Abstract
During fetal development, there is a continued demand for large numbers of primitive and mature hematopoietic cells. This demand may require that all potential hematopoietic stem cells (HSCs) migrate effectively to emerging hematopoietic sites and subsequently contribute to blood cell production, regardless of their cell cycle status. We recently established that umbilical cord blood cells in the G1 phase of the cell cycle have a repopulating potential similar to cells in G0, suggesting that cycling prenatal and neonatal HSCs may have the same functional capabilities described for quiescent, but not cycling, cells from adult sources. To establish the relationship between cell cycle status and hematopoietic potential at early stages of human ontogeny, the in vivo engraftment potential of mitotically defined fetal liver (FL) and fetal bone marrow (FBM) cells were examined in NOD/SCID recipients. Following transplantation of the same numbers of G0, G1, or S/G2+M CD34+ cells from FL, equivalent percentages of recipient mice were chimeric (55%, 60%, and 60%, respectively). FBM-derived CD34+ cells in all phases of the cell cycle engrafted in conditioned recipients and sustained human hematopoiesis, albeit at lower levels than their FL-derived counterparts. Multilineage differentiation was evident in all transplanted mice independent of the source or cell cycle status of graft cells. In addition, levels of chimerism in mice transplanted with fetal blood–derived G0or G1 CD34+ lineage-depleted cells were similar. These results support the assertion that mitotically quiescent and cycling fetal hematopoietic cells contain marrow-repopulating stem cells capable of multilineage engraftment in NOD/SCID mouse recipients.
Introduction
During embryonic and fetal life, various tissues sequentially lose and gain hematopoietic function. It has been suggested that hematopoietic stem cells (HSCs) are derived from the same ancestor cells early in development, which give rise to several different extra-embryonic (yolk sac) and embryonic tissues (embryonic mesoderm).1,2 During ontogeny, HSCs may migrate between these tissues, beginning in the yolk sac and the ventral wall of the dorsal aorta, followed by trafficking to the fetal liver (FL) and subsequently to fetal bone marrow (FBM).3,4 HSCs are found in the fetal blood (FB) from 5 weeks of gestation5 onward and are found transiently in the ventral wall of the dorsal aorta before hepatic hematopoiesis occurs.6 Hematopoiesis starts at 6 weeks of gestation in the FL7 and from 14 weeks of gestation in the FBM8; the latter continues to be the primary site of hematopoiesis throughout prenatal and adult life.
Unlike peripheral blood of adults, FB contains high concentrations of hematopoietic progenitors. Because it is accessible by intrauterine transfusion, FB may be a reasonable source during pregnancy for gene therapy intervention. Using a murine xenogeneic transplantation model, we recently established that at 12 to 18 weeks of gestation, circulating FB is a rich source of NOD/SCID repopulating cells and that these cells are intrinsically distinct from their counterparts in other fetal and postnatal sources of HSC.9 Not only do fetal tissues contain high frequencies of hematopoietic progenitor cells, they are also a rich source of putative HSCs.10-12 The percentage of CD34+ cells in FB at 12 to 18 weeks of gestation is as high as that seen in umbilical cord blood (UCB), whereas that of CD34+CD38− cells in FB is 3-fold higher than in UCB (0.3% and 0.09%, respectively).9
Adult mobilized peripheral blood (MPB) hematopoietic stem cells capable of engrafting NOD/SCID mice are predominantly found in the G0 phase of the cell cycle13 and exhibit a 16-fold enrichment in their repopulating potential compared to their counterparts residing in G1.13 We recently reported14 that unlike MPB, the repopulating capacity of mitotically active and resting cord blood CD34+ cells in NOD/SCID mice is similar.14 During ontogeny, normal hematopoietic development requires a considerable proliferative output from HSCs. It is possible that the continuous demand for rapidly increasing numbers of hematopoietic cells during mammalian development affords mitotically active HSCs in prenatal and neonatal tissue engraftment and hematopoietic potentials that are restricted to mitotically quiescent cells in adult tissues.13,14 In the context of gene transfer protocols, these mitotically active, yet engrafting, cells might be of great importance because of the need for cell cycle activation and progression through the cell cycle before efficient gene integration can be achieved in target cells. Fetal circulating blood hematopoietic progenitor cells have been shown to be more susceptible to retrovirus-mediated gene transfer than cells from adult tissues, most likely because of their mitotically active state.15 To investigate the relationship between cell cycle status and hematopoietic potential during the early stages of human ontogeny and to provide a static assessment of the distribution of engrafting stem cells across phases of the cell cycle, the engraftment capacity of mitotically defined fractions of fetal hematopoietic precursor cells was examined in NOD/SCID mice. In this report, we demonstrate that unlike adult mobilized peripheral blood and BM cells, but analogous to neonatal cord blood cells, fetal hematopoietic progenitors from blood, liver, and bone marrow residing in quiescent and active phases of the cell cycle contain putative stem cells capable of multilineage and sustained engraftment in NOD/SCID mouse recipients.
Materials and methods
Collection and purification of fetal liver and bone marrow CD34+ cells
Fetal liver and fetal bone marrow were obtained after informed consent from women undergoing elective termination of pregnancy for social reasons between 14 and 22 weeks of gestational age. Protocols for collection and cryopreservation of these tissues in Leiden and transfer of cryopreserved cells to Indiana were approved by the Institutional Review Boards of Leiden University Medical Center and Indiana University, respectively. Immediately after collection of FL or FBM, cell suspensions were prepared by mincing the liver thoroughly and flushing the long bones, and single-cell suspensions were cryopreserved in 10% dimethyl sulfoxide. Cells were thawed 24 hours before their use and were suspended in complete medium {consisting of Iscoves modified Dulbecco medium [IMDM] + 1% penicillin–streptomycin (10 000U/mL/10 000 μg/mL, respectively) + 10% fetal bovine serum + 1% l-glutamine} and were kept at 4°C until selection. CD34+ selection was performed using magnetic cell separation columns (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) and antibodies recognizing the CD34 epitope, QBEND/10, according to the manufacturer's procedure.
Collection and isolation of lineage-depleted CD34+fetal peripheral blood cells
Samples of human FB from 12- to 18-week elective abortus were obtained in conjunction with local ethics and biohazard authorities of the University of Western Ontario and the London Health Sciences Center. Fetal blood was procured in a manner that precluded the potential contamination of maternal blood and was similar to diagnostic methods of fetal blood sampling. Whole blood (1-3 mL) was collected in α-MEM (Gibco BRL, Grand Island, NY) or phosphate-buffered saline, and mononuclear cells were isolated by centrifugation over Ficoll-Hypaque (Pharmacia, Uppsala, Sweden). Lineage-depleted (Lin−) fetal blood cells were purified by negative selection using a StemSep device as described by the manufacturer (Stem Cell Technologies, Vancouver, British Columbia, Canada).
Cell cycle fractionation with Hoechst 33342 and Pyronin Y
To distinguish between FL, FBM, and FB progenitor cells in the G0 or G1 phase of the cell cycle, which have similar DNA but different RNA content, and between G0/G1 and S/G2+M cells, which have different DNA and RNA content, simultaneous DNA and RNA staining with Hoechst 33342 (Hst) and pyronin Y (PY), respectively, was performed as previously described16,17 (Figure1). Briefly, purified progenitor cells were resuspended in 1 μg/mL Hst (Molecular Probes, Eugene, OR) prepared in Hst buffer consisting of Hanks balanced salt solution (BioWhittaker, Walkersville, MD), 20 mM HEPES (BioWhittaker), 1 g/L glucose, 10% fetal calf serum (FCS; Hyclone, Logan, UT), and verapamil at 50 μM (Sigma, St Louis, MO). After 45-minute incubation at 37°C, PY (Polysciences, Warrington, PA) was added at a final concentration of 1 μg/mL. Cells were further incubated for another 45 minutes at 37°C, then washed once in chilled Hst buffer. FL or FBM CD34+ cells were incubated for 30 minutes with fluorescein isothiocyanate (FITC)-conjugated anti-CD34 monoclonal antibodies (PharMingen, San Diego, CA) at 4°C. Cells were washed again, resuspended in Hst buffer, and analyzed or sorted on a FACStar Plus (Becton Dickinson Immunocytometry Systems [BDIS], San Jose, CA). Gated FL and FBM CD34 FITC-positive cells were identified as in G0/G1 or S/G2+M based on their Hst staining profile. Cells in G0/G1were subdivided into those in G0 based on their minimal RNA content, whereas cells traversing into G1 were defined as those with high or maximal RNA staining.17 Cells in S/G2+M have higher than 2n DNA (high Hst) and high RNA (high PY) content. Fetal blood CD34+Lin−cells were first stained with Hst and PY and subsequently with allophycocyanin-conjugated CD34 monoclonal antibodies (BDIS). Cells were sorted on a Vantage SE (BDIS) to isolate G0 and G1 cells within the CD34+Lin− cell fraction. Sorting gates were designed as previously reported.13 18 This staining protocol allowed the isolation of viable FL and FBM CD34+ cells in G0 (G0CD34+), G1(G1CD34+), or S/G2+M (S/G2+MCD34+), and viable FB CD34+Lin− cells in G0(G0CD34+Lin−) or G1(G1CD34+Lin−). During sorting, cells were kept on ice to minimize dye leaking and were protected from light. Viability of sorted cells always exceeded 95%. Validation of cell cycle status of sorted cells was performed using propidium iodide staining (Figure 1).
Phenotypic differences between FL and FBM CD34+ cells.
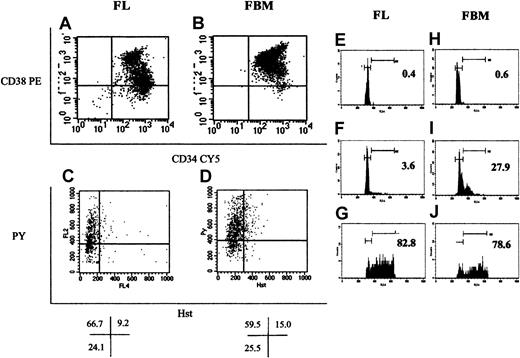
Selected FL and FBM cells were analyzed for the expression of CD34 (x-axis) and CD38 (y-axis), as shown in panels A and B, respectively. A larger group of CD34+CD38−cells was detected among FL than among FBM CD34+ cells. In addition, cells expressing higher levels of CD38 (CD38++) were more prominent among FBM cells. Most FL CD34+CD38+ cells coexpressed CD33+, whereas most of the corresponding FBM cells were CD19+(data not shown). Panels C and D depict Hst (x-axis) and PY (y-axis) staining of FL and FBM CD34+ cells, respectively. Percentages of cells in the different phases of the cell cycle (given as percentages in each quadrant under panels C and D) were similar for FL and FBM. The horizontal cursor separating G0 from G1 cells was positioned to separate PY staining into the lower one fourth containing G0 cells and the upper three fourths containing G1 cells. Data shown in panels A through D were derived from the analysis of FL and FBM cells from one fetus. FL (E-G) and FBM (H-J). CD34+ cells were sorted into G0, G1, and S/G2+M cells based on their Hst and PY staining pattern. Sorted cells were stained with propidium iodide and were analyzed on a FACScan for their cell cycle status, shown for G0 cells in panels E and H, for G1 cells in panels F and I, and for S/G2+M cells in panels G and J. Numbers inside panels E through J represent the percentages of cells detected in S/G2+M in each sorted group based on the positions of the gates to the right in each panel. Gates shown to the left in panels E through J denote the position of cells in G0/G1.
Phenotypic differences between FL and FBM CD34+ cells.
Selected FL and FBM cells were analyzed for the expression of CD34 (x-axis) and CD38 (y-axis), as shown in panels A and B, respectively. A larger group of CD34+CD38−cells was detected among FL than among FBM CD34+ cells. In addition, cells expressing higher levels of CD38 (CD38++) were more prominent among FBM cells. Most FL CD34+CD38+ cells coexpressed CD33+, whereas most of the corresponding FBM cells were CD19+(data not shown). Panels C and D depict Hst (x-axis) and PY (y-axis) staining of FL and FBM CD34+ cells, respectively. Percentages of cells in the different phases of the cell cycle (given as percentages in each quadrant under panels C and D) were similar for FL and FBM. The horizontal cursor separating G0 from G1 cells was positioned to separate PY staining into the lower one fourth containing G0 cells and the upper three fourths containing G1 cells. Data shown in panels A through D were derived from the analysis of FL and FBM cells from one fetus. FL (E-G) and FBM (H-J). CD34+ cells were sorted into G0, G1, and S/G2+M cells based on their Hst and PY staining pattern. Sorted cells were stained with propidium iodide and were analyzed on a FACScan for their cell cycle status, shown for G0 cells in panels E and H, for G1 cells in panels F and I, and for S/G2+M cells in panels G and J. Numbers inside panels E through J represent the percentages of cells detected in S/G2+M in each sorted group based on the positions of the gates to the right in each panel. Gates shown to the left in panels E through J denote the position of cells in G0/G1.
In vitro analysis of hematopoietic progenitor cells
To assay FL and FBM CD34+ cells for their original content of clonogenic hematopoietic progenitors, G0CD34+, G1CD34+, and S/G2+MCD34+ cells from one fetus were separated by cell sorting. For these assays, sorted G0, G1, or S/G2+MCD34+ cells were assayed in duplicate in plastic 35-mm tissue culture dishes in 1 mL IMDM containing, at the final concentration, 30% FCS, 1.3% methylcellulose, 5 × 10−5 M 2-mercaptoethanol (Sigma), 50 ng/mL stem cell factor, 10 ng/mL interleukin-3 (IL-3), 10 ng/mL interleukin-6 (IL-6), 5 ng/mL granulocyte macrophage–colony-stimulating factor (GM-CSF), and 2 U/mL erythropoietin. All cytokines were kind gifts from Amgen (Thousand Oaks, CA). Plates were cultured at 37°C in 100% humidified atmosphere containing 5% C02. After 2 weeks, plates were scored by means of an inverted microscope. To estimate the frequencies of long-term culture initiating cells (LTC-ICs) in the various fractions, LDA assays were performed as previously described19 with some modifications.20 M2-10B4 cells were irradiated at 80 Gy (GammaCell 40; Nordion International, Kanata, Ontario, Canada) and were plated in flat-bottom 96-well plates at a concentration of 15 × 103 cells/well in 100 μL long-term culture medium (LTCM). LTCM consisted of Myelocult (Stem Cell Technologies, Vancouver, BC, Canada) containing 10−6 mM hydrocortisone (Sigma). After 24 hours, test cells in 100 μL LTCM were added to the plated stromal cells in limiting dilution (64 to 8 cells per well, in a 2-fold dilution scheme using 48 wells per cell dose). Plates were maintained at 37°C in 100% humidified atmosphere containing 5% CO2, with weekly half-medium changes. After 5 weeks, 120 μL medium was removed from each well, followed by the addition of 150 μL IMDM containing methylcellulose and growth factors as described above. After 2 weeks, wells were scored for the presence or absence of hematopoietic colonies, and the frequency of LTC-IC was calculated using the maximum likelihood estimator.21
Transplantation of test cells into NOD/SCID mice
NOD/LtSz-scid/scid (NOD/SCID) mice22 used in these experiments were bred and housed at Indiana University or at the Robarts Research Institute. Mice used at Indiana University were kindly provided by Dr D. A. Williams (Indianapolis, IN). Animal experiments were performed in accordance with institutional guidelines approved by the Animal Care Committee of the Indiana University School of Medicine and the Robarts Research Institute/University of Western Ontario. Nine- to 12-week old NOD/SCID mice were sublethally irradiated with 3 Gy from a cesium Cs 137 source (GammaCell 40). Mice received by intravenous injection 107 nonadherent CD34−adult BM cells irradiated with 80 Gy as accessory cells. Two hours later, mice received transplants of 3.4 × 104 to 6 × 105 G0CD34+, G1CD34+, or S/G2+MCD34+cells from FL or FBM or G0CD34+Lin− or G1CD34+Lin− cells from FB. FL and FBM were obtained from the same fetus, and equal numbers of each group of cells were transplanted into each recipient. FB cells were obtained from different fetuses, and equal numbers of G0CD34+Lin− or G1CD34+Lin− cells were transplanted. After 8 weeks, blood was collected, the mice were killed by cervical dislocation, and the spleens and bone marrow were harvested. Bone marrow, spleen, and peripheral blood were analyzed for chimerism in recipients of FL and FBM grafts, whereas only the BM of mice that received FB transplants were analyzed for engraftment.
Flow cytometric analysis of engraftment
The level of chimerism in recipient mice was determined by flow cytometric assessment of the percentage of CD45+ cells contained in these animals. Single-cell suspensions of BM, spleen or blood were incubated with FITC-conjugated mouse anti-human CD45 or isotype control monoclonal antibodies (PharMingen) for 20 minutes at 4°C. Samples were analyzed on a FACScan (BDIS). Positive cells were identified by comparison with isotypic controls and with cells harvested from control (not those receiving transplants) NOD/SCID mice stained with the same antibodies. To determine the frequencies of subsets of human cells, BM and spleen cell suspensions, which contained more than 1% CD45+ cells, were also stained with phycoerythrin-conjugated CD19, CD20, CD33, CD34, and CD38 or FITC-conjugated CD61, CD3, CD4, and CD8 in different combinations and with Cy-5–conjugated anti-CD45 antibody (PharMingen) as the third color.
Progenitor cell analysis of mouse bone marrow
To enumerate different HPCs contained in the BM of mice 8 weeks after transplantation, murine BM cell suspensions that contained more than 1% human CD45+ cells were analyzed in progenitor cell assays, allowing preferential colony formation of human precursor cells. Total cell suspensions (between 1.1 × 104 and 7.5 × 104 total cells) containing 2 × 103CD45+CD34+ human cells (as determined by flow cytometric analysis of these samples) were assayed in duplicate in plastic 35-mm tissue culture dishes in 1 mL IMDM containing, at final concentration, 1.3% methylcellulose, 30% FCS, 5 × 10−5 M 2-mercaptoethanol, 50 ng/mL stem cell factor, 10 ng/mL IL-3, 10 ng/mL IL-6, 5 ng/mL GM-CSF, and 2 U/mL erythropoietin. Cultures were incubated at 37°C in 100% humidified atmosphere containing 5% CO2. Hematopoietic colonies were scored after 2 weeks using an inverted microscope. Only 2 granulocyte macrophage–colony-forming unit (CFU-GM)–derived colonies were detected when 4 × 104 control murine BM cells were plated under these conditions (n = 2).
Statistical analysis
A general linear model procedure (analysis of variance) was used to examine the association between percentage chimerism and position of cells in the cell cycle after adjusting the number of cells infused. The interaction between the cell cycle status of transplanted cells and the number of cells infused was also examined to assess whether the effect of cells infused on chimerism was similar between cells in different positions in the cell cycle. Least-squares means of chimerism are reported after they were adjusted for number of cells infused. Where applicable, mean ± SD or mean ± SEM of multiple measurements is reported. Data were analyzed using a Student t test, and differences yieldingP < .05 were considered statistically significant.
Results
Cell cycle fractionation of FL and FBM CD34+ cells and CD34+Lin− FB cells
To separate CD34+ cells in the G0phase of the cell cycle from those in G1 or S/G2+M, cells were stained with Hst and PY and were sorted as previously described.16 In addition, the size of the fraction of FL and FBM CD34+ cells expressing CD38 was examined (Figure 1). As can be seen in panels A and B of Figure 1, FL cells contained a larger percentage of CD34+CD38− cells than FBM cells. In addition, FBM CD34+ cells were uniformly bright for the expression of CD38, whereas 2 distinct populations of CD34+CD38bright and CD34+CD38dim were identified among FL CD34+ cells. Cell cycle distribution of FL and FBM CD34+ cells revealed that the percentages of cells residing in the different phases of cell cycle were comparable in both tissues (Figure 1; Table 1).
CFU content and LTC-IC frequency of different fractions of FBM, FL, and FB cells fractionated based on their cell cycle position
| Tissue source . | Fraction . | Size of each fraction* . | Total HPCs† . | LTC-ICs . |
|---|---|---|---|---|
| Fetal BM | G0CD34+ | 25.5 | 180 ± 41 (46)‡ | 5 |
| G1CD34+ | 59.5 | 250 ± 47 (150) | 4 | |
| S/G2 + M CD34+ | 15.0 | 240 (36) | 2 | |
| FL | G0CD34+ | 24.1 | 75 ± 15 (18) | 9 |
| G1CD34+ | 66.7 | 88 ± 23 (59) | 8 | |
| S/G2 + M CD34+ | 9.2 | 110 (11) | 7 | |
| FB | G0CD34+Lin− | 15.5 | 130 ± 26 (19) | ND |
| G1CD34+Lin− | 67.3 | 140 ± 15 (91) | ND |
| Tissue source . | Fraction . | Size of each fraction* . | Total HPCs† . | LTC-ICs . |
|---|---|---|---|---|
| Fetal BM | G0CD34+ | 25.5 | 180 ± 41 (46)‡ | 5 |
| G1CD34+ | 59.5 | 250 ± 47 (150) | 4 | |
| S/G2 + M CD34+ | 15.0 | 240 (36) | 2 | |
| FL | G0CD34+ | 24.1 | 75 ± 15 (18) | 9 |
| G1CD34+ | 66.7 | 88 ± 23 (59) | 8 | |
| S/G2 + M CD34+ | 9.2 | 110 (11) | 7 | |
| FB | G0CD34+Lin− | 15.5 | 130 ± 26 (19) | ND |
| G1CD34+Lin− | 67.3 | 140 ± 15 (91) | ND |
Sizes of G0, G1, and S/G2 + M fractions reported here are derived from the analysis of the FBM and FL samples shown in Figure 1. Similar results were obtained from the analysis of other samples used in these studies. Values obtained from the analysis of FB G0CD34+Lin− and G1CD34+Lin− fractions from one representative sample are also shown. In this particular sample, cells in S/G2 + M comprised 17.2% of CD34+Lin− cells analyzed.
Total number of clonogenic HPCs and frequency of LTC-ICs per 1 × 103 evaluated cells. Data are reported as mean ± SD of 2 to 4 samples for G0 and G1fractions of FBM and FL cells (assayed in duplicate) and 5 samples (assayed in triplicates) for FB. Only one sample each of FBM and FL CD34+ cells in S/G2 + M from the same fetus was assayed. LTC-IC data were collected from one set of FBM and FL tissues collected from the same fetus. No statistically significant differences were detected between groups.
Numbers in parentheses reflect the relative distribution of progenitors among different phases of the cell cycle. These values were obtained by multiplying the number of progenitor cells for every fraction by the percentage that fraction represents of total cells analyzed, as shown in Figure 1, panels C and D.
ND indicates not determined.
Progenitor cell content of FL and FBM G0CD34+, G1CD34+, and S/G2+M CD34+ cells
Whether fractionation of FL and FBM CD34+ cells based on their position in cell cycle results in the compartmentalization of HPCs was first examined in vitro. The numbers of clonogenic progenitor cells contained in 103 G0, G1, and S/G2+M fractions of FL and FBM CD34+ cells are shown in Table 1. Similar frequencies of colony-forming cells were detected in all 3 fractions of fetal BM cells. Relatively higher frequencies of progenitors were detected in G1 and S/G2+M fractions of FL CD34+ cells than in G0CD34+ cells. LTC-ICs were detected in the G0, G1, and S/G2+M fractions of CD34+ cells from FL and FBM (Table 1). Both G0 and G1 fractions of FB CD34+Lin− cells contained comparable frequencies of assayable progenitors (Table 1). These results indicated that equivalent frequencies of primitive hematopoietic cells were distributed among all phases of the cell cycle in these tissues and that cell cycle fractionation of FBM and FL CD34+ cells may not lead to efficient sequestration of HSCs. It is important to point out, however, that given the difference in distribution of cells among different phases of the cell cycle (Figure 1), the relative absolute numbers of progenitors in each compartment were different (Table1).
NOD/SCID repopulating ability of FL, FBM, and FB CD34+cells isolated in different phases of the cell cycle
The marrow-repopulating ability of G0, G1, and S/G2+MCD34+ cell populations from pooled (1-6 samples per experiment) cryopreserved FL and FBM samples and of G0 and G1CD34+Lin−cells from FB was assessed by transplanting test cells into conditioned NOD/SCID recipients. To confirm that verapamil had no adverse effects on the functional properties of test cells, the levels of chimerism in NOD/SCID mice transplanted with verapamil-treated and untreated sorted FB cells was assessed in a pilot study. In 2 experiments, chimerism rates in recipients of FB G0CD34+Lin− cells treated with verapamil were 12% and 12%, whereas those in recipients of untreated G0CD34+Lin− cells were 14% and 10%. Chimerism rates in recipients of verapamil-treated G1CD34+Lin− cells were 8% and 4%, and in mice receiving untreated cells they were 6% and 4.5%. Lack of adverse effects of cryopreservation on the isolation of engrafting cells in different phases of the cell cycle was also investigated in a pilot study using FB cells. Freshly isolated G0CD34+Lin− and G1CD34+Lin− cells promoted 12% ± 3% and 7% ± 3% (n = 4) chimerism, respectively. Cells isolated from the same samples after remaining cryopreserved for 1 week supported 14% ± 5% and 6% ± 3% chimerism for G0CD34+Lin− and G1CD34+Lin− cells, respectively.
In 7 separate experiments, a total of 26 mice received transplants of FL CD34+ cells, and 26 mice receiving transplants of FBM CD34+ cells were separated into G0, G1, or S/G2+M using between 3.4 × 104 and 6 × 105 cells per animal. Similarly, equal numbers of G0 or G1CD34+Lin− cells from FB were transplanted into 13 and 17 recipients, respectively, using between 103 and 25 × 103 cells per mouse. Human cell engraftment was evaluated 8 weeks later by flow cytometric determination of human CD45+ cells in cell suspensions of tissues collected from recipient mice, as described in “Materials and methods.” Of the 26 mice that received transplants of FL, 15 (58%) showed more than 1% engraftment of CD45+ cells in BM, whereas only 10 (38%) mice that received transplants of FBM cells were 1% or more chimeric. However, 22 of 26 (85%) of mice receiving FL cells and 19 of 26 (73%) of mice that received transplants of FBM cells contained more than 0.1% CD45+ cells in the marrow (Figure 2). As seen in recipients of FBM grafts, 10 (33%) mice transplanted with FB cells were chimeric at levels equal to or higher than 1%, whereas 57% of these recipient mice (17 of 30) were more than 0.1% chimeric (Figure 2).
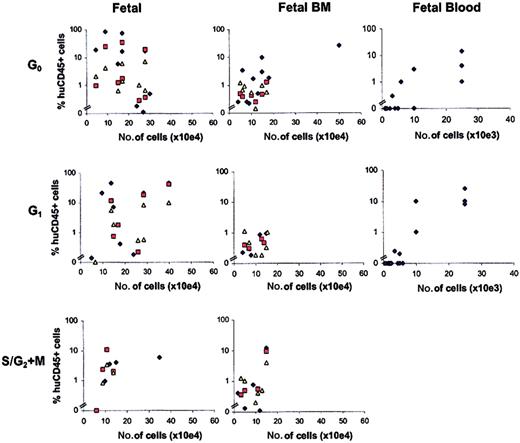
Relationship between number of transplanted FL, FBM, and FB hematopoietic cells and chimerism in NOD/SCID recipients.
Mice were transplanted with G0CD34+, G1CD34+, or S/G2+MCD34+cells from FL or FBM or with G0CD34+Lin− or G1CD34+Lin− cells from FB. Chimerism was defined as the percentage of human CD45+cells detected in BM (blue diamonds), spleen (red squares), or peripheral blood (yellow triangles) of recipient NOD/SCID mice 8 weeks after transplantation. Each point represents the level of chimerism in one tissue of one mouse. Note that the number of cells used in transplantation of fetal blood cells is different than that used for fetal liver and bone marrow, as is shown by the scale of the x-axis in the respective plots.
Relationship between number of transplanted FL, FBM, and FB hematopoietic cells and chimerism in NOD/SCID recipients.
Mice were transplanted with G0CD34+, G1CD34+, or S/G2+MCD34+cells from FL or FBM or with G0CD34+Lin− or G1CD34+Lin− cells from FB. Chimerism was defined as the percentage of human CD45+cells detected in BM (blue diamonds), spleen (red squares), or peripheral blood (yellow triangles) of recipient NOD/SCID mice 8 weeks after transplantation. Each point represents the level of chimerism in one tissue of one mouse. Note that the number of cells used in transplantation of fetal blood cells is different than that used for fetal liver and bone marrow, as is shown by the scale of the x-axis in the respective plots.
Similar percentages of recipient mice were chimeric following transplantation of FL G0, G1, or S/G2+MCD34+ cells (55%, 60%, and 60% of mice that received transplants contained 1% or more CD45+ cells in the marrow, respectively; Table 2). A more variable distribution of chimeric animals with 1% or more human CD45+ cells in BM was observed among recipients of FBM cells in different phases of the cell cycle (Table 2). When lower levels of engraftment (more than 0.1% CD45+) were included in the analysis, comparable numbers of engrafted animals were found (Table 2).
Percentages of NOD/SCID mice chimeric for human CD45+ cells after transplantation with human FL or FBM CD34+ cells isolated in G0, G1, or S/G2 + M phases of cell cycle
| Cell cycle status of graft . | Chimerism . | |||||
|---|---|---|---|---|---|---|
| Bone marrow . | Spleen . | Peripheral blood . | ||||
| FL . | FBM . | FL . | FBM . | FL . | FBM . | |
| G0 | 55 (n = 11) | 60 (n = 10) | 67 (n = 9) | 13 (n = 8) | 67 (n = 9) | 29 (n = 7) |
| (82)* | (90) | (89) | (75) | (89) | (100) | |
| G1 | 60 (n = 10) | 22 (n = 9) | 50 (n = 8) | 0 (n = 6) | 57 (n = 7) | 29 (n = 7) |
| (90) | (45) | (75) | (67) | (100) | (86) | |
| S/G2 + M | 60 (n = 5) | 29 (n = 7) | 75 (n = 4) | 20 (n = 5) | 50 (n = 4) | 50 (n = 6) |
| (80) | (86) | (75) | (80) | (75) | (100) | |
| Cell cycle status of graft . | Chimerism . | |||||
|---|---|---|---|---|---|---|
| Bone marrow . | Spleen . | Peripheral blood . | ||||
| FL . | FBM . | FL . | FBM . | FL . | FBM . | |
| G0 | 55 (n = 11) | 60 (n = 10) | 67 (n = 9) | 13 (n = 8) | 67 (n = 9) | 29 (n = 7) |
| (82)* | (90) | (89) | (75) | (89) | (100) | |
| G1 | 60 (n = 10) | 22 (n = 9) | 50 (n = 8) | 0 (n = 6) | 57 (n = 7) | 29 (n = 7) |
| (90) | (45) | (75) | (67) | (100) | (86) | |
| S/G2 + M | 60 (n = 5) | 29 (n = 7) | 75 (n = 4) | 20 (n = 5) | 50 (n = 4) | 50 (n = 6) |
| (80) | (86) | (75) | (80) | (75) | (100) | |
Except for n values, all numbers are percentages.
Data reflect percentages of chimeric animals (1% or more CD45+ cells) determined after analysis of each tissue (BM, spleen, or peripheral blood). Number of animals available for analysis is shown as n for every group.
Denotes percentage of chimeric animals at the 0.1% or higher CD45+ level in every group.
When G0 and G1CD34+Lin− cells derived from FB were compared, similar percentages of chimeric mice were detected among recipients of either cell fraction. A total of 39% and 30% of the mice that received transplants of either G0 or G1fractions of FB CD34+Lin− cells contained 1% or more CD45+ cells in BM, respectively (Figure 2). At levels greater than 0.1% CD45+ human cells, 62% and 53% of the animals engrafted after transplantation of G0 or G1 CD34+Lin− cells, respectively. NOD/SCID mice that received transplants of FB cells received significantly smaller numbers of cells than those that received transplants of FL or FBM cell fractions. Whether the lower level of chimerism detected in these mice, relative to that observed in recipients that received transplants of FBM and FL cells, resulted solely from the smaller number of graft cells they received or from the absence of lineage-committed CD34+ cells in these grafts could not be gleaned from these experiments.
Differentiation potential of repopulating FL and FBM cells
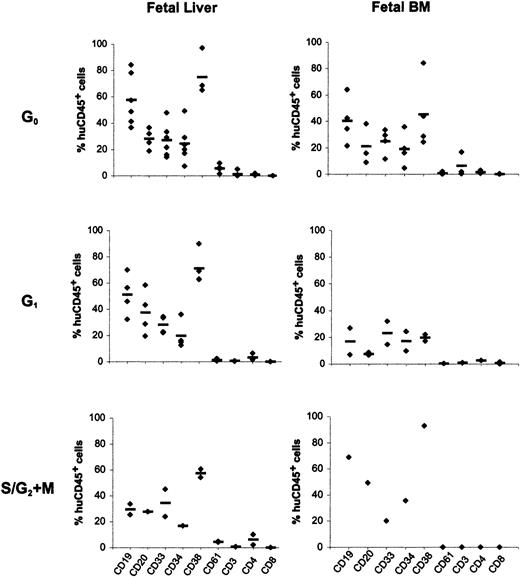
To compare the in vivo differentiation potential of total engrafting cells, the phenotypic profile of chimeric CD45+cells in bone marrow of recipient mice, resulting from the composite differentiation of all engrafting cells, was determined by 3-color immunostaining. Data generated from these analyses are shown in Figure3. A large fraction of human CD45+ cells in recipients of FL cells was also CD34+ (24% ± 14%, 20% ± 11%, and 17% ± 0.5% for cells in G0, G1, and S/G2+M, respectively). Among chimeric FL-derived cells, CD19 expression (58% ± 20% for G0 cells, 51% ± 16% for G1 cells, and 29% ± 6% for S/G2+M cells, as percentages of total CD45+ cells) and CD33 expression (27% ± 13% for G0 cells, 28% ± 7% for G1 cells, and 34% ± 15% for S/G2+M cells as percentages of total CD45+ cells) was identifiable, demonstrating that repopulating cells in all phases of the cell cycle were capable of lymphoid and myeloid differentiation. Although smaller numbers of chimeric mice could be analyzed after transplantation with FBM cells because of the lower engraftment levels, all chimeric mice contained CD45+ cells expressing CD34 and CD19 or CD33 cells, illustrating again that FBM-repopulating cells in all phases of the cell cycle were capable of multilineage differentiation. However, given the small numbers of mice that received transplants analyzed in some cases (FL and FBM S/G2+M cells and FBM G1cells), it is difficult to ascertain that no significant differences in the ability of these cells to sustain multilineage differentiation can be concluded. Phenotypic analysis of engrafting cells in recipients of FB grafts also documented multilineage differentiation capacity among recipients of G0 and G1CD34+Lin− cells (data not shown).
Differentiation potential of human cells in bone marrow of recipient mice.
Bone marrow cells from NOD/SCID mice transplanted with FL (left panels) and FBM (right panels) G0CD34+, G1CD34+, and S/G2+MCD34+ cells were analyzed for human hematopoietic lineages 8 weeks after transplantation. Each data point represents the percentage of human CD45+ cells positive for the expression of CD antigens indicated on the x-axis in one recipient. Bone marrow cells from mice receiving G0CD34+, G1CD34+, and S/G2+MCD34+ cells were analyzed for the expression of the 9 markers indicated. Horizontal bars for every marker denote the mean value of all measurements made for each antigen.
Differentiation potential of human cells in bone marrow of recipient mice.
Bone marrow cells from NOD/SCID mice transplanted with FL (left panels) and FBM (right panels) G0CD34+, G1CD34+, and S/G2+MCD34+ cells were analyzed for human hematopoietic lineages 8 weeks after transplantation. Each data point represents the percentage of human CD45+ cells positive for the expression of CD antigens indicated on the x-axis in one recipient. Bone marrow cells from mice receiving G0CD34+, G1CD34+, and S/G2+MCD34+ cells were analyzed for the expression of the 9 markers indicated. Horizontal bars for every marker denote the mean value of all measurements made for each antigen.
Human progenitor cells in the marrow of recipient NOD/SCID mice
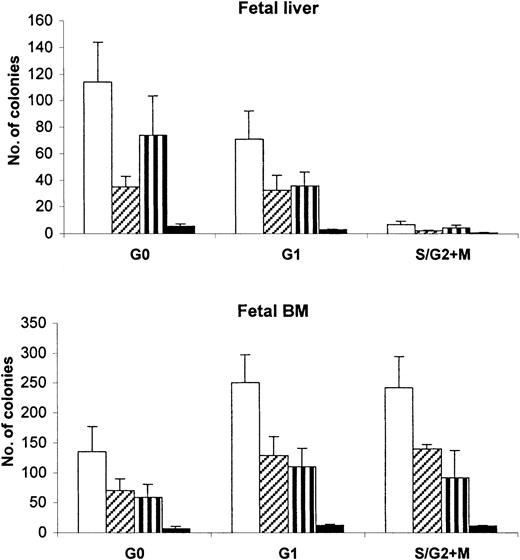
To compare the different classes of progenitors in bone marrow of recipient mice at 8 weeks after transplantation, BM cell suspensions from recipients demonstrating 1% or more CD45+ chimerism were assessed in progenitor cell assays (Figure4). The number of clonogenic progenitors contained in 2 × 103 CD45+CD34+cells derived from mice that received transplants of FL G0CD34+ cells was 110 ± 30, whereas that detected in an equivalent number of bone marrow cells from G1CD34+ recipients was 71 ± 22 and from S/G2+MCD34+ recipients it was 7 ± 2 (Figure4). The difference in the number of clonogenic progenitors contained in recipients of FL G0CD34+ cells and recipients of G1CD34+ cells did not reach statistical significance. In contrast to FL recipients, mice that received transplants of cycling FBM cells contained higher numbers of clonogenic cells than recipients of quiescent cells from the same source. Although 240 ± 52 and 250 ± 47 colonies were detected in the marrow of FBM S/G2+M and G1CD34+ recipients, respectively, only 140 ± 42 colonies were present in the BM of mice that received transplants of FBM G0CD34+ cells. However, these differences were not statistically significant.
Detection of different classes of clonogenic human progenitors contained in the marrow of chimeric transplantation recipients.
From the BM of mice receiving G0CD34+, G1CD34+, and S/G2+MCD34+ FL or FBM cells, 2 × 103 CD45+CD34+ cells were assayed in methylcellulose, as described in “Materials and methods.” The number of mice analyzed per group was different for different groups of cells as follows: FL, n = 12 for G0; n = 10 for G1; n = 6 for S/G2+M; FBM, n = 8 for G0; n = 4 for G1; n = 2 for S/G2+M. Data are presented as mean numbers ± SEM of clonogenic human progenitors contained in the marrow of chimeric transplantation recipients. The mean total number of colonies (clear bars) represent the arithmetic sum of derived colonies for erythroid burst-forming units (BFU-E, hatched bars), granulocyte/macrophage–colony-forming units (CFU-GM, striped bars); and granulocyte/erythroid/macrophage/megakaryocyte colony-forming units (CFU-GEMM, black bars).
Detection of different classes of clonogenic human progenitors contained in the marrow of chimeric transplantation recipients.
From the BM of mice receiving G0CD34+, G1CD34+, and S/G2+MCD34+ FL or FBM cells, 2 × 103 CD45+CD34+ cells were assayed in methylcellulose, as described in “Materials and methods.” The number of mice analyzed per group was different for different groups of cells as follows: FL, n = 12 for G0; n = 10 for G1; n = 6 for S/G2+M; FBM, n = 8 for G0; n = 4 for G1; n = 2 for S/G2+M. Data are presented as mean numbers ± SEM of clonogenic human progenitors contained in the marrow of chimeric transplantation recipients. The mean total number of colonies (clear bars) represent the arithmetic sum of derived colonies for erythroid burst-forming units (BFU-E, hatched bars), granulocyte/macrophage–colony-forming units (CFU-GM, striped bars); and granulocyte/erythroid/macrophage/megakaryocyte colony-forming units (CFU-GEMM, black bars).
Mathematical model for engraftment of human cells in NOD/SCID recipients
The relationship between the number of transplanted FL and FBM cells and the level of chimerism detected 8 weeks later was analyzed using a general linear models procedure, as previously applied to the analysis of the engraftment of umbilical cord blood cells in G0 or G1 in NOD/SCID mice.14 The relationship between the position of cells in the cell cycle and the number of cells infused can be analyzed using the equation:Y = α + β1 X1 + β2 X2 + β3 X3, where Y is the estimated level of chimerism in transplanted mice, X1 is an indicator variable equal to 1 if G1 cells are considered (zero otherwise),X2 is another indicator variable equal to 1 if S/G2 +M is considered (zero otherwise), andX3 is the number of cells infused in thousands. A constant derived from the linear model, α represents theY intercept. The value of β1 represents the expected difference between the levels of chimerism obtained with G0 versus G1 cells, whereas β2represents the expected difference between the levels of chimerism obtained with G0 versus S/G2 +M cells. β3 is the slope representing the expected incremental change in chimerism for every additional 103 cells contained in the graft. β1 = β2 = β3 = 0 represents the null hypothesis that there is no relationship between level of chimerism and cell cycle status or number of transplanted cells. If the analysis reaches statistical significance (P < .05), the hypothesis is rejected, indicating that a linear relationship exists between chimerism and cell cycle status or number of cells transplanted.
When FBM or FL cells were transplanted, no significant relationship was observed between chimerism in BM and either the number of cells infused or the position of transplanted cells in the cell cycle. These analyses indicated that fetal BM or FL-derived chimerism in the BM of recipient mice is independent of the position of graft cells in the cell cycle and the number of cells transplanted. For the analysis of chimerism after transplantation with FB cells, β2 was eliminated from the equation because no S/G2+M cells were used in these experiments.14 The interaction effect between position of FB cells in the cell cycle and the number of cells infused was significant (P = .03), indicating that the effect of the number of cells infused on chimerism is different, depending on the cell cycle position of transplanted cells. It is important to stress that this mathematical model confirms biologic data from transplantation studies and does not establish an independent model for the assessment of engraftment potential of test cells.
Discussion
While examining the hematopoietic repopulating potential of umbilical cord blood cells residing in different phases of the cell cycle, we recently14 proposed that the extensive demand for primitive and mature hematopoietic cells in the developing fetus may require that all potential HSCs contribute to blood cell production regardless of their position in the cell cycle. Such a requirement would necessitate that during embryonic development, mitotically quiescent cells and cells in active phases of the cell cycle home and sustain long-term hematopoiesis to support cell production and maintenance of the stem cell pool as hematopoiesis shifts from the yolk sac to the fetal liver and the BM. As a first step in examining this hypothesis, we recently showed that UCB cells in the G1phase of the cell cycle have a repopulating potential similar to that of cells in G0,14 suggesting that cycling prenatal and neonatal hematopoietic cells may have the same functional capabilities described for quiescent, but not cycling, adult BM and MPB cells.13 In this report, we demonstrate that as documented for UCB-derived repopulating cells,14 HSCs from earlier stages of human hematopoietic ontogeny, such as fetal liver, fetal blood, and fetal bone marrow, can be found in active phases of the cell cycle. These findings further support our contention that the prevailing dogma ascribing primitive hematopoietic functions to cells residing in deep dormancy in adults may not be true for cells derived from fetal tissues.
That fetal HPCs are functionally distinct from similar cells from adult tissues has been demonstrated. Lansdorp et al10demonstrated that FL cells had a higher proliferative capacity than cells derived from CB or adult BM and were capable of generating more CD34+ cells in culture. These authors concluded that the turnover rate and both the proliferative and differentiation potentials of HPCs decrease during ontogeny.10 More recently, it has been demonstrated that FL is enriched for NOD/SCID repopulating cells compared to CB cells.12,23 Under these conditions, the functional capacity assessed by these studies10,12 23 would have been contributed by resting and cycling fetal cells but only by quiescent cells derived from adult tissues.
It is intriguing that cells in the S/G2+M phases of the cell cycle from FL and FBM engrafted durably. Our previous studies examining the relationship between in vivo engraftment and the position of graft cells in cell cycle focused mainly on mobilized peripheral blood and UCB, sources of stem cells known to be predominantly in G0 or G1.13,14,24-26 Marrow CD34+ cells contain a small fraction of cells in S/G2+M, and that has precluded testing of these cells in the NOD/SCID model. Based on our results from BM and mobilized peripheral blood studies,13 it is unlikely that cells in S/G2+M from adult sources would engraft in vivo. This prediction, drawn from studies examining the marrow-repopulating potential of murine HSCs in active phases of the cell cycle,27-30 and the general belief that stem cells reside in deep dormancy within the BM microenvironment31 32 are in sharp contrast to our present observations.
Progression of cells into active phases of the cell cycle in ex vivo expansion cultures has long been suspected to be the primary reason expanded cells exhibit in vivo functions inferior to those of freshly isolated cells.33-36 However, mechanisms underlining this defect remain unresolved. In addition, it has been postulated that HSCs undergoing cell division in vitro may modulate adhesion molecules important for homing and engraftment, thus losing their repopulating potential.37-40 Cell cycle–associated modulation of expression of adhesion molecules by candidate stem cells has been previously reported.41 It was therefore speculated that cycling cells in vivo manifest limitations similar to those documented for cells cycling in vitro (loss of hematopoietic function) but that though the re-entry of cycling cells into mitotic quiescence and the reacquisition of function is possible in vivo, it is not achievable under conditions used for ex vivo expansion of HSCs. In a recent report, Glimm et al42 documented that ex vivo–expanded CB cells appear to fail to re-enter G0, as demonstrated by their inability to reconstitute NOD/SCID mice. Although in these studies limited numbers of G0 cells recovered from expansion cultures could be transplanted into immunodeficient mice, these grafts were large enough to contain sufficient numbers of engrafting cells (relative to unmanipulated cells) that might have returned to dormancy. Our present and previous14,20 studies indicate that whereas cycling stem cells from adult sources might suffer from in vivo loss of function, HSCs in fetal tissues retain their functional properties throughout the cell cycle. Whether mechanisms associated with loss of function of cells from mature hematopoietic tissues as they traverse the cell cycle are not operative in fetal cells has not yet been determined. Similarly, whether the modulation of adhesion molecules does not accompany the movement of fetal HSCs through the cell cycle or whether modulation of these markers is minimal or limited to molecules not involved in homing and engraftment remains to be established. Finally, it remains to be determined whether the repopulating ability observed in cycling fetal and neonatal cells14—but not their counterparts from adult tissues13—is affected by differences in telomere length43 or by differentiation status of these different cells as reflected by telomerase activity.44
Maintenance of engraftment potential of fetal HSCs as they progress through the cell cycle was also confirmed by analysis of engraftment with a general linear models procedure.14 These analyses indicated that for FL and FBM, no significant relationship was observed between chimerism in the BM and the position of cells in either G0 or G1, indicating that the level of chimerism in these recipients is independent of the position of graft cells in the cell cycle. Although for FB both phases of the cell cycle showed engraftment, the effect of the number of cells infused on chimerism was different, depending on the cell cycle position of transplanted cells. In part, this might have been caused by the low number of cells transplanted or by the fact that a subfraction of CD34+ cells was used in these studies.
Recently, we documented that a substantial fraction of adult human BM cells residing in the S/G2+M phases of the cell cycle undergo apoptosis shortly after their trafficking to the BM of conditioned NOD/SCID recipients.45 In these studies,45 a significantly smaller percentage of cells in G0/G1 detected in the BM of recipient mice were apoptotic, suggesting that whereas homing or trafficking of cycling adult hematopoietic cells may be intact, apoptosis may play a role in the loss of function of these cells. In view of these results, it would be interesting to examine whether cycling fetal hematopoietic cells are more resistant to apoptosis. It is unlikely that engraftment of cycling cells in these studies was enhanced by the recently described phenomenon46 attributed to the conditioning of recipient mice with apoptotic leukocytes (irradiated nonadherent BM CD34− cells in our studies) because this experimental step was used in our previous studies when cycling cells failed to engraft.13
Results obtained with FB may be of particular interest in view of possible prenatal correction of certain inborn errors. This source of HPCs has not been extensively investigated, but it represents a promising source of stem cells suitable for gene therapy.15 Our transplantation data presented here, and the results of gene transfer studies using FB cells,15demonstrate that these cells are not only enriched for marrow-reconstituting cells but are also capable of retaining in vivo hematopoietic functions following in vitro manipulations required for effective retroviral-mediated gene transfer. These results are encouraging for 2 reasons. First, they establish the usefulness of FB cells as a source of transplantable stem cells and as targets for effective retroviral-mediated gene transfer. Second, they illustrate that HSCs from fetal tissues may be less susceptible to the observed loss of function associated with ex vivo manipulation of cells from adult sources during transduction. That fetal HSCs traverse the cell cycle with minimal loss of function has been suggested in studies demonstrating the maintenance of SRC function in retrovirally marked clones expressed in NOD/SCID mice.15 However, it is important to stress that the loss of hematopoietic potential after ex vivo manipulation of fetal hematopoietic cells has been shown in earlier studies using different in vitro assays.10,47 48
In conclusion, these studies summarize our assessment of the hematopoietic potential of cells in different phases of the cell cycle throughout ontogeny. Although the engraftment potential of marrow-repopulating cells is restricted to quiescent cells in adult tissues, cycling and resting fetal and neonatal cells retain this functional property. Although the biologic significance of maintenance of engraftment potential in fetal HSCs at all phases of the cell cycle may lie in the continued demand for large numbers of hematopoietic cells and the possible migration of these cells from one site to another during fetal development, the significance of this property in reconstitutional therapies still has to be established.
Supported by National Institutes of Health grant R01 HL55716 (E.F.S.), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Center for Excellence in Molecular Hematology grant P50 DK49218, and the J. A. Cohen Institute for Radiopathology and Radiation Protection.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Edward F. Srour, Indiana University School of Medicine, R4-202, 1044 West Walnut St, Indianapolis, IN 46202-5121; e-mail: esrour@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal