that the only 2 MDS patients with LTC-CFC activity derived from CD34+CD38− population were those with most advanced MDS (RAEBt with 20% and 25% BM blasts, respectively).
LTC-IC activity of CD34+CD38−and CD34+CD38+ cells in MDS cases with +8.
Six-week LTC-CFC activity of MDS BM CD34+CD38−(A) and CD34+CD38+ (B) cells was evaluated as described in “Patients, materials, and methods.” CD34+CD38− and CD34+CD38+ cells from healthy subjects were included as controls in all experiments. After 6 weeks of culture, the number of CFCs produced was evaluated in methylcellulose. Results are the mean of 3-6 replicate wells from each cell population and patient. For patients #1 and #6 human allogeneic stroma was used while murine stromal feeders was used for patients #9, #10, #11, and #12. Note that the number of colonies found for the normal controls evaluated on human allogeneic stromas (patients #1 and #6) are significantly lower than those evaluated on murine stromal feeders (patients #9, #10, #11, and #12), reflecting the higher efficiency of the murine stromal feeders. Note also that no LTC-CFC activity was observed in the CD34+CD38+ populations of any MDS patient or in the CD34+CD38− population from patients #1, #6, #9, and #10 and that the only 2 MDS patients with LTC-CFC activity derived from CD34+CD38− population were those with most advanced MDS (RAEBt with 20% and 25% BM blasts, respectively).
Of the trisomy 8 cases that demonstrated a complete lack of LTC-IC activity, 3 were also investigated for potential in vivo HSC activity in the NOD-SCID transplantation assay. Whereas 6 of 17 mice that received transplants with normal BM CD34+(168 000-500 000) or CD34+CD38− (10-14 000) cells showed human lymphoid-myeloid reconstitution, no mice were reconstituted with comparable or higher numbers of CD34+ or CD34+CD38− cells from patients no. 1 and no. 9 (Table 3). Very high numbers of CD34+CD38+ cells from these patients also failed to reconstitute NOD-SCID mice. In patient no. 10, we were able to obtain a sufficient number of CD34+ cells to allow transplantation of as much as 2.5 million cells per mice, but again no reconstituting activity could be observed (Table 3). Thus, despite the content of large numbers of cells without +8, no normal HSC activity could be detected in any of the investigated patients with MDS in the in vitro LTC-IC assay or in the in vivo NOD-SCID repopulation model.
Deficient in vivo multilineage reconstitution ability of MDS BM CD34+ cells in NOD-SCID mice
| Cells . | Number of cells transplanted . | Mice3-150 . | Human reconstitution . | |
|---|---|---|---|---|
| % human engraftment . | Multilineage engraftment, positive mice . | |||
| Patient no. 1 | ||||
| CD34+ | 200 000 | N/S | 0 | 0/2 |
| CD34+CD38+ | 2 500 000 | N/S | 0 | 0/1 |
| CD34+CD38− | 17 500 | N/S | 0.233-151 | 0/3 |
| Patient no. 9 | ||||
| CD34+CD38+ | 200 000 | β2N/S | 0 | 0/2 |
| CD34+CD38− | 20 000 | β2N/S | 0 | 0/5 |
| Patient no. 10 | ||||
| CD34+ | 1 000 000 | β2N/S | 0 | 0/2 |
| CD34+ | 2 500 000 | N/S | 0 | 0/2 |
| Total MDS, patients 1, 9, 10 | — | — | 0/17 | |
| Normal BM populations | ||||
| First: CD34+ | 500 000 | N/S | 5.0 | 1/4 |
| Second: | ||||
| CD34+ | 168 000 | N/S | 2.8-0.8 | 2/5 |
| CD34+CD38− | 10 000 | N/S | 10.6 | 1/6 |
