Abstract
Defined angiographically, no-reflow (NR) manifests as an acute reduction in coronary flow in the absence of epicardial vessel obstruction. One candidate protein to cause coronary NR is tissue factor (TF), which is abundant in atherosclerotic plaque and a cofactor for activated plasma coagulation factor VII. Scrapings from atherosclerotic carotid arteries contained TF activity (corresponding to 33.03 ± 13.00 pg/cm2 luminal plaque surface). Active TF was sedimented, indicating that TF was associated with membranes. Coronary blood was drawn from 6 patients undergoing coronary interventions with the distal protection device PercuSurge GuardWire (Traatek, Miami, FL). Fine particulate material that was recovered from coronary blood showed TF activity (corresponding to 91.1 ± 62.16 pg/mL authentic TF). To examine the role of TF in acute coronary NR, blood was drawn via a catheter from coronary vessels in 13 patients during NR and after restoration of flow. Mean TF antigen levels were elevated during NR (194.3 ± 142.8 pg/mL) as compared with levels after flow restoration (73.27 ± 31.90 pg/mL; P = .02). To dissect the effects of particulate material and purified TF on flow, selective intracoronary injection of atherosclerotic material or purified relipidated TF was performed in a porcine model. TF induced NR in the model, thus strengthening the concept that TF is causal, not just a bystander to atherosclerotic plaque material. The data suggest that active TF is released from dissected coronary atherosclerotic plaque and is one of the factors causing the NR phenomenon. Thus, blood-borne TF in the coronary circulation is a major determinant of flow.
Introduction
The no-reflow (NR) phenomenon is defined as profound reduction in antegrade coronary flow1 in the absence of epicardial vessel obstruction.2,3 Despite a 0.6% to 2% incidence during percutaneous coronary interventions2,4 and a 15% mortality,2 the mechanisms underlying coronary NR are unclear. A common denominator of various disorders leading to NR appears to be microvascular damage.5
One candidate protein to cause coronary NR is tissue factor (TF), a 47-kd transmembrane glycoprotein and a member of the cytokine-receptor superfamily. TF initiates blood coagulation by binding activated coagulation factor VII (VIIa)6 with a dissociation constant of around 10 pM.7 The TF-VIIa complex proteolytically activates factors IX and X, triggering the coagulation system.8 The activity of TF is lipid dependent and is enhanced by a factor of 2000 to 3000 in the presence of phosphatidylserine/phosphatidylcholine,9 allowing almost immediate thrombus formation. Because TF-containing microparticles with procoagulant activity are abundant in atherosclerotic plaque,10-13 spontaneous or mechanical disruption of a coronary atherosclerotic plaque by angioplasty and/or stenting could lead to release of active TF into the coronary blood.
First, we demonstrate that ex vivo plaque disruption by scraping leads to release of membrane-bound active TF. Secondly, we show that in vivo plaque disruption by angioplasty and/or stenting causes shedding of active TF into the coronary blood. Third, we show that TF antigen levels during coronary NR are elevated compared with the levels that are measured in the same patients after restoration of flow. Finally, we demonstrate that intracoronary injection of atherosclerotic plaque material or purified TF causes microvascular thrombosis and NR in a porcine model.
Patients, materials, and methods
Harvest of carotid thromboendarterectomy specimens
Specimens were harvested in the operating room, and 1 cm2 of the intimal tissue flap was immediately scraped under sterile conditions and resuspended in cold Hepes-buffered saline (HBS) (10 mM Hepes, 0.1% bovine serum albumin [BSA], 5 mM CaCl2). Large particles were removed by centrifugation (1300g) before analysis of the supernatants.
Patient characteristics
Patients with acute coronary syndromes were admitted to the catheter laboratory with symptoms of longer than 30 minutes and shorter than 3 hours duration and with negative plasma creatine phosphokinase values. In some of these patients, acute myocardial infarction was diagnosed in the presence of troponin T values greater than 0.2 ng/mL and new Q waves longer than 0.03 seconds. Patients were heparinized at an activated coagulation time of 300 seconds or longer and were under full-dose aspirin. In addition, patients experiencing NR (Table 2) were treated with 100 to 500 μg intracoronary verapamil.
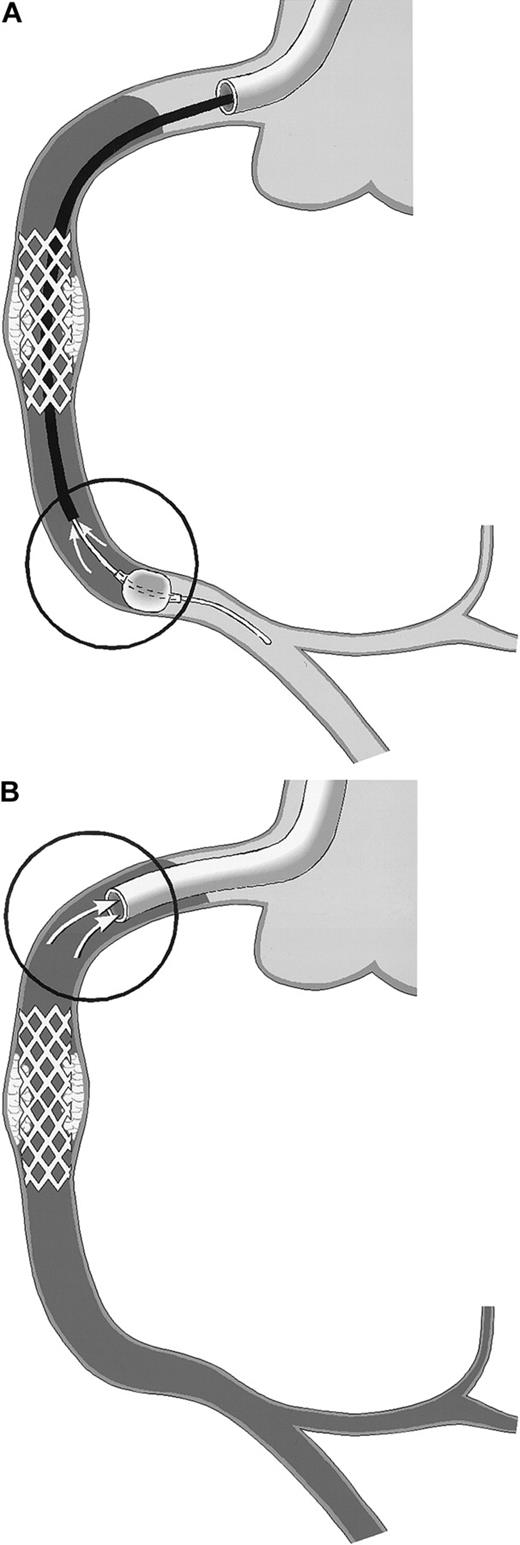
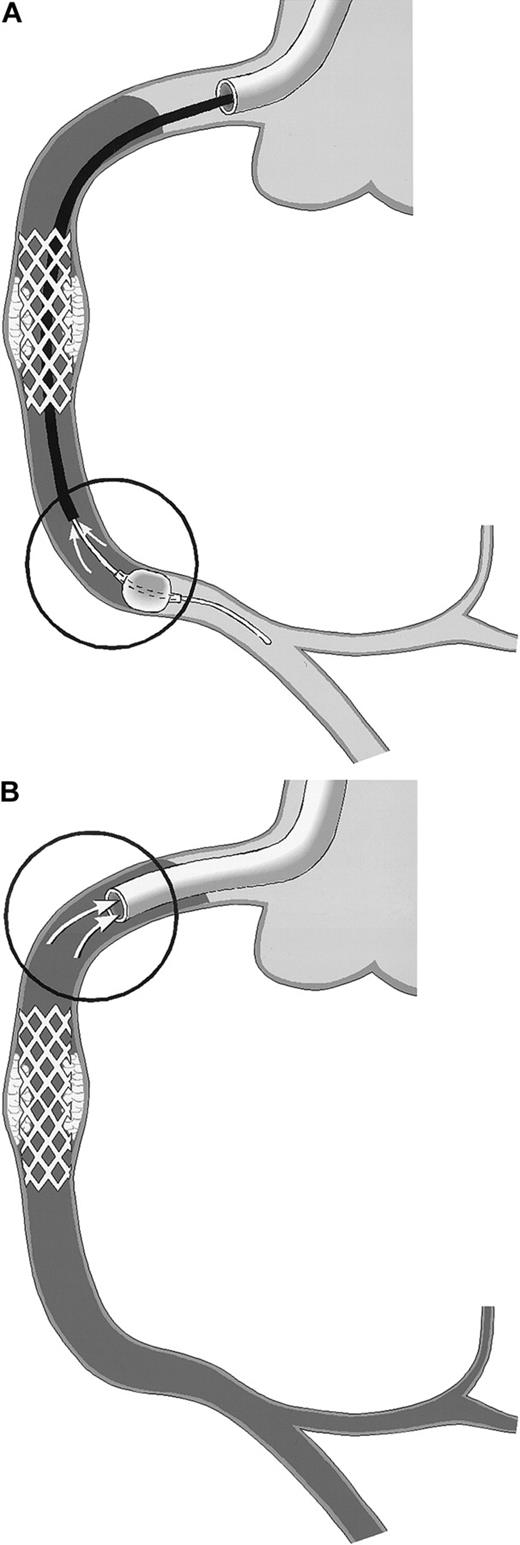
The PercuSurge GuardWire (Traatek, Miami, FL) protection device was used in 6 patients to prevent embolization of material into the capillary bed (Table 1). In these patients, the location of the target stenosis in venous grafts or in native vessel segments without side branches allowed its use. The system served the study in that it prevented losses of vessel wall–derived particles of any size into the distal vascular bed. The distal over-the-wire occlusion balloon was inflated shortly before the intervention and was held inflated during the intervention with the use of the hypotube as angioplasty guidewire. After removal of the intervention catheter, an aspiration catheter was advanced into the coronary artery and 40 mL blood was drawn from the coronary bed with the inflated distal balloon still in place (Figure1A) and with a syringe used to serve as a collection chamber. Then the distal balloon was deflated, and coronary flow was restored. The aspirated material was passed over a small filter unit (Falcon, 40-μm nylon) (Becton Dickinson, San Jose, CA). Material trapped in the filter was resuspended in 1 mL HBS, and stored at −70°C before TF measurement. For control, blood from healthy individuals was passed over a filter, and rinsing buffers were assayed in parallel.
Schematic drawing of coronary blood sampling.
The right coronary artery (RCA) is shown. Arrows within the circles point to the exact site of blood drawing. Stasis of the blood column is indicated by the use of gray. (A) PercuSurge distal protection device. Blood is aspirated from the tip of the export catheter, while the catheter is slowly withdrawn proximally. The harvested blood is passed over a filter. TF activity of the material on the filter is measured. (B) NR in the course of percutaneous transluminal coronary angioplasty (PTCA). Blood is aspirated from the static blood column at the tip of the guiding catheter, which is deeply intubated into the proximal vessel segment.
Schematic drawing of coronary blood sampling.
The right coronary artery (RCA) is shown. Arrows within the circles point to the exact site of blood drawing. Stasis of the blood column is indicated by the use of gray. (A) PercuSurge distal protection device. Blood is aspirated from the tip of the export catheter, while the catheter is slowly withdrawn proximally. The harvested blood is passed over a filter. TF activity of the material on the filter is measured. (B) NR in the course of percutaneous transluminal coronary angioplasty (PTCA). Blood is aspirated from the static blood column at the tip of the guiding catheter, which is deeply intubated into the proximal vessel segment.
In patients experiencing NR, blood was drawn through a no-sidehole guiding catheter from the target coronary artery (Figure 1B) after discarding 5-mL or 8-mL aspirates, respectively (with the use of a 6 or 7 French guiding catheter).
Flow was classified according to the Thrombolysis In Myocardial Infarction (TIMI) study.1 Flow was defined as TIMI 0 when no antegrade flow was present; as TIMI 1 when penetration of contrast was present without perfusion, ie, without opacification of the entire coronary bed; as TIMI 2 when the rate of entry of contrast material into the distal vessel or its rate of clearance from the distal bed was perceptibly slower than its flow into or clearance from comparable areas not perfused by the target vessel; and as TIMI 3 when coronary flow was normal, ie when the rate of entry of contrast material into the distal vessel or its rate of clearance from the distal bed was as rapid as flow into or clearance from a normal uninvolved coronary vessel.
Coronary blood samples from subjects undergoing coronary angiography for other indications, eg, valvular heart disease, or for a preoperative risk stratification and showing normal epicardial coronary arteries were collected as controls.
Sodium citrate (at a final concentration of 0.129 M) was added to blood samples, and platelet-poor plasma (PPP) was prepared by centrifugation (1300g, 4°C, 10 minutes). To expose membrane-associated TF,14 PPP samples were also subjected to 4 freeze-thaw cycles prior to antigen measurements. All human studies were approved by the Ethics Committee of the University of Vienna, Austria.
Differential centrifugation experiments
Rinsing buffers from carotid scrapings were centrifuged at 23 000g (15 minutes, 20°C) (Universal 30RF centrifuge) (Hettich, Tuttlingen, Germany) and at 250 000g (60 minutes, 20°C) (Beckman L-80 ultracentrifuge SW 50.1-Rotor, Beckman Coulter, Fullerton, CA), corresponding to a particle size of 0.5 to 2 μm and 10 to 15 nm, respectively. TF activity measurements were performed in the starting materials, 23 000g and 250 000g pellets, and their respective supernatants.
Reagents
Human recombinant factor VIIa was a kind gift from Novo-Nordisk (Copenhagen, Denmark) and human recombinant TF (TF1-243) was a gift from Robert Kelley (Genentech, San Francisco, CA), to Yale Nemerson. The phospholipids used for relipidation of TF consisted of 40% 1,2-dioleoyl-sn-glycero-3-phosphatidylserine and 60% 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (Avanti Polar Lipids, Alabaster, AL). Factor X was purified from human plasma.14 Spectrozyme-Xa was from American Diagnostica (Greenwich, CT).
Preparation of TF-phospholipid
TF activity assay
TF activity was measured by adding the sample to a solution containing 1 nM factor VIIa, 150 nM factor X, and 5 mM CaCl2. At intervals, samples were transferred to a microtiter plate in which each well contained 100 μL EDTA buffer (50 mM bicine, pH 8.5, 20 mM EDTA, 1 mg/mL BSA), which terminates production of factor Xa. Spectrozyme Xa (25 μL, 0.5 mM final concentration) (American Diagnostica) was added, and optical density was measured at 405 nm for 30 minutes by means of a kinetic enzyme-linked immunosorbent assay (ELISA) plate reader (BTK Microquant, Software KC4) (Biotek, Winooski, VT) at 35°C. A standard curve was generated with the use of relipidated recombinant human TF.
TF antigen assay
Imubind Tissue Factor ELISA Kit No. 845 (American Diagnostica) was used according to the manufacturer's instructions.
Immunoelectron microscopy
The PercuSurge specimens were fixed in 3% freshly depolymerized paraformaldehyde in phosphate-buffered saline (PBS) (pH 7.4) for 1 hour at 4°C, rinsed in PBS, and dehydrated in increasing concentrations of ethanol (30%, 50%, 70%, 95%, and 100%, 30 to 60 minutes each). The resin infiltration was achieved by increasing concentrations of HM20 resin (Lowicryl, Chemische Werke Lowi, Waldkraiburg, Germany) at −35°C. The resin was cured under UV light for 48 hours at −35°C and polymerized for 48 hours without UV light at room temperature. For immunostaining, 100-nm thin sections were mounted on grids and incubated in PBS with 3% BSA and 0.05% Tween-20, pH 7.4 (PBS-ST) to block unspecific binding sites. The primary antibody was monoclonal anti-TF (1:40, 2.5 hours at room temperature17) in PBS-ST, followed by 3 rinses in PBS and incubation with protein A–14-nm colloidal gold for 1 hour. The sections were rinsed in PBS and distilled water 3 times each and contrasted in aqueous 2% uranylacetate for 8 minutes and lead acetate for 2.5 minutes before examination in a Zeiss transmission electron microscope (Zeiss, Munich-Hallbergmoos, Germany).
Animal protocol
Ten domestic pigs (33 ± 6 kg) of both genders fed on a standard natural diet were studied. The animals were anesthetized with intravascular ketamine (30 mg/kg), acepromazin (12 mg/kg), thiopental (5 mg/kg), and robinul (0.025 mg/kg) and a continuous infusion of fentanyl (0.08 mg/kg). Following endotracheal intubation, the pigs were mechanically ventilated with a mixture of 20% pure oxygen and 80% room air. Arteriotomy of the carotid artery and insertion of a 7F sheath were performed under sterile conditions. Electrocardiogram, arterial blood pressure, and temperature were recorded throughout the procedure. After administration of 2000 IU heparin, the RCA was cannulated, and a 0.014-inch Doppler ultrasound guide wire (FloWire) (Cardiometrics, Mountain View, CA) was introduced to the midpart of the artery. The average peak flow velocity (APV) was assessed at baseline and during maximal hyperemia after intracoronary bolus injection of 18 μg adenosine (centimeters per second, peak APV), and the coronary flow reserve was calculated as a ratio of peak APV and baseline APV. We injected 1 mL carotid scraping material containing 33 pg/mL TF activity or 250 μL of 39 nmol relipidated human TF, respectively, as a bolus through a deeply intubated 7F Judkins standard RCA guiding catheter. Angiograms were performed before and during the first 30 minutes after injection. The pigs were killed by saturated potassium chloride. The protocol was approved by the Animal Subjects Committee of the University of Vienna.
Immunohistochemical analysis
Immediately after the pigs were killed, multiple transmural biopsies from the RCA-dependent left ventricular posterior wall and from the anterior wall myocardium were obtained. Tissues and PercuSurge material were fixed in 7.5% buffered formalin and were embedded in paraffin, and serial 3-μm sections were stained as described18 by means of mouse monoclonal antibodies against fibrin (1:50) (Biodesign International, Saco, ME) and TF (1:100, human TF1).17 In addition, for immunostaining of PercuSurge samples, anti-CD15 (1:25) (DAKO, Glostrup, Denmark) and anti-CD61 (1:25) (DAKO) mouse monoclonal antibodies were used.
Computer-assisted quantitative histological evaluation
Following immunohistochemistry, sections from all specimens were scanned in their full size by means of the AxioCam color digital camera (Zeiss). Computer-based planimetry of the area of microvascular thrombosis was performed by means of the AxioVision 2.05 software package (Zeiss). Fibrin stains and modified trichrome stains served to identify thrombus. Two observers who were blinded to the origin of the specimens independently analyzed 4 different areas of each section, and 4 sections per specimen.
Statistical analysis
Results are expressed as means ± SD. Comparisons were made with the Mann-Whitney U test and the paired ttest. P < .05 was considered significant.
Results
Mechanical plaque disruption leads to shedding of membrane particles with procoagulant activity
To simulate intervention-related mechanical plaque damage ex vivo, 6 carotid thromboendarterectomy specimens were scraped, and TF activity was determined in the rinsing buffers. Mean procoagulant activity corresponded to 33.03 ± 13.00 pg/mL authentic TF per square centimeter scraped carotid intimal flap area. The TF activity in fractions obtained after differential centrifugation of carotid atherosclerotic plaque scrapings were as follows: 180.16 ± 95.55 for the 23 000g pellet; 5.63 ± 2.32 for the corresponding supernatant; 98.23 ± 34.60 for the 250 000g pellet; and 0 for the corresponding supernatant. Thus, complete removal of active TF from the rinsing buffers was accomplished by ultracentrifugation at 250 000g, indicating that TF activity was membrane associated. P = .03 for the differences between the starting material and the respective pellets. Part of the plaque material was used for injection into porcine coronary arteries.
Coronary angioplasty and stenting are associated with shedding of procoagulant activity from the plaque into the coronary blood
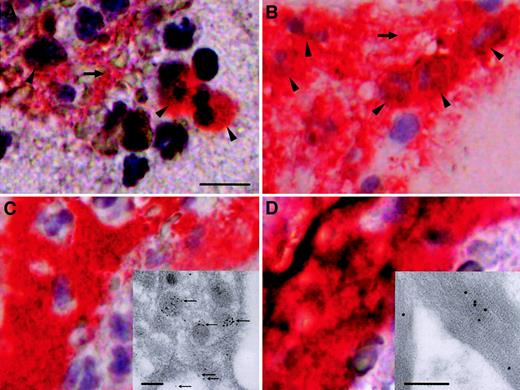
To confirm that intervention-related disruption of a coronary atherosclerotic plaque in vivo leads to release of active TF into the coronary blood, blood samples were harvested during angioplasty with a distal protection device, PercuSurge, in 6 patients (Table1). Fine particulate material was trapped in the filter and was recovered in rinsing buffer. By light microscopy, the filter material contained mainly leukocytes, platelets, and vast amounts of amorphous material with cholesterol crystals. Immunohistochemically, TF colocalized with leukocytes, platelets, and fibrin (Figure 2). Manual cell counts showed that only approximately 2% of CD15+ cells demonstrated immunoreactivity with anti-TF antibody. By immunoelectron microscopy, TF was identified within platelets (insert, Figure 2C) and found attached to fibrin (insert, Figure 2D).
Immunohistochemical and immunoelectron microscopical analyses of particulate material trapped in the PercuSurge filter.
Red immunoreactivity indicates positive staining in parallel sections. (A) TF in association with distinct cells (arrowheads) and the extracellular compartment (horizontal arrow). The scale bar represents 10 μm. (B) The majority of cells (arrowheads) and extracellular areas (horizontal arrow) stain with anti-CD15 antibody, a marker for mature granulocytes, and monocytes. (C) (D) Documentation of positive staining with anti-CD61 and antifibrin antibodies, which suggests that platelets and fibrin colocalize with TF immunoreactivity. Ultrastructural micrographs of a platelet (insert) and fibrin (insert) obtained from a PercuSurge filter, immunostained with monoclonal anti-TF antibody. The scale bars represent 250 nm. Positive immunoreactivity is visualized with immunogold. Panel C demonstrates the accumulation of TF within platelet granular structures and on the outer membrane (fine arrows). All immunohistochemistry panels were photographed at the original magnification × 400. All immunogold stains were photographed at the original magnification × 28 800.
Immunohistochemical and immunoelectron microscopical analyses of particulate material trapped in the PercuSurge filter.
Red immunoreactivity indicates positive staining in parallel sections. (A) TF in association with distinct cells (arrowheads) and the extracellular compartment (horizontal arrow). The scale bar represents 10 μm. (B) The majority of cells (arrowheads) and extracellular areas (horizontal arrow) stain with anti-CD15 antibody, a marker for mature granulocytes, and monocytes. (C) (D) Documentation of positive staining with anti-CD61 and antifibrin antibodies, which suggests that platelets and fibrin colocalize with TF immunoreactivity. Ultrastructural micrographs of a platelet (insert) and fibrin (insert) obtained from a PercuSurge filter, immunostained with monoclonal anti-TF antibody. The scale bars represent 250 nm. Positive immunoreactivity is visualized with immunogold. Panel C demonstrates the accumulation of TF within platelet granular structures and on the outer membrane (fine arrows). All immunohistochemistry panels were photographed at the original magnification × 400. All immunogold stains were photographed at the original magnification × 28 800.
The rinsing solutions contained procoagulant activity corresponding to 91.10 ± 62.16 pg per milliliter of authentic TF (Table 1). In addition, plasma TF was measured in the blood column proximal to the PercuSurge occlusion balloon (Figure 1A) and after release of the occlusion balloon with reflow (R) in the distal part of the vessel (Table 1). TF antigen was significantly lower under conditions of R (99.62 ± 39.16 pg/mL versus 48.78 ± 23.80 pg/mL,P = .028).
Coronary TF antigen is elevated during NR
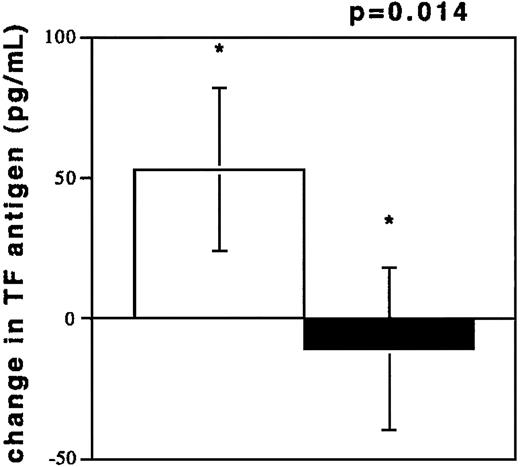
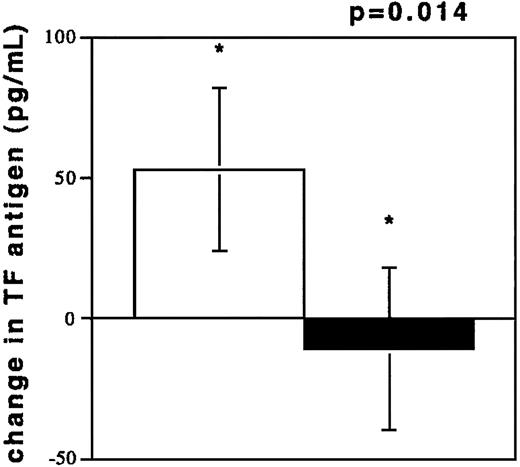
To investigate acute coronary NR, blood was drawn from coronary vessels in 13 patients during percutaneous interventions. The circumstances of NR are depicted in Table2. TF antigen levels were elevated during NR (194.3 ± 142.8 pg/mL) as compared with the TF levels in plasmas from control subjects (100.3 ± 60.7 pg/mL, P = .0333). TF antigen levels after freeze thawing of NR samples increased, while TF levels in plasmas from control subjects were unchanged after freeze thawing (Figure 3). TF was also elevated compared with samples obtained after flow restoration (73.27 ± 31.90 pg/mL, n = 7, P = .02). These values were not different from controls (P = .28). Flow restoration (TIMI 3) was achieved by sealing of culprit lesions with stents and by intracoronary pharmacological treatment (see “Patients, materials, and methods”) in patients no. 1 through 7. A dilution phenomenon through radiographic contrast did not occur because hematocrit values in the 2 samples were similar (40% ± 2% versus 39% ± 3%, NR/R).
TF antigen values (pg/mL) after freeze thawing of plasma samples.
Change of TF antigen values (pg/mL) after freeze thawing of plasma samples from individuals experiencing NR (n = 13, □) and from controls with TIMI 3 flow in angiographically normal coronary arteries (n = 13, ■).
TF antigen values (pg/mL) after freeze thawing of plasma samples.
Change of TF antigen values (pg/mL) after freeze thawing of plasma samples from individuals experiencing NR (n = 13, □) and from controls with TIMI 3 flow in angiographically normal coronary arteries (n = 13, ■).
Injection of TF induces NR in a porcine model
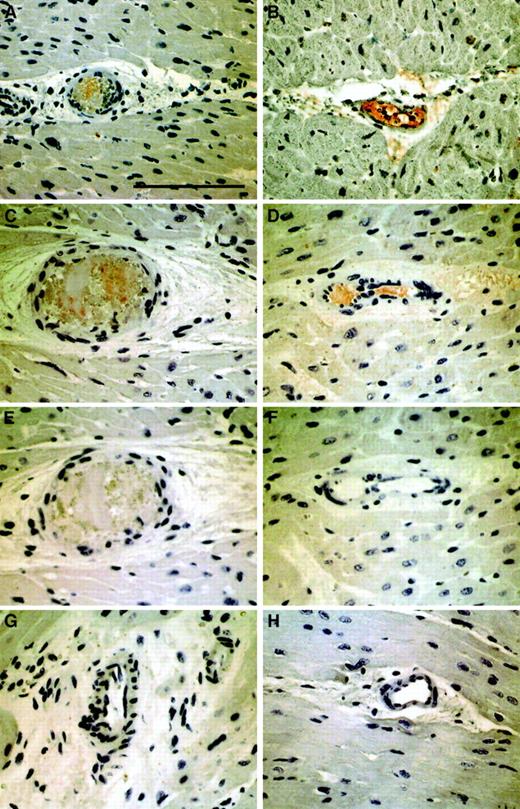
To demonstrate that TF is causal to NR and to exclude that TF accumulation in coronary blood is secondary to stasis, an animal model was established. Injection of atherosclerotic plaque material or TF into the porcine RCA compromised flow in 9 of 10 animals within 30 seconds. For quantification of myocardial blood flow, coronary flow reserve determinations were used. Adenosine-dependent coronary flow reserve after injection of atherosclerotic plaque material/TF immediately dropped from 2.0 ± 1 to 0.9 ± 0.6 in 9 pigs, with a marked decrease of baseline flow immediately after the injection. NR/slow-flow spontaneously resolved in 5 pigs over the following 20 minutes, while it persisted in the remaining animals. Two NR pigs spontaneously went into ventricular fibrillation. Histologically, positive TF staining (Figure4A,B) was identified in the microvasculature distal to the RCA that was filled with fibrin thrombi (Figure 4C,D). In plaque-injected animals, embolic obstruction was observed with surrounding thrombus (Figure 4A,C,E). No TF was detected on luminal endothelial cells.
Immunohistochemical detection of TF and fibrin in parallel sections of 2 representative myocardial specimens from experimentally induced NR.
Panels A, C, and E demonstrate arteriolar obstruction by atherosclerotic carotid plaque material and adjacent thrombus in a pig injected with carotid scraping (AM1). Panels B, D, and F show arteriolar thrombosis in pig TF2. Red immunoreactivity indicates positive staining with anti-TF (panels A,B) or antifibrin antibodies (panels C,D). Panels E and F represent negative controls stained with isotype antibodies. For comparison, panels G and H depict myocardial vessels from the same animals in uninjected territories stained with antifibrin antibodies. All panels were photographed at the original magnification × 400. The scale bar represents 100 μm.
Immunohistochemical detection of TF and fibrin in parallel sections of 2 representative myocardial specimens from experimentally induced NR.
Panels A, C, and E demonstrate arteriolar obstruction by atherosclerotic carotid plaque material and adjacent thrombus in a pig injected with carotid scraping (AM1). Panels B, D, and F show arteriolar thrombosis in pig TF2. Red immunoreactivity indicates positive staining with anti-TF (panels A,B) or antifibrin antibodies (panels C,D). Panels E and F represent negative controls stained with isotype antibodies. For comparison, panels G and H depict myocardial vessels from the same animals in uninjected territories stained with antifibrin antibodies. All panels were photographed at the original magnification × 400. The scale bar represents 100 μm.
There was no difference in the area of thrombosis in plaque-injected animals (4.0% ± 2.5% of the total field) versus TF-injected animals (3.8% ± 2.0% of the total field, P > .05) No fibrin deposition was detected in any noninjected myocardial vessel (Figure 4G,H). No correlation was observed between thrombus area and TIMI flow grade or coronary flow reserve.
Discussion
Our data provide important new insights into the mechanisms underlying NR inasmuch as injection of human TF-containing atherosclerotic plaque material or purified relipidated recombinant human TF into porcine RCAs led to flow impairment. Thus, we have established an experimental model that mimics human NR. Histological analysis revealed microthrombi in all animals, with 9 of 10 demonstrating TIMI flow of 2 or less (Table3). The most extensive area of microvascular fibrin thrombi was observed in animals with TIMI 0 flow. We speculate that NR occurs when the capacity of the regional coronary microvascular bed is exhausted; ie, when all vessels in a given domain are obstructed. Such obstruction may occur spontaneously, through shedding of particulate material and TF from ruptured plaque during acute coronary syndromes, or iatrogenically, from PTCA and stent implantation. However, other factors that are as yet unknown may exacerbate the flow disturbances.
Contribution of the coagulation system to the pathogenesis of coronary NR is a novel observation. Recent experimental work of Giesen et al19 has lent support to the concept that blood-borne TF is a powerful thrombogenic species. In the present work, we captured active TF in a distal protection device from patients undergoing coronary revascularization. Other studies found that 20- to 60-nm vesicles were recovered by scraping pig aorta,20demonstrating that the vessel wall itself may be a source of circulating TF. TF captured in the PercuSurge filter colocalized with leukocytes (Figure 3). Because circulating white blood cells upregulate TF only 24 to 48 hours after the percutaneous intervention,21 these TF-positive cells most likely originate from ruptured plaque, which is in line with previous findings.10 In addition, TF was found associated with platelets and fibrin (Figure 2C,D), which is in accord with recently published data.22 TF uptake by platelets and binding to fibrin may account for our observation that after restoration of flow, TF antigen returns to normal in plasma.
We found an increase of TF antigen after freeze thawing of PPP from NR (Figure 3), indicating some TF is in a protected state. It is known that TF incorporates into vesicles in such a way that half the molecules are facing outward and are therefore available to initiate coagulation while the other half are sequestered within the vesicle.15 Freeze thawing has been shown to reorient TF in such a way that half the sequestered molecules become available for coagulation. We have no information regarding the symmetry of the particles released following angioplasty, but the increase in immunologically detectable TF after freeze thawing suggests that some of the TF is sequestered. These molecules may, however, become available during thrombogenesis. There is currently no information regarding this hypothesis although it is being investigated in our laboratories.
It is known that TF antigen is elevated23 and that TF-positive microparticles have been captured on annexin V–coated plates from the blood of patients with unstable coronary artery disease.24 However, Mallat et al24investigated systemic venous blood samples up to 8 days following an acute ischemic episode. It is therefore possible that secondary immunological or inflammatory effects resulted in TF release from other sites. In contrast, the present study focuses on a single atherosclerotic target lesion or vessel as the culprit for the clinical syndrome. Former work has emphasized the roles of microvascular spasm5 and inflammation25 in the development of NR. The lack of a significant inflammatory response in our animal experiments is due to the short observation period. TF initiates the coagulation cascade within seconds, resulting in thrombin generation and fibrin deposition. Thrombin is able to induce vasospasm26,27 by activation of protease-activated receptors.28,29 In fact, inhibition of thrombin with hirudin was shown to limit infarct size in a rabbit coronary ligation model.30
Microvascular patency and consequently myocardial perfusion are predictors of mortality after thrombolytic therapy31 and after stent implantation in acute myocardial infarction.32The observation that administration of antibody directed against glycoprotein IIb/IIIa had no effect on peri-interventional slow-flow in a meta-analysis of the EPIC and EPILOG studies33 lends support to the concept that small tears liberating the lipid core of atherosclerotic plaque allow active TF to enter the coronary bed and cause coronary flow deceleration, culminating in NR. In this regard, it is noteworthy that unstable plaques contain more TF activity than stable plaques.12,13In the present study, 11 of 13 patients experiencing NR (Table 2) were unstable prior to the occurrence of NR. Active TF shed from dissected atherosclerotic plaque is associated with microvascular thrombosis and macrovascular slow-flow/NR. The concept that TF is a major player in the pathogenesis of NR is also supported by previous studies in a baboon model of middle cerebral artery occlusion, where NR was reversed with the administration of a TF antibody.34 In more recent experimental work, recombinant human, active site-blocked factor VIIa reduced infarct size and NR in rabbits.35 36 These observations support the view that clinical studies should be undertaken to investigate whether anti-TF agents are useful in the treatment of peri-interventional slow-flow and NR.
The authors are grateful to Dr Marin Guentschev for expert technical advice. The study would not have been possible without the support of the interventional coronary catheter laboratory staff and animal laboratory personnel of the University of Vienna.
Supported in part by the Austrian fellowship grants FWF P13834-MED and Nationalbank Jubiläumsfonds No. 7710 (I.M.L.); Hans und Blanca Moser-Stiftung (D.B.); and National Institutes of Health grants HL 29019 and HL 54469 (Y.N.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Irene M. Lang, Dept of Internal Medicine II, Division of Cardiology, University of Vienna, Währinger Gürtel 18-20, 1090 Vienna, Austria; e-mail:irene.lang@univie.ac.at.