Cord blood (CB) is used increasingly as a source of hematopoietic stem cells because of a lower risk of acute and chronic graft-versus-host disease (GVHD). However, there is some concern regarding the ability to adequately reconstitute host immune response due to the immaturity and naivety of CB T cells. This study was designed to evaluate T-cell reconstitution using combined approaches of phenotyping, analysis of αβ T-cell receptor (TCR) diversity, and assessment of ex vivo thymic function by measuring TCR rearrangement excision circles (TRECs). Ten patients who underwent CB transplantation for high-risk hematologic disorders were compared to a reference group of 19 age- and GVHD-matched patients who underwent transplantation with non-T cell-depleted bone marrow from an HLA-identical sibling donor. TREC values correlated with the relative number of naive T cells and with TCR repertoire polyclonality. During the first year after transplantation, TCR repertoires were highly abnormal and TREC values low in both groups. Notably, 2 years after transplantation onward TREC values as well as TCR diversity were higher in CB recipients than in recipients of bone marrow transplants. These data indicate an efficient thymic regeneration pathway from CB lymphoid progenitors despite the low number of cells infused compared to bone marrow, arguing for a complete clinical immune recovery after CB transplantation.
Introduction
More than 1500 umbilical cord blood transplantations (CBTs) have now been performed from related and unrelated donors for treatment of many high-risk hematologic disorders. Several reports have highlighted the practical advantages of cord blood (CB) as an alternative source of hematopoietic stem cells: relative ease of procurement, absence of risk to donors, reduced risk of transmitting infection, large donor pool, and faster allocation process.1,2 Recent studies suggested that children undergoing HLA-identical sibling CBT have a lower risk of acute and chronic graft-versus-host disease (GVHD).3 Some immunologic properties are particular to CB lymphocytes. They are all functionally and phenotypically naive compared with adult blood lymphocytes4 and they have a broad polyclonal T-cell receptor (TCR) repertoire.5 The immaturity and naivety of CB T cells could explain the lower GVHD risk, but they also raise some concern about the potential for immune reconstitution (IR) in patients receiving CB transplants.
After allogeneic hematopoietic stem cell transplantation (HSCT), all recipients experience a period of immunodeficiency. Regeneration of adequate T-cell number and repertoire diversity are key elements in the recovery of immune competence. In the absence of such recovery, susceptibility to opportunistic infections is increased. Defects in reconstituting a broad diversity of T-cell populations may also limit the T cell-based graft-versus-leukemia effect and predispose to relapse.
Regeneration of the T-cell population proceeds normally along 2 different pathways. The thymic-independent pathway includes transfer of graft-derived mature donor T cells followed by antigen- or cytokine-driven expansions especially in the case of non-T cell-depleted grafts. Although this provides the first wave of T-cell reconstitution after transplantation, these mature T cells have limited TCR diversity. The thymic-dependent pathway involves selection of graft-derived precursor cells, accounts for the more durable reconstitution of the T-cell compartment, and generates a more diverse TCR repertoire.6 7
Evaluation of IR after HSCT has improved through the development of direct methodologies for T-cell diversity analysis by the size of the β-chain complementarity determining region 3 (CDR3)8,9and for ex vivo evaluation of thymic function by quantitating TCR rearrangement excision circles (TRECs).10
In this study, IR was evaluated in a single institution series of patients treated for high-risk hematologic diseases with CBT and in a reference group of age- and GVHD-matched genoidentical sibling donor bone marrow transplantation (BMT) patients, the best clinical situation of IR. Our matching set-up provided the unique opportunity to study the impact of the hematopoietic stem cell (HSC) source on long-term IR. During the first year after transplantation, TCR repertoires were highly abnormal and TREC values low in both groups. In a longer follow-up (2 years or more after transplantation), TREC values and TCR diversity were increased, reaching values equal to age-matched healthy individuals in the CBT group.
Patients, materials, and methods
Patient characteristics and blood samples
We reviewed data on 29 patients treated with CBT (n = 10) or genoidentical sibling BMT (n = 19) at the Hematology and Bone Marrow Transplantation Unit, Hôpital Saint-Louis (Paris, France). The patient characteristics, treatments, and posttransplantation events are shown in Tables 1 and2. Patients were evaluated at the different time points indicated in Table3. Written informed consent was obtained from the patients or their parents and from the bone marrow (BM) donors. All umbilical CB samples were obtained at delivery from full-term healthy pregnancies after the mother's consent. Eleven CB samples and 15 healthy BM donors were used as reference values for TCR diversity analysis. Additional CB samples (n = 19) and peripheral blood mononuclear cells (PBMCs) from age-matched (mean age, 17.2 years; range, 4.5-25.9 years) healthy donors (n = 22) were obtained for reference TREC values. The investigation was approved by the Committee on Medical Ethics of the Hôpital Saint-Louis and was carried out according to the provisions of the Declaration of Helsinki. In the case of unrelated CB/recipient pairs, HLA typing included serology for HLA-A and -B and a high-resolution molecular typing for DRB1.
Flow cytometry analysis
The PBMCs were obtained after Ficoll-Hypaque (Pharmacia, Uppsala, Sweden) density gradient centrifugation and kept frozen in liquid nitrogen until use. Phenotypic analysis was performed on 0.5 to 1 × 104 gated lymphocytes (gate purity > 98%) by direct 2- or 3-color immunofluorescence flow cytometry using a FACScalibur analyzer (Becton Dickinson, San Jose, CA). The following conjugated monoclonal antibodies (mAbs) were used: CD45-fluorescein isothiocyanate (FITC), CD14-phycoerythrin (PE), CD3-peridinin chlorophyll-binding protein (PerCP) or FITC, CD4-FITC, CD8-PE, CD16+CD56-PE, CD28-PE, HLA-DR-PE, CD45RO-PE, CD19-FITC, CD27-PE (Becton Dickinson, Le Pont de Claix, France), CD45RA-PE, CD4-PE-cyanin 5.3 (PE-Cy5), CD8-PE-Cy5, CD5-PE-Cy5 (Immunotech Coulter Beckmann, Marseilles, France), and appropriate isotype-matched controls. The mAbs were used with following combinations: CD45/CD14, CD3/CD4/CD8, CD3/CD16+CD56, CD28/CD3/CD4, HLA-DR/CD3/CD4, CD45RO/CD3/CD4, and CD19/CD27/CD5. The proportions of competent CD28+, activated HLA class II DR+, memory CD45RObright (b)+, naive CD45ROb− in CD4+ and CD3+CD4− subsets, as well as natural killer (NK) cells, memory CD27+ and CD5+ B cells were assessed using standardized procedures combining a CD3, CD4, CD8b, or CD19 gate to the lymphocyte gate. Results are presented as an absolute count for each lymphocyte subset.
RNA extraction and TCR β-chain CDR3 size analysis
RNA and genomic DNA were purified using TriReagent (Molecular Research Center, Cincinnati, OH). Synthesis of complementary DNA (cDNA), polymerase chain reaction (PCR) amplifications of TCR beta chain variable (BV) and constant (BC) segments, BV-BC run-off using an internal BC fluorescent primer, gel running, and Immunoscope software analysis were performed as previously described.5
Statistical analysis of TCR diversity
Definition of the Immunoscope profiles as polyclonal, skewed, or negative was based on the identification of peaks that deviate from the normal distribution curve assuming that in a part of linear distribution, the height of the middle peak in a group of 3 adjacent peaks is the mean of the 2 outer peaks. First, we studied 11 normal CB samples to analyze the variation range of this ratio. We found that the mean (SD) value of the ratio was 0.95 (0.18). Ratios higher than 1.32 ( = mean + 2 SDs) were considered as oligoclonal peaks abnormally deviating from the linear distribution curve. Ratios lower than 0.65 ( = mean − 2 SDs) were assumed to indicate missing peaks (hole/gap). Between these ranges, peaks were considered to belong to linear (normal) distribution. BV families containing one or more abnormally deviating peaks were interpreted as skewed curve. Maximal raw peak intensities lower than 1000 arbitrary units were considered as negative. Results are presented as percentages of polyclonal, skewed (oligoclonal, monoclonal, missing peak), and negative BV families, and number of peaks. This method correlated well with the method developed by Gorochov et al.11 In our study population, the correlation coefficient between these 2 methods for calculation of polyclonality and extent of perturbation was 0.83 (P = .0001).
Quantification of TRECs by real-time PCR
After TriReagent extraction, DNA samples were adjusted to 250 ng/μL. Quantification of thymic signal-joint TCR delta excision circle (TREC) was done by real-time quantitative-PCR (ABI PRISM 7700, Applied Biosystems, Foster City, CA) according to Douek et al.12 A standard was created by cloning the signal-joint fragment in pCR2.1A using the TA cloning kit (Invitrogen, Groningen, The Netherlands). We determined the TREC value by quantitative PCR on genomic DNA from PBMCs, using an additional set of primers and probe to albumin to normalize for the genomic copy number.13 The amount of TREC and albumin copies present in a given cell sample was calculated by including a dilution series of these standards in B-LCL cell line (Cox) genomic DNA in each PCR experiment (107-102 copies/well for TREC and 5000-40 ng/well for albumin). The TREC values were corrected according to the percentage of CD3+ cells in the sample and then expressed as the numbers of TREC/μg CD3+ cell DNA.
Statistical analysis
Differences between patient groups were analyzed with the Mann-Whitney U test. Linear regression analysis was used for correlation analyses. P < .05 was considered significant. All statistical analyses were performed using the program StatView 5.0 for Windows (SAS Institute, Cary, NC).
Results
Clinical data
All patients were treated for malignant or severe hematologic disease (Table 1). Disease stages at the time of transplantation were more advanced in the CBT group. The median number of CD34+cells infused was 0.14 × 105/kg and 6.08 × 105/kg, respectively (P = .01). Conditioning protocols were broadly comparable, but 60% of recipients in the CBT group and about 30% of recipients in the BMT group received total body irradiation and antithymocyte globulin. Six recipients in the CBT group had 1 to 3 HLA mismatches with the CB and the other 4 patients received a transplant from a sibling with genoidentical CB.
A successful engraftment was observed in all analyzed patients. The median time to neutrophil engraftment was 31 days in the CBT group and 23 days in BMT group (P = .03; Table 2). Platelet engraftment was detected at day 87 after transplantation in CB transplant recipients and at day 26 in the BM transplant control group (P = .005).
Half the patients in the CBT group did not suffer from acute GVHD, 2 patients (20%) had grade I, and 3 other patients (30%) grade II acute GVHD. In the BMT group, 48% of recipients did not have GVHD, 26% of the patients had grade I, and 26% had a grade II GVHD. The median time to develop acute GVHD was 12 days (range, 8-40 days) and 21 days (range, 8-56 days), respectively. Sixty percent of those in the CBT group and 53% of patients in the BMT group did not develop chronic GVHD. Limited chronic GVHD was detected in 30% of patients receiving CB transplants and 42% of those receiving BM transplants. Only one recipient from each group suffered from extensive chronic GVHD. The median time to develop chronic GVHD was approximately the same, 210 days (range, 92-278 days) and 192 days (range, 155-386 days) in the CBT and BMT groups, respectively. Therefore, in these selected patients appearances of acute and chronic GVHD were comparable.
Before transplantation, 30% of CB transplant recipients, 68% of BM transplant recipients, and 58% of BM transplant donors were positive for cytomegalovirus (CMV). After transplantation, CMV infection occurred in 2 patients (20%) of the CBT group and in 5 patients (26%) of the BMT group. The mean number of life-threatening opportunistic infections during first year after transplantation was 1.20 versus 0.74/recipient (P = .28) in the CBT and BMT groups, respectively.
T-cell repertoire diversity
We followed the recovery of T-cell diversity using TCRBV CDR3 size analysis by the Immunoscope method.5 Data are presented in Table 3, and 2 representative examples of TCRBV repertoire analysis are shown in Figure 1. Before transplantation there was no statistically significant difference between the CBT and BMT groups in terms of diversity (49% and 65% of polyclonal profiles, respectively), which was lower than normal as reported in hematologic malignancies.14 Both groups reached the nadir of polyclonality between 2 to 6 months. Only about one third of the BV families were polyclonal at that time and the number of peaks had decreased of about 20%. At 1 year after transplantation, T-cell diversity was still low in both groups, and the majority (65% and 59%) of profiles were skewed or negative. Sixty percent in the CBT group and 53% in the BMT group still had less than one third of polyclonal BV families. On longer follow-up, reconstitution of TCR diversity appeared to be persistently improving. Follow-up duration was comparable in the CBT (mean, 48 months) and BMT (mean, 36 months) groups. The mean number of peaks was normalized and even higher than before transplantation. Two years or more after transplantation, the majority of BV families in the CBT group (n = 8) were polyclonal (89%) and only 8% were skewed. On the other hand, 56% of BV families in the BMT (n = 15) group were polyclonal and 36% were still skewed. We also analyzed the T-cell repertoire of 15 BM donors (data not shown). We found, as expected, a high level of polyclonality (89%) and very few skewed families in age-matched healthy donors. Taken together, this indicates that after 2 years following transplantation, patients receiving CB transplants had a fully reconstituted polyclonal αβ T-cell repertoire.
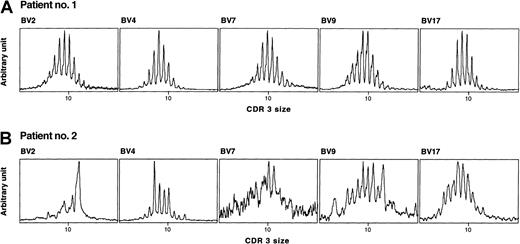
Representative examples of TCRBV CDR3 size distribution in 2 age-matched HSC recipients.
cDNA was amplified in PCR reactions primed by one BV subfamily and a BC-specific fluorescent primer. Patient no. 1 (age at transplantation, 17.7 years) was studied at 26 months after CBT, without episode of GVHD, had totally polyclonal BV families and a high TREC value (11 614/μg CD3+ DNA). Patient no. 2 (age at transplantation, 16.5 years), studied at 24 months after BMT, after an episode of acute GVHD, had several TCRBV disturbances (BV2, BV4, and BV9), an undetectable family (BV7), and a low TREC value (389/μg CD3+ DNA). Both patients were treated for acute lymphoblastic leukemia.
Representative examples of TCRBV CDR3 size distribution in 2 age-matched HSC recipients.
cDNA was amplified in PCR reactions primed by one BV subfamily and a BC-specific fluorescent primer. Patient no. 1 (age at transplantation, 17.7 years) was studied at 26 months after CBT, without episode of GVHD, had totally polyclonal BV families and a high TREC value (11 614/μg CD3+ DNA). Patient no. 2 (age at transplantation, 16.5 years), studied at 24 months after BMT, after an episode of acute GVHD, had several TCRBV disturbances (BV2, BV4, and BV9), an undetectable family (BV7), and a low TREC value (389/μg CD3+ DNA). Both patients were treated for acute lymphoblastic leukemia.
Thymic function and lymphocyte phenotypes in patients in the CBT and BMT groups
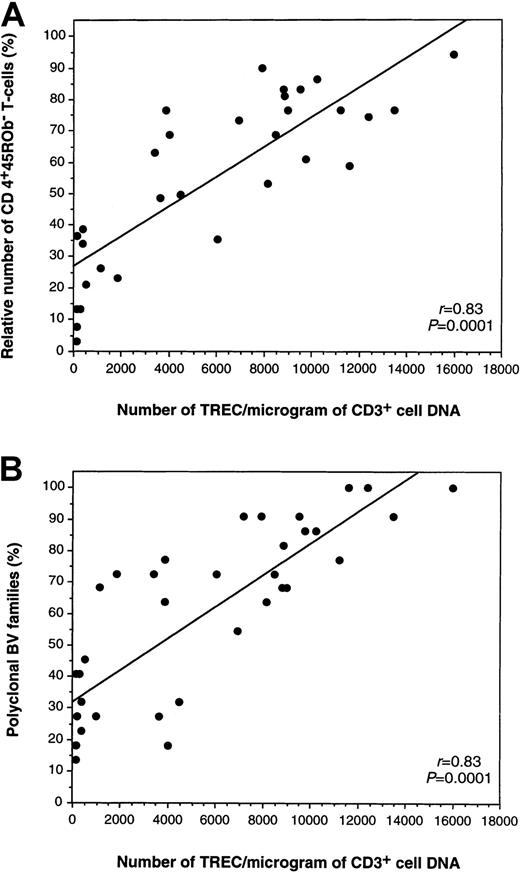
To directly estimate the ability of the thymus to produce new T cells, we measured the levels of TREC found in CD3+peripheral blood cells using quantitative PCR.12 In our hands, mean (SD) value for TREC per microgram CD3+ DNA was 15 428 (6248) for CB (n = 19) and 4138 (2705) for healthy young donors (n = 22), consistent with values from literature.12 Pretransplantation and early posttransplantation TREC levels (data not shown) were comparable in both groups. At 1 year, mean TREC values also reached similar levels of 3191 versus 3935/μg CD3+ cell DNA in the CBT and BMT groups (Table 4). Notably, at our latest follow-up, mean TREC values were significantly higher in the CBT group (n = 8) compared to age- and GVHD-matched BMT patients (n = 15), reaching values of 10 982 versus 6900/μg CD3+ cell DNA (P = .05; Table 4). One year after transplantation onward there was a positive correlation between the number of TRECs and the relative number of naive phenotype CD3+CD4+CD45ROb− T cells (r = 0.83, P = .0001; Figure2A), as well as with the percentage of polyclonal BV families (r = 0.83, P = .0001; Figure 2B). Although there was a trend toward a higher number of CD4+ naive T cells in the CBT group, this was not significant using the nonparametric Mann-Whitney U test. The only difference regarding phenotypes was the higher number of CD8+ activated (CD3+CD4−DR+) and memory (CD3+CD4−45ROb+) T cells in the BMT group, consistent with lower TREC values in this graft setting (Table 4).
Correlations between the number of TRECs, the relative number of CD4+ naive T-cells, and TCRBV diversity 1 year or more after transplantation.
(A) Correlations between the number of TREC/μg CD3+ DNA (x-axis) and relative number of CD4+ naive T cells (y-axis), and (B) between the number of TREC/μg CD3+ DNA (x-axis) and TCRBV diversity expressed as percentages of polyclonal BV families in the same sample (y-axis).
Correlations between the number of TRECs, the relative number of CD4+ naive T-cells, and TCRBV diversity 1 year or more after transplantation.
(A) Correlations between the number of TREC/μg CD3+ DNA (x-axis) and relative number of CD4+ naive T cells (y-axis), and (B) between the number of TREC/μg CD3+ DNA (x-axis) and TCRBV diversity expressed as percentages of polyclonal BV families in the same sample (y-axis).
Effects of GVHD on immune reconstitution
Acute and chronic GVHD are major factors affecting IR after allogeneic HSCT.15 To study this aspect we combined all recipients according to the occurrence of GVHD. At 1 year after transplantation, patients with acute GVHD (n = 9) had more skewed BV families (63% versus 41%, P = .04), reflecting peripheral T-cell expansions, than the patients without acute GVHD (n = 11). Lymphocyte cell surface marker and ex vivo thymic function analysis clearly showed the impact of acute GVHD on IR, even in relatively mild forms because no patient had grade III GVHD (Table5 and data not shown). At 1 year after transplantation, patients with a past episode of acute GVHD had a lower mean absolute lymphocyte (1454 versus 2681/μL, P = .02), CD3+CD4+CD28+ (197 versus 576/μL,P = .03), and naive CD3+CD4+45ROb− T-cell count (128 versus 407/μL, P = .10). CD19+ B-cell count (753 versus 239/μL, P = .01) and B-cell populations expressing the CD5+ (292 versus 96/μL,P = .05) or the CD27− markers (658 versus 218/μL, P = .01) were significantly higher in the group without acute GVHD. At 1 year after graft this effect on thymic function persisted (6755 versus 1231/μg CD3+ cell DNA,P = .01).
The impairment of IR by chronic GVHD was also clearly apparent because patients with chronic GVHD (n = 11) had, 1 year after graft, significantly lower TREC levels (2233 versus 6192/μg CD3+ cell DNA, P = .02) and more skewed BV families (60% versus 38%, P = .06) than patients without chronic GVHD (data not shown).
Discussion
Cord blood cells have been used for more than 10 years, with an increasing number of clinical indications, as a source of HSCs from sibling and unrelated donors.1,2 CBT has been associated with a reduced risk of developing severe GVHD,3 even when cells from partially HLA-mismatched donors are used. The lower risk of GVHD is commonly associated with the functional and phenotypic immaturity of CB cells.4 However, it is still unclear what the impact of such naive CB T cells is on posttransplantation IR, especially in the long-term on relapse rate and late side effects.
To date, relatively few studies have specifically described the state of a recipient's immune system after CBT.16-20 One of the main findings reported was an early recovery of NK and B-cell compartments19 especially for CD19+CD5+ subsets.21 Normal lymphocyte counts can be achieved during the first 6 months after transplantation, especially in the case of genoidentical BMT in children15,22; however, a closer analysis of the diversity of T-cell populations revealed the considerably longer delay required for complete T-cell recovery, in line with our observations.23-26 The groups of patients were carefully matched for age distribution and GVHD grade, 2 main factors affecting IR. Certain factors were present in the CBT group possibly impairing IR, such as a more advanced disease status at the time of transplantation, a more frequent irradiation to the thymic area, and T-cell depletion with antithymocyte globulin before transplantation.
Powerful techniques are now available, in addition to lymphocyte phenotyping, to directly assess the quality of IR. Spectratyping8 and Immunoscope9 are particularly suitable approaches in defining the diversity of αβ T cells. These techniques have evidenced the gradual improvement and the long delay in reconstituting a broad T-cell repertoire after allogeneic HSCT, especially if GVHD occurred.23-25 During the first 6 months after transplantation, reconstitution of the T-cell compartment by peripheral expansion restores T-cell number without reconstituting the full diversity of the TCR repertoire.7,27,28 This highlights the crucial role of the thymus for a durable reconstitution of T-cell compartments by diversifying the TCR repertoire and repairing disturbed TCR profiles.25 There are some limitations in using naive phenotype alone as a measurement of thymic function because a subset of CD8+CD45RA+ T cells are functionally memory T cells.29 Although TCRBV diversity reflects a broad naive T-cell repertoire,30 it is possible now to directly assess thymic function ex vivo by the TREC assay, which quantifies a stable, nonreplicating episomal DNA by-product of the TCR rearrangement process.10,31 Peripheral blood TREC levels could be theoretically affected both by changes in thymic output and by the division history of the cells. Because patient groups in this study are matched for GVHD grade distribution and are similar in a number of posttransplantation infectious complications that could both lower TREC values through peripheral stimulation, we may consider that TREC analysis will truly reflect here the potential of thymic function after engraftment. Indeed, we observed correlations between TREC and naive T-cell phenotype or diverse TCRBV repertoire (Figure 2). High TREC values in the CBT group could be associated with a persistent thymic rebound, a situation described after chemotherapy in children32 and in adults infected with human immunodeficiency virus.33
The data presented here could be interpreted in several ways. First, we confirmed the long time required to achieve broad TCR repertoire, even in the case of genoidentical non–T-depleted BMT. In fact, activated memory CD8+ T cells were still expanded in the periphery 2 years after transplantation in the BMT group as shown by phenotyping (Table 4) and Immunoscope (Table 3). We showed the persistent impact of acute and chronic GVHD, even clinically mild, not only on T-cell immune recovery,34 but also on the reconstitution of CD19+CD5+ B-cell compartments (Table 5). This is relevant to the analysis of previous reports describing expansion of such immature B cells after CBT21 and emphasizes the need of matching precisely for GVHD when comparing different HSCT situations. The main finding in this study is the high degree of T-cell diversity in the long-term after CBT ascertained independently by 2 different methods, Immunoscope and TREC assay. Interestingly, although we observed an overall correlation between TREC values and naive CD4+ T cells (Figure 2A), phenotypic comparisons showed a trend toward higher naive CD4+ T- and B-cell counts in the CBT group compared to the BMT group although this did not achieve statistical significance. This indicates that even in a relatively small series of patients, direct measurement of thymic function and T-cell diversity could be more sensitive and informative than cell surface markers alone. This suggests that TCR repertoire and TREC analysis could be incorporated routinely in the immune monitoring of HSCT. Indeed, the value of TREC as a predictive factor of T-cell IR has recently been outlined in the case of autologous HSCT.12
Finally, what factors underlie the reason long-term recovery of T-cell immunity could be so effective and complete after CBT? Even if subclinical differences in graft-versus-thymic effects cannot be ruled out between the 2 groups, other properties of CB cells could also be considered. One essential aspect to ponder is that the mature T cells in CB are all naive, with none of the memory T cells present in non-T cell-depleted BM. This would account for a less skewed TCR repertoire in CBT due to the lack of peripheral expansion of memory T cells after transplantation. The long-lasting delay of donor-derived naive T-cell production may be caused by the time needed to regenerate the thymic microenvironment and for effective thymic homing of hematopoietic progenitors. Evidence indicates that the characteristic properties of T-lymphoid progenitors in CB differ from BM CD34+ fractions in nonobese, diabetic, severe combined immunodeficiency fetal thymus organ cultures.35 At least 1 of 500 of CB-derived CD34+ cells is a T-cell progenitor, which is about 5-fold more than in BM and T-cell differentiation to CD4+CD8+ cells from adult BM CD34+fraction is also less efficient than from CB CD34+ fraction (10% versus 45%). In addition, other hematopoietic progenitors in CB could have characteristic properties; for example, dendritic cell36 or endothelial cell precursors37 38might contribute to improve homing properties in CBT.
In conclusion, these results indicate that despite the much lower number of CD34+ cells infused, recovery of normal T-lymphocyte diversity in CBT recipients was comparable, or even better, to that observed after HLA identical sibling BMT matched for age and GVHD. Particular properties of lymphoid progenitors in CB might favor a greater thymic contribution and prompt a more durable long-term reconstitution of the TCR diversity. These data provide an experimental rationale to the favorable clinical outcome of CBT once engraftment and hematologic recovery have been achieved.
The skillful technical assistance of P. Baret, D. Meret, and X. Fund is greatly acknowledged.
Supported by grants from the Finnish Medical Foundation, the Pediatric Research Foundation, the Blood Disease Research Foundation, Finland and Fondation Hôpital Saint-Louis (K.T.), Fondation de France (E.C.), Leukemia and Lymphoma Society of America translational research grant 6540-00 and AMFAR grant 02680-28-RGV (D.D.) and EUROCORD II (QLRT 1999-0380), EFG, LNCC, AP-HP (PHRC AOM97093), and FRM (ARS 2000) institutional grants.
K.T., E.C., and C.D. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Antoine Toubert, Laboratoire d'Immunologie et d'Histocompatibilité, Centre G. Hayem, Hôpital Saint-Louis, 1, Avenue Claude Vellefaux, F-75475 Paris, CEDEX 10, France; e-mail: toubert@histo.chu-stlouis.fr.