The clinical and pathologic features of classical Hodgkin lymphoma (cHL) reflect an abnormal immune response that is thought to be due to the elaboration of a variety of cytokines by the malignant Reed-Sternberg (RS) cells or surrounding tissues. The majority of cHL cases are characterized by expression of tumor necrosis factor receptor (TNFR) family members and their ligands, as well as an unbalanced production of Th2 cytokines and chemokines. Activation of TNFR members results in constitutive activation of nuclear factor-κB (NF-κB), a transcription factor important for the in vitro and in vivo growth of RS cell lines. The expression of Th2 cytokines and chemokines leads to the reactive infiltrate of eosinophils, Th2 cells, and fibroblasts characteristic of cHL, and can also contribute to a local suppression of Th1 cell–mediated cellular immune response. Another particularly important growth and survival factor for RS cell lines is the Th2 cytokine interleukin 13, which is also commonly expressed by primary RS cells. In approximately 40% of cHL cases, the presence of Epstein-Barr virus influences the Th1/Th2 balance toward the production of Th1 cytokines and chemokines, but this shift is apparently insufficient for the stimulation of an effective antitumor cell-mediated immune response. This review summarizes the current literature on cytokine expression by and activity on RS cell lines and primary cHL tissues, examines cytokine signaling pathways in RS cells, and discusses the role that cytokines play in the specific clinical and pathologic features of cHL.
Introduction
Classical Hodgkin lymphoma (cHL) is a lymphoid malignancy with characteristic features that distinguish it from nodular lymphocyte-predominant HL (NLPHL) and non-Hodgkin lymphomas (NHLs).1 2 The malignant cells in cHL, termed the Reed-Sternberg (RS) cells, constitute only a minor component of the tumor, whereas the majority of the malignancy is composed of a mixed inflammatory infiltrate variably composed of lymphocytes, eosinophils, fibroblasts, macrophages, and plasma cells. Patients with cHL also commonly present with constitutional symptoms such as fever, weight loss, and night sweats, and an apparent systemic defect in cell-mediated immune responses. Many of these distinctive clinical and histopathologic features of cHL reflect an abnormal immune response thought to be due largely to the effects of a wide variety of cytokines and chemokines that are primarily produced by the RS cells, but also secondarily by the surrounding reactive infiltrate. This review summarizes the data on cytokine production in cHL, examines cytokine signaling, and discusses the role of cytokines in the clinical and pathologic features of this disease.
Classical HL is now considered a distinct clinicopathologic entity from NLPHL and can be divided into 4 morphologic subtypes: nodular sclerosis cHL (NSHL), mixed cellularity cHL (MCHL), lymphocyte-rich cHL (LRCHL), and lymphocyte-depleted cHL (LDHL).2 There are few data on cytokine involvement in LRCHL and LDHL, because each disease comprises less than 5% of all cHL cases. As a result, nearly all of the data on the role of cytokines in cHL are derived from the study of NSHL and MCHL cases.
Cytokines are low-molecular-weight proteins with a wide variety of functions. They not only regulate immune and inflammatory responses but also contribute to hematopoiesis, wound healing, and other biologic processes. Cytokines are extremely potent molecules active in nanomolar to picomolar concentrations. Typically, a cytokine either acts in a paracrine manner to modulate the activity of surrounding cells, or in an autocrine fashion to affect the cell that produced it. In the context of cHL, cytokines produced by RS cells are thought to contribute to the pathogenesis of this disease both by acting as autocrine growth factors and by initiating and sustaining the reactive infiltrate. Alternatively, cytokines produced by surrounding reactive cells may be contributing to RS cell proliferation and survival.
The expression and activity of cytokines in cHL can be studied using cell lines derived from RS cells and in primary cHL tissues. Each approach has its limitations, so that a combination of both strategies provides the most comprehensive information on the activity of a specific cytokine. Cell lines derived from RS cells have been extremely useful in the study of cHL.3,4 However, outgrowth of such a cell line from cHL patient tissues is extremely rare, so that the few cell lines available may not represent the full spectrum of clinical and pathologic features of this disease. The RS cell lines that have been established are overrepresentative of patients with advanced stage disease, and those with disease involving pleural effusions, peripheral blood, or bone marrow. Furthermore, only one cell line, L-1236, has been definitively identified as being derived from RS cells.5,6 Unequivocal proof that the other cell lines are derived from RS cells is lacking, but extensive analysis of their morphologic, phenotypic, and genetic features has led the research community to generally regard most of them as being derived from RS cells.3 4
Analysis of short-term cultures of primary cHL biopsy material is hampered by the fragility of freshly isolated RS cells and contamination by the reactive infiltrate. Therefore, the activity of cytokines on primary RS cells is difficult to demonstrate directly and indirect evidence, such as the expression patterns of cytokines and their receptors, activation of downstream signaling molecules, and correlation of cytokine expression with expected pathologic features, must be relied on. Because cytokines are active at very low concentrations, methods for determining cytokine messenger RNA (mRNA) and protein expression in primary tissues must be very sensitive. Cytokine mRNA levels can be assessed in whole tissue extracts by Northern analysis, but this method does not allow localization of expression to specific cell populations, and may not be sufficiently sensitive if the cytokine is expressed by a small population of cells. In situ hybridization (ISH) techniques using radio-labeled RNA probes have been very useful for detecting cytokine mRNA levels in formalin-fixed, paraffin-embedded tissues. ISH is highly sensitive and allows retention of the morphology of positively staining cells. mRNA expression can also been studied on the single-cell level on RS cells isolated from cell suspensions.7 However, the presence of mRNA does not confirm the presence of the protein product, which requires examination by immunohistochemistry (IHC). A combination of ISH and IHC is therefore the most effective strategy for determining cytokine expression in tissue sections.
Th1 and Th2 cytokines in cHL
The human adaptive immune response can be divided into 2 distinct branches: the humoral immune response, which targets extracellular pathogens by stimulating B cells to produce antibodies, and the cell-mediated immune response, which targets intracellular pathogens by activating CD8+ cytotoxic T cells and macrophages. CD4+ helper T cells are crucial for regulating both types of responses and can be classed into 2 mutually exclusive subsets: T helper type 1 (Th1) and Th2 cells.8 Th1 cells, which require interleukin 12 (IL-12) for their differentiation, characteristically produce IL-2 and interferon-γ (IFN-γ), and facilitate the cell-mediated immune response by inducing inflammatory reactions and activating microbicidal functions of macrophages. Th1 cells also assist B cells to produce complement-fixing and opsonizing antibodies. Th2 cells require IL-4 for their differentiation and characteristically produce IL-4, IL-5, IL-6, IL-9, IL-10, and IL-13. These cells provide the help required for B cells to produce non–complement-fixing antibodies of the immunoglobulin (Ig) G4 and IgE classes. Th2 cells also promote the growth and differentiation of eosinophils important for clearing extracellular pathogens. The expression of Th2 and Th1 cytokines has been extensively studied in cHL (Table 1).
Th2 cytokines
IL-4 and IL-13.
Interleukin 4 and IL-13 are important cytokines that share many biologic activities.9 10 Both IL-4 and IL-13 regulate the humoral immune response by stimulating the proliferation and survival of B cells and triggering Ig class switching. IL-4 (but not IL-13) is required for the differentiation of Th2 cells.
The expression of IL-4 and IL-13 has been extensively examined in the context of cHL. In studies of RS cell lines, IL-4 was inconsistently expressed by 2, L-428 and HDLM-2, and not at all by 6 other lines.5,11-14 Klein et al detected small amounts of IL-4 (≤ 25 pg/mL) by enzyme-linked immunosorbent assay (ELISA) in L-428 and HDLM-2 cells11; however, IL-4 mRNA and protein could not be demonstrated in these cell lines using ISH, ELISA, or IHC in 3 subsequent studies.12-14 Furthermore, although exogenous IL-4 stimulated the growth of L-428 cells, anti–IL-4 antibody had no effect on their growth.15 In primary biopsy material of cHL-involved tissue, IL-4 expression has been consistently absent in RS cells and expressed at only low levels within the reactive infiltrate. IL-4 mRNA was detected in 2 of 12 cHL cases by Northern analysis, but examination by ISH revealed that the source was lymphocytes and not RS cells.16 In 4 subsequent studies that examined IL-4 expression in cHL by IHC or ISH, IL-4 could not be detected in RS cells from a total of 88 cases of cHL, and only rare (< 5%) small lymphocytes were positive.12-14 An IHC study of IL-4 expression in 27 cases of NSHL showed that the average case contained only about 5% of IL-4+ RS cells and reactive lymphocytes.17 In short, IL-4 is not expressed by primary RS cells and at very low levels within the reactive infiltrate, and there is no clear evidence that it acts as an RS cell growth factor.
In contrast, studies of IL-13 expression suggest that it is a key autocrine growth factor for RS cells. IL-13 is expressed by 4 of 5 RS cell lines.14,18 Using ISH, Kapp et al first identified IL-13 expression in primary RS cells from 4 of 4 NSHL cases,18 a result confirmed in a subsequent ISH study of 36 cHL cases in which 86% contained IL-13+ RS cells.19 IL-13 expression in primary RS cells was subsequently confirmed at the protein level by Ohshima et al, who demonstrated IL-13 in 100% of cHL cases by IHC.20 These studies found that IL-13 was expressed almost exclusively by the RS cell population, and only rarely by cells within the reactive infiltrate. IL-13 was not expressed by lymphocytic and histiocytic cells in NLPHL and was expressed in a minority of cases of anaplastic large cell lymphoma (ALCL) and T cell–rich B-cell lymphoma (TCRBCL).19
For cytokine signals to be received, the target cell must express the appropriate cytokine receptor. The IL-13 receptor consists of the IL-4Rα chain and an IL-13–specific receptor chain, IL-13Rα1. IL-4Rα expression has been demonstrated in 3 RS cell lines21 but has not been investigated in primary cHL samples. IL-13Rα1 is expressed by 6 RS cell lines examined19,21 and by RS cells in 95% of cHL cases as determined by ISH or IHC.19,20 The expression of both IL-13 and its receptor by RS cells is consistent with a role for IL-13 as an autocrine growth factor for these cells. Indeed, antibody-mediated neutralization of IL-13 in the IL-13+ RS cell lines HDLM-2 and L-1236 led to a dose-dependent inhibition of proliferation in both cell lines and the induction of apoptosis in L-1236 cells (but not in HDLM-2 cells).14,18 21 However, treatment of the IL-13+ RS cell lines L-428 and KM-H2 with the same anti–IL-13 antibody had no effect on cell proliferation.
IL-5.
Interleukin 5 is a Th2 cytokine essential for the growth and differentiation of eosinophils. It is also a B-cell growth factor in mice (but not in humans).22 IL-5 is expressed by 2 of 6 RS cell lines11,18 and has been identified in primary RS cells from tumors with tissue eosinophilia.23 The proliferation of 2 IL-5+ RS cell lines (L-428, KM-H2) was not affected by antibody-mediated neutralization of IL-5, suggesting that IL-5 is not an autocrine growth factor for cHL.18
IL-6.
Interleukin 6 was first identified as a T cell-derived cytokine that induces the maturation of B cells into antibody-producing plasma cells. IL-6 also induces hematopoiesis from stem cells and acute phase reactions by hepatocytes.24 IL-6 expression has been widely studied in RS cell lines and in primary cHL cases by Northern analysis, ISH, and IHC. IL-6 was expressed by 5 of 7 RS cell lines,5,11,12,25 and in RS cells from 65% to 100% of cHL cases.12,16,25-28 IL-6 was also occasionally expressed by lymphocytes and macrophages within the reactive infiltrate. Interestingly, IL-6 expression was significantly higher in Epstein-Barr virus (EBV)+ cases of cHL versus EBV− cases (84% versus 51%).27
Components of the IL-6 receptor have been identified in cultured and primary RS cells. The IL-6 receptor complex consists of an IL-6–specific receptor chain (IL-6R) and the gp130 chain that is shared with several other cytokines, including IL-11, leukemia inhibitory factor, oncostatin M, ciliary neurotrophic factor, and cardiotrophin-1.24 Both IL-6R and gp130 are commonly expressed in RS cell lines and in primary RS cells,11,25,27 giving IL-6 the potential to act as an autocrine growth factor. However, treatment of 6 RS cell lines with neutralizing antibodies to IL-6 or IL-6R had no effect on cellular proliferation, arguing against a role for IL-6 as an autocrine growth factor in cHL.11,12 29
IL-9.
Interleukin 9 is a T-cell and mast cell growth factor that can also potentiate IL-4–induced IgG4 and IgE production.30Although IL-9 mRNA was not detected in unstimulated L-428 cells, it was identified in 5 of 12 (42%) cases of cHL by Northern analysis.31 In 5 of these cases, including 2 that were IL-9− by Northern analysis, IL-9 mRNA was identified by ISH in both the RS cell population and reactive lymphocytes. Thus, the true incidence of IL-9 expression in cHL is more than 50%. IL-9 was also expressed in one ALCL cell line and in 2 of 6 cases of ALCL as shown by Northern analysis.31 Exogenous IL-9 had a moderate stimulatory effect on the proliferation of KM-H2 cells, suggesting that it may be acting as an RS cell growth factor.32
IL-10.
Interleukin 10 is a Th2 cytokine with strong anti-inflammatory properties. IL-10 inhibits T-cell growth, blocks IL-2 and IFN-γ production by Th1 cells, and down-regulates proinflammatory cytokine production by lipopolysaccharide-stimulated monocytes. In addition, IL-10 is a growth and differentiation factor for B cells.33 IL-10 mRNA and protein have been detected in 2 of 7 RS cell lines,5,13 and in RS cells in 21% to 36% of primary cHL samples.13,28 IL-10 was expressed in 66% of EBV+ cHL cases but in only 16% of EBV−cases.13 All 27 cases of NSHL examined using IHC expressed IL-10, but the number of IL-10+ RS cells per case varied widely from 3% to 52%.17 Again, EBV+ cases of NSHL contained a significantly higher percentage of IL-10+ RS cells than the EBV− cases.
Th1 cytokines
IL-12.
Interleukin 12 is required for Th1-cell differentiation.34In the context of cHL, IL-12 has been detected by IHC in 28 of 33 (85%) cases, expressed by reactive cells but not by RS cells.35 The level of IL-12 expression varied from tumor to tumor, ranging from a few or absent IL-12+ cells in some cases to clusters of positive cells around RS cells in others. IL-12+ cells could be detected within the reactive infiltrate in all 22 EBV+ cases, but in only 5 of 10 EBV− cHL cases.35
IL-2.
Interleukin 2 is a principal growth factor for T cells, and also augments the cytolytic activity of natural killer (NK) cells.36 IL-2 was not expressed by any of 7 RS cell lines examined,5,11,37,38 and expression of IL-2 in primary cHL cases has been extremely variable. Eight cHL cases were negative for IL-2 mRNA by Northern analysis.16 More recently, Dukers et al found that less than 5% of RS cells and reactive lymphocytes were IL-2+ in 27 cases of NSHL tested using IHC.17However, Hsu et al could demonstrate IL-2+ RS cells in 10 cases of NSHL by IHC, with a range of 30% to 75% IL-2+ RS cells.37
Three IL-2 receptor chains have been identified: IL-2Rα (CD25, Tac), IL2Rβ, and the common γ (γc) chain. These chains combine to form 3 different IL-2 receptor complexes distinguished by their binding affinity for IL-2: the low-affinity (α chain only), intermediate-affinity (α and β chains), and high-affinity (α, β, and γc chains) receptors. The cytoplasmic domain of the β chain is essential for signal transduction in response to IL-2, such that only the intermediate- and high-affinity receptors are able to transduce IL-2 signals.39 IL-2Rα expression has been demonstrated in 5 of 6 RS cell lines11,37,38,40,41 and in primary RS cells by IHC in 75% of cHL cases.37,40-44IL-2Rβ chain expression, however, is less consistent in both cultured and primary RS cells. Using IHC, Hsu et al37 could not detect IL-2Rβ on 2 RS cell lines (HDLM-2 and KM-H2) or on primary RS cells from 10 cases of cHL. However, Tesch et al used the same antibody to detect IL-2Rβ protein by IHC on 5 RS cell lines (including HDLM-2 and KM-H2) and in primary RS cells from 7 of 13 cases of cHL.41 Similarly, Trumper et al detected transcripts for IL-2Rβ in 50% to 75% of RS cells from all 6 cHL cases in which mRNA expression levels were determined on single RS cells isolated from cell suspensions.7 Several investigators have shown that recombinant IL-2 does not affect the proliferation of cultured RS cells,37,45,46 even in a cell line shown to express high-affinity IL-2R.41 In summary, the weight of evidence indicates that RS cells do not express IL-2 and variably express IL-2Rβ, and that exogenous IL-2 has no effect on RS cell growth. Therefore, IL-2 is unlikely to be an autocrine growth factor in cHL.
IFN-γ.
Interferon γ supports cell-mediated immunity primarily through its effects on the monocyte/macrophage population, enhancing their ability to phagocytize and kill microorganisms and secrete the proinflammatory cytokines tumor necrosis factor α (TNF-α) and IL-1. IFN-γ also promotes production of several growth factors, notably transforming growth factor β (TGF-β), which stimulates fibroblast proliferation and collagen synthesis.47 In the context of cHL, IFN-γ was expressed in RS cell lines L-540 and L-1236 but was absent in L-428.5,48 Treatment of HDLM-2 and KM-H2 cells with recombinant IFN-γ had no effect on their proliferation.46 In primary cHL material, Northern analysis revealed the expression of IFN-γ mRNA in 3 of 8 cHL cases,16 and IHC studies showed that 14 of 30 (47%) cHL cases contained 1% to 90% IFN-γ+ RS cells.48 In 27 cases of NSHL, an average of approximately 15% of RS cells and reactive cells expressed IFN-γ as assessed by IHC.17 Interestingly, IFN-γ appears to be expressed at higher levels in MCHL cases compared to NSHL cases.49
Chemokines in cHL
Chemokines are chemoattractant cytokines that regulate the selective migration of leukocytes through binding to specific chemokine receptors differentially expressed on various leukocyte populations. Approximately 50 chemokines and 20 chemokine receptors have been identified in humans50 but only a few have been studied in cHL (Table 2).
Most of the chemokines that have been studied in cHL are associated with either a humoral or cell-mediated immune response, based on the expression pattern of their receptors on Th1 and Th2 cells.51 Th1 cells express the chemokine receptors CXCR3 and CCR5 and therefore are attracted to sites of production of their respective ligands. The ligands for CXCR3 include inducible protein 10 (IP-10) and monokine induced by IFN-γ (Mig); the ligands for CCR5 include macrophage inflammatory protein (MIP)–1α and MIP-1β. CCR5 is also expressed by monocytes, the precursors of activated macrophages that are crucial for cell-mediated immunity. MIP-1α and MIP-1β therefore not only recruit Th1 cells but also recruit the monocytes that will be activated in response to Th1 cytokines. Th2 cells express the chemokine receptors CCR3, CCR4, and CCR8 and thus respond to chemokines that use these receptors. Eotaxin is a ligand for CCR3, whereas thymus- and activation-regulated chemokine (TARC) and macrophage-derived chemokine (MDC) are ligands for CCR4.52Eosinophils, which play an important role in the humoral response, express high levels of CCR3 and are therefore attracted along with CCR3+ Th2 cells. RANTES (regulated on activation, normal T-cell expressed and secreted) is a ligand for both CCR5 and CCR3.
Th2 chemokines
Expression of TARC in cHL was first discovered using a serial gene expression analysis of RS cell lines.53 TARC was expressed by 4 RS cell lines examined, but absent in B-lymphoblastoid and NHL cell lines. TARC was also expressed by RS cells in 88% of cHL cases as determined by ISH or IHC.53,54 Cases of NSHL demonstrated stronger staining by IHC compared to MCHL cases. TARC was not expressed in NLPHL, and the majority of NHL cases were TARC−, except for a small number of ALCL and TCRBCL cases.53,54 Although moderate to strong levels of CCR4 mRNA could be detected by reverse transcription–polymerase chain reaction (RT-PCR) in 4 RS cell lines, CCR4 expression could not be detected in primary RS cells by ISH. However, a high proportion of the lymphocytes surrounding the RS cells were CCR4+, consistent with an activated Th2-cell phenotype.53
Although levels of MDC expression in cHL tissues as a group were not significantly different from those in normal lymphoid tissues, MDC expression among cHL subtypes was significantly higher in NSHL cases compared to MCHL cases.49 By IHC, 87% of cHL cases demonstrated MDC expression, primarily localized to RS cells.55
Eotaxin expression is higher in cHL tissues than in normal lymphoid tissues, as demonstrated by RT-PCR.49 Within cHL subtypes, elevated eotaxin expression was preferentially associated with NSHL cases. Eotaxin was detected by IHC in RS cells, fibroblasts, macrophages, and lymphocytes within cHL tissues, with more intense staining in NSHL than MCHL cases. Moreover, expression of eotaxin correlated with the number of eosinophils in the cHL tissue, suggesting that eotaxin contributes to eosinophil recruitment to cHL tissues. In contrast to these findings, Jundt et al detected eotaxin mRNA in only 1 of 5 RS cell lines and could not detect eotaxin expression in primary RS cells by IHC or ISH.56 They found that fibroblasts were the major source of eotaxin with a minor contribution by macrophages. Eotaxin expression could be induced in cultured fibroblasts separated from cocultured RS cells by a micropore membrane, indicating that soluble factors produced by the cultured RS cells stimulated the up-regulation of eotaxin expression. The RS cell line L-1236 produces high levels of TNF-α,5 a cytokine known to stimulate eotaxin production. Studies of antibody-mediated neutralization of TNF-α indicated that this cytokine was largely responsible for the stimulation of eotaxin production by fibroblasts cocultured with L-1236 cells.56
Expression of CCR3 could not be demonstrated on RS cell lines by flow cytometry or on primary RS cells by IHC.57 However, strong expression of CCR3 could be detected by IHC on about half of the cells within the cHL reactive infiltrate, compared to very rare CCR3 expression in control lymph nodes. Flow cytometry indicated that CCR3 was expressed not only on T cells in cHL tissue but also on B cells, an unexpected finding because normal B cells do not express CCR3.57 Whether this observation represents a cHL-specific dysregulation of CCR3 expression or a more general phenomenon is unclear.
Th1 chemokines
The Th1-associated chemokines IP-10, Mig-1, MIP-1α, MIP-1β, and RANTES are expressed at higher levels in cHL tissues compared to benign lymphoid tissues.49,57 In contrast to eotaxin, TARC and MDC, which are associated with the nodular sclerosis (NS) subtype of cHL, expression levels of IP-10, Mig-1, and MIP-1α were higher in MCHL cases.49 Elevated expression of these chemokines was also associated with EBV+ cHL cases. IP-10 and Mig-1 immunoreactivity was strongest in RS cells but also present in endothelial cells, macrophages, lymphocytes, and fibroblasts. CCR5, the receptor for MIP-1α and MIP-1β, was not present on cultured or primary RS cells but was expressed on approximately half of the cells within the reactive infiltrate, including CD4+ T cells and B cells.57 The expression of CXCR3, the receptor for IP-10 and Mig-1, was moderately up-regulated in the CD4+ T-cell population.57
IL-8
Interleukin 8 is a potent neutrophil recruitment factor that binds to the chemokine receptors CXCR1 and CXCR2 on neutrophils. IL-8 mRNA was detected by ISH in 20 of 33 cases of cHL and its level correlated with the density of tissue neutrophilia.58 IL-8 was predominantly expressed by cells within the reactive infiltrate and could be detected in RS cells in only 3 cases.
Tumor necrosis factor receptor and ligand families in cHL
Members of the tumor necrosis factor (TNF) family of receptors and ligands play important roles in the pathogenesis of cHL. Approximately 25 TNF receptor (TNFR) members have been identified in normal tissues, including TNFRI, TNFRII, CD40, CD30, CD27, OX40, receptor activator of nuclear factor κB (RANK), and FAS.59 Members of the TNFR family share similar signaling characteristics that are important for cell growth and survival. These molecules coordinate the assembly of the lymphoid organs, maximize the immune response to pathogens, act as costimulatory factors for B and T cells, and mediate the acute inflammatory response.59 The ligands of TNFR family members exist primarily as membrane-bound molecules, although a few, such as TNF-α, lymphotoxin α (LT-α), and FAS, are functional in a soluble form. Several of the TNFR family members and their ligands have been studied in the context of cHL (Table3) and are discussed in this review.
TNF-α and LT-α
Originally, TNF-α was identified as a macrophage product mediating cytotoxicity against certain cell types, especially transformed cell lines. Subsequently, TNF-α has been shown to play important roles in inflammation, tissue remodeling, and wound healing.60 TNF-α enhances the phagocytic and microbicidal activities of macrophages and stimulates the production of other proinflammatory cytokines including IL-1 and IL-6. In the context of cHL, TNF-α expression at the mRNA and protein levels has been demonstrated in 7 RS cell lines.5,11,26,61,62 TNF-α was detected by Northern analysis in all 19 cHL cases examined,63 and by IHC or ISH in primary RS cells in 69% of cHL cases examined in 6 studies.26,61,62,64-66 Cells within the reactive infiltrate, including lymphocytes and macrophages, were also TNF-α+. TNF-α can use 2 receptors called TNFRI (p55) and TNFRII (p75).60 These receptors have been studied in only a small number of primary tumors and not at all in RS cell lines. Ryffel et al used IHC to examine malignant lymphomas (including 4 cases of cHL) for the expression of TNFRI and TNFRII. Two cHL cases contained TNFRII+ RS cells and one had TNFRI+ RS cells.67 Treatment of the RS cell lines HDLM-2 and KM-H2 with recombinant TNF-α did not stimulate or suppress cellular proliferation.46
Lymphotoxin α shares approximately 50% structural homology with TNF-α. LT-α exists either as a membrane-bound form complexed with LT-β, which uses the LT-β receptor, or as a secreted form that uses TNFRI and TNFRII. This sharing of receptors with TNF-α results in overlapping biologic activities.60 LT-α is expressed by 5 of 6 RS cell lines,26,61,62 and is commonly expressed by primary RS cells. LT-α mRNA was detected in 17 of 19 cHL cases by Northern analysis63 and in another 24 cases by ISH.26 LT-α expression was almost exclusively limited to RS cells, with more than 60% of RS cells positive for LT-α in each case.26 Expression of LT-β and LT-βR have not been studied in cHL.
CD40
CD40/CD40L-mediated contacts between B and T cells are required for the generation of T cell–dependent humoral immune responses. Activation of CD40 on B cells stimulates proliferation and mediates Ig class switching in conjunction with IL-4 and IL-13.68Surface CD40 expression was detected in 4 RS cell lines examined by flow cytometry.32,69 O'Grady et al first studied CD40 expression in primary cHL by IHC and found strong membrane or cytoplasmic staining of RS cells in 26 of 37 (70%) cases.70 In contrast, CD40 expression was much weaker in normal lymphoid tissues and present in only 3 of 23 cases of B-cell NHL. Strong CD40 expression as determined by IHC was subsequently reported in primary RS cells in a total of 190 cHL cases.32,69,71,72 However, CD40 expression was not exclusive to cHL, because 105 of 127 B-cell NHLs stained weakly positive for CD40.32
The activation of CD40 on a cell requires contact with CD40L expressed on the surface of a neighboring cell. CD40L is absent on cultured and primary RS cells, but is present on CD4+ T cells within cHL tissues.69,71,72 Numbers of CD40L+ T cells were increased in cHL tissues compared to normal lymphoid tissues. The CD40L+ T cells were usually located in close proximity to RS cells, with an average of 2.5 to 5 CD40L+ T cells surrounding a single RS cell.71 In contrast, the malignant cell population and reactive component of cases of CD40+B-cell NHL failed to express significant levels of CD40L. Treatment of RS cell lines with soluble trimeric CD40L to activate CD40 stimulated the release of IL-8, enhanced secretion of IL-6, TNF-α, and LT-α, and increased expression of intercellular adhesion molecule 1 (ICAM-1) and B7-1.69 CD40 activation had no mitogenic effect on RS cell lines in this study; however, Carbone et al found that soluble CD40L increased the clonogenic growth of RS cell lines L-428 and KM-H2 and increased their survival in vitro.71 CD40L activation also protected HDLM-2 cells from Fas-induced apoptosis.73
CD40 signaling pathways can be activated by the latent membrane protein-1 (LMP-1) of EBV. Approximately 40% of cHL cases from immunocompetent patients in Western countries are associated with EBV infection and viral sequences can be detected within RS cells.74-76 RS cells show a type II pattern of EBV latency antigen expression, with expression of LMP-1, LMP-2, and Epstein-Barr nuclear antigen-1 (EBNA-1). EBV LMP-1 can mimic CD40 signaling in a ligand-independent and constitutive manner77 because the cytoplasmic portions of CD40 and LMP-1 recruit many of the same signal-transducing molecules, including members of the TNFR-associated factor (TRAF) family78 79 (discussed below in NF-κB signaling).
CD30
CD30 is expressed on RS cells from virtually all cases of cHL.80 Several cells within cHL tumors express CD30L, allowing potential activation of CD30 on neighboring RS cells. CD30L expression could not be detected in cultured RS cells by Northern analysis,81 flow cytometry,82 or RT-PCR.83 By IHC, however, weak expression of CD30L could be detected in primary RS cells in all cHL cases examined.82,83 However, RS cells demonstrated cytoplasmic staining without membrane reactivity; therefore, it remains unclear whether primary RS cells express CD30L on their surfaces. However, several other cell populations within the reactive infiltrate express CD30L, including eosinophils84 and mast cells.85 Circulating eosinophils from cHL patients expressed higher CD30L levels than cells from healthy donors.84
Activation of CD30 induces significantly different responses in different CD30+ lymphoma cell lines. This was first demonstrated by Smith et al who showed that CD30L activation stimulated proliferation of the CD30+ RS cell line HDLM-2 but had no effect on the proliferation of the CD30+ RS cell lines KM-H2 and L-428.86 In contrast, CD30L stimulation of the CD30+ ALCL cell line KARPAS-299 induced cytotoxic cell death. These investigations were extended by Gruss et al who found that CD30 could also stimulate the proliferation of the RS cell line L-540, but had cytotoxic effects on 6 of an additional 7 CD30+ALCL cell lines,87 results subsequently confirmed by other groups.84,85 Hsu and Hsu also found that activation of CD30 induced increased proliferation of L-428 and KM-H2 cells and of enriched RS cells from primary patient material.83 Like CD40 activation, CD30 activation increased the secretion of IL-6, TNF-α, and LT-α by RS cell lines, as well as the expression of ICAM-1.88
RANK
RANKL is a member of the TNF ligand family that plays a role in osteoclast differentiation, activation of mature osteoclasts, and interactions between T cells and dendritic cells. RANKL binds to 2 receptors, RANK and osteoprotegerin (OPG). RANK, RANKL, and OPG are all expressed by L-428, KM-H2, and HDLM-2 cells.89 RANK can be also detected by IHC in primary RS cells from both NSHL and MCHL cases. Activation of RANK in RS cell lines by soluble RANKL up-regulates the expression of several cytokines, including IFN-γ, IL-9, IL-13, and IL-15. However, treatment of these RS cell lines with soluble RANKL, or inhibition of RANK-RANKL interactions between cultured RS cells, has no effect on the proliferation or survival of these cells.89
Other cytokines in cHL
Several cytokines that do not belong to the previously discussed groups, including IL-1, TGF-β, and hematopoietic growth factors, have also been studied in cHL (Table 4).
IL-1
Interleukin 1 is a potent proinflammatory cytokine.90IL-1 has 2 distinct forms (IL-1α and IL-1β) that bind to the same receptor and have identical biologic activities. With respect to cHL, 3 of 6 RS cell lines were positive for IL-1,11,91 and IL-1 has been detected in cHL tissues in 58% of cases examined by IHC or ISH.64-66,92,93 In most instances, IL-1 was expressed predominantly by RS cells, but was also seen in lymphocytes, granulocytes, and macrophages. Recombinant IL-1 had no effect on the proliferation of HDLM-2 or KM-H2 cells.46
TGF-β
Transforming growth factor β has potent anti-inflammatory effects. It suppresses B- and T-cell proliferation and the cytolytic activity of NK cells, and stimulates fibroblast proliferation and collagen synthesis.94 The RS cell line L-428 produces TGF-β mRNA and secretes a high-molecular-weight form of TGF-β protein that is active at physiologic pH.95 Using IHC, Kadin et al first identified TGF-β in primary RS cells from 6 of 8 cases of NSHL but not in 4 cases of MCHL or 1 case of LDHL.96 TGF-β+ RS cells were typically located near zones of necrosis and at the margins of collagen bands. Subsequently, ISH was used to show that TGF-β transcripts were present in eosinophils from cases of NSHL but absent in RS cells, suggesting that eosinophils are the major source of TGF-β in cHL and that the RS cell immunoreactivity in the previous study represented secondary binding and uptake by RS cells.97 Interestingly, Newcom and Gu were able to detect TGF-β in RS cells of predominantly NSHL cases by both IHC and ISH.98 In short, although the cellular source of TGF-β is unclear, this cytokine is characteristically expressed in the NS subtype of cHL.
Hematopoietic growth factors
Hematopoietic growth factors include IL-3, IL-7, granulocyte-macrophage colony stimulating factor (GM-CSF), and macrophage-CSF (M-CSF).99 IL-3 stimulates colony formation of erythroid, megakaryocyte, eosinophil, basophil, and monocyte lineages, whereas IL-7 is a growth factor for progenitor B and T cells and mature T cells. GM-CSF is a differentiation factor for granulocytic and monocytic cells, whereas M-CSF is a growth and differentiation factor for macrophages and their progenitors.
Expression of IL-3 protein was not detected in 6 RS cell lines examined,11 and IL-3 mRNA was detected in only 2 of 8 cases of cHL by Northern analysis.16 Exogenous IL-3 had no effect on the proliferation of HDLM-2 or KM-H2 cells.46IL-7 mRNA was detected in RS cells from 24 of 31 cases of cHL, but identified in only 1 of 10 cases of lymphoblastoid lymphoma and in no cases of chronic B-lymphocytic leukemia.100 GM-CSF expression was detected in 2 of 6 RS cell lines11,18 but not in 8 cHL cases examined by Northern analysis.16Exogenous GM-CSF had no effect on the proliferation of HDLM-2 or KM-H2 cells.46 L428 and 2 sublines expressed M-CSF and its receptor, the product of the proto-oncogene c-fms. However, only one of the sublines showed any evidence that M-CSF could act as an autocrine growth factor.101 Although M-CSF was detected by IHC in primary RS cells from 100% of cHL cases in one study,102 and 75% of cHL cases in another,103 c-fms mRNA expression could not be detected in primary RS cells.7 104
Cytokine signaling in cHL
STAT signaling
The interaction of cytokines with their specific cell surface receptors triggers the activation of intracellular signaling cascades that ultimately have effects on multiple cellular functions. The earliest event is the activation of Janus kinase (Jak) family members, which then phosphorylate substrates crucial for the transduction of cytokine signals. One of the most important substrates is the family of signal transducer and activator of transcription (STAT) proteins.105 STATs are latent transcription factors residing in the cytoplasm that become activated by phosphorylation on a single tyrosine residue. This phosphorylation is carried out by Jak family members activated in response to cytokine receptor stimulation. Tyrosine phosphorylation leads to STAT dimerization and translocation of the activated transcription factor to the nucleus, where it stimulates transcription resulting in changes to gene expression. Seven members of the STAT family have been identified, STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6, and each is activated by a distinct set of cytokines (reviewed by Leonard and O'Shea105).
STAT6.
Interleukin 4 and IL-13 are the primary activators of STAT6,106 and its pivotal role in signaling of these cytokines has been demonstrated in mice deficient for STAT6, which show impaired proliferation of B and T cells in response to IL-4, and abrogation of Ig class switching and Th2 cytokine production.107-109 Additionally, STAT6 is involved in the expression of Th2 chemokines, including eotaxin, TARC, and MDC.110,111 All 5 RS cell lines examined demonstrated constitutive STAT6 phosphorylation, which was shown to be due to autocrine secretion of IL-13 in HDLM-2 and L-1236 cells.14Nuclear staining of phospho(P)-STAT6 was present in primary RS cells from 78% of cHL cases, whereas it was rarely detected in the reactive infiltrate.14 In cHL subtypes, STAT6 activation was present in 95% of NSHL cases, but in only 50% of MCHL cases. P-STAT6 expression was rare in normal lymphoid tissue, and among NHLs it was present only in a minority of ALCL and TCRBCL cases. P-STAT6 expression was associated with IL-13 expression in cHL cases, consistent with the active signaling of IL-13 in primary RS cells that would be expected for an autocrine growth factor.
STAT3.
STAT3 plays a role in oncogenesis, being involved in both hematolymphoid and solid tumors (reviewed by Bowman et al112). A wide range of cytokines activates STAT3, including the IL-6 family of cytokines, IL-10, and cytokines whose receptors use the γc chain (IL-2, IL-7, IL-9, IL-15).105EBV LMP-1 and CD40 engagement can also lead to STAT3 activation.113,114 Several of these activators are commonly expressed in RS cells. High levels of constitutively activated STAT3 were found in 5 of 6 RS cell lines but also in cell lines derived from acute myeloid leukemia, multiple myeloma, and ALCL.29The constitutive STAT3 phosphorylation in RS cell lines was not dependent on IL-6 signaling, because antibody-mediated neutralization of IL-6 or IL-6 receptor did not alter STAT3 activation.29Furthermore, neither incubation of cHL cells in starvation medium nor fresh medium exchange reduced STAT3 activity, suggesting that STAT3 activation does not depend on factors in the culture medium or factors produced by cultured RS cells. Treatment of RS cell lines with the Jak inhibitor AG490115,116 reduced the level of constitutive STAT3 activation and inhibited cell proliferation, providing indirect evidence that STAT3 may be contributing to the growth of RS cell lines. However, AG490 is not specific for STAT3 and may be inhibiting other pathways involved in cellular proliferation. With respect to cHL patients, constitutive STAT3 phosphorylation has been shown in primary RS cells in 87% of cases.14 However, STAT3 activation was not specific to RS cells, because a majority of NHL cases and a large proportion of the reactive infiltrate within cHL cases also demonstrated nuclear P-STAT3 expression.
STAT5.
STAT5 is activated by several cytokines, including those that use the γc chain in their receptors (IL-2, IL-4, IL-7, IL-9, IL-15), those that use the common β chain (IL-3, IL-5, GM-CSF), as well as erythropoietin, prolactin, and growth hormone.105 Some of these cytokines, particularly IL-2 and IL-9, have been postulated to be RS cell growth factors. However, whereas STAT5 was constitutively phosphorylated in 3 of 5 RS cell lines, it was only detectable in RS cell nuclei from 26% of cHL cases.14 These data do not support a role for STAT5-dependent cytokines as autocrine growth factors in the majority of cHL cases.
NF-κ B signaling
One of the most important pathways of cell survival mediated via signaling through the TNFR family is the activation of NF-κB. The NF-κB family of transcription factors plays a pivotal role in inflammation, proliferation, and prevention of apoptosis.
A functional NF-κB molecule is a heterodimer composed of members of the Rel family of proteins, which includes RelA (p65), RelB, c-Rel, p50, and 52.117 The major form of NF-κB that is rapidly induced after stimulation is the RelA/p50 complex, and this dimer is what is commonly referred to as NF-κB. NF-κB is maintained in an inactive form in the cytoplasm by the inhibitor IκB, which binds to NF-κB and masks its nuclear localization signal.117There are several IκB proteins (IκB-α, IκB-β, IκB-ε) that are regulated differently and have different affinities for individual NF-κB complexes. IκB-α is the best characterized. IκB proteins contain several domains important for their function. The N-terminal region is essential for signal-mediated phosphorylation and subsequent degradation, the internal region contains multiple ankyrin repeats that are involved in binding to NF-κB complexes, and the C-terminal region is required for inhibiting DNA binding by NF-κB.
Activation of NF-κB through TNFR family members is mediated by the TRAF molecules. TRAFs are adaptor proteins that promote intracellular signal transduction linked to NF-κB activation by binding to receptors and recruiting other proteins to form an active signaling complex that ultimately triggers the activation of the IκB kinase complex (IKK).78 79 IKK then phosphorylates 2 serine residues in the N-terminus of IκB, allowing for the ubiquitinization and degradation of IκB, and release of NF-κB. NF-κB is then able to translocate to the nucleus where it stimulates the transcription of a wide variety of genes, including cytokines, cell adhesion molecules, and acute phase response proteins, which are involved in proliferation and survival as well as the inflammatory response.
Six distinct TRAF molecules have been identified, termed TRAF1 through TRAF6.78,79 Signal transduction via TRAF2, TRAF5, and TRAF6 is linked to NF-κB activation. TRAF2 and TRAF5 bind to many of the same receptors, including TNFRII, CD30, CD40, and RANK, and can also transduce signals from EBV LMP-1. TRAF6 binds to a more restricted group of receptors that includes CD40 and RANK. With respect to cHL, TRAF1, TRAF2, and TRAF6 are expressed in L-428 and KM-H2 cells,118 and TRAF1 and TRAF2 are commonly expressed in primary RS cells.118-120
Given the role of NF-κB in cell proliferation and survival, it is not surprising that constitutive NF-κB signaling has been implicated in oncogenesis (reviewed by Rayet and Gelinas121). Chromosomal amplification and rearrangements of genes encoding NF-κB family members have been identified in many hematolymphoid and solid tumors, including NHL, multiple myeloma, and carcinomas of the lung and breast.121 Constitutive nuclear NF-κB activity has been identified not only in cHL (discussed below), but also in activated B cell–like diffuse large B-cell lymphomas,122 acute lymphoblastoid leukemias,123 and in a wide variety of cell lines derived from solid tumors such as breast and prostate carcinomas.121
NF-κB and cHL.
Constitutive NF-κB activity is common in cultured and primary RS cells and is associated with proliferation and survival. Constitutive DNA binding of RelA/p50 dimers has been demonstrated in all RS cell lines examined.124-127 Constitutive NF-κB activity has also been seen in a multiple myeloma cell line, but was absent in ALCL and other NHL cell lines. In primary RS cells, NF-κB activation has been studied using an antibody that detects the nuclear localization epitope of RelA, meaning that it recognizes RelA only in its free form and not when it is bound to IκB. Nuclear and cytoplasmic staining of RelA was detected in the majority of RS cells from all cases of cHL, including both NS and MC subtypes.127 128 In contrast, activated RelA could not be detected in cases of NHL.
The consequences of constitutive NF-κB activity in RS cell lines were studied by overexpressing a dominant-negative form of IκB-α (IκB-αΔN). This molecule contains an N-terminal truncation which allows it to bind to NF-κB but not release it. Overexpression of IκB-αΔN in stably transfected RS cells reduced constitutive NF-κB DNA binding activity and markedly decreased cell proliferation.128 Although there was no effect on spontaneous cell death, IκB-αΔN–expressing cultured RS cells underwent apoptosis in response to serum starvation, whereas mock-transfected cells were resistant to apoptosis under the same conditions. Additionally, IκB-αΔN–expressing RS cells showed strongly diminished tumor growth in severe combined immunodeficiency mice.128 Adenovirus-mediated expression of IκB-αΔN allowed for even more efficient inhibition of NF-κB, and resulted in massive spontaneous apoptosis of RS cell lines.129 NF-κB activation promotes RS cell proliferation and survival by up-regulating expression of the cell-cycle regulator cyclin D2 and the antiapoptotic proteins A1, c-IAP2, TRAF1, and bcl-xL.129 Indeed, overexpression of bcl-xL in IκB-αΔN–expressing L428 cells partially rescued them from apoptosis. Bcl-xL is also expressed in primary RS cells in a large majority of cHL cases.130Another prosurvival target of NF-κB activation in cHL is CD40. A positive feedback loop may be established because activation of CD40 by CD40L leads to NF-κB activation.129
Two mechanisms of constitutive NF-κB activation in RS cells have been identified: constitutive stimulation of receptors that lead to NF-κB activation, and dysregulation of IκB-α–mediated control through mutations of the IKBA gene. HDLM-2 and L-1236 cells demonstrate constitutive NF-κB activation but do not have mutations of their IKBA genes.131,132 The high turnover rate of wild-type IκB-α in these cells suggests that the constitutive NF-κB activation in these cell lines is due to continuous upstream signaling through NF-κB activating pathways.126 TNFR family molecules and EBV LMP-1 are commonly expressed in primary RS cells and are known to activate NF-κB in various cell systems. In particular, activation of CD40133 and RANK89 has been shown to increase NF-κB DNA-binding activity in RS cell lines.
Mutations of IKBA gene.
Mutations of the IKBA gene leading to impaired regulation of NF-κB have been identified in a minority of cultured and primary RS cells. Mutations leading to C-terminally truncated IκB-α proteins have been identified in 2 of 6 RS cell lines.131,132 134In L-428 cells, a point mutation generating a premature stop codon in the IKBA gene potentially encodes a 266 amino acid mutant IκB-α protein missing the C-terminal domain. A mutant IκB-α protein of 30 kd was identified in L-428 cells and no wild-typeIKBA allele or normal IκB-α protein (38 kd) was detected. In KM-H2 cells, the IKBA gene contains a 213 nucleotide deletion that generates a mutant IκB-α protein missing the ankyrin repeats and C-terminal regions. This mutant IκB-α protein of 18 kd can only be detected when the cells are pretreated with proteasomal inhibitors, indicating that the mutant IκB-α protein is undergoing extremely rapid degradation. No wild-typeIKBA allele or normal IκB-α protein was detected in KM-H2 cells.
Mutations of the IKBA gene have also been identified in primary RS cells. Deletions within the IKBA gene were detected in enriched CD30+ primary RS cells from 2 of 8 cHL samples.131 In both cases, the IKBA gene contained deletions removing most of the ankyrin repeat region, thereby rendering the IκB-α protein incapable of binding to NF-κB. However, this work was done using only partially purified primary RS cells, so it was not conclusively shown that the IKBAmutations were resident in the malignant cell population. In 2 subsequent studies, IKBA mutations were demonstrated in primary RS cells using single-cell PCR. Emmerich et al sequenced theIKBA genes of RS cells from 10 cases of cHL.134In one case, one IKBA allele contained a point mutation introducing a stop codon such that a C-terminally truncated IκB-α protein would be produced; the other IKBA allele was wild-type. The IKBA gene from cells within the reactive infiltrate was also wild-type, indicating that the IKBAmutation was restricted to the RS cells. Jungnickel et al sequenced theIKBA gene of RS cells from 3 EBV− and 2 EBV+ cases of cHL.132 Mutations identified in the IKBA genes from 2 of the EBV− cases were restricted to the RS cells and absent in the reactive infiltrate. RS cells from one case had deletions of one nucleotide in oneIKBA allele and 2 nucleotides in the other allele. Both deletions led to frameshifts, precluding the synthesis of a full-length IκB-α protein by these cells. RS cells in the other case of EBV− cHL contained a deletion of 2 nucleotides in oneIKBA allele, whereas the other allele was wild-type. These data indicate that mutations of the IKBA gene are present in primary RS cells from a minority of cHL cases; however, the significance of monoallelic mutation is unclear, because loss of IκB-α activity would presumably require inactivation of both alleles.
The role of cytokines in pathologic and clinical features of cHL
Cytokines and RS cell proliferation and survival
Abnormal activation of NF-κB due to the action of TNFR-related molecules or mutations of the IKBA gene is clearly important for the proliferation and survival of RS cell lines. These mechanisms also likely occur in primary RS cells, because these cells usually express TNFR family molecules, are surrounded by cells expressing ligands for these receptors, and demonstrate nuclear localization of activated RelA. Additionally, IL-13 has been identified as an autocrine growth and survival factor for RS cell lines, and the expression of IL-13, IL-13Rα1, and nuclear P-STAT6 in primary RS cells indicates that IL-13 signaling is operational in vivo. Given that IL-13 and CD40 (a TNFR family member) promote the proliferation and survival of normal B-cells,135-137 a function for IL-13 and CD40 (and other TNFR members) as autocrine growth factors for RS cells is not surprising, because RS cells are derived from B cells.
Activation of NF-κB and IL-13 may promote cHL development by rescuing RS cell precursors from apoptosis. RS cells from the vast majority of cHL cases are thought to arise from B cells, demonstrating rearranged but nonproductive Ig genes,138,139 a situation that would normally lead to apoptosis.140 Moreover, these rearranged Ig genes show evidence of somatic hypermutation, indicating that RS cells are derived from B cells during germinal center formation. Normal B cells within the germinal center are destined to die unless they receive survival signals to rescue them from apoptosis.141Those germinal center B cells that acquire Ig gene mutations resulting in improved high-affinity antigen receptors will bind antigen on follicular dendritic cells, providing one of these survival signals. Additional survival signals are provided via CD40L expressed by activated, antigen-specific Th2 cells, the activity of which is enhanced by cytokines, especially IL-4. In cHL, RS cells lose Ig expression and therefore cannot receive survival signals through antigen binding, a situation that should lead to apoptosis. However, signaling through CD40L/CD40 and IL-4 or related molecules may rescue them from apoptosis.
Under physiologic conditions, survival signals delivered by T cells within the germinal center are very short-lived. Surface expression of CD40L on T cells is rapidly down-regulated on binding to CD40142 and IL-4 production by activated T cells is transient.143 CD40 stimulates proliferation in part through NF-κB activation. Constitutive activation of NF-κB in RS cell precursors can be achieved in a ligand-independent fashion through the action of EBV LMP-1 or by mutations of the IKBA gene leading to inactivation of IκB-α protein. In cHL cases that are negative for EBV and IKBA mutations, autocrine secretion of TNF-α and LT-α, activation of CD30 by surrounding CD30L+ eosinophils and mast cells, or activation of RANK by RANKL on RS cells are additional potential activators of NF-κB. Constitutive expression of IL-13 also provides another B-cell survival signal. IL-13 and CD40 activation promote B-cell survival through induction of bcl-xL,144 an antiapoptotic molecule that is commonly expressed by primary RS cells.130 Constitutive activation from these signals may prevent apoptosis and sufficiently prolong the survival of RS cell precursors such that other transforming events can occur. Even in fully developed cHL, it appears that RS cells continue to be dependent on these signals for their growth and survival.
Cytokines and the reactive infiltrate
Eosinophils.
Lymph nodes involved by cHL often show evidence of an eosinophilic infiltrate. Cytokines and chemokines may contribute to this eosinophilia both by increasing the production of eosinophils and stimulating their recruitment to the site of cHL involvement. IL-3, IL-5, and GM-CSF regulate eosinophilopoiesis in the bone marrow.145 IL-5 is particularly important, inducing terminal differentiation of immature eosinophils, stimulating the release of eosinophils into the circulation, and prolonging eosinophil survival. In the context of cHL, the expression of IL-5 correlates with tissue eosinophilia.23 Chemokines may also contribute to the recruitment of eosinophils to lymph nodes involved by cHL. As mentioned above, expression levels of eotaxin, a potent recruitment factor for eosinophils, correlate with tissue eosinophilia in cHL.49 Importantly, eotaxin expression in fibroblasts and airway epithelial cells in vitro can be stimulated by IL-13, an effect that shows synergy with TNF-α.146,147 Similarly, in vivo overexpression of IL-13 in the lung causes increased eotaxin production and eosinophilic infiltration.148 It is thus likely that both IL-5 and eotaxin (via IL-13 and TNF-α) make overlapping contributions to the eosinophilia commonly seen in cHL.
T cells.
T cells constitute a significant component of the reactive infiltrate in cHL. In immunocompetent cHL patients, the CD4/CD8 ratio within the reactive infiltrate is elevated to 4:1 to 6:1.149-152Moreover, the majority of CD4+ T cells in the infiltrate show features of Th2 cells.153 The increase in CD4+ T-cell numbers in cHL tumors is associated with a decrease in CD4+ T cells in the peripheral blood, consistent with a displacement of these cells from the circulation into the involved tissues.154 The CD4+ T cells forming rosettes around RS cells are not directed against a common antigen,155 and may be nonspecifically recruited to the site by chemoattractants such as TARC, MDC, and eotaxin produced by cHL tissues. The expression of these chemokines can be stimulated by IL-13 and TNF-α146,147,156-158 and is dependent on STAT6 signaling.110 111
CD8+ T cells make up a small proportion of the T-cell infiltrate and are typically not in close contact with RS cells.149 They do not appear to be directed against a common antigen.159 The numbers of CD8+ T cells are higher in EBV+ cases of cHL than in EBV−cases.160-162 Although EBV-specific cytotoxic T lymphocytes (CTLs) can be detected in the peripheral blood of patients with EBV+ cHL, no CTLs targeting EBV proteins expressed by RS cells (including LMP-1 and LMP-2) can be identified within the tumors.163 164 These data indicate that EBV-specific CTLs that could potentially target the RS cells either fail to penetrate the tumor site or fail to function within the tumor microenvironment. It is possible that anti-inflammatory cytokines produced by the surrounding cHL tissues are responsible for this effect (see below).
Fibrosis.
Nodular sclerosis HL is characterized by the presence of collagenous bands dividing the tumor into nodules. Newcom and O'Rourke showed that soluble factors from NSHL tissues may contribute to this fibrosis, because supernatants of cell cultures from primary NSHL cases can potentiate the growth of fibroblasts in vitro.165 The most likely candidate is TGF-β, a potent stimulator of fibroblast proliferation and collagen synthesis in vitro. As discussed previously, TGF-β is expressed by RS cells and eosinophils and is most consistently associated with the NSHL subtype. Other factors contributing to fibrosis may include basic fibroblast growth factor (bFGF), a molecule expressed by both RS cells and cells within the reactive infiltrate. Like TGF-β, bFGF expression is more prominent in NSHL cases than in MCHL cases.166 Indeed, a combination of TGF-β and bFGF causes persistent fibrosis in a murine model of cutaneous fibrosis.167
An emerging hypothesis for the pathogenesis of fibrosis in multiple diseases suggests that an unbalanced production of Th2 over Th1 cytokines causes an abnormal reaction to tissue damage that leads to fibrosis.168 The Th2 cytokine IL-13 has been implicated in the pathogenesis of fibrosis in several disease states, including pulmonary and hepatic fibrosis.148,169,170 In cHL, IL-13 is produced by a higher percentage of RS cells in NSHL compared to MCHL, and fibroblasts expressing IL-13Rα1 protein are present in higher numbers in NSHL cases.20 A role for IL-13 in fibrosis would be consistent with its ability to stimulate collagen synthesis in vitro.169,171 IL-13 is also a potent stimulator and activator of TGF-β in vivo, and mediates pulmonary fibrosis in a mouse model largely through activation of TGF-β.172
Cytokines and clinical symptoms
Patients with cHL often present with “B” symptoms, including fever, weight loss, and night sweats. Although the exact cause of these symptoms is unclear, it is thought that one or more cytokines elaborated by cHL tumors may be responsible. Several studies have examined the relationship between IL-6 expression in either serum or cHL tissues and the presence of “B” symptoms, with conflicting results. Two studies found that patients with “B” symptoms had higher serum IL-6 levels compared to asymptomatic patients,173,174 whereas 3 other studies found no such correlation.175-177 Foss et al did not detect a difference in the levels of IL-6 expression in cHL tissues between symptomatic and asymptomatic patients.26 Ree et al found that most patients with small- to medium-sized IL-1+ cells within the reactive infiltrate of their tumors had “B” symptoms; however, most patients with “B” symptoms did not have detectable numbers of these cells.93 Another study did not find a correlation between the presence of IL-1+ RS cells and “B” symptoms.66 Blay et al found that cHL patients had increased serum levels of IL-1 compared to healthy patients, but there was no correlation with “B” symptoms.176 Two other studies found elevated serum IL-1 levels to be rare in cHL patients.173,174 Benharroch et al found a correlation between expression of TNF-α by primary RS cells and the presence of “B” symptoms66; however, this was not found in another study.26 No correlation between serum TNF-α levels and “B” symptoms could be detected in cHL patients.173,174 176 In short, although the known biologic activities of many of these cytokines are consistent with constitutional symptoms seen in cHL patients, there is no firm evidence implicating any particular cytokine in their pathogenesis.
Cytokines and impairment of cellular immunity
Patients with untreated cHL frequently exhibit a systemic defect in cell-mediated immunity (reviewed by Slivnick et al178). The defect occurs in patients of all stages of disease and can persist in long-term survivors. Humoral immunity, on the other hand, is intact and often overactive, as exemplified by increased serum IgE levels in 30% to 50% of untreated cHL patients.179 180 The impairment in cell-mediated immunity is manifested as delayed hypersensitivity to new and recall antigens and increased susceptibility to bacterial, fungal, and viral infections. T-cell proliferation in response to T-cell mitogens and autologous mixed lymphocyte reactions is depressed, and in vitro synthesis of the Th1 cytokines IL-2 and IFN-γ is reduced. It is unclear whether the impaired cellular immune response is due to a primary cellular defect or the action of anti-inflammatory cytokines produced by cHL tissues. TGF-β (produced primarily by the NS subtype) and IL-10 (produced by EBV+ cHL cases) are both potent anti-inflammatory cytokines. The unbalanced production of Th2 cytokines could also prevent the development of an effective Th1-driven cell-mediated immune response.
Another potential mediator of the defect in cell-mediated immunity is soluble IL-2Rα. Many soluble receptors have the potential to act as antagonists preventing ligand binding to membrane-associated receptors.181 Soluble IL-2Rα is commonly produced by RS cell lines and can be detected in the serum of a majority of cHL patients. The presence of soluble IL-2Rα correlates strongly with disease stage, tumor burden, and a poor prognosis.182,183Serum levels of soluble IL-2Rα are also elevated in infectious, autoimmune, and other malignant disorders, where this molecule may play a role in immune suppression.184-186 Sera from patients with cHL demonstrate a suppressive effect on normal lymphocytes, inhibiting mitogen-induced T-cell proliferation. This effect was apparently due to soluble IL-2Rα because the suppression could be partially lifted by the addition of exogenous IL-2 and the removal of soluble IL-2Rα by immune absorption.187
Concluding remarks
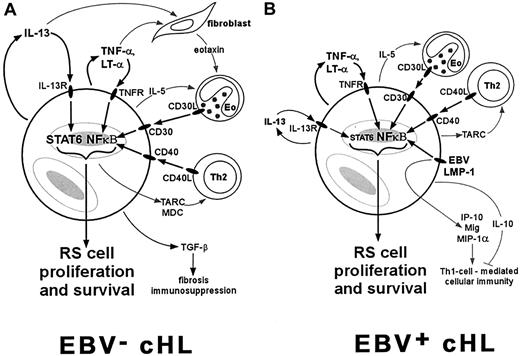
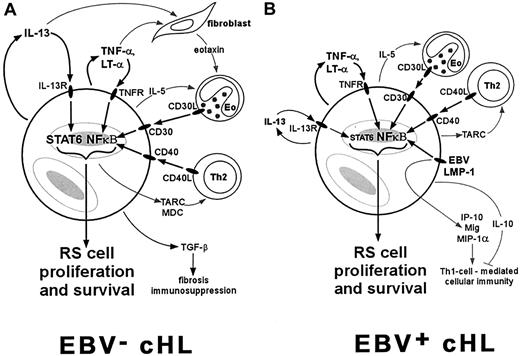
Most cases of cHL are characterized by constitutive activation of NF-κB and an overproduction of Th2 cytokines and chemokines, including IL-13, IL-5, TARC, MDC, and eotaxin (Figure1A). Activation of NF-κB in RS cells can occur through several mechanisms, including ligand-dependent signaling through various TNFR family members, EBV LMP-1 signaling, or inactivating mutations of the IKBA gene. NF-κB activators and IL-13 have been identified as important growth factors for RS cells, possibly rescuing RS cell precursors within the germinal center from apoptosis long enough for other transforming events to occur. The persistent activation of NF-κB and autocrine stimulation by IL-13 can also lead to the production of chemokines such as TARC, MDC, and eotaxin, which promote chronic pathophysiologic dysfunction characterized by an influx mainly of Th2 cells and eosinophils. These reactive cells are then able to contribute to RS cell proliferation by activating NF-κB via engagement of CD30L (on eosinophils) and CD40L (on Th2 cells). Other Th2 cytokines also support the reactive infiltrate, as exemplified by the effects of IL-5 on eosinophils. The overexpression of Th2 cytokines and TGF-β promotes the fibrosis characteristic of NSHL and suppresses cell-mediated immunity.
A scenario for the role of cytokines in EBV− and EBV+ cHL.
RS cells commonly express TNFR family members such as TNFR, CD30, and CD40, and Th2 cytokines such as IL-13 and IL-5. (A) In cases of NSHL, which are typically EBV−, activation of NF-κB through TNFR family members and activation of STAT6 through IL-13 can contribute to RS cell proliferation and to the production of chemokines such as eotaxin, MDC, and TARC. These chemokines recruit Th2 cells and eosinophils (Eo) that constitute the characteristic reactive infiltrate. These reactive cells also contribute to RS cell proliferation through CD30L/CD30 and CD40L/CD40 interactions. (B) In EBV+ cases, which are predominantly MCHL, RS cell proliferation and recruitment of the reactive infiltrate are stimulated by many of the same pathways. The presence of EBV, however, provides another pathway for NF-κB activation through LMP-1 expression, and may lead to the development of a Th1 cell–mediated immune response through up-regulation of IP-10, Mig, and MIP-1α. However, this cell-mediated response appears to be ineffective, perhaps due to the production of IL-10 by surrounding cHL tissues.
A scenario for the role of cytokines in EBV− and EBV+ cHL.
RS cells commonly express TNFR family members such as TNFR, CD30, and CD40, and Th2 cytokines such as IL-13 and IL-5. (A) In cases of NSHL, which are typically EBV−, activation of NF-κB through TNFR family members and activation of STAT6 through IL-13 can contribute to RS cell proliferation and to the production of chemokines such as eotaxin, MDC, and TARC. These chemokines recruit Th2 cells and eosinophils (Eo) that constitute the characteristic reactive infiltrate. These reactive cells also contribute to RS cell proliferation through CD30L/CD30 and CD40L/CD40 interactions. (B) In EBV+ cases, which are predominantly MCHL, RS cell proliferation and recruitment of the reactive infiltrate are stimulated by many of the same pathways. The presence of EBV, however, provides another pathway for NF-κB activation through LMP-1 expression, and may lead to the development of a Th1 cell–mediated immune response through up-regulation of IP-10, Mig, and MIP-1α. However, this cell-mediated response appears to be ineffective, perhaps due to the production of IL-10 by surrounding cHL tissues.
The above scenario fits well with the clinical features of NSHL, but is more problematic for MCHL, a subtype more commonly associated with EBV compared to NSHL. The presence of EBV may be altering the expression of cytokines and chemokines (Figure 1B). Constitutive NF-κB activation is consistently present in RS cells from MCHL; however, although RS cells in MCHL commonly express IL-13, STAT6 activation is seen in only half of MCHL cases. Presumably other cytokines may be contributing to the growth of RS cells in this subtype. Moreover, although Th2 cytokines (IL-13, IL-5, IL-6) and Th2 chemokines (TARC) are usually expressed within MCHL tissues, there is an apparent attempt to mount a Th1-driven cell-mediated immune response. The expression of IL-12, which is responsible for Th1-cell differentiation, and chemokines that support a Th1 response (IP-10, Mig, MIP-1α) are expressed at higher levels in EBV+ cHL cases than in EBV− cases. Accordingly, CD8+ T cells are more numerous in the reactive infiltrate of EBV+cases. However, this attempt at a cell-mediated immune response in EBV+ cases appears to be ineffective, because there is a local suppression of cytotoxic T cells specifically targeting EBV antigens. This suppression may be due to the presence of IL-10, a potent anti-inflammatory cytokine frequently produced by RS cells in EBV+ cHL cases.
Detailed understanding of the different cytokines and chemokines that contribute to the pathogenesis of cHL has provided insights into the pathophysiology, immune dysfunction, and symptomatology associated with this disease. It is hoped that this understanding of the role of cytokines will translate into the development of novel treatment strategies for patients with cHL. Therapeutics that target those cytokines and signaling pathways that contribute to the proliferation and survival of RS cells, or rectify the cell-mediated immune defect that causes an impaired antitumor response, may be helpful in these patients. However, given the important role of cytokines in the overall function of normal immune and inflammatory reactions, such therapeutic strategies must be carefully chosen as to not significantly impair the overall immune function of these patients during treatment.
The authors thank Dr Randy D. Gascoyne and Dr Joseph M. Connors for critical review of the manuscript and Dr Mary Saunders for scientific editing.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2002-01-0099.
Supported in part by grants from the Canadian Institute of Health Research and the National Cancer Institute of Canada.
References
Author notes
Tak W. Mak, Amgen Research Institute/Ontario Cancer Institute, 620 University Ave, Ste 706, Toronto, ONT, Canada M5G 2C1; e-mail: tmak@oci.utoronto.ca.