| Third: CD34+CD38− | 14 000 | β2N/S | 0.2-0.5 | 2/2 |
| Total, normal BM | — | — | 6/17 | |
| Cells . | Number of cells transplanted . | Mice3-150 . | Human reconstitution . | |
|---|---|---|---|---|
| % human engraftment . | Multilineage engraftment, positive mice . | |||
| Patient no. 1 | ||||
| CD34+ | 200 000 | N/S | 0 | 0/2 |
| CD34+CD38+ | 2 500 000 | N/S | 0 | 0/1 |
| CD34+CD38− | 17 500 | N/S | 0.233-151 | 0/3 |
| Patient no. 9 | ||||
| CD34+CD38+ | 200 000 | β2N/S | 0 | 0/2 |
| CD34+CD38− | 20 000 | β2N/S | 0 | 0/5 |
| Patient no. 10 | ||||
| CD34+ | 1 000 000 | β2N/S | 0 | 0/2 |
| CD34+ | 2 500 000 | N/S | 0 | 0/2 |
| Total MDS, patients 1, 9, 10 | — | — | 0/17 | |
| Normal BM populations | ||||
| First: CD34+ | 500 000 | N/S | 5.0 | 1/4 |
| Second: | ||||
| CD34+ | 168 000 | N/S | 2.8-0.8 | 2/5 |
| CD34+CD38− | 10 000 | N/S | 10.6 | 1/6 |
| Third: CD34+CD38− | 14 000 | β2N/S | 0.2-0.5 | 2/2 |
| Total, normal BM | — | — | 6/17 | |
Irradiated nonobese diabetic–severe combined immunodeficiency (NOD-SCID) mice received transplants of the indicated bone marrow (BM) cell types and numbers from patients with myelodysplastic syndrome (MDS) and healthy controls, and were investigated for human reconstitution 6 to 8 weeks later (“Patients, materials, and methods”). Data are presented as the number of mice positive for human reconstitution with both B cells and myeloid cells. Also shown is the percent of human engraftment (representing total human reconstitution independent of whether both myeloid and lymphoid reconstitution were observed). Note that no multilineage engraftment was seen in any mouse that received a transplant with CD34 subpopulations from patients with MDS, in spite of having received a transplant with as high or a higher number of cells than was used in parallel for normal BM CD34+ populations (positive control).
N/S indicates NOD-SCID mice and β2N/S indicates β2-microglobulin–deficient NOD-SCID mice.
No myeloid reconstitution, only CD45+CD19+ cells with disomy 8, most likely representing normal B cells.
MDS cases with trisomy 8 have an intrinsic HSC deficiency, also involving HSC disomic for chromosome 8
The functional studies described above clearly suggested that in all trisomy 8 cases investigated, the large numbers of CD34+CD38− cells with disomy 8 were also functionally deficient. To obtain more direct evidence for this notion and to address whether the deficiency was intrinsic to the HSCs, we next performed 2 lines of experiments. In the first, we addressed whether the presence of MDS cells might negatively affect potentially coexisting normal HSCs. Toward this aim, CD34+CD38− cells were isolated from 2 patients with MDS in whom the CD34+CD38− cells had no LTC-IC activity (Table 4). These CD34+CD38− cells were next mixed with CD34+CD38− cells isolated from a healthy donor and cultured in the long-term culture assay. In both cases (one trisomy 8 and one del[20q]), the presence of MDS CD34+CD38− cells, even at 10- to 100-fold higher numbers than normal CD34+CD38− cells, had no effect on the activity of LTC-IC derived from the normal cells (Table 4). As expected, FISH analysis demonstrated that the LTC-CFCs were without aberrations and derived from the healthy donor (opposite sex) (L.N. and S.E.W.J., unpublished data, November 2001).
The presence of excess MDS CD34+CD38− cells does not influence HSC activity of normal CD34+CD38− cells
| Patient . | Number of CD34+CD38− cells seeded . | LTC-CFC . | |
|---|---|---|---|
| Normal . | MDS . | ||
| Patient no. 10 | 0 | 75 | 0 |
| 0 | 750 | 0 | |
| 0 | 7500 | 0 | |
| 75 | 0 | 29 | |
| 75 | 75 | 31 | |
| 75 | 750 | 30 | |
| Patient with del(20q) MDS | 0 | 75 | 0 |
| 0 | 750 | 0 | |
| 75 | 0 | 17 | |
| 75 | 75 | 13 | |
| 75 | 750 | 19 | |
| Patient . | Number of CD34+CD38− cells seeded . | LTC-CFC . | |
|---|---|---|---|
| Normal . | MDS . | ||
| Patient no. 10 | 0 | 75 | 0 |
| 0 | 750 | 0 | |
| 0 | 7500 | 0 | |
| 75 | 0 | 29 | |
| 75 | 75 | 31 | |
| 75 | 750 | 30 | |
| Patient with del(20q) MDS | 0 | 75 | 0 |
| 0 | 750 | 0 | |
| 75 | 0 | 17 | |
| 75 | 75 | 13 | |
| 75 | 750 | 19 | |
Purified CD34+CD38− cells from patient no. 10 with MDS and +8 and a patient with MDS with del(20q) (see “Patients, materials, and methods”) were seeded at different cell numbers, either alone or together with CD34+CD38− cells from a healthy donor onto murine stromal feeders (see “Patients, materials, and methods”). Normal CD34+CD38− cells were also cultured in the absence of MDS cells. After 6 weeks of weekly half medium exchange, the LTC-CFC activity was evaluated and colonies were picked to establish the presence of + 8 or del(20q). In patient no. 10, 66% of the starting CD34+CD38− cells were + 8, whereas in the other patient, 96.5% of the sorted CD34+CD38− cells had del(20q). Colony numbers represent the mean of 3 to 6 replicates.
MDS indicates myelodysplastic syndrome; HSC, hematopoietic stem cell; LTC-CFC, long-term culture–colony forming cells.
These data suggested that in the investigated trisomy 8 cases the vast majority of CD34+CD38− cells without this aberration had an intrinsic deficiency. In an effort to address directly whether these cells were part of the MDS clone, we analyzed cases that in addition to trisomy 8 had a clonal marker for the disease that could be present more ubiquitously in the HSC pool. Since we had previously demonstrated that 5q deletions usually occur in the CD34+CD38− HSC pool, we identified 4 patients who had both of these aberrations. In one of these patients we were able to sort the CD34+CD38− HSC population and by dual FISH analysis demonstrate that although virtually all cells were 5q deleted, only 66% of these carried the +8 aberration, and no +8 cells without the 5q deletion were observed (Table5 and Figure4). Similar findings were observed in the CD34+CD38+ progenitor pool as well as in the granulocytic and erythroid lineages. Noteworthy, in this patient the majority of the CD34+CD38− cells harbored both del(5q) and +8, suggesting a competitive advantage over cells with only the 5q deletion in the HSC pool. In contrast, in both the CD34+CD38+ progenitor pool and the erythroid and granulocytic lineages, the majority of cells had only del(5q), indicating that the acquisition of trisomy 8 in this case was accompanied by a more deficient differentiation potential.
Dual-FISH analysis of BM populations of an MDS case with combined del(5q) and trisomy 8
| Cell population . | FISH results, % of total cells . | |||
|---|---|---|---|---|
| Normal5-150 . | +8 alone . | 5q- alone . | 5q- and +8 . | |
| CD34+CD38−(HSCs) | 0 | 0 | 34 | 66 |
| CD34+CD38+(progenitors) | 0 | 0 | 80 | 20 |
| Granulocytic5-151 | 2 | 0 | 81 | 17 |
| Erythroid (normoblasts) | 12 | 0 | 82 | 6 |
| Cell population . | FISH results, % of total cells . | |||
|---|---|---|---|---|
| Normal5-150 . | +8 alone . | 5q- alone . | 5q- and +8 . | |
| CD34+CD38−(HSCs) | 0 | 0 | 34 | 66 |
| CD34+CD38+(progenitors) | 0 | 0 | 80 | 20 |
| Granulocytic5-151 | 2 | 0 | 81 | 17 |
| Erythroid (normoblasts) | 12 | 0 | 82 | 6 |
A patient with myelodysplastic syndrome (MDS) with both 5q deletion and trisomy 8 (patient no. 10) was investigated by dual fluorescence in situ hybridization (FISH) with DNA probes hybridizing to 5q31 and to chromosome 8. Note that regardless of the cell population investigated, the del(5q) was found in the majority but trisomy 8 only in a fraction of the cells. There were no cells with trisomy 8 alone (without concomitant 5q deletion). Hematopoietic stem cell (HSC) and progenitor populations were obtained by fluorescence activated cell sorting (FACS) purification whereas granulocyte and erythroid cell lineages were investigated by morphologic identification after May-Grünwald-Giemsa (MGG) staining.
Normal indicates cells with normal chromosome 5 and 8 status.
Includes all stages of granulocytic differentiation in the bone marrow (dysplastic and nondysplastic), except blasts.
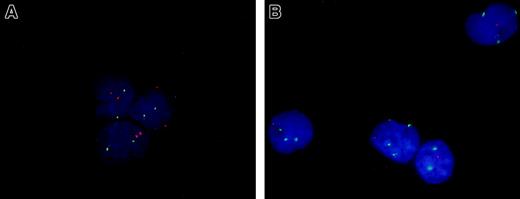
Dual FISH analysis of CD34+CD38− cells from an MDS case with both del(5q) and trisomy 8.
Purified BM CD34+CD38− cells from a healthy donor (A) and an MDS case with both del(5q) and trisomy 8 (B) were subjected to dual FISH analysis. The nuclei of cells with normal chromosome 5 and 8 status display 2 orange (5q) and 2 green (8) signals (all 3 cells in panel A). In panel B all 4 nuclei had del(5q) and 3 of these displayed +8 in addition. Original magnification approximately × 1000.
Dual FISH analysis of CD34+CD38− cells from an MDS case with both del(5q) and trisomy 8.
Purified BM CD34+CD38− cells from a healthy donor (A) and an MDS case with both del(5q) and trisomy 8 (B) were subjected to dual FISH analysis. The nuclei of cells with normal chromosome 5 and 8 status display 2 orange (5q) and 2 green (8) signals (all 3 cells in panel A). In panel B all 4 nuclei had del(5q) and 3 of these displayed +8 in addition. Original magnification approximately × 1000.
There were 3 additional patients (no. 3, no. 5, and no. 13) who were investigated by dual FISH combined with MGG staining (Figure5). Also in all of these patients, all cells either carried only del(5q) or both del(5q) and +8, whereas no cells with +8 alone were observed. Thus, trisomy 8 appears to be a secondary or late event in MDS, and although this might occur outside of the HSC pool, the primary transformation appears to occur at the CD34+CD38− HSC level.
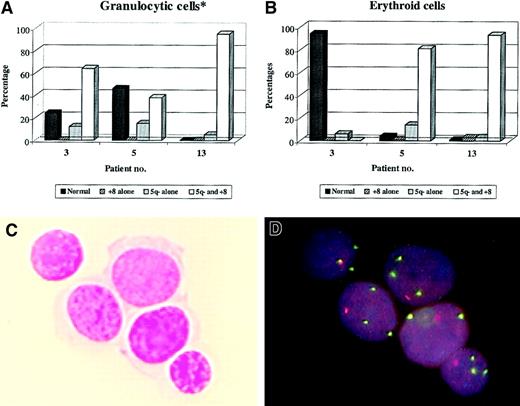
Dual FISH analysis of BM smears from MDS cases with both del(5q) and trisomy 8.
MGG-stained BM smears from 3 MDS cases known to have both del(5q) and trisomy 8 were investigated by dual FISH analysis for presence of the 2 abberations in the granulocytic (A) and erythroid (B) lineages (as described in Table 5). Data are presented as percentage of total cells within each lineage that had a normal chromosome 5 and 8 status or different combinations of 5q deletion and/or trisomy 8 (cutoff value at 2%-3% marked with horizontal lines). *See Table 5. Also shown is an example of MGG-stained erythroid cells (C) from patient no. 5 and the subsequent dual FISH analysis of the same cells (D). Note that all cells have both del(5q) and trisomy 8. Original magnification approximately × 1000.
Dual FISH analysis of BM smears from MDS cases with both del(5q) and trisomy 8.
MGG-stained BM smears from 3 MDS cases known to have both del(5q) and trisomy 8 were investigated by dual FISH analysis for presence of the 2 abberations in the granulocytic (A) and erythroid (B) lineages (as described in Table 5). Data are presented as percentage of total cells within each lineage that had a normal chromosome 5 and 8 status or different combinations of 5q deletion and/or trisomy 8 (cutoff value at 2%-3% marked with horizontal lines). *See Table 5. Also shown is an example of MGG-stained erythroid cells (C) from patient no. 5 and the subsequent dual FISH analysis of the same cells (D). Note that all cells have both del(5q) and trisomy 8. Original magnification approximately × 1000.
Discussion
Allogeneic bone marrow transplantation represents the only curative treatment for MDS but is rarely a realistic therapeutic option, due to the advanced age of most patients and the limited availability of donors.1,3 From the search for new therapeutic modalities, autologous transplantation has emerged as a new and potentially useful approach for treating MDS.3,40Thus, the potential involvement and status of the HSC pool in patients with MDS could be of considerable importance for the clinical outcome of autologous transplantation. As such, autologous HSC transplantation has been implicated as a potentially useful approach in patients with MDS with trisomy 8, because the CD34+Thy1+ HSC pool in these patients was found not to carry the +8 aberration,24 therefore potentially representing residual normal HSCs. At variance with this, we had previously shown that the CD34+CD38− HSC pool is invariably part of the MDS clone in patients with 5q deletions12 and findings in MDS cases with monosomy 7 also support that MDS may arise at a lymphomyeloid stem cell level.41 These diverging findings could potentially reflect the extensive heterogeneity in MDS. However, combining phenotypic, cytogenetic, and functional characterization of the CD34+CD38− HSCs in a number of trisomy 8 patients, we here present several lines of evidence suggesting that the CD34+CD38− HSC pool is frequently part of and therefore probably the origin of the MDS clone also in cases with trisomy 8.
Whereas none of the 12 previously investigated MDS cases with del(5q) had more than 6% of cells in the CD34+CD38−HSC pool with a normal chromosome 5 status,12 the level of involvement was considerably lower in all cases with trisomy +8. In fact, 20% to 100% of the CD34+CD38− (or CD34+CD38−Thy-1+) cells were disomic for chromosome 8. Because only a small fraction of CD34+CD38− cells are thought to represent true HSCs,38 this could mean that the functionally, rather than the phenotypically, defined HSC pool may not belong to the +8 clone in these patients, and therefore also potentially not be part of the disease. However, because normal human HSCs cannot be defined reliably based on phenotypic features and/or absence of known cytogenetic aberrations alone, it is crucial to identify them also by their unique function, as reflected by their ability to reconstitute long-term cultures in vitro as well as immune-deficient mice in vivo.19,20 22 Using both of these approaches we could detect little or no normal HSC activity in the CD34+CD38− (or CD34+CD38+) population of all investigated MDS cases with trisomy 8, suggesting that the CD34+CD38− pool is part of the MDS clone.
Interestingly, in MDS cases with trisomy 8 at earlier stages of disease, the CD34+CD38− (or CD34+CD38+) cells failed to reconstitute hematopoiesis in vitro or in vivo, reflecting the ineffective hematopoiesis of these patients. In contrast, the purified CD34+CD38− (but not CD34+CD38+) population from the 2 patients with most advanced MDS (RAEB-t) showed LTC-IC activity, but also in these cases no normal HSC activity was observed, because all LTC-CFC activity was derived from the trisomy 8 clone. Noteworthy, these findings were in agreement with those of del(5q) cases, where also CD34+CD38− (and CD34+CD38+) cells isolated from patients prior to transformation failed to reconstitute long-term cultures, whereas 5q-deleted CD34+CD38− (but not CD34+CD38+) cells from a transformed MDS case with del(5q) showed high but abnormal reconstituting activity.12 In addition to reflecting the advanced/transformed disease stage of these patients with MDS, the findings also support that the minor CD34+CD38− population in MDS cases with del(5q) as well as with trisomy 8 represent the MDS stem cell population, although representing less than 1% of the total BM cells in these patients. Several other lines of evidence support this conclusion. Thy-1, which has been shown to be another marker for normal human HSCs,17,33 was coexpressed on CD34+CD38− but not on CD34+CD38+ cells from investigated MDS cases with del(5q). A higher level of Thy-1 expression was also observed on CD34+CD38− cells in some trisomy 8 cases, although some cases lacked detectable Thy-1 expression in the CD34+CD38− population. Interestingly, in AML, Thy-1 expression has been demonstrated to be down-regulated,34-36 and it is thus tempting to speculate that this occurs as MDS HSCs progress toward transformation to AML.
As previously demonstrated for MDS cases with del(5q), CD34+CD38− cells from most trisomy 8 cases showed a low but typical HSC in vitro cytokine response pattern,29 in particular by revealing responsiveness to the early-acting cytokines MGDF, FL, and KL.
There are 2 lines of evidence that strongly support that the absence of detectable normal HSC activity in CD34+CD38−cells with disomy 8 was not due to indirect suppressive effects of MDS cells, but rather intrinsic to the CD34+CD38−HSC population. First, the in vitro reconstituting activity of CD34+CD38− cells isolated from healthy donors was not affected by coculture with a large excess of CD34+CD38− cells from 2 patients with MDS who had no detectable LTC-IC activity themselves. Second, in 4 investigated MDS cases carrying both del(5q) and trisomy 8, no CD34+CD38− cells, granulocytic cells, or erythroid cells were found to have trisomy 8 without also having del(5q). In contrast, cells with del(5q) and normal chromosome 8 status (disomy 8) could be observed in all patients (all CD34+CD38− cells carried the 5q deletion), supporting that trisomy 8 frequently is a late event in the MDS transformation process.25 This demonstrates that the CD34+CD38− HSC pool is part of the MDS clone and functionally deficient in these patients, despite a large fraction of cells not carrying the +8 aberration. This illustrates the difficulties with using absence of known cytogenetic aberrations as indicators of lack of involvement of the HSC pool. Although it is likely that residual normal HSCs exist in the investigated and other patients with MDS with clinical symptoms, these studies also clearly show that they must be present at very low numbers, and that it will be difficult to identify and isolate them based on their phenotype only, in particular since we here also demonstrate that the MDS stem cells have a similar phenotype as normal HSCs.
We thank Drs Janet Nichol and Graham Molineux (both at Amgen) and Stewart Lyman (at Immunex) for generous contributions of cytokines for these studies, and Drs. P. D. Jensen (University of Aarhus), P. G. Nilsson (Department of Hematology in Lund), and I. Turesson (Department of Hematology in Malmö) for providing crucial BM samples. The assistance of Anna Fossum, Zhi Ma, and Gunilla Gärdebring in cell enrichment and sorting, Lilian Wittman in mouse work (all at the Department of Stem Cell Biology in Lund) and Bodil Strömbeck (Department of Clinical Genetics in Lund) for some FISH analyses, are highly appreciated. We also thank all patients and bone marrow donors for their contributions; the staff at the Department of Hematology for assistance; and Inge Olsson, Jan Westin, Bengt Sallerfors, Tor Olofsson, Theo de Witte, and Stefan Karlsson for helpful discussions and critical review of the manuscript.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2001-12-0188.
Supported by grants from the Swedish Cancer Society, the Cancer Society in Stockholm, The Crafoord Foundation, Georg Danielsson Foundation, Nilsson′s Cancer Foundation, Åke Wiberg Foundation, John and Augusta Persson Foundation, John Persson Foundation, Tobias Foundation, Government Public Health (ALF) Grants, Skåne Landsting, and the Medical Faculty, University of Lund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sten Eirik W. Jacobsen, Department of Stem Cell Biology, Biomedical Center, Klinikgatan 26, BMC B12, SE-221 85 Lund, Sweden; e-mail: sten.jacobsen@stemcell.lu.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal