Abstract
Multiple myeloma (MM) is a plasma cell malignancy that occurs mainly in bone marrow. As MM cells proliferate slowly, it would seem essential to find means of preventing their growth and accumulation inside bone marrow. The present study used an antisense strategy to elucidate the respective roles of Bcl-2, Bcl-xL, and Mcl-1 proteins in myeloma cell survival. Each antisense oligonucleotide (ASO; Bcl-2, Bcl-xL, or Mcl-1 ASO) introduced into human myeloma cell lines by electroporation induced a marked reduction in the level of the corresponding protein. Mcl-1 ASO triggers an important decrease of viability in all myeloma cell lines tested and in 2 primary myeloma cells, whereas neither Bcl-2 nor Bcl-xL ASO affected the viability of myeloma cells. The decrease of cell viability induced by Mcl-1 ASO treatment was associated with an induction of apoptosis that occurred through the disruption of mitochondrial membrane potential ΔΨm and the activation of executioner caspase-3. Furthermore, we have shown that interleukin 6 cannot prevent the Mcl-1 ASO-induced apoptosis. Finally, although Bcl-2 ASO treatment alone has no effect, it can sensitize myeloma cell lines to dexamethasone (Dex), whereas Bcl-xL ASO in combination with Dex still had no effect. As MM remains an incurable disease despite intensive chemotherapy, these results suggest that Mcl-1 antisense strategy rather than Bcl-2 antisense strategy could be of considerable importance in the treatment of MM.
Introduction
Multiple myeloma (MM) is a disorder in which malignant plasma cells accumulate within bone marrow where they proliferate slowly and display a weak apoptotic index in vivo.1 This situation suggests that their accumulation could be due to a defect related to the apoptotic process. Proteins of the Bcl-2 family play a key role in apoptosis and are classified as antiapoptotic (eg, Bcl-xL, Bcl-2, Mcl-1, A1) or proapoptotic (eg, Bcl-xS, Bax, Bad, Bag, Bak).2 Bcl-2 protein is known to confer resistance to apoptosis. The role of this protein is still unclear in MM, but our previous results suggest that Bcl-2 could be involved in regulation of the cell cycle.3 Data in the literature indicate that Bcl-2 contributes to an antiapoptotic effect by reducing cell proliferation.4,5 Bcl-xL is also known to promote cell survival, and a report suggests that expression of this protein in MM may reflect disease severity and serve as an indicator of patient chemoresistance.6 The role of Mcl-1 in supporting cell survival was demonstrated initially in Chinese hamster ovary cells, and studies suggest that this protein plays an important role in B cells, particularly during the late stages of differentiation.7 Moreover, our work showed that Mcl-1 and Bcl-xL are coregulated by interleukin 6 (IL-6) in human myeloma cells.8 The signal transduction pathway leading to Bcl-xL and Mcl-1 up-regulation seems to depend on a common mechanism involving the janus kinase (JAK)–signal transducers and activators of transcription (STAT) pathway.9,10 In fact, a JAK2 inhibitor and subsequently a dominant-negative STAT3 protein inhibited Bcl-xL expression in U266 myeloma cells and induced apoptosis.10 A previous study by our group showed that a JAK2 inhibitor is able to abolish the effect of IL-6 on Mcl-1 up-regulation in myeloma cells.9 On the whole, these results indicate that Bcl-2, Bcl-xL, and Mcl-1 are important antiapoptotic proteins. The role of Bcl-2, on one hand, and of Bcl-xL and Mcl-1, on the other hand, are complementary in controlling apoptosis in MM.
In the present study, antisense oligonucleotides (ASOs) designed to inhibit the expression of Bcl-2, Bcl-xL, or Mcl-1 were used to show that each of these proteins has a definitive and specific antiapoptotic role in myeloma cells. The behavior of human myeloma cell lines was analyzed as well as that of malignant plasma cells from patients with MM after inhibition of Bcl-2, Bcl-xL, or Mcl-1 by a specific ASO.
Patients, materials, and methods
Human myeloma cell lines and culture conditions
LP-1, OPM-2, and L363 human myeloma cell lines (HMCLs) were purchased from DSM (Braunschweig, Germany), and the U266 line was obtained from the American Type Culture Collection (Rockville, MD). The XG-6 IL-6–dependent HMCL has been previously established in our laboratory and are cultured in the presence of 6 ng/mL recombinant IL-6 (Novartis, Basel, Switzerland). All these HMCLs were found to be free of mycoplasmas. Cells were maintained in RPMI-1640 medium supplemented with 5% fetal calf serum (FCS), 2 mM glutamine, antibiotics (100 IU/mL penicillin and 100 μg/mL streptomycin), and 10 μM 2-β-mercapto-ethanol.
Patients
Two tumor samples (pleural effusions) from patients with progressive disease were used in the study. The first came from a 72-year-old man and the second from a 60-year-old woman, both presenting immunoglobulin Ak MM at the time of extramedullary relapse. Invasion of both samples by malignant plasma cells was more than 90%.
Monoclonal antibodies and reagents
Dexamethasone (Dex) was obtained from Alexis (San Diego, CA), z-VAD-fmk from Calbiochem (La Jolla, CA), and anti–IL-6 blocking monoclonal antibody (mAb) B-E8 from Diaclone Research (Besançon, France). Anti–Bcl-x and anti–Mcl-1 rabbit affinity-purified polyclonal antibodies were purchased, respectively, from Transduction Laboratories (Interchim, Montluçon, France) and DAKO (Glostrup, Denmark). Anti–Bcl-2 mAb was obtained from DAKO, Bad mAb from Transduction Laboratories, and Bax mAb from Beckman Coulter (Roissy CDG, France).
ASO synthesis
The 2′-O-methoxyethyl-deoxynucleotide ASOs used in all experiments had a uniform phosphorothioate backbone.11ASOs were synthesized by using an Applied Biosystems 380B automated DNA synthesizer (Foster City, CA) and purified as previously described.11 These ASOs were selected to obtain the most efficient down-regulation of the related protein. The Bcl-2 or Bcl-xL control ASOs were mismatched on some bases (their sequences are shown in Table 1). The Mcl-1 control ASO, ISIS 29848, was synthesized as a mixture of A (adenine), G (guanosine), T (thymine), and C (cytosine) bases to provide a preparation containing an equimolar mix of all possible 4th to 19th oligonucleotides. The oligochemistry of the control was the same as that of the other oligonucleotides (Table 1). Bcl-xL and Mcl-1 ASOs were previously described in other cellular models.12 13
Sequence of Bcl-2, Bcl-x, Mcl-1, and control antisense oligonucleotides
| Target . | Name . | Sequence (5′-3′) . |
|---|---|---|
| Bcl-2 | ISIS 21 939 | ATGCGGAATTGCCCAGGGAC |
| Bcl-2 control | ISIS 23 983 | ATTCGTAATTACCCTGAGAC |
| Bcl-x | ISIS 16 009 | CTACGCTTTCCACGCACAGT |
| Bcl-x control | ISIS 16 967 | CTCCAATGTCCCCTCAAGGT |
| Mcl-1 | ISIS 20 408 | TTGGCTTTGTGTCCTTGGCG |
| Target . | Name . | Sequence (5′-3′) . |
|---|---|---|
| Bcl-2 | ISIS 21 939 | ATGCGGAATTGCCCAGGGAC |
| Bcl-2 control | ISIS 23 983 | ATTCGTAATTACCCTGAGAC |
| Bcl-x | ISIS 16 009 | CTACGCTTTCCACGCACAGT |
| Bcl-x control | ISIS 16 967 | CTCCAATGTCCCCTCAAGGT |
| Mcl-1 | ISIS 20 408 | TTGGCTTTGTGTCCTTGGCG |
The sequence is shown for antisense oligonucleotides targeting Bcl-2, Bcl-x, or Mcl-1 messenger RNA. The 2-O-methoxyethyl-modified nucleotides are represented by boldface letters and the 2-deoxynucleotides by nonboldface letters. Oligonucleotides were further modified by phosphorothioate linkage.
Electroporation
After overnight incubation in 2% FCS, myeloma cell lines (2 × 107) were suspended in 1 mL RPMI-1640 medium. Cells (360 μL) were mixed with a mismatched control (40 μL) or ASOs at the concentration of interest. The mix was then placed directly into 0.4-cm gap electroporation chambers and electroporated with an EasyJect Plus (Eurogentec, Seraing, Belgium) at 250 V and 1050 microfarad (μF). Cells were then resuspended in fresh medium supplemented with 2% FCS. Myeloma cells from patients and XG-6 HMCL were electroporated at 210 V, 900 μF, and resuspended in fresh medium supplemented with 10% FCS.
Cell viability
Cell viability was determined by vital dye (0.4% eosin) exclusion and assessed by visual inspection in a hemocytometer.
Western blot
Cells (4 × 106) were resuspended in 150 μL lysis buffer (10 mM Tris, pH 7.6, 150 mM NaCl, 5 mM EDTA, and 1% Triton X-100) containing 2 mM phenylmethylsulfonyl fluoride and 2 mg/mL aprotinin. After 40 minutes on ice, lysates were cleared by centrifugation at 12 000g for 30 minutes at 4°C. The protein concentration of lysates was determined by using bicinchoninic acid (BCA protein assay; Pierce, Rockford, IL). Equal amounts of protein (Bcl-2, Bcl-xL, or Mcl-1) were separated by 10% sodium dodecyl sulfate–polyacrylamide gel and then electrotransferred to polyvinylidene fluoride membranes. Western blot analysis was performed by standard techniques with enhanced chemiluminescence detection (Pierce). The blot was quantified by using 1D Image Analysis Software (Eastman Kodak, Rochester, NY).
Apoptosis detection
The percentage of apoptotic cells was determined by flow cytometry with the use of APO 2.7 mAb coupled to phycoerythrin (Beckman Coulter). Cells (2.5 × 105) were sampled in each condition and washed with phosphate-buffered saline before incubation with APO 2.7 mAb (2.5 μL) for 20 minutes. In these conditions, APO 2.7 staining detected delayed apoptosis. Alternately, cell cycle distribution was analyzed, in which case 2 × 105 cells were incubated for 40 minutes at 37°C in a solution of Triton X-100 0.1%, sodium citrate 0.1%, and 5 IU RNAse before staining with propidium iodide 50 mg/L. Flow cytometry analysis was performed on a FACSCalibur with the use of the CELLQuest program (Becton Dickinson). Data were gated on the FL2-area versus FL2-width cytogram to exclude doublets and aggregates, and a minimum of 2 × 104 gated cells was collected per sample. The cell cycle was analyzed by using the Modfit LT program V2.01 for Mac (Verity Software House). Early apoptotic cells were detected as a subdiploid peak, as described previously.14
Measurement of mitochondrial membrane potential Δψm
Disruption of mitochondrial membrane potential was measured by using a specific fluorescent probe JC-1 (Calbiochem, Meudon, France) that was incubated at 37°C with the cells at the concentration of 5 mg/mL for 20 minutes. After incubation with JC-1, cells were analyzed by flow cytometry in fluorescence channel FL-2. JC-1 emits a red fluorescence (JC-1 aggregates, high ΔΨm) when sequestered in the mitochondrial membrane of healthy cells. Mitochondrial membrane depolarization is associated with a large shift in emission at 590 nm (red fluorescence).15
Caspase-3 activity
Caspase-3 activity was measured with a caspase-3 colorimetric assay (R&D Systems, Abingdon, United Kingdom). Cells (3 × 106) were collected for each lysate. Caspase activity was determined as indicated by the manufacturer.
Results
ASOs inhibit the protein of interest specifically
Five HMCLs (U266, L363, LP-1, OPM-2, and XG-6) were chosen for their different endogenous levels of the 3 antiapoptotic proteins (Bcl-2, Bcl-xL, and Mcl-1) and their growth status. XG-6 is an IL-6–dependent HMCL; U266 is a well-characterized IL-6 autocrine HMCL; and L363, LP-1, and OPM-2 are independent of IL-6 for their growth. XG-6, U266, and L363 exhibited both high Bcl-xL and Mcl-1 endogenous levels, whereas LP-1 and OPM-2 displayed a lower level of both Bcl-xL and Mcl-1. Bcl-2 was highly expressed in all 5 HMCLs.8 Because HMCLs are difficult to transfect, electroporation was used to introduce ASOs. Cells were electroporated in the presence of ASO or the related mismatched control, and the expression of the corresponding protein was then analyzed by Western blot at different times after electroporation. Each ASO (Bcl-2, Bcl-x, and Mcl-1) induced dose- and time-dependent depletion of the corresponding protein, whereas the mismatched control had no effect, indicating the specificity of the ASO (Figure1). Because of the variable endogenous levels of each protein of interest, the ASO concentration used differed and was calculated to obtain maximal inhibition of the protein with the lowest nonspecific effects. Bcl-2 ASO was used at a concentration of 10 μM, and maximum protein inhibition was observed at 72 hours. Bcl-2 ASO induced a depletion of more than 90% in L363 and OPM-2 cells and of 75% and 65% in U266 and LP-1, respectively (Figure 1 and data not shown). With Bcl-xL ASO, the strongest effect was observed at 40 hours with 20 μM ASO. In these conditions, Bcl-x ASO was responsible for an 80% down-regulation of Bcl-xL protein in L363 and LP-1 and a 50% down-regulation in U266 (Figure 1). On the basis of the endogenous levels of Mcl-1, Mcl-1 ASO was used at a concentration of 20 μM in LP-1, OPM-2, and XG-6 and 30 μM in U266 and L363 HMCLs. The down-regulation of Mcl-1 in the presence of ASO was observed as early as 6 hours, becoming maximal between 12 and 18 hours. This difference in kinetics seems attributable to the presence of 2 regions enriched in proline, glutamate, serine, and threonine (PEST sequences) in the Mcl-1 sequence, which could target Mcl-1 for rapid turnover via proteasome-mediated degradation.16Mcl-1 ASO induced major down-regulation of Mcl-1 protein level, ie, a depletion of at least 70% in U266, LP-1, and OPM-2 and a lesser depletion in L363 (53%) and XG-6 (50%) (Figure 1 and data not shown). As shown in Figure 1, depletion of each protein by the related ASO did not alter the level of any of the other antiapoptotic proteins evaluated by Western blot, except Bcl-xL, which slightly decreased in the presence of Mcl-1 ASO. It is noteworthy that the levels of some proapoptotic (Bad and Bax) proteins were not modified, regardless of the ASO used (data not shown).
Down-regulation of Bcl-2, Bcl-xL, and Mcl-1 after treatment by ASOs.
Three HMCLs were studied (L363, U266, and LP-1), and specific ASOs against Bcl-2, Bcl-xL, and Mcl-1 were used. Cells were electroporated without ASO or with either control mismatched or specific ASO. A control consisting of nonelectroporated cells (ie, no treatment) is shown. Cells were recovered at the time corresponding to maximum inhibition of the related protein (18, 40, and 72 hours for Mcl-1, Bcl-xL, and Bcl-2 ASO, respectively). The cells were then lysed, and protein lysates were quantified. Total protein (20 μg) was loaded per lane for immunoblot analysis.
Down-regulation of Bcl-2, Bcl-xL, and Mcl-1 after treatment by ASOs.
Three HMCLs were studied (L363, U266, and LP-1), and specific ASOs against Bcl-2, Bcl-xL, and Mcl-1 were used. Cells were electroporated without ASO or with either control mismatched or specific ASO. A control consisting of nonelectroporated cells (ie, no treatment) is shown. Cells were recovered at the time corresponding to maximum inhibition of the related protein (18, 40, and 72 hours for Mcl-1, Bcl-xL, and Bcl-2 ASO, respectively). The cells were then lysed, and protein lysates were quantified. Total protein (20 μg) was loaded per lane for immunoblot analysis.
Mcl-1 but not Bcl-2 or Bcl-xL down-regulation induces rapid and strong apoptosis of human myeloma cells
To analyze the biologic effects of ASO-induced depletion of Bcl-2, Bcl-xL, or Mcl-1, cell viability and apoptosis were determined at different times after electroporation in U266, L363, and LP1 HMCLs. Apoptosis was estimated by using either APO 2.7 staining or cell cycle analysis. Treatment by Bcl-2 ASO or Bcl-xL had no effect on cell viability in any of the 3 HMCLs studied (Figure2A). In contrast, Mcl-1 ASO triggered a decrease of cell viability in all 3 HCMLs tested (Figure 2A) and induced a significant apoptosis in U266 and LP1 cells (Figure 2B). Of note, LP-1 displayed the most dramatic induction of apoptosis (a 3-fold increase) when Mcl-1 protein level was decreased. Apoptosis was detected as early as day 2 after introduction of the ASO, with a maximum effect at days 4 and 6 (Figure 2B). This rapid apoptosis was also revealed by the occurrence of a subdiploid peak (50%) as early as 18 hours after introduction of Mcl-1 ASO in U266 (Figure 2C). Down-regulation of Mcl-1 was lowest for L363. Nonetheless, Mcl-1 ASO induced a small but significant apoptosis as shown by the appearance of a subdiploid peak of 35% in L363 cells treated by Mcl-1 ASO versus 12.5% in control cells at day 1 (Figure 2C). Finally, in a fourth cell line tested OPM-2, Mcl-1 ASO triggers apoptosis (2-fold increase) (data not shown). Altogether, these results show that specific disruption of Mcl-1 expression results in a rapid entry into apoptosis in myeloma cells, whereas the specific disruption of Bcl-xL or Bcl-2 expression has no effect on cell viability or apoptosis.
Analysis of cell viability and apoptosis following Bcl-2, Bcl-xL, or Mcl-1 ASO delivery.
Cells were electroporated with either Bcl-2, Bcl-xL, or Mcl-1 ASO or the mismatched related control. Then cells were resuspended in RPMI 1640 with 2% FCS. A control involving cells electroporated without ASO was performed. (A) Cell viability was determined by vital dye (eosin) exclusion, as assessed by visual inspection in a hemocytometer. (B) Apoptosis was determined by the percentage of APO 2.7–positive cells analyzed by flow cytometry. Apoptosis is expressed as the mean percentage of APO 2.7–positive cells ± SD (n = 3 at least for each condition). (C) Cell cycle analysis following electroporation of cells with Mcl-1 ASO. DNA fluorescence histograms of propidium iodide-stained myeloma cells. The cell cycle was analyzed by using the Modfit LT program (Verity Software House, Topsham, ME). The percentage of apoptotic cells represented by a sub-G1 peak is indicated.
Analysis of cell viability and apoptosis following Bcl-2, Bcl-xL, or Mcl-1 ASO delivery.
Cells were electroporated with either Bcl-2, Bcl-xL, or Mcl-1 ASO or the mismatched related control. Then cells were resuspended in RPMI 1640 with 2% FCS. A control involving cells electroporated without ASO was performed. (A) Cell viability was determined by vital dye (eosin) exclusion, as assessed by visual inspection in a hemocytometer. (B) Apoptosis was determined by the percentage of APO 2.7–positive cells analyzed by flow cytometry. Apoptosis is expressed as the mean percentage of APO 2.7–positive cells ± SD (n = 3 at least for each condition). (C) Cell cycle analysis following electroporation of cells with Mcl-1 ASO. DNA fluorescence histograms of propidium iodide-stained myeloma cells. The cell cycle was analyzed by using the Modfit LT program (Verity Software House, Topsham, ME). The percentage of apoptotic cells represented by a sub-G1 peak is indicated.
Down-regulation of either Bcl-xL or Bcl-2 proteins does not sensitize myeloma cells to apoptosis induced by Mcl-1 ASO
We compared the efficiency of Mcl-1 ASO in combination or not with either Bcl-2 or Bcl-xL ASO on the induction of apoptosis in U266 and L363 cells. We demonstrated that the down-regulation of either Bcl-xL or Bcl-2 proteins does not sensitize U266 and L363 cells to apoptosis induced by Mcl-1 ASO (Figure3). These results may be explained by the strong efficiency of Mcl-1 ASO and above all by the rapid induction of apoptosis that characterized Mcl-1–induced apoptosis.
Analysis of apoptosis following the combination of Bcl-2 and Mcl-1 ASO or Bcl-xL and Mcl-1 ASO delivery.
Cells were electroporated with either Bcl-2 and Mcl-1 ASOs or Bcl-xL and Mcl-1 ASOs. Then, cells were resuspended in RPMI 1640 with 2% FCS. Apoptosis was determined by the percentage of APO 2.7–positive cells analyzed by flow cytometry. Apoptosis is expressed as the percentage of APO 2.7–positive cells (a representative experiment of 3).
Analysis of apoptosis following the combination of Bcl-2 and Mcl-1 ASO or Bcl-xL and Mcl-1 ASO delivery.
Cells were electroporated with either Bcl-2 and Mcl-1 ASOs or Bcl-xL and Mcl-1 ASOs. Then, cells were resuspended in RPMI 1640 with 2% FCS. Apoptosis was determined by the percentage of APO 2.7–positive cells analyzed by flow cytometry. Apoptosis is expressed as the percentage of APO 2.7–positive cells (a representative experiment of 3).
Bcl-2 or Mcl-1 down-regulation sensitizes myeloma cells to Dex-induced apoptosis
Dex is known to be effective in the treatment of MM, and our work and that of other groups have shown that IL-6 protects myeloma cells from Dex-induced apoptosis in vitro.17 18 In this context, further investigations were performed to determine whether Bcl-2, Bcl-xL, or Mcl-1 depletion sensitizes HMCLs to glucocorticoids. In U266 cells, which are primarily resistant to Dex, the addition of Bcl-2 ASO to Dex triggered a decrease of cell viability and an increase of apoptosis (80% increase at days 6 and 8) (Figure4A,B). Bcl-2 ASO also sensitized L363 cells to Dex, producing 80% and 120% increases of apoptosis at days 3 and 6, respectively, (Figure 4B). However, treatment of LP-1 cells by Dex in the presence of Bcl-2 ASO did not sensitize cells to glucocorticoids (Figure 4A,B). The same observation was made with OPM-2 (data not shown). On the whole, these results indicate the efficiency of Bcl-2 down-regulation in sensitizing some myeloma cells to apoptosis in the presence of Dex. In contrast to Bcl-2 ASO, addition of Bcl-x ASO did not sensitize myeloma cells to Dex-induced apoptosis in any of the 3 cell lines tested (Figure 4B). The combination of Dex and Mcl-1 ASO induced only a small increase of apoptosis in cell lines (LP1 and U266) in which Mcl-1 ASO alone induced already an important apoptosis. However, in L363 cells in which Mcl-1 ASO alone is poorly efficient the combination of Dex and Mcl-1 ASO triggered a dramatic decrease of viable cells and an important increase of apoptosis (Figure4A,B).
Analysis of cell viability and apoptosis following Bcl-2, Bcl-xL, or Mcl-1 ASO delivery in the presence of Dex.
Cells were electroporated with either Bcl-2, Bcl-xL, or Mcl-1 ASO, or the mismatched related control. Then cells were resuspended in RPMI 1640 with 2% FCS in the presence or not of Dex (10−6 M). A control involving cells electroporated without ASO was performed. (A) Cell viability was determined as indicated in Figure 2. (B) The percentage of APO 2.7–positive cells was determined by flow cytometry. Results are expressed as the mean ± SD of 3 experiments.
Analysis of cell viability and apoptosis following Bcl-2, Bcl-xL, or Mcl-1 ASO delivery in the presence of Dex.
Cells were electroporated with either Bcl-2, Bcl-xL, or Mcl-1 ASO, or the mismatched related control. Then cells were resuspended in RPMI 1640 with 2% FCS in the presence or not of Dex (10−6 M). A control involving cells electroporated without ASO was performed. (A) Cell viability was determined as indicated in Figure 2. (B) The percentage of APO 2.7–positive cells was determined by flow cytometry. Results are expressed as the mean ± SD of 3 experiments.
Effect of IL-6 on apoptosis induced by Mcl-1 ASO
Because IL-6 is the major growth factor of myeloma cells, we tested the effect of IL-6 on apoptosis induced by ASO either by neutralizing autocrine IL-6 in U266 or by testing the effect of Mcl-1 ASO in XG-6, an IL-6–dependent cell line. When IL-6 was neutralized by the addition of B-E8 mAb in U266, Bcl-2 ASO induced a weak but significant increase of apoptosis (50% increase at day 8) (Figure5A). Furthermore, the inhibition of IL-6 by B-E8 intensified the apoptosis induced by Dex (75%, 100%, and 60% increase at day, 6, 8, and 10, respectively) (Figure 5A). The inhibition of IL-6 autocrine did not allow us to detect any effect of Bcl-x ASO and did not significantly modify the apoptosis induced by Mcl-1 ASO (Figure 5A). Mcl-1 ASO triggered an important decrease of cell viability in XG-6 HCMLs cultured with 6 ng/mL IL-6 and 10% FCS, showing that IL-6 is unable to prevent apoptosis following Mcl-1 depletion (Figure 5B). Altogether, these results indicate that Mcl-1 depletion results in entry into apoptosis in myeloma cells even in the presence of IL-6.
Effect of IL-6 on the apoptosis induced by ASO.
(A) U266 cells were electroporated with either Bcl-2, Bcl-xL, or Mcl-1 ASO or the mismatched related control. Then cells were resuspended in RPMI 1640 with 2% FCS in the presence or not of Dex (10−6 M). B-E8 was added as indicated at the concentration of 50 ng/mL. (B) Cell viability of XG-6 myeloma cells after Mcl-1 ASO delivery in the presence of 6 ng/mL IL-6.
Effect of IL-6 on the apoptosis induced by ASO.
(A) U266 cells were electroporated with either Bcl-2, Bcl-xL, or Mcl-1 ASO or the mismatched related control. Then cells were resuspended in RPMI 1640 with 2% FCS in the presence or not of Dex (10−6 M). B-E8 was added as indicated at the concentration of 50 ng/mL. (B) Cell viability of XG-6 myeloma cells after Mcl-1 ASO delivery in the presence of 6 ng/mL IL-6.
Characterization of the Mcl-1 ASO-induced apoptosis
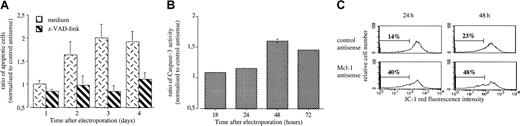
To unravel the mechanisms by which Mcl-1 depletion induced apoptosis, we examined the involvement of caspase pathway either using a broad-spectrum irreversible caspase inhibitor (z-VAD-fmk) or measuring caspase-3 activity. The addition of 100 μM z-VAD-fmk in LP-1 totally blocks the apoptosis induced by Mcl-1 ASO, indicating a caspase-dependent process (Figure 6A). This finding was also confirmed by the increase of caspase-3 activity induced by Mcl-1 treatment as shown in Figure 6B. Finally, LP-1 cells were probed with JC-1 to visualize disruption of mitochondrial membrane potential. When cells were treated with Mcl-1 ASO, an important shift in red fluorescence was observed, indicating loss of mitochondrial membrane potential (Figure 6C).
Characterization of Mcl-1 ASO-induced apoptosis.
(A) LP-1 cells were electroporated with either Mcl-1 ASO or the mismatched related control. Then cells were resuspended in RPMI 1640 with 2% FCS in the presence or not of z-VAD-fmk (100 μM). The percentage of APO 2.7–positive cells was determined by flow cytometry. Results are expressed as the mean ± SD of 3 experiments. (B) LP-1 cells were electroporated with either Mcl-1 ASO or the mismatched related control. Cells were recovered at the indicated time, and caspase-3 activity was determined in cell lysates by using colorimetric assay. (C) Disruption of mitochondrial membrane potential in LP-1 treated or not by MCl-1 ASO. The percentage of cells with a low ΔΨm is indicated.
Characterization of Mcl-1 ASO-induced apoptosis.
(A) LP-1 cells were electroporated with either Mcl-1 ASO or the mismatched related control. Then cells were resuspended in RPMI 1640 with 2% FCS in the presence or not of z-VAD-fmk (100 μM). The percentage of APO 2.7–positive cells was determined by flow cytometry. Results are expressed as the mean ± SD of 3 experiments. (B) LP-1 cells were electroporated with either Mcl-1 ASO or the mismatched related control. Cells were recovered at the indicated time, and caspase-3 activity was determined in cell lysates by using colorimetric assay. (C) Disruption of mitochondrial membrane potential in LP-1 treated or not by MCl-1 ASO. The percentage of cells with a low ΔΨm is indicated.
Mcl-1 down-regulation induces apoptosis in freshly expanded myeloma cells
Studies were then conducted to determine the effect of Mcl-1 ASO in primary myeloma cells. The reduction of Mcl-1 levels after electroporation by Mcl-1 ASO in primary myeloma cells from 2 patients showed a decrease in the Mcl-1 level of 70% and 50%, respectively, for patients 1 and 2 (Figure 7A). Further studies of cell survival after Mcl-1 delivery indicated that Mcl-1 down-regulation triggered an induction of apoptosis in both patients. The increase of apoptosis at day 4 after ASO treatment was greater in patient 1 (200%) than in patient 2 (169%), which is consistent with the most marked depletion of Mcl-1 level in patient 1 (Figure 7B). Of note, although Bcl-2 ASO treatment had induced a significant down-regulation of Bcl-2 protein in patient 1, this depletion had no effect on cell viability (result not shown).
Mcl-1 ASO triggered apoptosis in myeloma cells from 2 patients.
(A) Mcl-1 protein was down-regulated after treatment by Mcl-1 ASO. Cells from patients were electroporated in the presence 20 μM Mcl-1 ASO and recovered at 18 hours. Cells were then lysed, and 20 μg total protein was loaded per lane for immunoblot analysis. (B) Determination of apoptosis following Mcl-1 ASO treatment. A control involving cells electroporated without ASO was performed. After electroporation, cells were resuspended in RPMI 1640 with 10% FCS. Apoptosis induced by ASO was determined by the percentage of APO 2.7–positive cells.
Mcl-1 ASO triggered apoptosis in myeloma cells from 2 patients.
(A) Mcl-1 protein was down-regulated after treatment by Mcl-1 ASO. Cells from patients were electroporated in the presence 20 μM Mcl-1 ASO and recovered at 18 hours. Cells were then lysed, and 20 μg total protein was loaded per lane for immunoblot analysis. (B) Determination of apoptosis following Mcl-1 ASO treatment. A control involving cells electroporated without ASO was performed. After electroporation, cells were resuspended in RPMI 1640 with 10% FCS. Apoptosis induced by ASO was determined by the percentage of APO 2.7–positive cells.
Discussion
This study used antisense strategy to define the specific role of 3 major antiapoptotic proteins (Bcl-2, Bcl-xL, and Mcl-1) in the survival of human myeloma cells. Mcl-1 proved to be a key determinant in ensuring the survival of myeloma cells. Furthermore, we demonstrated that apoptosis triggered by Mcl-1 depletion occurred through the disruption of mitochondrial membrane potential ΔΨm and the activation of executioner caspase-3.
Our investigations and those of other groups indicate that Mcl-1 and Bcl-xL, unlike Bcl-2, are regulated by survival and apoptotic stimuli such as IL-6, which accounts for their role in the regulation of myeloma cell survival.7,9,10 Moreover, our earlier work showed that Mcl-1 and Bcl-xL are coexpressed in myeloma cells.8 On the whole, these data suggest that Bcl-xL and Mcl-1, but not Bcl-2, regulate apoptosis in a similar way. Nonetheless, our study clearly demonstrates that Mcl-1 rather than Bcl-xL is an essential survival protein in MM. We show here that specific disruption of Mcl-1 expression either in HMCLs or primary myeloma cells results in a rapid entry into apoptosis. Moreover, we demonstrated that the combination of IL-6 and high concentration of serum does not prevent this apoptosis. Surprisingly, the depletion of Bcl-xL did not induce apoptosis in any of the conditions tested, although it cannot be excluded that the residual Bcl-xL level (< 20%) interfered with our observations. Moreover, the combination of Mcl-1 and Bcl-xL ASOs does not potentiate the effect of Mcl-1 ASO. The explanation for the differential behavior of these 2 proteins is currently unknown, and further investigations of their interactions with proapoptotic proteins would seem of particular interest. Finally, our study is the first to demonstrate that the maintenance of Mcl-1 expression in cells exhibiting it physiologically is the essential mechanism for preventing apoptosis. Moreover, the level of Bcl-2 remained stable after Mcl-1 treatment but provided no protection from apoptosis, indicating that Mcl-1 and Bcl-2 play distinct roles in apoptosis regulation. Finally, the strong and rapid apoptosis induced by Mcl-1 ASO cannot be maximized by Bcl-2 ASO. Apoptosis induction by Mcl-1 ASO triggered a weak down-regulation of Bcl-xL expression that is prevented by the addition of z-VAD-fmk caspase inhibitor. This finding is in agreement with the negative coregulation of Mcl-1 and Bcl-xL induced by withdrawal of survival factors.8 It is noteworthy that an increasing number of studies have dealt with the importance of the protection afforded by Mcl-1. In particular, it has been shown that Mcl-1 induction is required to prevent apoptosis during the differentiation of U937.19 The importance of Mcl-1 in B-cell chronic lymphocytic leukemia has also been stressed in the study of Kitada et al,20 showing that protein kinase inhibitors induce apoptosis by down-regulating Mcl-1 levels. Finally, up-regulation of Mcl-1 was found to prevent apoptosis of hepatoma cells by IL-6.21
The functional effect of Bcl-2 in MM remains unclear. Some studies suggest that chemotherapeutic agents enhance Bcl-2 levels and contribute to chemoresistance,22 whereas others claim that Bcl-2 expression has no relation to short-term survival in MM, implying that Bcl-2 does not play a major role in influencing response to treatment.23 Our study and others found an inverse correlation between Bcl-2 expression and the proliferation rate of both normal and malignant plasma cells, thus indicating that Bcl-2 could also play a role in regulating the cell cycle.3,24 Our current data show that down-regulation of Bcl-2 cannot induce apoptosis in myeloma cells, even in combination with Bcl-xL ASO (data not shown), although it sensitizes these cells to Dex-induced apoptosis. These results are consistent with our previous data, indicating that the Bcl-2–dependent cell cycle position controls the regulation of chemosensitivity and with the notion that G1-phase–dependent expression of Bcl-2 messenger RNA and protein correlates with the chemoresistance of human cancer cells.25
MM remains an incurable disease, despite intensive chemotherapy. Although Bcl-2 antisense therapeutic strategy is feasible without toxicity26,27 and has already been proposed for MM patients,28 our results suggest that an approach involving Mcl-1 deserves more thorough testing. This approach is of special interest because the presence of IL-6 cannot prevent the Mcl-1 ASO-induced apoptosis.
Supported by grants from the Ligue contre le Cancer de Loire-Atlantique. S.D. is supported by the Association pour la Recherche sur le Cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Martine Amiot, U463, Institut de Biologie, 9, quai Moncousu 44 093 Nantes cedex 01, France; e-mail:mamiot@nantes.inserm.fr.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal