Abstract
Digestive tract damage during graft-versus-host reaction (GVHR) causes high morbidity and mortality. Diagnosis is often late because biopsies are performed when clinical signs are severe and pathologic markers of early inflammatory lesions are lacking. Eosinophils are inflammatory cells, cytotoxic in vitro to digestive epithelium; they are found in biopsy specimens taken during acute flare-ups of inflammatory bowel disease. We performed systematic duodenal biopsies immediately after digestive symptoms occurred and found a digestive GVHR incidence of 73.1% (n = 93), higher than that found when digestive biopsies were performed immediately after severe clinical signs. Eosinophils were only present when there were histologic signs of GVHR; eosinophil presence correlated with GVHR severity. Electron microscopy with immunogold staining showed pathologic signs of in situ eosinophil activation, such as cytoplasmic granule alterations, and eosinophil peroxidase release in all patients. Interleukin-5 presence in activated eosinophils suggests eosinophil recruitment in digestive GVHR is an autocrine mechanism. Eosinophil density also correlated with GVHR severity, whether in acute or chronic clinical phases. Tissue eosinophils could thus be a marker of acute inflammatory flare-ups in GVHR. Systematic duodenal biopsy performed at the onset of digestive symptoms should allow early GVHR detection, and pathologic signs of GVHR, together with eosinophil density, might help modulate immunosuppressive therapy.
Introduction
Eosinophils are inflammatory cells that play an important role in defending the host against parasitic pathogens. Although eosinophils are circulating leukocytes, they primarily reside in the epithelial lining of the gastrointestinal (GI) and respiratory tracts.1 On activation, they release preformed cationic granule proteins that exert cytotoxic effects on epithelial cells in vivo.2
Interleukin-5 (IL-5) is the main cytokine, contributing to activation and recruitment of eosinophils. In vitro, IL-5 exerts a wide range of effects on eosinophils. It supports the proliferation and terminal differentiation of eosinophil precursors and can prolong the survival of mature eosinophils.3,4 In addition, IL-5 is chemotactic for eosinophils and contributes to their activation.5 IL-5 is secreted by lymphocytes, mast cells, and eosinophils.6,7 In eosinophilic heart disease, tissue eosinophils are activated and are able to synthesize IL-5. IL-5 then contributes to the recruitment and activation of other eosinophils through an autocrine mechanism.8 IL-3 and granulocyte macrophage–colony-stimulating factor have complementary effects with IL-5 on eosinophil maturation in vitro.9 Another eosinophil chemoattractant, eotaxin, has been characterized in a guinea pig model of asthma.10 More recently, eotaxin genes from mice, rats, and humans have been cloned. Eotaxin exhibits potent and specific chemotactic activity for eosinophils in all these species, both in vivo and in vitro.10 When delivered to mice, eotaxin induces a potent and rapid eosinophil recruitment that is enhanced by IL-5.11 Targeted disruption of eotaxin in knockout mice partially reduces antigen-induced tissue eosinophil accumulation and eosinophil traffic to Peyer patches.12 13
In the GI tract, the number of tissue eosinophils increases during acute phases of Crohn disease and celiac disease.14,15Combined ultrastructural and immunogold studies have shown in these abnormalities that tissue eosinophils are able to synthesize IL-5 and to contribute to the recruitment of other eosinophils by an autocrine mechanism.16,17 In acute phases of Crohn disease, endothelial and epithelial cells synthesize eotaxin, which further contributes to eosinophil recruitment.10 Moreover, in acute phases of Crohn disease and celiac disease, tissue eosinophils show signs of activation (ie, degranulation and release of their toxic cationic proteins in the lamina propria).18,19 These cytotoxic proteins are found in intestinal fluids and may cause mucosal damage to the digestive tract.20 In the liver, eosinophils are found in portal tracts during acute liver allograft rejection.21-23 In this context of allograft rejection, in the liver as in the kidney, tissue eosinophils show signs of activation with deposits of cationic protein,24 and their density has a predictive value for steroid-resistant rejection.25 26
The GI tract, liver, and skin are the main target organs of graft-versus-host disease (GVHD). At the cellular level, epithelial cells are the targets of the graft-versus-host reaction (GVHR), inducing them to undergo apoptosis.27 In animal models, the enteropathy of GVHD produced by CD4+ T cells is mediated by soluble factors rather than by cytotoxity.28,29 Moreover, the GI tract is critical for the propagation of the so-called cytokine storm.30,31Therefore, Nikolic et al32 suggest a cytokine-directed approach for treating GVHD.
Initially, human digestive GVHD was studied in necropsy series and later in rectal or colonic biopsy specimens.33,34 Snover et al35 found a discrepancy in the GVHD assessment in colonic and upper GI tract biopsy specimens in patients with digestive symptoms who have undergone bone marrow transplantation (BMT). Two other studies reported that GI tract biopsy is more sensitive than rectal biopsy for the diagnosis of digestive GVHD, even when diarrhea is a prominent symptom.36, 37 In a series of 469 patients, Weisdorf et al37 found that the incidence of upper GI GVHD is at least 22% and that it is underestimated when clinical features alone are taken into account.
The role of eosinophils in GVHD has also been studied in blood and skin. In acute GVHD, blood eosinophils are activated.38Blood eosinophilia, occurring after total body irradiation or busulphan and cyclophosphamide preparative regimens, has a predictive value for the subsequent development of chronic GVHD.39 In fasciitis, a chronic form of skin involvement in GVHD resistant to any conventional treatment, degranulating eosinophils were found in the sclerotic tissue, and blood eosinophilia was present in half the patients.40 In a murine model of lethal GVHD and hypereosinophilia, eosinophilia in the lung is correlated with the severity of pulmonary fibrosis.41
Therefore, we studied upper GI tract eosinophils in patients with gastrointestinal GVHD. Our aims were to assess the relation between eosinophil recruitment and activation and to assess the incidence and severity of allogeneic lesions.
Patients, materials, and methods
Patients
Between January 1997 and May 1999, 93 patients with digestive symptoms underwent GI tract exploration with multiple biopsies. All patients underwent allogeneic hematopoietic stem cell transplantation (HSCT) in the Bone Marrow Transplantation Department of the Hospital Saint-Louis in Paris. This text has been approved by the institutional review board of the Institut Universitaire d'Hématologie, Hospital Saint-Louis.
Clinical characteristics of the patients are shown in Table1. Ages ranged from 5 to 60 years (median, 33 years). Thirty-three patients underwent HSCT for acute leukemia, 36 for chronic myelogenous leukemia, 7 for non-Hodgkin lymphoma, 8 for myelodysplastic syndrome, and 10 for aplastic anemia. Sixty-six patients received marrow from an HLA-identical sibling donor, 22 received it from an unrelated donor (UD), and 9 underwent umbilical cord blood transplantation. All living patients were followed up for at least 1 year after transplantation.
Conditioning regimens consisted of chemotherapy in 43 patients, whereas 50 patients received chemotherapy and a total body irradiation. Prophylaxis against GVHD included short-course methotrexate and cyclosporine treatment. Horse antithymocyte globulin, prednisone, and ex vivo T-lymphocyte depletion were also used for HSCT and for unrelated donor HSCT. GVHD was assessed with standard clinical criteria and was graded according to the conventional Seattle scale.42
All patients received oral broad-spectrum antibiotics from the time of admission through the time of engraftment. All patients also received prophylaxis against fungal infections and Pneumocystis. Searches for viruses—including cytomegalovirus, adenovirus, rotavirus, and astrovirus—as Clostridium difficile toxin were systematically made in the stools.
Gastrointestinal endoscopy and biopsy
Digestive GVHD was suspected in patients who had nausea, vomiting, anorexia, pain or burning, or food intolerance and diarrhea. Systematic GI tract explorations with multiple biopsies were performed. Each patient signed an informed consent statement. Histologic grading of digestive lesions was made according to the criteria of Sale et al,43 Epstein et al,44 and Washington et al.45
For each patient, 6 biopsies were systematically performed in the duodenum, of which 2 were transmitted to the microbiology department and studied further for bacterial, viral, or fungal pathogens. For pathologic study, one biopsy specimen was immediately snap frozen in liquid nitrogen. Another biopsy specimen was immediately immersed in 1% glutaraldehyde in cacodylate buffer for 1 hour at 4°C. After 2 cacodylate rinses, half the specimens were embedded in Epon and were further processed for conventional transmission electron microscopy. The other half was embedded in Lowicryl HM20 for immunocolloidal gold labeling, according to previously published procedures.46Two biopsy specimens were oriented, fixed in formaldehyde, and further processed for paraffin embedding. Paraffin blocks were sectioned at 4 μm and were stained with hematoxylin-eosin and May-Gruenwald-Giemsa.
Immunohistochemistry on cryocut sections
Antibodies.
Monoclonal mouse antibody directed against the eosinophil peroxidase was purchased from Oncogene Science (Uniondale, NY), monoclonal mice antibody directed against human eotaxin was purchased from R&D Systems (Abingdon, United Kingdom), and polyclonal rabbit antibody directed against human IL-5 was purchased from Genzyme (Cambridge, MA).
Immunohistochemical methods.
Cryocut sections were incubated for 45 minutes with primary reagents (ie, eosinophil peroxidase, at 1/100 dilution; IL-5, at 1/250 dilution; and eotaxin at 1/800 dilution) at room temperature. After incubation, slides were washed for 5 minutes in Tris HCl-buffered saline (20 mM Tris HCl, 0.5 M NaCl) pH 7.4 (TBS). Binding was detected by means of streptavidin complex (LSAB2 kit, alkaline phosphatase; DAKO, Copenhagen, Denmark). Enzymatic complex, alkaline phosphatase anti–alkaline phosphatase was added at a 1/50 dilution and was developed with phosphate substrate and neofuscin (Sigma Chemical, St Louis, MO).
Controls.
The specificity of immunohistochemistry was tested by omitting the first antibody and by substituting it with an irrelevant antibody of the same isotype but with a different specificity (anti–Echinococcus granulosus).
The indirect immunohistochemistry method included alkaline phosphatase to avoid false-positive results from endogenous eosinophil peroxidase when horseradish peroxidase was used.
Ultrastructural immunogold labeling on Lowicryl sections
Antibodies.
One-nanometer, gold-conjugated, goat anti–rabbit immunoglobulin G (IgG) and anti–mouse IgG were purchased from British Bio Cell (Cardiff, United Kingdom). Danscher silver lactate hydroquinone was purchased from Sigma (St Quentin Fallavier, France).
Immunogold labeling methods.
Ultrathin sections on nickel grids were incubated for 10 minutes on a drop of TBS containing 5% (wt/vol) ovalbumin, supplemented with 1% heat-inactivated normal goat serum. This was followed by incubation with either polyclonal rabbit antibody to human IL-5 or monoclonal mouse antibody to human eosinophil peroxidase used as primary reagents. The grids were rinsed with TBS-ovalbumin and incubated on a drop of the 1-nm, gold-conjugated, goat anti–rabbit IgG (1/100) or anti–mouse IgG (1/50). After 1-hour incubation at room temperature, sections were thoroughly washed with TBS, postfixed for 10 minutes in distilled water containing 1% glutaraldehyde, and washed again with distilled water. Finally, the sections were subjected to silver enhancement according to a modification of Danscher silver lactate hydroquinone physical developer. Lowicryl sections (Electron Microscopy Sciences, Fort Washington, PA) were contrasted with uranyl acetate (Electron Microscopy Sciences) and lead citrate (Electron Microscopy Sciences) before examination with a Zeiss EM 10 electron microscope (Welwyn Garden City, Hertfordshire, United Kingdom).
Controls.
The specificity of immunostaining was tested by omitting the first antibody and by substituting the specific antibodies with preimmune serum or with unrelated antibodies whose labeling had been analyzed previously. To exclude nonspecific binding to proteoglycans, an additional control was performed with a monoclonal antibody directed against a toxoplasmic amylopectin used as primary antibodies. No gold deposit was observed.
Methods of analysis
Clinical graft-versus-host disease.
According to the conventional Seattle criteria, the skin, liver, and GI tract were clinically staged and graded at the time of GI tract biopsy.42
Histologic criteria of digestive GVHD.
Histologic criteria of digestive GVHD were assessed on biopsy specimens of the upper GI tract. Sale et al43 proposed 4 grades for histologic criteria of digestive GVHD. Epstein et al44added criteria of early GVHD, demonstrating that crypt cell degeneration, even without crypt dilatation and crypt abscess, is characteristic of GVHD. Washington et al45 showed that crypt cell degeneration corresponded to epithelial cell apoptosis or single cell necrosis with karyorrhectic debris and that it was the most useful marker of acute GVHD in the GI tract. Therefore, histologic grading was as follows: grade 1, crypt cell degeneration or epithelial cell apoptosis, without crypt loss; grade 2, loss of up to 3 contiguous crypts; grade 3, loss of 4 or more crypts without sloughing; grade 4, total sloughing. The pattern of mucosal changes was determined blindly by 2 different pathologists, without knowledge of the clinical data, on similar areas in the different biopsy specimens.
Inflammatory reaction and number of tissue eosinophils.
The number of inflammatory cells within the lamina propria was systematically assessed on paraffin sections stained with hematoxylin-eosin in 3 different areas. Cell counts were based on an individually defined mucosal tissue unit consisting of a 4-μm thick and a 500-μm block of tissue overlying 200 μm muscularis mucosae. Counts were performed on an Olympus Provis AX 70 microscope, with wide-field eyepiece number 26.5. At × 400 magnification, this wide-field eyepiece provided a field size of 0.344 mm2. Results are expressed as the mean number of cells per field (1+ to 4+) at × 400 magnification.
Analytic counts were performed for lymphocytes, neutrophils, and macrophages. The number of eosinophils was assessed on paraffin sections, stained with May-Gruenwald-Giemsa, in 3 areas, at a × 400 magnification. To express the results semi-quantitatively, we graded the density of tissue eosinophils per field as follows: 0-5 eosinophils per field, 0; 5-10 per field, 1; 10-15 per field, 2+; 15-20 per field, 3+; and more than 20 per field, 4+ at × 400 magnification. Edema was assessed according to intensity, from 0 to 3+, at × 400 magnification.
Signs of tissue eosinophil activation.
Eosinophil degranulation was assessed on paraffin sections stained with May-Gruenwald-Giemsa. A systematic ultrastructural study was performed for each biopsy with tissue eosinophils. It focused on alterations of eosinophil fine structure (ie, alterations of cytoplasmic granules or total cell lysis with scattered extracellular granules).
The release of eosinophil cationic protein was studied on cryosections and ultrathin sections. On cryocut sections, an antibody directed against eosinophil peroxidase was used with an indirect immunologic method and was revealed by alkaline phosphatase to avoid false-positive results from endogenous eosinophil peroxidase. Combined ultrastructural and immunogold labeling allowed us to study the distribution of this preformed cationic protein and its possible association with specific cell structures.
Identical immunohistochemical and immunoelectron microscopic methods were used to study the expression of IL-5, a cytokine involved in recruitment and activation of eosinophils.
Statistical analysis
Statistical comparisons between the severity of histologic digestive GVHD and the density of tissue eosinophils were analyzed using χ2 analysis. P < .05 was considered statistically significant.
Results
Clinical manifestations of GVHD
Acute and chronic GVHD.
Upper GI tract biopsy was performed in all 93 patients with digestive symptoms. Biopsy specimens were collected in 68 (73.1%) patients within 100 days of BMT, and 25 (26.8%) were collected after day 100.
One patient had a complication resulting from the endoscopic procedure: hemorrhage of the duodenal wall. The subsequent hematoma lasted for 4 weeks.
Fifty-five of 68 (80.8%) patients who underwent biopsy by day 100 had clinical acute GVHD at the time of biopsy. The maximum clinical grades of acute GVHD were grade 1 for 4 patients, grade 2 for 23 patients, grade 3 for 13 patients, and grade 4 for 16 patients. Five of 25 (20%) patients who underwent biopsy by day 100 after BMT had extensive clinical chronic GVHD.
Extraintestinal GVHD.
Twenty-five of 68 (36.7%) patients who underwent biopsy by day 100 after BMT had either skin or liver involvement at the time of biopsy. Eight of 25 (32%) patients had clinical involvement of other organs (skin, mouth, or liver).
Abnormalities of the digestive tract
Gastrointestinal tract symptoms.
Within 100 days, 13 patients had either nausea or vomiting, and 55 patients had diarrhea. The GI tract involvement was clinically staged at the time of digestive biopsy using the conventional Seattle criteria. Among 55 patients with diarrhea, 39 (70.9%) had digestive clinical stage 0 or 1 (lower than 500 mL diarrhea per day), and 16 (29.1%) patients had digestive clinical stage 2 to 4 (more than 500 mL diarrhea per day). After day 100, 5 of 25 (20%) patients had either pain or vomiting, and 20 (80%) had diarrhea.
Gastrointestinal tract disease.
Histologic digestive GVHD (Figure 1) was found in 68 of 93 (73.1%) patients. Thirty-three (48.5%) patients had grade 1, 21 (30.8%) patients had grade 2, 11 (16.2%) patients had grade 3, and 3 (4.5%) patients had grade 4 histologic digestive GVHD.
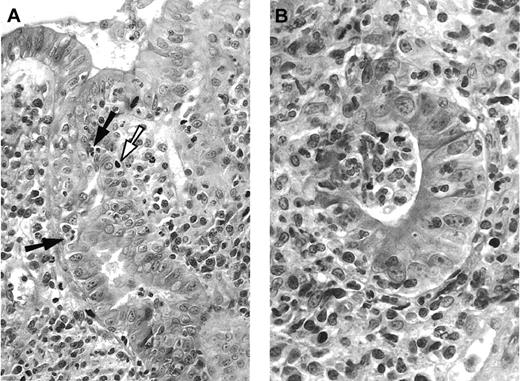
Pathologic aspect of digestive GVHD.
(A) Duodenal biopsy with apoptotic epithelial cells (white arrow), some of them in close contact with intra-epithelial mononuclear cells (black arrows), and dense infiltrate in the lamina propria, composed mononuclear and polynuclear cells. Hematoxylin-eosin, × 250. (B) Higher magnification of damaged glandular epithelium “exploding crypt” in the lamina propria. Hematoxylin-eosin, × 400.
Pathologic aspect of digestive GVHD.
(A) Duodenal biopsy with apoptotic epithelial cells (white arrow), some of them in close contact with intra-epithelial mononuclear cells (black arrows), and dense infiltrate in the lamina propria, composed mononuclear and polynuclear cells. Hematoxylin-eosin, × 250. (B) Higher magnification of damaged glandular epithelium “exploding crypt” in the lamina propria. Hematoxylin-eosin, × 400.
Before day 100, 15 of 16 (93.7%) patients with diarrhea of more than 500 mL per day had histologic digestive GVHD. This was also the case for 31 of 39 (79.4%) patients with diarrhea lower than 500 mL per day and for 7 of 13 (53.9%) patients with only nausea or vomiting (Table 2). After day 100, 15 of 25 (60%) patients had histologic digestive GVHD.
Twelve of the 93 patients who had duodenal biopsies also had stomach biopsies. The histologic grade of GVHD was similar in the stomach and in the duodenum for the 12 patients. It is notable that no patient with histologic signs of GVHD in the stomach was without effects in the duodenum.
Tissue eosinophils
Presence of eosinophils.
For all 93 digestive biopsies, the number of tissue eosinophils per field at × 400 magnification was distributed as follows: 0 to 5 eosinophils in 57 (61.3%) biopsies, 5 to 10 eosinophils in 23 (24.8%) biopsies, 10 to 15 eosinophils in 10 (10.7%) biopsies, and 15 to 20 eosinophils in 3 (3.2%) biopsies. We considered eosinophils present when more than 5 tissue eosinophils per field at × 400 magnification were found in a biopsy specimen. According to this criterion, tissue eosinophils were found during digestive biopsy in 36 of 93 (38.7%) patients.
When present, eosinophils were found in the lamina propria on duodenal biopsy and in the glandular epithelium as isolated cells, but they were not grouped in crypt abscesses. Systematic ultrastructural and immunohistochemical studies showed that eosinophils with signs of activation were present in all 36 biopsies with eosinophil infiltrate.
Signs of tissue eosinophil activation.
When studied by electron microscopy and in paraffin sections with May-Gruenwald-Giemsa staining, half the tissue eosinophils were degranulated. Electron microscopy revealed that more than 80% of the tissue eosinophils had alterations of cytoplasmic granules (Figure2A) with an inverted density of the central cores or total lysis with necrotic nucleus, cytoplasmic degeneration, and scattered extracellular granules.
Ultrastructural study of tissue eosinophils on digestive biopsy with histologic GVHD.
(A) Eosinophils showing moderate signs of activation. Most cytoplasmic granules are altered and have characteristic crystal-shaped central cores that either disappear or are of diminished intensity (white arrows) compared with the few remaining normal central cores (black arrows). However, the cytoplasm is not disintegrated, and there are no cytoplasmic granules in the extracellular space. Epon embedding; bar = 1 μm. (B) Eosinophil showing signs of important in situ activation; the cytoplasm is partially disintegrated, and eosinophil granules are found in the intercellular space. Staining with an antibody directed against eosinophil peroxidase shows gold particles in the matrix of eosinophil granules, whether intracytoplasmic (white arrow) or extracellular (black arrow). Lowicryl HM20 embedding, indirect immunogold staining with an antibody directed against eosinophil peroxidase; bar = 1 μm. (C) Activated eosinophil synthesizing IL-5. Immunogold labeling with an antibody directed against IL-5 shows gold particles in the matrix of cytoplasmic granules (white arrows) in the extracellular space (black arrows) and in damaged extracellular granules. Lowicryl HM20 embedding, indirect immunogold staining with an antibody directed against IL-5; bar = 1 μm.
Ultrastructural study of tissue eosinophils on digestive biopsy with histologic GVHD.
(A) Eosinophils showing moderate signs of activation. Most cytoplasmic granules are altered and have characteristic crystal-shaped central cores that either disappear or are of diminished intensity (white arrows) compared with the few remaining normal central cores (black arrows). However, the cytoplasm is not disintegrated, and there are no cytoplasmic granules in the extracellular space. Epon embedding; bar = 1 μm. (B) Eosinophil showing signs of important in situ activation; the cytoplasm is partially disintegrated, and eosinophil granules are found in the intercellular space. Staining with an antibody directed against eosinophil peroxidase shows gold particles in the matrix of eosinophil granules, whether intracytoplasmic (white arrow) or extracellular (black arrow). Lowicryl HM20 embedding, indirect immunogold staining with an antibody directed against eosinophil peroxidase; bar = 1 μm. (C) Activated eosinophil synthesizing IL-5. Immunogold labeling with an antibody directed against IL-5 shows gold particles in the matrix of cytoplasmic granules (white arrows) in the extracellular space (black arrows) and in damaged extracellular granules. Lowicryl HM20 embedding, indirect immunogold staining with an antibody directed against IL-5; bar = 1 μm.
The release of a preformed cationic protein, eosinophil peroxidase, was detected in the lamina propria by immunohistochemistry (Figure3A). At high magnification, it could be seen that polynuclear cells with bilobed nuclei were stained. Combined ultrastructural examination with immunogold staining showed that eosinophil peroxidase was located in the central core of eosinophil cytoplasmic granules. There was a lower concentration of gold particles when the central cores were altered (Figure 2B). This preformed cationic protein was present on the central cores of free eosinophil granules and in the extracellular spaces. No staining was observed in the controls, when the first antibody was omitted, or when a nonspecific first antibody was used.
Indirect alkaline phosphatase staining on frozen sections of digestive biopsy specimens with histologic GVHD.
(A) In the infiltrate of the lamina propria, 2 of 3 labeled cells have bilobulated nuclei. Antibody directed against eosinophil peroxidase, × 300 magnification. (B) Staining is only found on inflammatory cells of the lamina propria, and not on epithelial glandular cells. Mononuclear and polynuclear cells are labeled. Antibody directed against IL-5, × 250. (C) Some inflammatory cells and mononuclear and polynuclear cells are stained in the inflammatory infiltrate of the lamina propria. Antibody directed against eotaxin, × 300 magnification.
Indirect alkaline phosphatase staining on frozen sections of digestive biopsy specimens with histologic GVHD.
(A) In the infiltrate of the lamina propria, 2 of 3 labeled cells have bilobulated nuclei. Antibody directed against eosinophil peroxidase, × 300 magnification. (B) Staining is only found on inflammatory cells of the lamina propria, and not on epithelial glandular cells. Mononuclear and polynuclear cells are labeled. Antibody directed against IL-5, × 250. (C) Some inflammatory cells and mononuclear and polynuclear cells are stained in the inflammatory infiltrate of the lamina propria. Antibody directed against eotaxin, × 300 magnification.
The presence of IL-5 and eotaxin was also assessed by immunohistochemical reactions with antibodies directed against human IL-5 and eotaxin. Stained cells were observed in the lamina propria of all 36 digestive biopsies with tissue eosinophils. At high magnification, mononuclear and polynuclear cells were stained with anti–IL-5 antibody (Figure 3B), whereas only mononuclear cells were stained with eotaxin (Figure 3C). No staining was observed in the controls, when the first antibody was omitted, or when a nonspecific first antibody was used. Combined ultrastructural examination with immunogold staining localized the expression of IL-5 on cytoplasmic granules of tissue eosinophils. When the cytoplasmic granules were not altered, dense deposits were found within the matrix surrounding the central core, which was not labeled. Dense deposits were also localized in free eosinophil granules in the extracellular spaces (Figure 2C). No staining was observed in the controls, when the first antibody was omitted, or when a nonspecific antibody was used.
Relation between presence of tissue eosinophils and occurrence of GVHD.
Of the 93 digestive biopsies, tissue eosinophils were observed in 36 (38.7%), whereas for 57 (61.3%) patients no tissue eosinophils were found on digestive biopsy. All 36 patients with tissue eosinophils also had histologic digestive GVHD. In the 57 patients without tissue eosinophils, 32 (56.1%) had histologic digestive GVHD, and 25 (43.9%) had no histologic GVHD. In the cohort of 93 patients, there was a significant correlation between the presence of eosinophils in digestive biopsy specimens and the presence of histologic digestive GVHD (P < .001).
Relation between presence of tissue eosinophils and severity of histologic digestive GVHD.
For these 93 patients, the distribution of tissue eosinophils according to histologic grade of digestive GVHD was studied. For histologic digestive GVHD grade 1 (n = 33), only 7 patients had more than 5 tissue eosinophils per field, and 26 had fewer. For grade 2 (n = 21), 15 patients had more than 5 tissue eosinophils, whereas 6 patients had fewer. For grades 3 (n = 11) and 4 (n = 3), all patients had more than 5 tissue eosinophils per field (Figure4).
Histologic grade of digestive GVHD and density of eosinophil infiltration.
n = 93 patients.
Histologic grade of digestive GVHD and density of eosinophil infiltration.
n = 93 patients.
There was a significant correlation between the severity of histologic digestive GVHD (grade 1 vs grades 2-4) and the presence of eosinophils on digestive biopsy (P < .001). Moreover, there was a significant correlation between the severity of histologic digestive GVHD (grade 1 vs grades 2-4) and the density of tissue eosinophils (5 to 10 tissue eosinophils per field at × 400 magnification vs more than 10) (P < .001).
Discussion
In this systematic study of upper GI tract biopsy for digestive symptoms in 93 patients undergoing allogeneic HSCT, the incidence of pathologically proven digestive GVHD was 72%. Taking into account traditional clinical separation between acute GVHD (occurring before day 100) and chronic GVHD (occurring after day 100), the incidence of histologic digestive GVHD was 77.9% before day 100 and 60% after day 100.
Systematic pathologic study of the GI tract has been carried out in 2 series of patients after BMT.37,47 Weisdorf et al37 examined the incidence of upper GI syndrome and of histologic digestive GVHD in 469 patients during the first year after BMT. In this study, 139 (28.1%) patients had upper GI syndrome; nearly half of them (46.7%) had histologic signs of digestive GVHD. The study of Roy et al47 was different in that only patients with digestive symptoms underwent biopsy, and the limit of the study period was day 100, not 1 year after BMT. In this cohort of 77 patients, the incidence of histologic signs of digestive GVHD was 44.1%.
The incidence of digestive GVHD might have been higher in our series because biopsies were systematically performed before any addition of corticosteroids, whereas in the other series, some of the patients underwent biopsy before immunosuppressive treatment and some during immunosuppressive treatment. For the type of digestive symptoms necessitating biopsy, upper GI tract symptoms (anorexia, nausea, and vomiting) can be distinguished from lower GI tract symptoms (diarrhea). For the 469 patients in the Weisdorf et al study,37 the incidence of digestive GVHD was given globally. For upper GI tract symptoms, Roy et al47 found the incidence of histologic digestive GVHD to be 79% (58 patients), whereas in our series it was 53.9% (13 patients).
For lower GI tract symptoms, Weisdorf et al37 and Roy et al47 performed upper GI tract biopsy when the patients had more than 500 mL diarrhea; in our study, upper GI tract biopsy was performed immediately after any diarrhea. We found the incidence of histologic digestive GVHD to be 79.4% (39 patients) for diarrhea less than or equal to 500 mL. Therefore, we confirm that the incidence of histologic digestive GVHD, as pointed out by Weisdorf et al,37 is underestimated. When upper GI tract biopsy is performed as soon as diarrhea occurs, the incidence of histologic digestive GVHD increases.
Regardless of when upper GI tract GVHD occurred in our series, the incidence of tissue eosinophil infiltration was 52.9% in the 68 patients with histologic digestive GVHD. When present, eosinophils were located in the lamina propria, but they also invaded the crypt epithelium as isolated cells. Similar eosinophil infiltration with epithelial invasion is observed in hypereosinophilic syndrome,48 in eosinophilic gastroenteritis,49 and in other inflammatory digestive diseases. It is particularly common when patients undergo biopsy at the moment of acute flare-up of disease and before the administration of immunosuppression therapy.15,20 Eosinophil infiltration was found in a series of 10 patients with acute outbreak of celiac disease.15 This was also the case in 9 patients with early recurrence of Crohn disease.17 In our study, there was a significant correlation between the presence of tissue eosinophils and the incidence of pathologic digestive GVHD. Thus, the presence of tissue eosinophils could represent a sign of inflammatory activity accompanying outbreaks of digestive GVHD.
In all 36 patients with tissue eosinophils, we found the release of a cationic protein, eosinophil peroxidase, and other pathologic features considered signs of eosinophil activation, such as cytoplasm vacuoles, alterations in the size and number of granules, and numerous free granules in the extra-cellular space.1,50 Eosinophil peroxidase is a cationic protein normally localized within the matrix of eosinophil granules released in the extracellular space when eosinophils are activated, as shown in an in vitro anaphylactic model.51,52 Similarly, an in vivo ultrastructural study of human gut eosinophils showed granular changes with the disappearance of the central core linked to cationic protein release.18Such evidence of eosinophil activation was described in outbreaks of other inflammatory digestive diseases, such as celiac disease and Crohn disease.14,15 The release of eosinophil peroxidase into the extracellular space has cytopathogenic effects on the digestive epithelium20 and on the conjunctival epithelium.2 In allergic rhinitis, there is a correlation between the rate of eosinophil lysis and the severity of epithelial shedding.2 In our study, activated eosinophils could participate in digestive mucosal damage of GVHD for 2 reasons. First, there was a significant correlation between the presence of an eosinophil infiltrate and the severity of digestive GVHD. Second, there was a significant correlation between the density of tissue eosinophils and the severity of digestive GVHD.
IL-5 is the main cytokine that stimulates eosinophil production in bone marrow. It is a major mediator that recruits, activates, and prolongs eosinophil survival in vivo.53 It inhibits eosinophil apoptosis for at least 12 to 14 days in vitro. Tissue eosinophils can also regulate their own survival through an autocrine pathway.7,54,55 In all 36 digestive GVHD biopsy specimens with tissue eosinophils, combined ultrastructural study and immunogold labeling showed the constant expression of IL-5. The precise localization of IL-5 was within eosinophil granules, whether situated within the cytoplasm of eosinophils or in the extracellular space. This constant expression of IL-5 confirms other signs of in situ eosinophil activation. It also suggests an autocrine mechanism for eosinophil recruitment and activation in acute flare-ups of digestive GVHD. Similar autocrine recruitment and eosinophil activation in acute flare-ups of digestive inflammatory diseases (eg, celiac disease and Crohn disease) have been described.19,56 It was also found in biopsy and necropsy specimens of heart lesions in patients with hypereosinophilic syndromes.8
On sequential biopsies of digestive tract diseases with eosinophils, IL-5 expression was the first marker to disappear under treatment.15,16 In vitro, glucocorticosteroids prevent the increased survival of eosinophils mediated by IL-3, IL-5, and granulocyte macrophage–colony-stimulating factor,57 and they promote eosinophil apoptosis.58 Therefore, because we found IL-5 in all biopsy specimens of patients with digestive GVHD, eosinophil presence could be linked to the fact that all biopsies were performed before any steroid treatment.
Eotaxin expression was also found by immunohistochemistry in all biopsy specimens of the 36 patients with digestive GVHD and eosinophils. As with IL-5, eotaxin can recruit tissue eosinophils in allergic diseases.10,59 At the molecular level, it acts on a single receptor, CCR3, found at a high density of eosinophils.60Eotaxin is also expressed by activated eosinophils because blood eosinophil cultured in the presence of IL-3 expresses eotaxin mRNA.10
To confirm whether activated eosinophils on digestive biopsy of patients with GVHD could be a marker of acute flare-up of inflammation, and thus a marker for modulating immunosuppression treatment, we studied biopsy specimens taken after day 100, a stage considered clinically as chronic. In the cohort of 25 patients with digestive symptoms for which biopsy was performed 100 days or more after BMT, 60% (15 patients) had histologic digestive GVHD, 46.6% (7 patients) had a severe form of histologic digestive GVHD, and all but one had tissue eosinophils. Thus the presence of tissue eosinophils could represent a marker of inflammatory activity accompanying outbreaks of digestive GVHD, whether at the acute or the chronic clinical phases. Therefore, systematic duodenal biopsy at the onset of digestive symptoms should allow early GVHR detection, and pathologic signs of GVHR, together with eosinophil density, might help modulate immunosuppression therapy.
To our knowledge, this is the first study showing that tissue eosinophil density might be a biologic marker of GVHD severity. However, confirmatory results by prospective studies from other groups are needed before it can be accepted as a prognostic marker.
We thank Marcel Brus-Ramer for providing assistance in this study, and we thank the photographic team of the University Institute of Hematology, Alain Plockyn and François Rocher, for technical assistance.
Supported by grants from Programme Hospitalier de Recherche Clinique AOM 96120, Etablissement Français des Greffes, and Association pour la Recherche contre le Cancer.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anne Janin, Laboratoire de Recherche Universitaire de Pathologie EA 2378, Institut Universitaire d'Hématologie, Hôpital Saint-Louis, 1 Ave Claude Vellefaux, 75475 Paris, France; e-mail: anne_janin@yahoo.com.