Blockade of B7/CD28 costimulation allows human haploidentical bone marrow transplantation without graft-versus-host disease. This study shows that blockade of B7/CD28 in anergizing mixed lymphocyte reaction (MLR) cultures of peripheral blood mononuclear cells results in the generation of alternatively activated macrophages (AAMΦ). In contrast, priming MLR cultures result in generation of classically activated macrophages (CAMΦ). AAMΦ had enhanced expression of CD14, major histocompatibility complex class II, and CD23; produced alternative macrophage activation-associated CC-chemokine 1 (AMAC-1) chemokine; and displayed increased phagocytotic activity but decreased ability for antigen presentation. Suppression subtractive hybridization revealed that although AAMΦ had undergone terminal maturation and differentiation, they entered a distinct gene expression program as compared with CAMΦ and selectively expressed β2-microglobulin, lysozyme, ferritin heavy and light chain, and the scavenger receptors macrophage mannose receptor and sortilin. Anergic T cells isolated from cultures that led to the development of AAMΦ produced low amounts of interleukin-2 (IL-2), IL-4, and interferon-γ, but high amounts of IL-10. Addition of anti–IL-10 neutralizing monoclonal antibody in anergizing cultures reversed the functional characteristics of AAMΦ, indicating that at least one mechanism involved in the generation of AAMΦ was mediated by IL-10. Importantly, when added in MLR cultures, AAMΦ suppressed T-cell responses. Therefore, besides direct inhibition of T-cell costimulation, blockade of B7/CD28 may facilitate induction of T-cell unresponsiveness by generating AAMΦ. Because in healthy individuals, AAMΦ are found in the placenta and lung, where they protect from unwanted immune reactivity, the results suggest that AAMΦ may play a critical role in the induction of transplantation tolerance.
Introduction
The success of allogeneic bone marrow transplantation (ABMT) is limited by the restricted donor pool and the morbidity of graft-versus-host disease (GVHD). Donor T cells present in the graft are mediators of GVHD. These donor T cells recognize host alloantigens and initiate a cascade of pathophysiologic events, which result in the development of the clinical syndrome of GVHD.1 Although GVHD can be prevented or ameliorated by nonspecific immunosuppression or T-cell depletion, these approaches are associated with increased infection and relapse rates and, in the case of T-cell depletion, with increased graft failure and lymphoproliferative disease.2
Studies in murine and subhuman primate models3-7 and more recently a clinical trial in humans8 have provided great promise that induction of T-cell anergy by blockade of costimulation may prevent GVHD without nonspecific immunosuppression or T-cell depletion. Anergy in vitro and its in vivo counterpart, tolerance, are defined as the inability of viable, antigen-specific T cells to produce interleukin-2 (IL-2) or clonally expand when rechallenged with fully competent antigen-presenting cells (APCs) delivering T-cell receptor (TCR) and costimulatory signals.9 Anergy can be induced when T cells are stimulated via TCR without costimulation or IL-2.9,10 The B7-1/B7-2:CD28 pathway is the dominant costimulatory pathway and has a critical role in the regulation of T-cell viability, cytokine production, clonal expansion, and effector function.11 The in vivo biologic significance and the clinical relevance of this pathway have been validated in multiple models for allogeneic renal, cardiac, and bone marrow transplantation.3-5 We have shown that ex vivo blockade of this pathway can inactivate the ability of donor haploidentical T cells to recognize host alloantigens, thereby significantly reducing the incidence of GVHD in patients undergoing haploidentical bone marrow transplantation.8
T lymphocytes are the mediators of immunity, but their function is under the control of APCs. Monocytes, the most abundant APCs, develop from hematopoietic precursors, enter the bloodstream, and follow divergent differentiation programs to form the wide variety of morphologically and functionally distinct macrophages. The activation and differentiation program of monocytes into macrophages is determined by the presence of specific cytokines in selected microenvironments in vivo or in culture supernatants in vitro. The balanced macrophage-activation hypothesis states that in parallel with the Th1/Th2 paradigm, macrophages can be divided into “classically” activated (CAMΦ) and “alternatively” activated macrophages (AAMΦ).12-14 Classic activation of macrophages is induced by proinflammatory cytokines such as interferon (IFN)–γ and bacterial lipopolysaccharide (LPS), whereas alternative activation of macrophages is induced by anti-inflammatory agents such as IL-4, IL-10, IL-13, transforming growth factor (TGF)–β, and glucocorticoids. AAMΦ preferentially express the receptors of innate immunity with broad specificity for foreign antigens, such as the mannose receptor15 and other scavenger receptors,16-18 and high levels of CD14, which has an important role in phagocytosis.19,20 In contrast to CD64 (FcγRI), CD32 (FcγRII), and CD16 (FcγRIII), expressed by CAMΦ, AAMΦ express high levels of CD23 (FcεRII) and major histocompatibility complex (MHC) class II. They have enhanced capacity for phagocytosis and scavenging, but decreased capacity for nitric oxide (NO) and O2 production and antigen processing.21,22 Moreover, they lack expression of the inflammatory macrophage cytokine macrophage inflammatory protein 1α (MIP-1α) but express the novel β-chemokine alternative macrophage activation-associated CC-chemokine 1 (AMAC-1) (also termed DC-CK1).23,24 Functionally, AAMΦ are immunosuppressive and inhibit mitogen-induced proliferation of CD4+ T cells.13,25 In healthy individuals, AAMΦ are preferentially found in placenta and lung, where they function to protect from unwanted immune reactivity,26-28 and in wound tissue during the healing phase, where they down-regulate inflammatory reactions.29These observations strongly suggest that mechanisms that regulate the alternative activation program of macrophages have an important role in the maintenance of the immune quiescent state in vivo.
In our recent clinical study, histoincompatible bone marrow grafts infused to patients after ex vivo blockade of the B7/CD28 pathway with cytotoxic T lymphocyte antigen 4 (CTLA4)–Ig contained donor T cells that exceeded by 1 or 2 orders of magnitude the suggested threshold dose for minimizing GVHD.8 These patients had additional high-risk factors for GVHD, including mismatching of the donor and recipient, recurrent or persistent disease, irradiation-based conditioning, and cytomegalovirus positivity.8 Strikingly, the incidence of GVHD by this treatment approach not only was not increased, but was significantly reduced as compared with that observed after classic treatments. To determine whether besides inhibition of CD28 signals, additional mechanisms may contribute to the immunosuppression of host-specific donor T cells by this treatment approach, we examined the immunophenotypic, molecular, and functional properties of macrophages present in anergizing mixed lymphocyte reaction (MLR) cultures of peripheral blood mononuclear cells (PBMCs).
Here we show that blockade of the B7/CD28 pathway results in the generation of AAMΦ, which have high expression of MHC class II, CD14, and scavenger receptors and increased phagocytotic capacity. Although these macrophages have increased phagocytotic activity, they have reduced capacity to process antigen and they suppress T-cell responses when added in MLR cultures. These results suggest that besides a direct inhibitory effect on T-cell activation, blockade of B7/CD28 costimulation may facilitate T-cell immunosuppression by generating AAMΦ.
Materials and methods
Mixed lymphocyte reactions
PBMCs were prepared from buffy coat leukapheresis residues obtained from the blood banks of the Dana-Farber Cancer Institute and the Brigham & Women's Hospital. Mononuclear cells were isolated by Ficoll/paque (Amersham-Pharmacia Biotech, Piscataway, NJ) gradient centrifugation. Stimulator PBMCs were irradiated at 3300 cGy and were cultured in 1:1 ratio with responder PBMCs in medium containing RPMI 1640 with 10% heat-inactivated fetal calf serum (FCS), 2 mM glutamine, 1 mM sodium pyruvate, penicillin (100 U/mL), streptomycin (100 U/mL), and gentamicin sulfate (5 μg/mL). Where indicated, enrichment for CD4+ cells was done by positive selection using anti-CD4 MicroBeads and MACS columns (Miltenyi Biotec, Auburn, CA). For proliferation assays, 105 responder cells per well were cultured with 105 irradiated stimulator cells per well in 96-well round-bottom plates, and 3H-thymidine incorporation was assessed for the last 16 hours of the indicated total interval of culture. CTLA4-Ig,8 anti–B7-1, and anti–B7-2 monoclonal antibodies (mAbs) (Genetics Institute, Cambridge, MA) were added at a final concentration of 10 μg/mL. Anti–IL-10 neutralizing mAb was purchased from R&D Systems (Minneapolis, MN) and was used at a final concentration of 5 μg/mL. For secondary MLR cultures, responder PBMCs were initially cultured in primary MLR with irradiated stimulators at 1:1 ratio in 24-well plates for 7 days, and subsequently viable cells were recovered by Ficoll/paque separation and restimulated with new irradiated stimulators in 96-well round-bottom plates. Tuberculin-purified protein derivative (PPD) loading of macrophages and antigen presentation experiments were done as described previously.30 Briefly, CD14+ cells were isolated from the indicated cultures by positive selection using CD14 MicroBeads and MACS columns (Miltenyi Biotec) and loaded with PPD (25 ng/mL) by incubating at 37°C for 3 hours during constant end-to-end mixing. Loaded APCs were then irradiated at 3300 cGy, excess peptide was removed, and cells at 105/well were cultured with autologous T cells at 1:1 ratio in 96-well plates. Culture was continued for 5 days, and 3H-thymidine incorporation was assessed for the last 18 hours. For RNA preparation and for collection of culture supernatants, 1 × 106 responder cells were cocultured with 1 × 106 irradiated stimulators in 24-well plates for the indicated time intervals and in the presence of medium either alone or with the indicated factors.
Immunofluorescence and flow cytometry
For immunophenotyping, cells were isolated from the various culture conditions at the indicated time intervals and examined for the expression of surface markers using mouse antihuman mAbs specific for MHC class II (I3)–phycoerythrin (PE), CD14 (Mo2)-fluorescein isothiocyanate (FITC), CD14(Mo2)-PE, CD23-PE, CD32-PE, and CD16-FITC (all from Coulter, Hialeah, FL); CD64-PE (DAKO, Carpinteria, CA); CD163 (Research Diagnostics, Flanders, NJ); and CD83-PE (BD/Pharmingen, Toryanna, CA). Anti–B7-1 and anti–B7-2 mAbs were developed in our laboratory and were described previously.31 Irrelevant isotype-matched antibodies were used as negative controls. Cells were initially stained with CD14-specific mAb, and expression of MHC class II, CD23, CD32, CD16, CD64, and CD163 on CD14+ cells was examined by double staining using the appropriate combination of FITC- and PE-conjugated mAbs. Samples were analyzed in a Coulter Elite flow cytometer (Coulter Electronics, Hialeah, FL), and data were acquired in listmode files. At least 5000 positive events were measured for each sample.
Carboxyfluorescein diacetate succinimidyl ester labeling and analysis
Carboxyfluorescein diacetate succinimidyl ester (CFSE) was purchased from Molecular Probes (Eugene, OR) and was reconstituted and used according to the manufacturer's protocol. Briefly, CFSE was diluted to a final concentration of 1 μM, and cells at a concentration of 107/mL were incubated at 37°C for 15 minutes in nonsupplemented RPMI (Gibco-BRL). The labeled cells were washed once with phosphate-buffered saline (PBS) containing 10% FCS, washed 3 times with PBS, and set to the indicated final concentration before initiation of MLR cultures.
FITC-dextran uptake
Uptake of FITC-dextran (molecular weight 70 000; Sigma, St Louis, MO) was evaluated as described previously.32Briefly, CD14+ cells isolated from the indicated cultures were resuspended at 1 × 106 cells/mL in RPMI containing 10% FCS and 25 mM HEPES and incubated with FITC-dextran (1 mg/mL) for the indicated time intervals. The quantitative uptake of FITC-dextran was determined by flow cytometry.
Cytokine enzyme-linked immunosorbent assay
Culture supernatants were harvested at various time intervals of primary and secondary cultures and analyzed by enzyme-linked immunosorbent assay (ELISA) for cytokine levels of IL-2, IFN-γ, IL-4, IL-10, and TGF-β (R&D Systems). Assessment of total NO was done by colorimetric enzymatic assay according to the manufacturer's protocol (R&D Systems).
Reverse transcription–polymerase chain reaction
Before RNA extraction, CD4+ and CD14+ cells isolated from MLR cultures by positive selection using anti-CD4 or -CD14 MicroBeads and MACS columns (Miltenyi Biotec), RNA was prepared by the RNAsol kit (Tel-Test, Friendswood, TX), and 2 μg RNA was used for reverse transcription as described previously.33 Polymerase chain reaction (PCR) amplification of cDNA was performed using specific oligonucleotides for IL-10, 5′-AAGCTGAGAACCAAGACCCAGACATCAAGGCG-3′ (forward) and 5′-AGCTATCCCAGAG CCCAGATCCGATTTTGG-3′ (reverse); glyceraldehyde-3-phosphate dehydrogenase (G3PDH), 5′-GTGAAGGTCGGAGTCAACG-3′ (forward) and 5′-ACCAGGAAATGAGCTTGACAAA-3′ (reverse); and AMAC-1, 5′-AAGCCCCAGCTCACTCTGAC-3′ (forward) and 5′-ACCTGGCTTGGGGCACT-3′ (reverse). Fifteen microliters of each of the final reaction products was analyzed by electrophoresis on 2% agarose gel containing ethidium bromide.
Suppression subtractive hybridization
Suppression subtractive hybridization34 was done with the use of the PCR-select cDNA subtraction kit (Clontech, Palo Alto, CA) according to the manufacturer's protocol. Briefly, cDNA from CD14+ cells isolated from anergizing cultures was used as “tester” (in which differential gene expression is searched), and cDNA from CD14+ cells isolated from priming cultures was used as “driver” (to which gene expression is compared). Tester cDNA was ligated to 2 different cDNA adaptors, tester and driver cDNAs were hybridized twice, and hybrid sequences were removed. Consequently, the remaining unhybridized cDNAs represented genes that are expressed in the tester but are absent from the driver mRNA. Subsequently, only unhybridized molecules containing adaptor sequences (and therefore originating from tester cDNA) were exponentially amplified by PCR using adaptor-specific primers. After a subtracted cDNA library was obtained, confirmation that individual clones were indeed differentially expressed was done by differential screening using the PCR-select differential screening kit (Clontech), as indicated by the manufacturer's protocol.
Results
Blockade of B7/CD28 costimulation results in the generation of macrophages with immunophenotypic markers of AAMΦ
Freshly isolated PBMCs from HLA-mismatched individuals were cultured in 1:1 ratio with irradiated PBMCs as stimulators with either medium, CTLA4-Ig, anti–B7-1 plus anti–B7-2 mAbs, control Ig, or control mAb. At various time intervals of culture (24 hours to 7 days), cells were isolated and rechallenged with the original stimulators to determine the time interval of primary culture that resulted in hyporesponsiveness on rechallenge. Response of primary MLR was reduced by CTLA4-Ig, consistent with previous observations,8 35and more significantly by the combination of anti–B7-1 and anti–B7-2 mAbs (data not shown). Also consistent with previous observations, treatment of primary culture with either CTLA4-Ig or the combination of anti–B7-1 plus anti–B7-2 mAbs also resulted in hyporesponsiveness on rechallenge (data not shown). As reported previously, control Ig or control mAb neither reduced primary MLR nor resulted in unresponsiveness on rechallenge (data not shown). Hyporesponsiveness on rechallenge was more significant when primary culture was conducted for 3 days or longer, and these time intervals of primary cultures were used for comparative studies in the subsequent experiments.
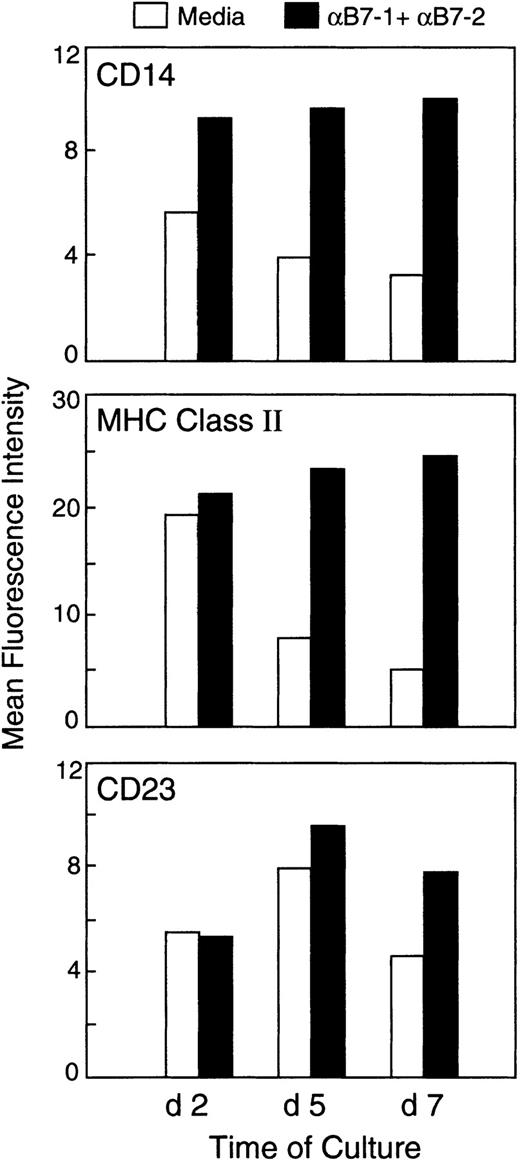
To determine whether blockade of the B7/CD28 pathway with either anti-B7 mAbs or CTLA4-Ig influenced the maturation and differentiation program of monocytes present in the anergizing cultures, we first examined surface markers that had been previously determined to be differentially expressed in classically and alternatively activated macrophages.13,14 We examined the expression of CD14, MHC class II, CD23, CD32, CD64, CD16, and CD163. At 48 hours of culture, there was a detectable difference in the expression of CD14 but not in the expression of any of the other markers (Figure1, top panel; and data not shown). At days 5 and 7, a significantly enhanced expression of CD14 and MHC class II was observed on monocytes isolated from cultures treated with anti-B7 mAbs as compared with monocytes isolated from cultures with medium alone (Figure 1, top and middle panels) or with control mAbs (data not shown). Similar results were observed when CTLA4-Ig was used instead of the combination of anti–B7-1 plus anti–B7-2 mAbs to induce anergy (data not shown). Further analysis of markers expressed on CD14+ cells in each population revealed that CD14+ cells isolated from anergizing cultures treated with either the combination of anti–B7-1 plus anti–B7-2 mAbs or with CTLA4-Ig had increased expression of CD23 (Figure 1, lower panel; and data not shown). The expression levels of CD64 and CD32 were only slightly lower on CD14+ cells isolated from anergizing cultures than on CD14+ cells isolated from cultures with medium alone (data not shown). In contrast to previous reports,18 no detectable differences were observed in the expression of CD16 and CD163 on the CD14+ cells in the different culture conditions (data not shown).
Differential expression of immunophenotypic markers in macrophages isolated from anergizing and priming MLR cultures.
PBMCs from HLA-mismatched individuals were cultured in 1:1 ratio with irradiated PBMCs as stimulators with either medium or anti–B7-1 plus anti–B7-2 mAbs. At various time intervals of culture (24 hours to 7 days), cells were isolated and examined for the expression of CD14 using anti–CD14-FITC conjugated mAb. CD14+ cells were subsequently analyzed for coexpression of MHC class II or CD23, as described in “Materials and methods.” An identical pattern of results as shown here was observed when control mAb was used instead of medium alone in primary culture. Results show the findings at 3 of 6 time points examined and are representative of 3 experiments.
Differential expression of immunophenotypic markers in macrophages isolated from anergizing and priming MLR cultures.
PBMCs from HLA-mismatched individuals were cultured in 1:1 ratio with irradiated PBMCs as stimulators with either medium or anti–B7-1 plus anti–B7-2 mAbs. At various time intervals of culture (24 hours to 7 days), cells were isolated and examined for the expression of CD14 using anti–CD14-FITC conjugated mAb. CD14+ cells were subsequently analyzed for coexpression of MHC class II or CD23, as described in “Materials and methods.” An identical pattern of results as shown here was observed when control mAb was used instead of medium alone in primary culture. Results show the findings at 3 of 6 time points examined and are representative of 3 experiments.
Because B7-1 (CD80) is induced and the very low constitutive levels of B7-2 (CD86) are up-regulated on monocytes after activation,36 37 we examined whether CD14+cells isolated from the anergizing and priming cultures had different expression of B7-1 or B7-2. At all time intervals of culture tested, equivalent levels of B7-1 and B7-2 were induced on CD14+cells in the anergizing and priming cultures (data not shown). Analysis of the dendritic cell–specific marker CD83 showed no detectable levels of CD83 expression in our cultures at any time point tested (data not shown). This is most likely due to the specific culture requirements for dendritic cell growth, which were not established in these classic MLR cultures.
Distinct cytokine profile in anergizing cultures contributes to the generation of AAMΦ
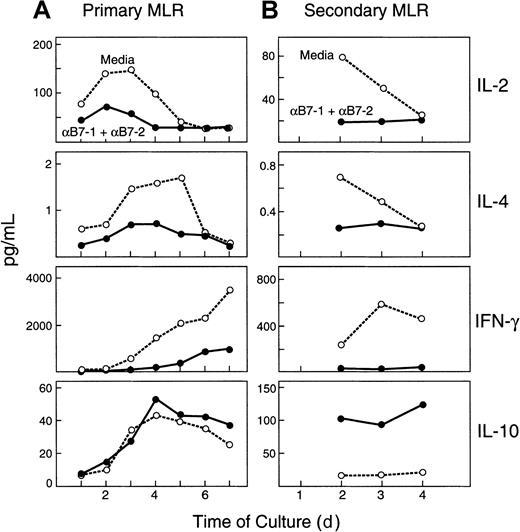
These observations suggested that several immunophenotypic features previously reported to be present in AAMΦ were detected in CD14+ cells isolated from anergizing MLR cultures. Previous studies have suggested that the differentiation program of macrophages is significantly influenced by cytokines present in distinct microenvironments in vivo or in cultures in vitro.13,14,32,38,39 Specifically, it has been shown that classic activation of macrophages is induced by proinflammatory cytokines such as IFN-γ, whereas alternative activation of macrophages is induced by anti-inflammatory cytokines such as IL-4 and IL-10.13,14 It is well documented that the B7/CD28 costimulatory pathway dramatically augments cytokine production by T cells, and blockade of this pathway leads to inhibition of cytokine production.40 Moreover, studies in a different experimental system have shown that although production of IL-2 and IFN-γ is blocked in anergizing MLR cultures, production of IL-10 is augmented.41 Therefore, we examined the expression of cytokines at various time intervals of our MLR cultures. Because the functional characteristics of anergic cells are identified on rechallenge, expression of cytokines was also assessed during rechallenge (secondary) cultures. Blockade of the B7/CD28 pathway with the combination of anti–B7-1 plus anti–B7-2 mAbs during primary MLR resulted in dramatic inhibition of IL-2, IL-4, and IFN-γ production in both primary and secondary cultures (Figure2A,B). In contrast, addition of anti–B7-1 plus anti–B7-2 mAbs during primary MLR resulted in increased production of IL-10. Although there was only a slight increase in IL-10 levels during primary culture in the presence of anti–B7-1 plus anti–B7-2 mAbs (Figure 2A), there was a dramatic increase in IL-10 levels in rechallenge, secondary cultures of cells that had been treated with anti-B7 mAbs during primary MLR (Figure 2B). TGF-β was not detected in any of the conditions of primary or secondary MLR cultures (data not shown). The observation that IL-4 production was inhibited by blockade of B7-mediated costimulation with anti-B7 mAbs provides an explanation for the lack of significant down-regulation of CD32 and CD64 on macrophages isolated from anergizing cultures. IL-4 is the critical factor that mediates down-regulation of CD32 and CD64, and this effect is counteracted by IFN-γγ.21 Therefore, the presence of IFN-γ in priming cultures in medium prevents IL-4–mediated down-regulation of these receptors, whereas in anergizing cultures, none of these cytokines is present to mediate an effect.
Distinct cytokine profiles in anergizing and priming MLR cultures.
(A) For primary MLR, responder cells were cocultured in 1:1 ratio with irradiated stimulators in the presence of medium alone or with anti–B7-1 plus anti–B7-2 mAbs. At the indicated time intervals of culture, supernatants were collected and cytokine levels were analyzed by ELISA. (B) For secondary MLR, PBMCs isolated from primary cultures were rechallenged in 1:1 ratio with irradiated original stimulators, and culture supernatants were collected at the indicated time intervals and analyzed for cytokine concentrations by ELISA. Results are representative of 3 experiments. A similar pattern of results as shown here was observed when control mAb was used instead of medium alone in primary MLR. Lowest limits of detection were as follows: IL-2, 6 pg/mL; IL-4, 0.13 pg/mL; IFN-γ, 8 pg/mL; IL-10, 3.9 pg/mL.
Distinct cytokine profiles in anergizing and priming MLR cultures.
(A) For primary MLR, responder cells were cocultured in 1:1 ratio with irradiated stimulators in the presence of medium alone or with anti–B7-1 plus anti–B7-2 mAbs. At the indicated time intervals of culture, supernatants were collected and cytokine levels were analyzed by ELISA. (B) For secondary MLR, PBMCs isolated from primary cultures were rechallenged in 1:1 ratio with irradiated original stimulators, and culture supernatants were collected at the indicated time intervals and analyzed for cytokine concentrations by ELISA. Results are representative of 3 experiments. A similar pattern of results as shown here was observed when control mAb was used instead of medium alone in primary MLR. Lowest limits of detection were as follows: IL-2, 6 pg/mL; IL-4, 0.13 pg/mL; IFN-γ, 8 pg/mL; IL-10, 3.9 pg/mL.
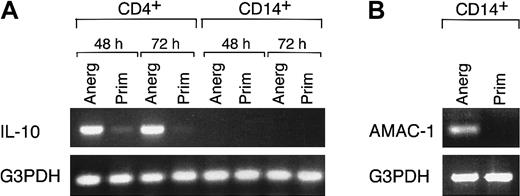
It has been proposed that IL-10 leads to the generation of AAMΦ,32,38,39 whereas other studies have shown that IL-10 is produced by AAMΦ.25 Therefore, we sought to identify the cell population that produced IL-10 in our system. CD4+ and CD14+ cells were isolated by positive selection from rechallenge, secondary cultures in which the highest amounts of IL-10 were detected, and then examined for IL-10 mRNA expression by semiquantitative reverse transcription (RT)–PCR. As shown in Figure 3A, significant amounts of IL-10 mRNA were detected in CD4+ populations isolated from 48 and 72 hours of secondary culture of cells that had been treated with anti–B7-1 plus anti–B7-2 mAbs during primary MLR. At the same time intervals, CD4+ cells isolated from priming cultures also produced IL-10, albeit at much lower levels, consistent with the results of the IL-10 protein assessment by ELISA (Figure 2B). In contrast, IL-10 was not produced by CD14+ cells in any culture conditions (Figure 3A). Thus, IL-10 was exclusively produced by CD4+ cells in our experimental system. These observations suggest that blockade of the B7/CD28 pathway in MLR cultures resulted in the generation of IL-10–producing CD4+ T cells, which subsequently influenced the activation and differentiation of macrophages.
IL-10 is produced by CD4+ cells isolated from anergizing MLR cultures.
(A) After the indicated time intervals of rechallenge in secondary MLR cultures, CD4+ and CD14+ cells were isolated by positive selection and examined for expression of IL-10 mRNA by RT-PCR. “Anerg” and “Prim” denote the culture conditions of primary MLR cultures. Results are representative of 4 experiments. (B) CD14+ cells isolated from 48 hours of secondary MLR as described in (A) were examined for expression of AMAC-1 by RT-PCR. Results are representative of 4 experiments.
IL-10 is produced by CD4+ cells isolated from anergizing MLR cultures.
(A) After the indicated time intervals of rechallenge in secondary MLR cultures, CD4+ and CD14+ cells were isolated by positive selection and examined for expression of IL-10 mRNA by RT-PCR. “Anerg” and “Prim” denote the culture conditions of primary MLR cultures. Results are representative of 4 experiments. (B) CD14+ cells isolated from 48 hours of secondary MLR as described in (A) were examined for expression of AMAC-1 by RT-PCR. Results are representative of 4 experiments.
AMAC-1 is a cytokine closely related to MIP-1α.23,24 It has been shown that in contrast to MIP-1α, which is produced by macrophages activated under inflammatory conditions, production of AMAC-1 is a hallmark of alternative activation of macrophages.23 As shown in Figure 3B, RT-PCR analysis of CD14+ cells revealed that AMAC-1 was selectively produced by macrophages isolated from anergizing MLR cultures treated with anti–B7-1 plus anti–B7-2 mAbs, and not by macrophages isolated from priming MLR cultures in medium alone.
AAMΦ isolated from anergizing MLR cultures have increased phagocytotic activity, but reduced ability to present antigen
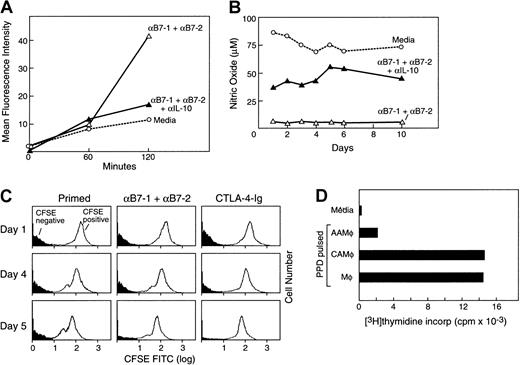
These observations suggested that features previously reported to be present in AAMΦ were detected in CD14+ cells isolated from anergizing MLR cultures. Previous studies have shown that although AAMΦ have reduced capacity to process antigens, they have increased phagocytotic activity.32 38 To determine whether macrophages isolated from anergizing cultures had gained this functional feature of AAMΦ, we examined their phagocytotic ability. CD14+ cells were isolated by positive selection from viable responder PBMCs and were incubated with FITC-dextran for various time intervals. Subsequently, endocytotic capacity of macrophages was determined by flow cytometry. As shown in Figure4A, macrophages isolated from cultures treated with anti–B7-1 plus anti–B7-2 mAbs showed 4 times greater endocytosis of FITC-dextran as compared with cells isolated from priming cultures in medium alone or with control mAb (data not shown). Increased phagocytotic capacity was also observed when CTLA4-Ig was used instead of anti–B7-1 plus anti–B7-2 mAbs in anergizing MLR cultures (data not shown).
Increased phagocytotic activity but decreased ability for antigen processing and antigen presentation in macrophages isolated from anergizing MLR cultures.
(A) CD14+ cells isolated from the indicated culture conditions were incubated with FITC-dextran for various time intervals, and endocytotic capacity was determined by flow cytometry. Results are representative of 3 experiments. (B) Following the indicated conditions and time intervals of MLR cultures, supernatants were isolated and examined for production of NO by colorimetric enzymatic assay. Results are representative of 2 experiments. (C) CFSE-labeled responder PBMCs were cultured with irradiated unlabeled (CFSE-negative) allogeneic stimulators. At various time intervals of culture, labeled CFSE-positive responder and unlabeled CFSE-negative stimulator populations were examined by flow cytometry. Results are representative of 4 experiments. (D) PBMCs from rigorous PPD responder individuals were cultured in 1:1 ratio with irradiated allogeneic PBMCs as stimulators with either medium or anti–B7-1 plus anti–B7-2 mAbs. After 7 days of culture, CD14+ cells were isolated, loaded with PPD, and used to stimulate autologous purified T cells. As controls, freshly isolated CD14+ cells were loaded with PPD and used as stimulators of autologous T cells in the same experiment. Response was determined by 3H-thymidine incorporation for the last 18 hours of the 5-day culture period.
Increased phagocytotic activity but decreased ability for antigen processing and antigen presentation in macrophages isolated from anergizing MLR cultures.
(A) CD14+ cells isolated from the indicated culture conditions were incubated with FITC-dextran for various time intervals, and endocytotic capacity was determined by flow cytometry. Results are representative of 3 experiments. (B) Following the indicated conditions and time intervals of MLR cultures, supernatants were isolated and examined for production of NO by colorimetric enzymatic assay. Results are representative of 2 experiments. (C) CFSE-labeled responder PBMCs were cultured with irradiated unlabeled (CFSE-negative) allogeneic stimulators. At various time intervals of culture, labeled CFSE-positive responder and unlabeled CFSE-negative stimulator populations were examined by flow cytometry. Results are representative of 4 experiments. (D) PBMCs from rigorous PPD responder individuals were cultured in 1:1 ratio with irradiated allogeneic PBMCs as stimulators with either medium or anti–B7-1 plus anti–B7-2 mAbs. After 7 days of culture, CD14+ cells were isolated, loaded with PPD, and used to stimulate autologous purified T cells. As controls, freshly isolated CD14+ cells were loaded with PPD and used as stimulators of autologous T cells in the same experiment. Response was determined by 3H-thymidine incorporation for the last 18 hours of the 5-day culture period.
Intracellular processing of phagocytosed antigen requires enzymatic activation of nitric oxide reductase, which subsequently generates NO and O2.42 It has been shown that although AAMΦ have increased endocytotic capacity, they have reduced ability to process antigen and produce reduced levels of NO.21 22Therefore, we examined whether macrophages differentiated in anergizing MLR cultures had differential ability to produce NO as compared with macrophages differentiated in priming MLR cultures. Supernatants were collected at the indicated days of MLR cultures, and NO levels were assessed. Cultures with anti–B7-1 plus anti–B7-2 mAbs had much lower NO levels as compared with cultures in medium alone (Figure 4B) or with control mAb (data not shown). Addition of CTLA4-Ig, but not control Ig, also resulted in lower NO levels in culture supernatants (data not shown). Taken together, these results indicate that although macrophages generated in anergizing cultures in the presence of anti–B7-1 plus anti–B7-2 mAbs or in the presence of CTLA4-Ig had higher endocytotic activity as determined by FITC-dextran uptake, they had significantly reduced capacity for NO production, indicating a reduced capacity for antigen processing.
To determine the in vivo biologic significance of these findings, we examined whether macrophages in priming and anergizing culture conditions might have differential phagocytotic ability against cell populations in the MLR culture. CFSE-labeled responder PBMCs were cultured with unlabeled allogeneic stimulators. This approach allowed us to discriminate these 2 populations and to follow the fate of each one during culture. Strikingly, culture in the presence of anti–B7-1 plus anti–B7-2 mAbs or with CTLA4-Ig resulted in a significant decrease of the unlabeled (CFSE-negative) stimulator cells as compared with those detected in cultures with medium alone (Figure 4C). This event became detectable after 3 days of culture and was further augmented by day 7. This observation indicates that a higher degree of scavenging and clearance of the unlabeled (CFSE-negative) stimulator cells occurred in anergizing culture conditions.
As mentioned earlier, previous studies have shown that AAMΦ have increased phagocytotic activity but reduced ability to present antigen. Our results indicated that AAMΦ generated in anergizing MLR cultures had increased phagocytotic capacity but reduced capacity to process antigen and produce NO. On the basis of these observations, we examined whether these AAMΦ also had reduced capacity for antigen presentation. To address this question, we used PBMCs isolated from donors known to mount a rigorous response to PPD. Using these PBMCs as responders, we established MLR cultures with either medium alone, B7-1 and B7-2, or control mAb. Subsequently, CD14+ cells were isolated from these cultures, loaded with PPD, and used to stimulate autologous purified T cells. As controls, freshly isolated CD14+ cells were also loaded with PPD and used as stimulators of autologous T cells in the same experiment. As shown in Figure 4D, PPD-pulsed AAMΦ isolated from anergizing MLR cultures induced a diminished response from autologous T cells, as compared with the response generated by CAMΦ isolated from priming MLR cultures and the response generated by freshly isolated control macrophages. These results confirm that macrophages isolated from anergizing MLR cultures have reduced ability for antigen presentation, another functional feature of AAMΦ.
IL-10 has an active role in the generation of AAMΦ in anergizing MLR cultures
Previous studies have shown that IL-10 promotes maturation of monocytes into macrophages with increased phagocytotic capacity and mediates the generation of AAMΦ.13 32 To examine whether IL-10 might be responsible for the enhanced phagocytotic activity observed in macrophages isolated from anergizing cultures, we added anti–IL-10 neutralizing mAb in anergizing MLR cultures treated with anti–B7-1 plus anti–B7-2 mAbs. Culture under these conditions significantly reduced the phagocytotic capacity of macrophages as determined by FITC-dextran uptake (Figure 4A). Moreover, anti–IL-10 neutralizing mAb increased the levels of NO produced in the culture treated with anti–B7-1 plus anti–B7-2 mAbs (Figure 4B). Thus, IL-10 mediates at least one mechanism involved in the generation of macrophages with increased phagocytotic capacity in anergizing MLR cultures. Taken together, all of these results strongly suggest that during induction of T-cell anergy by blockade of the B7/CD28 pathway, a distinct activation program is initiated in macrophages, leading to immunophenotypic and functional properties of AAMΦ.
Differential gene expression in macrophages isolated from anergizing and priming cultures
To examine whether AAMΦ that were generated in anergizing MLR cultures had differential expression of other macrophage-specific genes as compared with CAMΦ generated in priming cultures, we performed suppression subtractive hybridization using PCR-select cDNA subtraction. RNA was extracted from CD14+ cell populations isolated from anergizing and priming cultures, and cDNA was prepared by RT-PCR. cDNA from cells isolated from anergizing cultures was used as “tester” and cDNA from priming cultures was used as “driver” cDNA, and suppression subtractive hybridization was performed as described in “Materials and methods.”34 After a subtracted cDNA library was obtained, confirmation that individual clones were indeed differentially expressed was done by differential screening, using as probes either cDNA prepared from anergizing cultures (forward probe) or cDNA prepared from priming cultures (reverse probe). This approach allows the identification of genes in the subtracted library that are selectively detected by the forward probe and therefore are selectively expressed in the cDNA population isolated from anergizing cultures.
Among 200 recombinant clones tested, 11 clones represented genes that were selectively or differentially expressed in macrophages isolated from anergizing cultures (data not shown). Here, we present a number of genes related to previously determined functional properties on macrophages. Two receptors with known scavenging activity were selectively expressed on macrophages isolated from anergizing cultures:macrophage mannose receptor and sortilin. Macrophage mannose receptor was previously identified as a marker of alternative immunologic macrophage activation.15 This is an important phagocytotic receptor mediating binding and ingestion of microorganisms with surface mannose residues and soluble mannose-containing glycoproteins. In fact, it has been reported that macrophage mannose receptor has the most important role in mediating endocytosis and scavenging by macrophages. It is expressed in alveolar macrophages, and its expression is increased by steroids and inhibited by IFN-γ.15 Sortilin is a type I receptor that is mainly located at the Golgi apparatus and vesicles, but also on the cell membrane. Sortilin functions both as a sorting receptor in the Golgi compartment and as a clearance receptor on the cell membrane.43 As a surface receptor, sortilin scavenges a diverse set of extracellular ligands, such as lipoprotein lipase and neurotensin, and mediates their endocytosis and degradation.43 Therefore, the presence of sortilin is linked to scavenging activity.
Besides these 2 receptors, which are directly linked to the phagocytic and scavenging properties of macrophages, a number of genes previously associated with the maturation and differentiation program of monocytes were differentially expressed between macrophages isolated from anergizing and from priming MLR cultures. β2-Microglobulinand lysozyme, both of which are down-regulated during classic activation of macrophages by LPS,44 were selectively and highly expressed in macrophages isolated from the anergizing MLR cultures. This observation indicates that in contrast to priming culture conditions, which induce a classic activation program on macrophages and down-regulate lysozyme and β2-microglobulin, the activation program initiated on macrophages in anergizing culture conditions is distinct and does not lead to these events. However, this is not due to the lack of activation and differentiation signals, because ferritin heavy and light chain, which are expressed in mature terminally differentiated macrophages,44 45 were highly expressed in macrophages isolated from anergizing cultures.
These results provide molecular evidence that distinct activation and differentiation gene programs are initiated on macrophages during anergizing and priming culture conditions. These distinct gene programs result in distinct functional properties of macrophages.
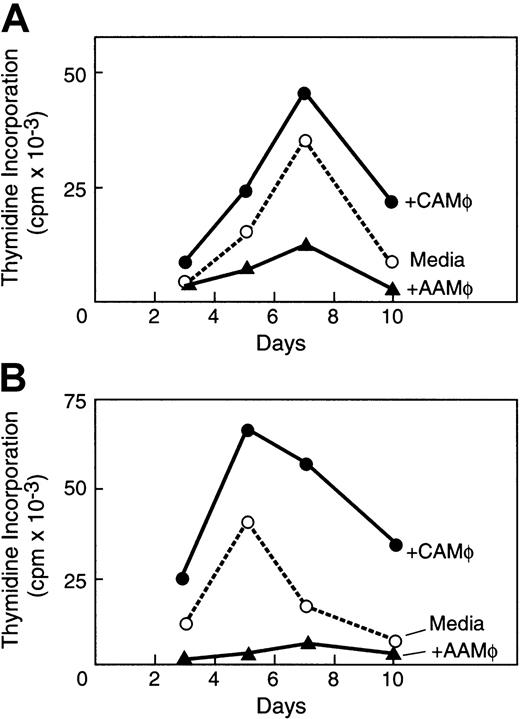
Macrophages isolated from anergizing MLR cultures mediate suppression of T-cell responses
Previous studies have shown that APCs generated in the presence of IL-10 do not induce T-cell responses but are rather immunosuppressive.25,32,38 39 To examine whether AAMΦ generated in anergizing cultures have immunosuppressive properties, we isolated CD14+ cells from anergizing and priming cultures by positive selection. These CD14+ cells were subsequently added in a new primary MLR culture and in a secondary MLR culture of purified CD4+ original responder cells and original PBMC stimulators. CD14+ cells isolated from anergizing primary cultures, which have phenotypic, functional, and molecular properties of AAMΦ, reduced alloresponses of CD4+ original responder cells in a new primary MLR culture (Figure5A) and in a secondary MLR culture (Figure 5B). This inhibitory effect was dose dependent (data not shown). Moreover, AAMΦ also reduced alloresponses in primary and secondary MLR cultures of different responder CD4+ cells (data not shown). In contrast, CD14+ cells isolated from priming cultures in medium alone, which have phenotypic and molecular properties of CAMΦ, augmented primary and secondary MLR responses of original CD4+ responder cells (Figure 5A,B). These CAMΦ also augmented primary and secondary responses of different CD4+ responder cells (data not shown).
AAMΦ from anergizing MLR cultures suppress T-cell alloresponses.
AAMΦ isolated from anergizing MLR cultures and CAMΦ isolated from priming MLR cultures were added in (A) a new primary MLR and (B) in a new secondary MLR of the purified original CD4+ responders and original PBMC stimulators. The effects of AAMΦ and CAMΦ were compared with the response of MLR in medium alone and were determined by 3H-thymidine incorporation for the last 16 hours of the indicated time intervals of culture.
AAMΦ from anergizing MLR cultures suppress T-cell alloresponses.
AAMΦ isolated from anergizing MLR cultures and CAMΦ isolated from priming MLR cultures were added in (A) a new primary MLR and (B) in a new secondary MLR of the purified original CD4+ responders and original PBMC stimulators. The effects of AAMΦ and CAMΦ were compared with the response of MLR in medium alone and were determined by 3H-thymidine incorporation for the last 16 hours of the indicated time intervals of culture.
Discussion
T cells play a central role in the generation of GVHD after ABMT. Although T cells are mediators of immunity, their function is under the control of APCs. Therefore, APCs may regulate the activation of host-specific donor T cells, which are responsible for the generation of GVHD. Our present results show that blockade of the B7/CD28 pathway in anergizing priming MLR cultures leads to a distinct program of activation and differentiation of monocytes into macrophages, which acquire distinct gene expression and functional properties as compared with macrophages generated in MLR cultures in medium alone. This altered macrophage differentiation program closely resembles what has been defined as “alternative activation of antigen-presenting cells.”13 14 Macrophages isolated from anergizing cultures have increased expression of CD14, MHC class II, CD23, and scavenger receptors and increased phagocytotic activity, but reduced ability to process and present antigen. Moreover, these macrophages mediate immunosuppressive effects to T cells.
In our recent clinical trial that allowed successful haploidentical BMT, recipient PBMCs were collected, irradiated, and cocultured with donor bone marrow ex vivo in the presence of CTLA4-Ig to block B7-1– and B7-2–mediated costimulation, washed, and infused into the patient.8 All treated patients rapidly achieved engraftment and developed full donor chimerism and no clinically detectable GVHD. Although in vitro analysis during that study indicated that anergy had been induced to host-specific donor T cells, our present results indicate that additional mechanisms may be responsible for the clinical outcome of this treatment. As we show here, anergizing cultures result in the generation of AAMΦ, which have increased phagocytotic capacity and immunosuppressive properties. These results suggest that blockade of B7/CD28 contributes to tolerance induction not only by directly inhibiting T-cell activation, but also by subsequently regulating the function of macrophages.
The cytokine profile of the anergic cells is characterized by defective production of IFN-γ, a proinflammatory cytokine that mediates macrophage activation. At the same time, IL-10 is secreted, thereby leading to the generation of microenvironmental conditions that promote alternative activation of macrophages.13,14 Our studies showed that in anergizing cultures, IL-10 is produced by T cells. However, in this experimental system, it is impossible to clarify whether all T cells in anergizing cultures are IL-10 producers. It is possible that initially only the anergic T-cell subset within this population secretes IL-10, which subsequently facilitates the generation of regulatory Tr1-type cells that produce IL-10,46 further contributing in the generation of AAMΦ.
An additional mechanism responsible for the generation of AAMΦ in anergizing cultures may be related to the increased apoptosis. CD28-mediated costimulation up-regulates bcl-xL and provides a critical antiapoptotic signal that protects T cells from suicidal Fas-mediated apoptosis during TCR-mediated activation.47 Therefore, blockade of B7/CD28 costimulation in anergizing cultures leads to increased Fas-mediated apoptosis. It has been shown that APCs that capture apoptotic cells induce tolerance in vivo.48-50 Ingestion of apoptotic cells by macrophages inhibits proinflammatory cytokine production and induces suppressive properties of macrophages.51 Moreover, it has been proposed that Fas-mediated apoptosis of lymphoid cells results in rapid production of IL-10. Subsequent uptake of apoptotic cells by APCs under these conditions mediates immune deviation and differentiation of Th2-type cells, which further produce high levels of IL-10.52 Therefore, lack of CD28-mediated viability signals will result in increased numbers of apoptotic cells, which will be rapidly captured by viable APCs, resulting in the generation of tolerogenic APCs in an IL-10–dependent and IL-10–independent manner.
Several studies have shown an important role of IL-10 in the regulation of the differentiation and activation program of APCs.13,32,38 IL-10 prevents the differentiation of monocytes to dendritic cells and promotes their maturation into macrophages.32 These macrophages that develop in the presence of IL-10 have increased endocytotic activity but defective ability for antigen presentation. Although IL-10 blocks the differentiation of CD14+ monocytes to dendritic cells induced by granulocyte macrophage colony-stimulating factor and IL-13, the macrophages that develop under these conditions have cytochemical and phenotypic features of mature macrophages. Thus, the presence of IL-10 does not simply prevent activation of monocytes, but initiates an activation program that preferentially leads to their differentiation into mature macrophages instead of dendritic cells. Our present studies show that the defective ability of macrophages differentiated in the presence of IL-10 to induce T-cell activation is linked to their defective ability to process and present antigen. At the same time, AAMΦ may express ligands of immunoinhibitory receptors such as PD-L153 and/or PD-L2,54 resulting in an altered balance of positive signals mediated via the TCR and negative signals mediated by immunoinhibitory receptors.
Consistent with these functional findings, biochemical evidence from another study supports the notion that IL-10 does not simply deactivate monocytes, but leads to activation of selective signaling pathways.55 IL-10 blocks LPS-mediated activation of p56lyn, phosphorylation of vav, and activation of Ras in monocytes, but does not inhibit p56lyn-mediated induction of c-jun and c-fos.55 Thus, selective predominance of signaling pathways during monocyte activation in the presence of IL-10 results in distinct gene transcription and distinct functional properties.
These developing observations about the significance of the distinct differentiation programs of APCs in the regulation of the immune response13,48 may have important implications in the field of transplantation. Clinical data and results from in vivo animal models have shown that allografts of granulocyte colony-stimulating factor (G-CSF)–mobilized peripheral blood stem cells (PBSCs) have an unexpected immunologic behavior.56,57 Transplantation of allogeneic G-CSF–mobilized PBSCs, which contain 10-fold higher numbers of T cells as compared with bone marrow grafts, did not result in a higher incidence of GVHD.58,59 Moreover, such grafts achieved better engraftment across the HLA barrier.60,61Potentially, CD14+ cells contained in the G-CSF–mobilized PBMCs have an active role in the reduced responsiveness of the CD4+ cells contained in this population.62These CD14+ cells are involved in the impaired transactivation of the CD28 response element (CD28RE) of G-CSF–mobilized CD4+ cells.63 It was shown that such CD14+ cells produce high levels of IL-10 and mediate their effects in an IL-10–dependent manner.64
Studies in animal models have indicated that T cells from G-CSF–treated animals preferentially produce IL-4 and IL-10 and are associated with diminished ability to induce GVHD.57,65Consistent with these experimental data, G-CSF–mobilized PBSCs from healthy donors contain high numbers of type-2 dendritic cells (DC2), which prime T cells to produce IL-4 and IL-10.66Interestingly, umbilical cord blood, another source of allogeneic stem cells for transplantation associated with a low incidence of acute GVHD, contains DC2 and not DC1-type cells.67 These studies suggest that donor APCs regulate both GVHD induction and engraftment and have a critical role in the clinical outcome of allogeneic transplantation. Consistent with these observations, in our system IL-10 was produced by CD4+ and not by CD14+cells. Moreover, our experiments showed a reciprocal regulation between T cells and macrophages. Anergic T cells generated during blockade of B7/CD28 interaction produce high amounts of IL-10, which mediates generation of AAMΦ with increased phagocytotic activity. Increased phagocytosis results in increased scavenging of antigen from the external milieu, which in conjunction with defective antigen presentation by AAMΦ25 39 contributes to T-cell immunosuppression.
An additional interesting finding of our study with potential clinical significance is the observation that blockade of B7/CD28 interactions leads to the generation of AAMΦ, which have molecular findings of mature macrophages. The maturation stage of the monocytic cells determines their ability to enter tissues and engraft.68Using distinct populations of freshly isolated and ex vivo expanded monocytes, it was shown that subsets with characteristics of mature macrophages rapidly engrafted and preferentially repopulated areas in which irradiation had depleted local macrophages.68 Our present results showed that AAMΦ generated during blockade of B7/CD28 costimulation had characteristics of mature macrophages and increased scavenging activity. Thus, after infusion to the patient, these macrophages may rapidly enter and repopulate the recipient's marrow, where they can phagocytose recipient's hemopoietic cells undergoing apoptosis after irradiation, facilitating the establishment of hemopoiesis of donor origin. Indeed, rapid engraftment and hemopoietic reconstitution of completely donor origin were observed in all our patients who received histoincompatible transplantation after ex vivo anergy induction with CTLA4-Ig.8
In conclusion, our results show that induction of T-cell anergy by blockade of B7/CD28 costimulation influences the maturation and differentiation process of macrophages, which subsequently have an inhibitory role on T-cell responses. This novel and unexpected observation that anergic T cells mediate the generation of AAMΦ, which have immunosuppressive properties, provides new insights into the mechanisms by which manipulation of the B7/CD28 pathway may facilitate ABMT.
Supported by National Institutes of Health grants AI 43552, AI 41584, and HL 54785.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Vassiliki A. Boussiotis, Dana-Farber Cancer Institute, Mayer 547, 44 Binney St, Boston, MA 02115; e-mail:vassiliki_boussiotis@dfci.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal