Abstract
Cb2 is a novel protooncogene encoding the peripheral cannabinoid receptor. Previous studies demonstrated that 2 distinct noncoding first exons exist: exon-1A and exon-1B, which both splice to protein-coding exon-2. We demonstrate that in retrovirally induced murine myeloid leukemia cells with proviral insertion inCb2, exon-1B/exon-2 Cb2 messenger RNA levels have been increased, resulting in high receptor numbers. In myeloid leukemia cells without virus insertion in this locus, low levels of only exon-1A/exon-2 Cb2 transcripts were present and receptors could not be detected. To elucidate the function of Cb2 in myeloid leukemia cells, a set of in vitro experiments was carried out using 32D/G-CSF-R (granulocyte colony-stimulating factor receptor) cells transfected with exon-1B/exon-2 Cb2 complementary DNA and a myeloid cell line carrying a virus insertion in Cb2(ie, NFS 78). We demonstrate that a major function of the Cb2 receptor is stimulation of migration as determined in a transwell assay. Exposure of Cb2-expressing cells to different cannabinoids showed that the true ligand for Cb2 is 2-arachidonoylglycerol (2-AG), which may act as chemoatractant and as a chemokinetic agent. Furthermore, we observed a significant synergistic activity between 2-AG and interleukin-3 or G-CSF, suggesting cross-talk between the different receptor systems. Radioactive-ligand binding studies revealed significant numbers of Cb2 receptors in normal spleen. Transwell experiments carried out with normal mouse spleen cells showed 2-AG–induced migration of B220-, CD19-, immunoglobulin M–, and immunoglobulin D–expressing B lymphocytes. Our study demonstrates that a major function of Cb2 receptor expressed on myeloid leukemia cells or normal splenocytes is stimulation of migration.
Introduction
Two distinct cannabinoid receptors have previously been identified: the central1 (Cb1) and the peripheral (Cb2) cannabinoid receptor.2 In retrovirally induced murine leukemia, proviral insertions frequently occur in the gene encoding Cb2, suggesting that the peripheral cannabinoid receptor is an oncoprotein.3,4 The cannabinoid receptors belong to the superfamily of 7-transmembrane G protein–coupled receptors (GPCRs). Several GPCRs have been shown to be involved in cell growth and oncogenesis as the result of aberrant expression.5-7 Examples of GPCRs with transforming ability are the α1B-adrenergic,8 thrombin,9 and serotonin 1c receptors10 and the receptor encoded byMAS oncogene.11,12 Our previous observation that Cb2 is a common virus integration site suggests that aberrant expression of this 7-transmembrane receptor may be a critical event in transformation in certain cases of leukemia.3 The protein-coding region of Cb2 is located on a single exon (exon-2) of approximately 4 kilobases. Recently we identified 2 distinct 5′ noncoding exons (ie, exon-1A and exon-1B) previously designated exon-1 and exon-1′, respectively.3 In the study presented here we first carried out experiments to investigateCb2 messenger RNA (mRNA) transcripts and protein expression in leukemic cells with or without retroviral insertion in theCb2 locus. Secondly, we performed studies to determine the function of the peripheral cannabinoid receptor when overexpressed in myeloid cells.
GPCRs have been related to many functions, including cell proliferation, maturation, survival, apoptosis, or migration.6,13,14 In the present study, we investigated the function of the peripheral cannabinoid receptor when overexpressed on myeloid cells (ie, 32D/G-CSF-R [granulocyte colony-stimulating factor receptor]) in which we overexpressed exon-1B/exon-2Cb2 splice variant and a myeloid leukemia cell line containing a virus insertion in the Cb2 locus, NFS 78. We also wished to determine which of the large panel of Cb2 ligands that have been identified previously is the true agonist of the receptor. We investigated the effects of natural (δ8-tetrahydrocannabinol [δ8-THC],15δ9-THC,16 cannabinol,17 and cannabidiol16), synthetic (WIN 55212-2 18and CP55940 19), and endogenous (2-arachidonoylglycerol [2-AG],20,21 anandamide [AEA],22,23 N-palmitoylethanolamine [PEA],24 and N-acylethanolamine [POEA]24) cannabinoids. We show that 2-AG is the most potent agonist for the Cb2 receptor and that a major function of 2-AG is stimulation of migration. We further studied whether 2-AG acts as a chemotactic or chemokinetic agent and whether cytokines, such as interleukin-3 (IL-3) or G-CSF, increase the stimulatory effect of 2-AG. Because the other cannabinoid ligands are also able to bind to the peripheral cannabinoid receptor, we investigated whether these compounds either synergize or antagonize with the stimulatory effect of 2-AG. Next, we investigated Cb2 receptor expression and function in normal spleen and thymus. We show that Cb2 receptors are normally expressed on B220+ splenocytes and that the major function of the peripheral cannabinoid receptor on these B lymphocytes is regulation of migration as well.
Materials and methods
Cell lines
The myeloid cell lines NFS 58, 61, 70, and 78 were established from Cas-Br-M murine leukemia virus–initiated primary tumors.25 The 32D/G-CSF-R cell line26 was kindly donated by Prof Dr I. P. Touw (Erasmus University Rotterdam). The cell lines were cultured in RPMI 1640 medium (Life Technologies, Breda, The Netherlands) supplemented with penicillin (100 IU/mL), streptomycin (100 ng/mL), murine IL-3 (10 ng/mL), and 10% fetal calf serum (Life Technologies).
Ligands and cannabinoid ligands
Recombinant human stromal cell-derived factor (SDF-1α) was obtained from R&D Systems (Uithoorn, The Netherdands). The Cb2 ligands used were 2-AG, AEA, WIN 55212-2, cannabinol, cannabidiol, δ8-THC, and δ9-THC from Sigma (Zwijndrecht, The Netherlands). PEA and POEA are from ICN Biomedicals (Zoetermeer, The Netherlands) and CP55940 from Pfizer (Groton, CT). Cb1-specific antagonist SR141716 and Cb2-specific antagonist SR144528 were kindly donated by Dr Casellas (Sanofi Recherche, Montpellier, France).
Cb2 expression constructs and transfection of 32D/G-CSF-R cells
Cb2 exon-1B plus exon-2, which was hemagglutinin (HA)–tagged at the 5′ end (EcoRI/NcoI), was cloned as HindIII/BamHI fragment intoHindIII/BglII sites of pLNCX. The expression construct was transfected into 32D/G-CSF-R by electroporation (230V, 100 microfarads, and 1000 milliseconds). Following gene transfer, cells were cultured in RPMI 1640 medium supplemented as above for 48 hours and then selected in neomycin at concentrations of 0.8 mg/mL. Neomycin-resistant clones were expanded. To study Cb2 mRNA expression, ribonuclease (RNase) protection analysis was applied. Because mouse Cb2-specific antibodies are not yet available and HA antibodies are not capable of detecting HA-Cb2, Cb2 protein expression was analyzed by ligand binding (see below).
RNase protection analysis
RNase protection analysis was performed as described previously.27 Total cellular RNA was prepared from kidney, heart, spleen, and thymus by homogenizing tissue cells in 4 M guanidium thiocyanate, followed by phenol-chloroform extraction and isopropanol precipitation.28 RNA from NFS cell lines and 32D/G-CSF-R–transfected cells was isolated using 4 M guanidium thiocyanate or Trizol Reagent (Life Technologies). The RNA samples were subjected to an RNase protection assay, essentially as described by the supplier (Promega, Leiden, The Netherlands). A 249–base pair (bp) fragment (bp −147 to bp +102 of Cb2 complementary DNA [cDNA], probe P) (Figure 1A) was cloned into a pBluescript II SK+ vector and linearized withHindIII. A radiolabeled GAPDH RNA fragment was used as a control.29
Expression of distinct
Cb2 mRNA splice variants and determination of receptor binding sites in mouse myeloid leukemia cell lines. (A) Genomic organization of mouse Cb2. The gray box represents the Cb2 protein-coding region. Two distinct splice variants have been identified: exon-1A/exon-2 and exon-1B/exon-2. The 249 nucleotide RNA probe (probe P) representing exon-1B/exon-2 that was used for RNase protection analysis is indicated. (B) RNase protection analysis on distinct myeloid cell lines using probe P (see panel A). (C) Cb2 receptor density and receptor affinity for CP55940 on the NFS cell lines was assessed by saturation radioligand receptor binding experiments using [3H]CP55940. Kdis added to the columns.
Expression of distinct
Cb2 mRNA splice variants and determination of receptor binding sites in mouse myeloid leukemia cell lines. (A) Genomic organization of mouse Cb2. The gray box represents the Cb2 protein-coding region. Two distinct splice variants have been identified: exon-1A/exon-2 and exon-1B/exon-2. The 249 nucleotide RNA probe (probe P) representing exon-1B/exon-2 that was used for RNase protection analysis is indicated. (B) RNase protection analysis on distinct myeloid cell lines using probe P (see panel A). (C) Cb2 receptor density and receptor affinity for CP55940 on the NFS cell lines was assessed by saturation radioligand receptor binding experiments using [3H]CP55940. Kdis added to the columns.
Membrane preparation and [3H]CP55940 binding assays
Frozen cell and tissue pellets were kept at −80°C until use. Pellets were thawed and suspended in assay buffer (50 mM Tris-HCl [pH 7.0], 1 mM ethylenediaminetetraacetic acid, 3 mM MgCl2, 100 μM phenylmethylsulfonyl fluoride [pH 7.4] containing 0.1% bovine serum albumin [Serva, Heidelberg, Germany]), and membrane suspensions were homogenized and centrifuged at 10 000g for 10 minutes (4°C). Pellets were then resuspended in 5 mL in assay buffer, homogenized using a Potter-Elvejhem homogenizer, and resuspended in assay buffer at a final membrane concentration equivalent to 106 cells per milliliter. For binding experiments, 160 μL membrane suspension (106 cells/mL) was incubated in 96-well plates (flat-bottom plates, Greiner) with 20 μL [3H]CP55940 (DuPont-New England Nuclear) in concentrations ranging from 0.2 nM to 1.2 nM and 20 μL assay buffer for total binding or assay buffer containing 10−5 M nonlabeled CP55940 to assess nonspecific binding. Mixtures (200 μL final volume) were incubated at 30°C for 50 minutes, after which suspensions were filtrated over Unifilter GF/B plates using a Filtermate-196 Harvester (Packard) and washed twice for 5 seconds with 200 μL ice-cold washing buffer (50 mM Tris-HCl [pH 7.0] containing 0.25% bovine serum albumin). Filtration plates were sealed at the bottom, 25 μL scintillation fluid (Microscint-O, Packard) was added per well, and radioactivity was counted in a TopCount scintillation counter (Canberra Packard). Saturation curves produced identical affinities for [3H]CP55940 (affinity dissociation constant [Kd] ranged from 0.25 to 0.5 nM). Saturation plots were constructed by plotting specific binding (ie, total binding minus nonspecific binding) against label concentration, ranging from 0.2 nM to 1.2 nM, after which nonlinear curve fitting was done to estimate Bmax and affinity. In parallel, Scatchard plots were constructed by plotting the ratio-specific binding over free-label concentration against specific binding. Cb2 binding sites with spleen cells or thymocytes (Figure7B) were assessed in triplicate using one radioligand concentration of CP55940 (1 nM). Nonspecific binding was determined in the presence of excess nonradioactive CP55940 (10−6M), and specific binding was expressed as femtomoles per 106 cells (Figure 7C).
Migration assay
Migration assays were performed using 6.5 mm–diameter transwells with 5 μm pore size (Corning Costar, Amsterdam, The Netherlands). The cells used for the migration assay were NFS 58, 61 and 78 cells, transfected 32D/G-CSF-R cells, and spleen and thymus cells from male FVB mice. Mice were killed by inhalation of CO2. Spleen and thymus were isolated immediately and placed on RPMI 1640 medium. Single-cell suspension was prepared using 70-μm nylon cell strainer (Falcon, NJ). For migration assay, 1 × 105 or 2 × 105 cells were washed twice with Hanks balanced salt solution medium, resuspended in 100 μL migration medium (Iscoves modified Dulbecco medium plus 0.5% bovine serum albumin), and placed in the upper chamber of the transwells with or without presence of ligand. In the lower chamber, 600 μL migration medium with or without ligand was placed. After 4 hours' incubation at 37°C and 5% CO2, the upper chamber was removed and the number of migrated cells was determined using a CASY1/TTC cell counter (Schärfe System, Germany).
Flow cytometric analysis
Spleen cells that migrated to the lower chamber in the migration assay were immunophenotyped using a FACSCalibur flow cytometer (Becton Dickinson, Mountain View, CA). The following rat monoclonal antibodies were used in indirect immunofluorescence assays: RA3-6B2 (anti-B220/CD45R), Ter119 (LY-76), 59-AD2.2 (anti–Thy-1), and KT3 (anti-CD3). A fluorescein isothiocyanate–conjugated goat anti–rat immunoglobulin (Ig) (Nordic Immunological Labs, Tilburg, The Netherlands) was used as a second-step reagent. Immunophenotyping with double or triple labeling was performed combining the mentioned antibodies with allophycocyanin-conjugated anti-B220/CD45R (RA3-6B2), phycoerythrin-conjugated anti-IgD (Southern Biotechnology, Birmingham, AL), anti-CD19 (1D3), and anti-CD11c (HL3), as well as biotinylated anti-IgM (II/41), using streptavidin-allophycocyanin as a second step (Pharmingen, San Diego, CA).
Results
Cb2 expression analysis in myeloid leukemic cell lines
The protein-coding region for the peripheral cannabinoid receptor is located on exon-2 of the Cb2 gene (Figure 1A). Two distinct Cb2 splice variants have been identified, which both comprise the Cb2 receptor coding exon but contain different nonprotein-coding first exons: exon-1A or exon-1B (Figure1A). To determine which splice variant is present in myeloid cell lines, RNase protection was performed using a 249-bp polymerase chain reaction product (probe P) overlapping exon-1B (101 nucleotides) and exon-2 (148 nucleotides) (Figure 1A). A protected band of 249 nucleotides corresponding to exon-1B plus exon-2 Cb2 mRNA was identified in the myeloid cell line NFS 78 (Figure 1B), which contains a retroviral insertion in Cb2, whereas a 148 nucleotide exon-2–protected fragment was identified in the other myeloid cell lines (Figure 1B). As demonstrated previously, this latter protected fragment represents exon-1A/exon-2 Cb2transcript.3 27 Ligand binding studies using [3H]CP55940 and Scatchard plot analysis revealed the presence of significant numbers of cannabinoid binding sites on NFS 78 cells, whereas receptors were not measurable on the other IL-3–dependent myeloid cell lines (Figure 1C).
The endocannabinoid 2-AG is the true ligand for Cb2 receptor and stimulates migration of Cb2-expressing myeloid cells
Next, we wished to determine the function of the peripheral cannabinoid receptor when overexpressed in myeloid cells and investigate which of the large panel of molecules capable of binding is the true ligand for Cb2. For this purpose we used the Cb2-overexpressing myeloid cell line NFS 78 as well as 2 clones of the 32D/G-CSF-R cell line in which we introduced exon-1B/exon-2Cb2 cDNA. High exon-1B/exon-2 Cb2 mRNA expression was demonstrated by RNase protection (Figure2A), and ligand binding studies demonstrated excess of [3H]CP55940 binding sites on exon-1B/exon-2 Cb2-transfected cells (Figure 2B). No exon-1B/exon-2 Cb2 mRNA or ligand binding sites were detected in the vector control 32D/G-CSF-R cells (Figure 2).
Messenger RNase protection analysis and ligand binding studies on transfected 32D/G-CSF-R cells.
(A) RNase protection analysis using probe P on 10 μg total mRNA from 4 distinct transfected 32D/G-CSF-R clones: 2 32D/G-CSF-R clones transfected with Cb2 exon-1B/exon-2 cDNA and 2 clones with control vector pLNCX. (B) Cb2 receptor density and receptor affinity for CP55940 on 2 Cb2 exon-1B/exon-2 clones and 2 empty vector control clones. Density and affinity were assessed by saturation radioligand receptor binding experiments using [3H]CP55940. Kd is added to the columns.
Messenger RNase protection analysis and ligand binding studies on transfected 32D/G-CSF-R cells.
(A) RNase protection analysis using probe P on 10 μg total mRNA from 4 distinct transfected 32D/G-CSF-R clones: 2 32D/G-CSF-R clones transfected with Cb2 exon-1B/exon-2 cDNA and 2 clones with control vector pLNCX. (B) Cb2 receptor density and receptor affinity for CP55940 on 2 Cb2 exon-1B/exon-2 clones and 2 empty vector control clones. Density and affinity were assessed by saturation radioligand receptor binding experiments using [3H]CP55940. Kd is added to the columns.
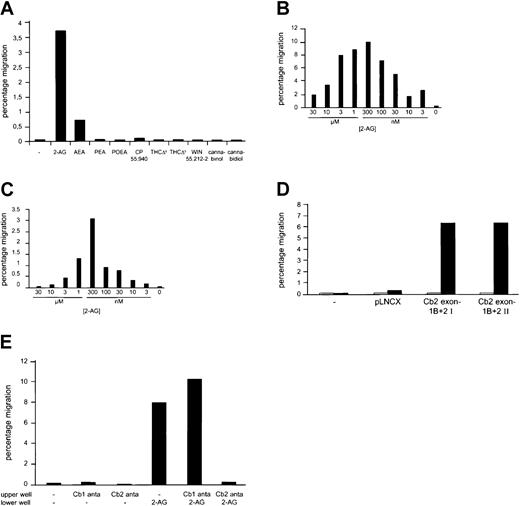
Because G protein–coupled receptors may function in cell mobility, we investigated in vitro migration in transwell assays. The following cannabinoid ligands were added to the lower chamber to test their capacity to induce chemotactic migration (1 μM): AEA, 2-AG, PEA, POEA, CP55940, δ8-THC, δ9-THC, WIN 55212-2, cannabinol, and cannabidiol; 2-AG and, to a lesser extent, AEA stimulated migration of Cb2-transfected 32D/G-CSF-R (Figure3A). None of the other ligands were capable of stimulating migration of these Cb2-expressing cells (Figure3A). In fact, in separate experiments using different concentrations of each of these ligands (1 nM to 10 μM) 2-AG was the only potent inducer of migration at a optimal concentration of 300 nM (Figure 3B-C). Migration was shown to be specific because 32D/G-CSF-R parental cells as well as vector control–transfected 32D/G-CSF-R cells did not migrate in response to 2-AG (Figure 3D). Neither did we observe any migration using the cell lines NFS 58 and NFS 61 (data not shown), which were revealed to be negative in the [3H]CP55940 binding assay (Figure 1C). Moreover, addition of SR144528 (100 nM), a Cb2-specific antagonist, to the upper chamber abolished the 2-AG–mediated transwell migration of 32D/G-CSF-R/Cb2 cells completely, whereas the Cb1-specific antagonist SR141716 did not affect 2-AG–induced migration (Figure 3E).
In vitro migration of Cb2-expressing cells following exposure to cannabinoids.
(A) Comparison of the chemoattractive effect of different cannabinoid ligands (1 μM) on Cb2 exon-1B/exon-2–expressing 32D/G-CSF-R cells. Ligands were added to the lower chamber, and cells that migrated to the lower well were counted after 4 hours of incubation. Y-axis shows the percentage of migrated cells from an input of 1 × 105. (B) Effect of different concentrations of 2-AG when added to the lower chamber on the in vitro migration ofCb2-expressing 32D/G-CSF-R cells. Y-axis shows the percentage of migrated cells from an input of 1 × 105. (C) 2-AG titration experiment using the myeloid cell line NFS 78. Y-axis shows the percentage of migrated cells from an input of 2 × 105. (D) Chemoattractive effect of 300 nM 2-AG (▪) or nothing (■) on exon-1B/exon-2 Cb2-expressing cells versus non-Cb2–expressing cells. Y-axis shows percentage of migrated cells from an input of 2 × 105cells. (E) Cb2-expressing 32D/G-CSF-R cells were exposed to medium with or without 300 nM 2-AG added to the lower well; 100 nM Cb1-specific antagonist, SR141716, Cb2-specific antagonist, SR144528, or cells without antagonist as a control were placed on the upper well. Y-axis shows percentage of migration from an input of 1 × 105 cells.
In vitro migration of Cb2-expressing cells following exposure to cannabinoids.
(A) Comparison of the chemoattractive effect of different cannabinoid ligands (1 μM) on Cb2 exon-1B/exon-2–expressing 32D/G-CSF-R cells. Ligands were added to the lower chamber, and cells that migrated to the lower well were counted after 4 hours of incubation. Y-axis shows the percentage of migrated cells from an input of 1 × 105. (B) Effect of different concentrations of 2-AG when added to the lower chamber on the in vitro migration ofCb2-expressing 32D/G-CSF-R cells. Y-axis shows the percentage of migrated cells from an input of 1 × 105. (C) 2-AG titration experiment using the myeloid cell line NFS 78. Y-axis shows the percentage of migrated cells from an input of 2 × 105. (D) Chemoattractive effect of 300 nM 2-AG (▪) or nothing (■) on exon-1B/exon-2 Cb2-expressing cells versus non-Cb2–expressing cells. Y-axis shows percentage of migrated cells from an input of 2 × 105cells. (E) Cb2-expressing 32D/G-CSF-R cells were exposed to medium with or without 300 nM 2-AG added to the lower well; 100 nM Cb1-specific antagonist, SR141716, Cb2-specific antagonist, SR144528, or cells without antagonist as a control were placed on the upper well. Y-axis shows percentage of migration from an input of 1 × 105 cells.
2-AG stimulates chemotaxis as well as chemokinesis of Cb2-expressing myeloid cells
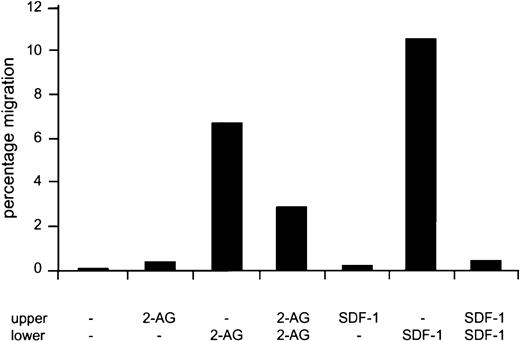
To investigate whether 2-AG is a chemokinetic as well as a chemotactic agent, in vitro migration experiments were carried out with 2-AG added to the lower chamber, the upper chamber, or both. When 2-AG was added to the upper and lower chamber simultaneously, approximately 50% migration was observed as compared with the chemotactic experiment (ie, with 2-AG added to the lower well only) (Figure4). No cells migrated when 2-AG was added to the upper well only. In comparison, 32D/G-CSF-R/Cb2 cells, which express the CXC chemokine receptor 4 (CXCR4) endogenously, migrated when the CXCR4 ligand SDF-1α was added to the lower chamber. However, no chemokinetic mobility was observed when SDF-1α was added to both chambers (Figure 4).
Chemotaxis versus chemokinesis of Cb2-expressing cells in response to 2-AG or SDF-1 using a transwell assay.
Cb2-transfected 32D/G-CSF-R cells, which naturally express CXCR4, were placed in the upper well with or without 2-AG or SDF-1α. Migration medium with or without ligand was placed in the lower well. Y-axis shows percentage of migration from an input of 1 × 105 cells.
Chemotaxis versus chemokinesis of Cb2-expressing cells in response to 2-AG or SDF-1 using a transwell assay.
Cb2-transfected 32D/G-CSF-R cells, which naturally express CXCR4, were placed in the upper well with or without 2-AG or SDF-1α. Migration medium with or without ligand was placed in the lower well. Y-axis shows percentage of migration from an input of 1 × 105 cells.
Natural and synthetic cannabinoids inhibit 2-AG–induced migration, whereas endocannabinoids have no effect
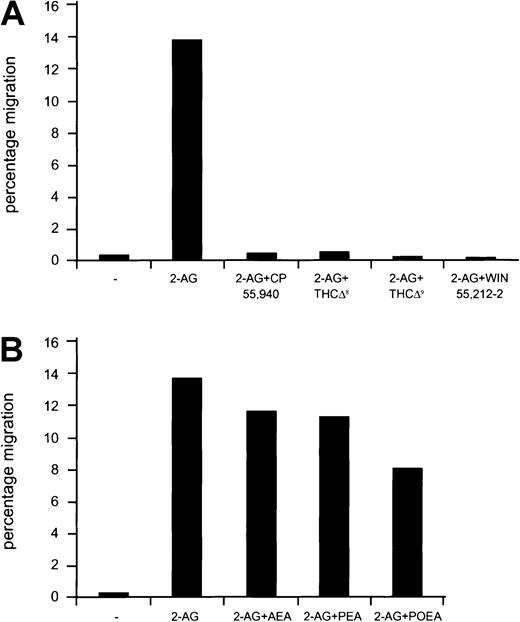
Because the endocannabinoids (AEA, PEA, POEA) as well as the natural (δ8-THC, δ9-THC) and the synthetic cannabinoid ligands (CP55940, WIN 55212-2) are capable of binding to the peripheral cannabinoid receptor, we wondered whether these compounds could either synergize with 2-AG or antagonize the effect of this ligand in a migration assay. The synthetic cannabinoids CP55940 and WIN 55212-2 as well as δ8-THC and δ9-THC completely abolished 2-AG–induced chemoattraction of Cb2-transfected 32D/G-CSF-R cells when added either to the lower (Figure 5A) or the upper chamber in a transwell assay (data not shown). On the other hand, AEA, PEA, and POEA did not affect 2-AG–induced chemoattraction (Student t test, P > .05) of cells when either added to the lower (Figure 5B) or to the upper chamber in a migration assay (not shown).
Effect of endocannabinoids and natural and synthetic cannabinoid ligands on the 2-AG–induced mobilization of cells.
(A) Cb2-transfected 32D/G-CSF-R cells were exposed to 2-AG or 2-AG plus CP55940, δ9THC, δ8THC, or WIN55212-2 in a chemotactic experiment. Y-axis shows percentage of migration from an input of 2 × 105 cells. (B) Cb2-transfected 32D/G-CSF-R cells were placed in the upper well, and migration medium containing 2-AG or 2-AG in combination with AEA, PEA, and POEA was added to the lower chamber. Y-axis shows percentage of migration from an input of 2 × 105 cells.
Effect of endocannabinoids and natural and synthetic cannabinoid ligands on the 2-AG–induced mobilization of cells.
(A) Cb2-transfected 32D/G-CSF-R cells were exposed to 2-AG or 2-AG plus CP55940, δ9THC, δ8THC, or WIN55212-2 in a chemotactic experiment. Y-axis shows percentage of migration from an input of 2 × 105 cells. (B) Cb2-transfected 32D/G-CSF-R cells were placed in the upper well, and migration medium containing 2-AG or 2-AG in combination with AEA, PEA, and POEA was added to the lower chamber. Y-axis shows percentage of migration from an input of 2 × 105 cells.
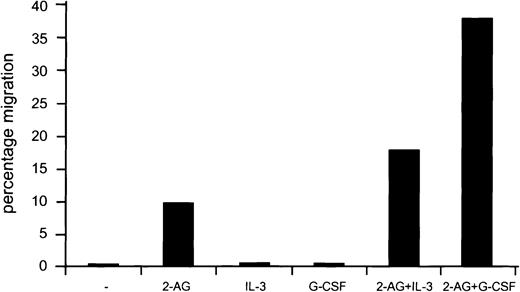
Effects of 2-AG in combination with IL-3 or G-CSF on migration of Cb2-expressing cells
To study whether Cb2 receptor ligands may synergize with other ligands that can activate 32D/G-CSF-R cells, we carried out migration experiments using 2-AG in combination with IL-3 or G-CSF. No migration was observed when 32D/G-CSF-R/Cb2 cells were exposed to IL-3 or G-CSF alone in a transwell assay. However, addition of 2-AG with IL-3 or G-CSF showed a significant increase in the migration rate of these cells as compared with experiments using 2-AG as a single agent (Figure6). The same effect was observed when NFS 78 cells were exposed to 2-AG and IL-3 (data not shown).
Synergy between 2-AG and IL-3 or G-CSF in migration stimulation.
Cb2-transfected 32D/G-CSF-R cells were added to the upper chamber in a migration assay. Cells were exposed to migration medium present at the lower well containing 2-AG with or without IL-3 or G-CSF. Y-axis shows percentage of migration from an input of 2 × 105 cells.
Synergy between 2-AG and IL-3 or G-CSF in migration stimulation.
Cb2-transfected 32D/G-CSF-R cells were added to the upper chamber in a migration assay. Cells were exposed to migration medium present at the lower well containing 2-AG with or without IL-3 or G-CSF. Y-axis shows percentage of migration from an input of 2 × 105 cells.
Distinct Cb2 mRNA splice variants and protein levels in mouse spleen and thymus
The functional assays carried out so far were performed with cells that overexpress Cb2 receptor. Before elucidating whether naturally expressing Cb2 cells migrate following 2-AG exposure as well, we first investigated which hematopoietic organs express Cb2. RNase protection analysis was carried out on thymus and spleen mRNA using probe P (Figure 1A). A protected band of 249 nucleotides, corresponding to exon-1B plus exon-2 Cb2 mRNA, was identified in spleen (Figure 7A), whereas a 148 nucleotide exon-2–protected fragment, representing exon-1A/exon-2 Cb2transcript, was identified in spleen and thymus (Figure 7A).Cb2 mRNA was not detectable in the other tissues investigated27 (Figure 7A). To study the presence of Cb2 binding sites in spleen and thymus, a binding assay was carried out using a saturated concentration of 1 nM [3H]CP55940 (determined on NFS 78 [Figure 1C]). The experiment in Figure 7B demonstrates that [3H]CP55940 binding is observed in spleen and not in thymus of normal FVB mice, which is in agreement with previous studies.30 31
Cb2 expression and function in normal spleen and thymus.
(A) RNase protection on 10 μg total mouse mRNA isolated from several organs. The protected fragments were 249 nucleotides (exon-1B/exon-2Cb2 mRNA-protected) or 148 nucleotides (exon-2Cb2 mRNA-protected, representing exon-1A/exon-2). AGAPDH probe was used for normalization of the signals. (B) Receptor density (femtomoles per 106 cells) on spleen cells and thymocytes was assessed by measuring specific binding of [3H]CP55940 (1 nM) (see “Materials and methods”). (C) Effect of Cb1-specific antagonist SR141716 or Cb2-specific antagonist SR144528 on spontaneous or 2-AG–induced migration of spleen cells. Antagonists and 2-AG were placed in the wells as indicated under the figure.Y-axis shows percentage of migration from an input of 2 × 105 spleen cells. (D) Immunophenotyping of 2-AG–induced migrated spleen cells versus spontaneous spleen-migrated cells using flow cytometry.
Cb2 expression and function in normal spleen and thymus.
(A) RNase protection on 10 μg total mouse mRNA isolated from several organs. The protected fragments were 249 nucleotides (exon-1B/exon-2Cb2 mRNA-protected) or 148 nucleotides (exon-2Cb2 mRNA-protected, representing exon-1A/exon-2). AGAPDH probe was used for normalization of the signals. (B) Receptor density (femtomoles per 106 cells) on spleen cells and thymocytes was assessed by measuring specific binding of [3H]CP55940 (1 nM) (see “Materials and methods”). (C) Effect of Cb1-specific antagonist SR141716 or Cb2-specific antagonist SR144528 on spontaneous or 2-AG–induced migration of spleen cells. Antagonists and 2-AG were placed in the wells as indicated under the figure.Y-axis shows percentage of migration from an input of 2 × 105 spleen cells. (D) Immunophenotyping of 2-AG–induced migrated spleen cells versus spontaneous spleen-migrated cells using flow cytometry.
Migration of Cb2-expressing spleen cells following 2-AG stimulation
To investigate whether naturally expressing Cb2 cells migrate following 2-AG exposure as well, spleen cells from FVB mice were studied using a transwell assay. A titration experiment showed optimal migration of spleen cells in the presence of 300 nM 2-AG (data not shown). Moreover, this migration could be blocked by addition of Cb2-specific antagonist to the upper well but not by addition of Cb1-specific antagonist (Figure 7C). No significant migration was evident of thymocytes in transwells (data not shown). Immunophenotyping by flow cytometric analysis of the 2-AG–induced migrated spleen cells compared with spontaneously migrated spleen cells revealed that the cells were B220+. Double staining revealed that these B220+ cells expressed CD19, IgM, and IgD (Figure 7D). In addition, these cells were CD11c−, indicating that the spleen-migrated cells were B lymphocytes and not B220+/CD11c+ dentritic cells (Figure7D).
Discussion
In the study presented here, we demonstrate that 2 distinctCb2 mRNA splice variants exist in the mouse, each composed of the same protein-encoding exon-2 but with a different nonprotein-coding first exon. In most myeloid leukemia cell linesCb2 exon-1A/exon-2 is expressed, which correlates with low protein expression. On the other hand, in mouse leukemia NFS 78, retroviral insertion has occurred 5′ of exon-1B,3,32resulting in the expression of high levels of Cb2exon-1B/exon-2 mRNA and the appearance of Cb2 proteins on the cell surface. Cb2 receptors are also present on normal spleen cells, which express both Cb2 splice variants: exon-1A/exon-2 and exon-1B/exon-2. On the other hand, thymocytes only expressCb2 exon-1A/exon-2 splice variants, which are not accompanied by the presence of detectable numbers of ligand binding sites. These data suggest that in the cells studied here, Cb2 protein may be translated from exon-1B/exon-2 transcripts rather than from exon1A/exon2 mRNAs. These results may be explained by a regulatory mechanism of translation involving the noncoding first exons ofCb2. Although several mechanisms of translational control involving noncoding mRNA have been described,33-36 the function of the 2 first exons in Cb2 is currently unknown. Whether and how these nonprotein-coding exons may regulate protein expression is subject to future investigations.
Oncogenic transformation by GPCRs may be caused either by structural alteration of the receptor itself or by deregulated presentation of the ligands.8,37,38 We previously demonstrated that retroviral insertion in the Cb2 locus occurs frequently in myeloid leukemias in mice.3,32 The data presented here suggest that proviral insertion in Cb2 in myeloid leukemia cells may result in the selective expression of particular splice variants and overexpression of the peripheral cannabinoid receptor. GPCRs have been related to a variety of cellular functions, including cell proliferation, differentiation, survival, and migration.39,40 To study the role of the peripheral cannabinoid receptor when overexpressed on myeloid cells, we investigated the effects of cannabinoid ligands on the myeloid cell line NFS 78 and on 32D/G-CSF-R cells transfected with theCb2 exon-1B/exon-2 splice variant. We demonstrate that stimulation of Cb2-overexpressing cells by its potent agonist 2-AG induces migration in vitro at nanomolar concentrations. Given the role of Cb2 receptor in migration in vitro, we investigated whether 2-AG may act as a chemotactic as well as a chemokinetic agent. In contrast to SDF-1α, which is a chemoattractant that acts via CXCR4,41-45 2-AG may stimulate both chemotaxis (directional migration) and chemokinesis (random migration), a behavior that has been described for other receptors as well.46,47Interestingly, a significant increase of migration was observed when Cb2-expressing 32D/G-CSF-R cells were exposed to 2-AG in combination with IL-3 as well as with G-CSF. This set of experiments demonstrates that activation of the peripheral cannabinoid receptor by the endocannabinoid 2-AG may occur in many ways, such as by chemoattraction, chemokinesis, and in synergy with cytokines. Therefore, aberrant Cb2 expression on myeloid leukemia cells may result in changes in mobilization and, subsequently, altered homing of the leukemic precursors in vivo. In future experiments we plan to investigate the in vivo mobility of Cb2-expressing bone marrow cells using Cb2 transgenic mice48 or bone marrow precursor cells that have been infected with viral vectors carrying the Cb2 gene.
We previously demonstrated that AEA is a potent inducer of proliferation in synergy with IL-3, granulocyte-macrophage CSF, or G-CSF.27 However, we and other investigators suggested that although AEA may be an activator of Cb2, this fatty acid has also a nonreceptor-mediated stimulating effect.27,49-51 We could not detect an effect of 2-AG on survival or proliferation of Cb2-expressing cells (data not shown). However, this does not exclude the possibility that under different conditions or in combination with other cytokines this receptor may have an effect on these functions. In fact, synergy between cytokines and chemokines has been previously observed in proliferation, differentiation, and survival assays as well as in migration experiments.27 52-55 Our findings showing increased migration when cells were stimulated with 2-AG and IL-3 as well as 2-AG and G-CSF strengthen the idea to further investigate the role that 2-AG may have in combination with cytokines in proliferation, differentiation, or survival of leukemic progenitor cells.
Several cannabinoid ligands from different origin (natural, synthetic, and endogenous) have been proposed as the true cannabinoid ligands.19,20,22,56 In the present study we compared the effects of 2-AG with that of the other endocanabinoids (AEA, PEA, POEA), the natural cannabinoids (δ8-THC, δ9-THC, cannabinol, cannabidiol), and the synthetic molecules (WIN55212-2, CP55940). Among the endocannabinoids, 2-AG was the most potent inducer of migration, supporting the idea that 2-AG is the true endogenous ligand for the peripheral cannabinoid receptor.56-59 AEA, which was the first endogenous cannabinoid ligand isolated,22 shows similar binding affinity to the Cb2 receptor as 2-AG does.59 However, AEA only weakly stimulated migration of Cb2-expressing cells. This observation is in agreement with other studies demonstrating that AEA acts at the most as a partial agonist for the Cb2 receptor.59 The fact that the other endocannabinoids (PEA, POEA) did not stimulate migration or interfere with 2-AG–induced migration may be explained by much lower affinity of these compounds for the peripheral cannabinoid receptor.60 Neither CP55940, which has been considered a potent cannabinoid ligand and an inducer of migration,31,61 nor any of the other synthetic or natural cannabinoids were capable of stimulating cell mobility. In fact, we observed that most of the synthetic and natural cannabinoids interfere with 2-AG–stimulated Cb2 receptor activation and function. This observation may be in agreement with previous findings suggesting that cannabinoids may one way or the other impair the immune response.62 This interference may include impairment of macrophage functions,63,64 induction of an imbalance in T-cell CD4/CD8 ratio,65 alteration of immunoglobin production,66-68 down-regulation of natural killer cell activity,69,70 or perturbation in macrophage/T-cell cooperation.64,71 It will therefore be of relevance to exactly define which cells express Cb2 and respond to its endocannabinoid and further dissect the function of Cb2 receptor in the immune response. In this study we demonstrate that spleen cells migrating in response 2-AG were B220+, CD19+, IgM+, and IgD+, which is an agreement with previous studies showing that Cb2 receptor is mainly expressed on B lymphocytes.31,72,73 Furthermore, our data show that the 2-AG–induced migration could be completely blocked by SR144528, a Cb2-specific antagonist, but not by SR141716, a Cb1-specific antagonist, indicating that stimulation of migration occurs exclusively via Cb2 receptors. Previously, the function of the peripheral cannabinoid receptor has also been related to B-cell differentiation, as proposed by Carayon et al.31 These observations together with our findings suggest a role of the peripheral cannabinoid receptor in attraction, mobilization, or activation of B cells during the immune response.
We thank Dr R. Hendrix for excellent assistance during immunophenotyping of spleen-migrated cells and P. van Hennik for technical assistance in migration assays. We also thank Karola van Rooyen for preparation of the figures and Dr P. Casellas (Sanofi Recherche, Montpellier, France) for donation of the Cb1 and Cb2 receptor-specific antagonists SR141716 and SR144528, respectively.
Supported by the Dutch Cancer Foundation Koningin Wilhelmina Fonds, The Netherlands Organisation for Scientific Research (NWO), and the Royal Dutch Academy of Sciences (KNAW).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ruud Delwel, Institute for Hematology, Erasmus University Rotterdam, Dr Molewaterplein 50, 3015GE Rotterdam, The Netherlands; e-mail: delwel@hema.fgg.eur.nl.

![Fig. 1. Expression of distinct. / Cb2 mRNA splice variants and determination of receptor binding sites in mouse myeloid leukemia cell lines. (A) Genomic organization of mouse Cb2. The gray box represents the Cb2 protein-coding region. Two distinct splice variants have been identified: exon-1A/exon-2 and exon-1B/exon-2. The 249 nucleotide RNA probe (probe P) representing exon-1B/exon-2 that was used for RNase protection analysis is indicated. (B) RNase protection analysis on distinct myeloid cell lines using probe P (see panel A). (C) Cb2 receptor density and receptor affinity for CP55940 on the NFS cell lines was assessed by saturation radioligand receptor binding experiments using [3H]CP55940. Kdis added to the columns.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/8/10.1182_blood.v99.8.2786/5/m_h80822431001.jpeg?Expires=1769234305&Signature=zwDyz4dTYCHAdeKmftV1qp8qT~nAh0vcTd2pdIgJDi7f-HBWqzN3aoMmYCr-M1im75R7ftyHthBZ7jAB7Fhw8LhrgMmJqLnXFR3tj9YP8j90-JZLyfhGML9P-TUp8qS7meRmuTw2ntqiGjqcKDWwFkm3jKA4Zml3nnHtXnwSnQxvEgFfbfVtwYtgdzMvWYlC6fEWHk1b0cI40PVg0RtbouvW4L~1hsEz9LnSrpRKV6np4iPCjdW79NlK5LTp-Z0W3EI2vEcndk6D454RwLsm9SS2fUtAd4lmArIEdKKR3SFa~cz8k6Nkn4Gxe6WYKsRwkOOXxuyIGZ9O9-3eQZryOg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 2. Messenger RNase protection analysis and ligand binding studies on transfected 32D/G-CSF-R cells. / (A) RNase protection analysis using probe P on 10 μg total mRNA from 4 distinct transfected 32D/G-CSF-R clones: 2 32D/G-CSF-R clones transfected with Cb2 exon-1B/exon-2 cDNA and 2 clones with control vector pLNCX. (B) Cb2 receptor density and receptor affinity for CP55940 on 2 Cb2 exon-1B/exon-2 clones and 2 empty vector control clones. Density and affinity were assessed by saturation radioligand receptor binding experiments using [3H]CP55940. Kd is added to the columns.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/8/10.1182_blood.v99.8.2786/5/m_h80822431002.jpeg?Expires=1769234305&Signature=kZRPtAPRpMmkt3GUx9jRt40g-aB-4MJKLZOqVTXu07RIuEK75hPZZxgcUpxPRd5HMyvyxJUhtFEcu35SnxQ8UdpoQwmnbxjoEFO4zA83eeSxw3Yv~dGEm2uHGKe43FSp9ng80vqIByGlhkdlOmVP~b6lNnWI6eT~EzLMwyJlLdgleATZBZVB1HevcrFCLMFfNJ9DpOel0e10~8X6Sv589lYdLQmYXzHsnwGtRBOJlsl8t7iFu6WvY0CkNtfUVOMgjGv0EN1GXPTMT9m~7dl-GbAbyI0amQbAzuJftLZhhCVNyy0tX~fWwa3EOFXTGvn03QXVDYt6TfqoxKLyLi~QTg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. Cb2 expression and function in normal spleen and thymus. / (A) RNase protection on 10 μg total mouse mRNA isolated from several organs. The protected fragments were 249 nucleotides (exon-1B/exon-2Cb2 mRNA-protected) or 148 nucleotides (exon-2Cb2 mRNA-protected, representing exon-1A/exon-2). AGAPDH probe was used for normalization of the signals. (B) Receptor density (femtomoles per 106 cells) on spleen cells and thymocytes was assessed by measuring specific binding of [3H]CP55940 (1 nM) (see “Materials and methods”). (C) Effect of Cb1-specific antagonist SR141716 or Cb2-specific antagonist SR144528 on spontaneous or 2-AG–induced migration of spleen cells. Antagonists and 2-AG were placed in the wells as indicated under the figure.Y-axis shows percentage of migration from an input of 2 × 105 spleen cells. (D) Immunophenotyping of 2-AG–induced migrated spleen cells versus spontaneous spleen-migrated cells using flow cytometry.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/8/10.1182_blood.v99.8.2786/5/m_h80822431007.jpeg?Expires=1769234305&Signature=B3t3hdxrvYYgatf7~SpUTy2LzwjhcZSFIUpnEpeDSG-VGnwdU~3Ny4QyBHW6mRLzrpFBpVqu6LJVMJFLTVsCD4NEvj0UNpXa2mG5EI264qEhkkRdu6OlZd9N9LzRImvi7UhReQkJlNWlysTgHLem-~autROXUWEcVOrQNlZIL8VHpF~aaxyXrMsi2~Zg61VDeuREJMercR-~iumAOmZZ9CTu5WRT6zDij7gX3GaWBkxOOtUmwklFWJy3HC670wIWJJ2vzI5iY4BGXJUbPxqnh6PyV0q~MmxMUBrMb7BK6VgFFoO5dkqTpDaTvmChlW3O1CGFd1anieqk3X9vv7A0Dw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal