The cytolytic function of natural killer (NK) cells is induced by the engagement of a series of activating receptors and coreceptors some of which have recently been identified and collectively termed natural cytotoxicity receptors (NCRs). Here, we analyzed the cytolytic function of NK cells obtained from patients with acute myeloid leukemia (AML). In sharp contrast with healthy donors, in most (16 of 18) patients with AML the majority of NK cells displayed low NCR surface density (NCRdull). This phenotype correlated with a weak cytolytic activity against autologous leukemic cells that could not be reversed by the monoclonal antibody-mediated disruption of HLA class I/killer immunoglobulinlike receptor interaction. The remaining 2 patients were characterized by NK cells having an NCRbright phenotype. Surprisingly, although displaying NCR-mediated cytolytic activity, these NCRbright NK cells were unable to kill autologous leukemic blasts. Importantly, the leukemic blasts from these 2 patients were also resistant to lysis mediated by normal NCRbrightallogeneic NK cells. Our study suggests that in most instances the inability of NK cells to kill autologous leukemic blasts is consequent to low NCR surface expression. In few cases, however, this failure appears to involve a mechanism of tumor escape based on down-regulation of ligands relevant for NCR-mediated target cell recognition.
Introduction
Human natural killer (NK) lymphocytes are potent effector cells involved in clearance of virus-infected cells and tumors. Their cytolytic function is regulated by the expression of a series of surface receptors that either block or enhance the NK-mediated cytotoxicity. Under physiologic conditions, target cells are protected from NK-mediated cytotoxicity because they express an adequate amount of HLA class I molecules. Indeed, NK cells express at the cell surface HLA-specific inhibitory receptors (including killer immunoglobulinlike receptors [KIRs] and CD94/NKG2A heterodimers) that on recognition of the ligands on normal target cells down-regulate the NK-mediated cytolytic activity.1,2 In the absence of these inhibitory interactions, target cells become susceptible to NK-mediated killing.3-5 As recently demonstrated, this susceptibility is due to the expression by NK cells of different non–HLA-specific activating receptors that are involved in the induction of cytotoxicity.4 Three of these molecules, NKp46, NKp30, and NKp44 (collectively termed natural cytotoxicity receptors [NCRs]), are selectively expressed by NK cells, and their engagement results in a strong enhancement of the NK-mediated cytolytic activity.6 Importantly, NCRs play a crucial role in NK-mediated recognition and killing of most target cells because monoclonal antibody (mAb)–mediated disruption of the NCR/ligand(s) interactions may abrogate the NK-mediated killing of tumor-transformed cells of autologous, allogeneic, or (in the case of NKp46) xenogeneic origin.7 Interestingly, the NCR surface density, as measured by the brightness of immunofluorescence, varies among individuals. Thus, NK cells from some donors express a homogeneously high density of NCR (NCRbright), whereas in other individuals 2 subsets of NK cells expressing either high (NCRbright) or low (NCRdull) receptor densities were detected.7,8 Importantly, a strict correlation exists between NCR density and NK-mediated cytolytic activity.7Thus, although NCRbright NK cell clones display strong cytolytic activity, those expressing low NCR surface density (NCRdull) are poorly or even noncytolytic against most target cells.
Another surface receptor playing a role in the induction of NK-mediated cytotoxicity is represented by NKG2D, a receptor that, different from NCRs, is also expressed by virtually all T-cell receptor (TCR)γδ+ and CD8+ TCRαβ+cells.9-11 Its expression does not correlate with that of NCRs because similar levels of NKG2D are detected in both NCRbright and NCRdull NK cells.4It has been recently demonstrated that NKG2D plays a role in the NK cell–mediated cytotoxicity against certain target cells either by acting in concert with NCR (in NCRbright NK clones) or by representing the main receptor (in NCRdull NK cells).11 Although NCR-specific ligands expressed on tumor cells are still unknown, at least some of the NKG2D-specific ligands have been characterized. These ligands include the MICA and MICB stress-inducible molecules12 and the ULBP (UL16-binding protein) major histocompatibility complex class I–related molecules.13
An altered HLA class I phenotype has been observed in most human tumors,5,14,15 More particularly, a deficient HLA class I expression has been described in leukemia,16-18 with a quite variable level of expression for specific alleles.19Moreover, allogeneic bone marrow transplantation suggests that NK cells may have potent antileukemia activity.20-23 Previous reports have focused on the role of NK cells in the control of chronic myelogenous leukemia (CML).24-26 For example, it has been shown that in CML the NK cell number and the NK cell function progressively decrease during the spontaneous course of the disease27 and that both recover on interferon-α treatment.28 Moreover, activated autologous NK cells suppress primitive CML progenitors in long-term culture,26whereas BCR-ABL fusion protein does not prevent NK-mediated apoptotic cell death.29 The acute myelogenous leukemias (AMLs) are life-threatening hematologic malignancies in which immunotherapy could contribute to better clinical outcome. It has been shown that in patients with AML the NK cell activity correlates positively with the relapse-free survival in patients in complete remission.30Moreover, NK cells adhere to bone marrow fibroblasts competing with myeloid, but not with lymphoid, leukemic blasts for binding to the microenvironment, suggesting that they may inhibit leukemic cell growth.31 In a mice model, NK cells were shown to inhibit radiation-induced lymphomagenesis32 and to control the tumorigenesis induced by human T-cell leukemia virus.33 34
Altogether these data suggest that NK cells may play an important role in the control and clearance of leukemic cells. So far, however, little is known regarding the direct role of the various activating receptors/coreceptors in the NK-mediated control of leukemia.
The aim of the present study was to analyze the NK cell function in patients affected by AML and, in particular, to define the role of the various activating receptors in the recognition and killing of leukemic cells.
Materials and methods
Cells
Peripheral blood samples were obtained from patients or healthy blood donors after informed consent. Approval was obtained from the Institutional Review Board (Institut Paoli-Calmettes) for these studies. Informed consent was provided according to the Declaration of Helsinki. Peripheral blood samples from patients were obtained before specific antileukemic therapy and were part of diagnostic procedures. Slides were independently reviewed by 2 morphologists (D.S. and C.A.). Diagnosis was established by cytologic criteria based on the French-American-British (FAB) classification. We analyzed 18 AMLs from M1 to M5 FAB subtypes.
Peripheral blood mononucleated cells (PBMCs) from healthy donors or leukemic patients were isolated on Ficoll-Hypaque gradients and viably frozen in liquid nitrogen until use. For cytotoxicity experiments using leukemia cells as targets, only samples with 95% or more leukemic cells were used; the purity of the preparation was assessed by flow cytometry analysis and Giemsa stain of cytocentrifuge preparations.
Monoclonal antibodies
The following mAbs were produced in our laboratory (A.M.; Molecular Immunology Laboratory): E59-109 (immunoglobulin G1 [IgG1], anti-HLA-I), JT3A (IgG2a, anti-CD3), c127 (IgG1, anti-CD16), BAB281 (IgG1, anti-NK p46), AZ20 (IgG1, anti-NK p30), Z231 (IgG1, anti-NK p44), PP35 (IgG1, anti-2B4), XA185 (IgG1, anti-CD94), Z270 (IgG1, anti-NKG2A), BAT221 (IgG1, anti-NKG2D), A6-136 (IgM, anti HLA class I), KS38 (IgM, anti-NKp44), and KL247 (IgM, anti-NK p46). Commercially available fluorescein isothiocyanate (FITC)– or phycoerythrin (PE)–labeled mAbs were used to control cell lineage; anti-CD19, -CD20, -CD14, -CD33 (Immunotech, Marseille, France).
Flow cytometry analysis
When using our own produced mAbs, we performed indirect staining; briefly, cells were incubated with the appropriate mAb followed by PE- or FITC-conjugated isotype-specific goat antimouse secondary reagent (Southern Biotechnology Associated, Birmingham, AL). Directly coupled commercially available mAbs were used in one-step direct staining. Samples were analyzed by one- or 2-color cytofluorimetric analysis (FACScan; Becton Dickinson, Mountain View, CA). In all experiments, isotype-matched controls were used to set up the negative values.
NK cell lines derived from healthy and leukemic patients
To obtain NK cell lines from healthy donors or leukemic patients, PBMCs were depleted of plastic-adherent cells. Then, CD3−CD4−DR− cells were obtained by incubating peripheral blood lymphocytes with anti-CD3 (JT3A), anti-CD4 (HP2.6), and anti-HLA DR (D1.12) mAbs (30 minutes at 4°C) followed by immunomagnetic depletion with goat antimouse-coated Dynabeads (Dynal, Oslo, Norway) (30 minutes at 4°C). CD3−CD4−DR− cells were cultured on irradiated feeder cells in the presence of 100 U/mL recombinant interleukin 2 (rIL-2; Proleukin; Chiron, Emeryville, CA) and 1.5 ng/mL phytohemagglutinin (PHA; Gibco, Paisley, Scotland) to obtain activated polyclonal NK cell population.8
Cell culture and cytolytic activity
Cell cultures were performed in RPMI 1640 (Bioproducts, Walkersville, MD) with 10% fetal bovine serum (Bioproducts). The cytotoxic assays were performed as previously described8 by means of a 4-hour51chromium-release assay. The concentrations of the various mAbs were 10 μg/mL for the masking experiments and 0.5 μg/mL for the redirected killing experiments. All experiments were performed in triplicate in at least 2 independent experiments.
Results
Phenotypic analysis of NK cells derived from patients with AML at diagnosis
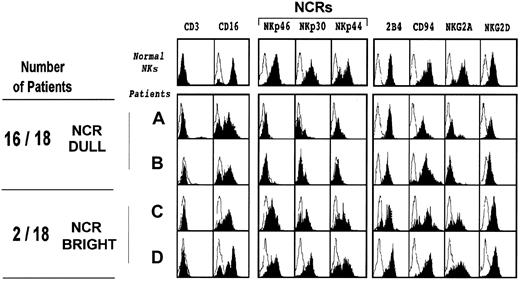
Fresh NK cells were isolated from 18 patients with AML at diagnosis and before any chemotherapy. In most patients these cells (CD56+/CD3−) represented less than 1% of the PBMC population. Purified CD3− cells were cultured in vitro in the presence of rIL-2 to obtain polyclonal-activated NK cell populations, thereafter termed AML-NK cells. These cells were then analyzed for surface phenotype by indirect immunofluorescence and cytofluorimetric analysis (Figure 1). No significant differences could be observed between AML-NK cells and NK cells derived from healthy donors regarding the expression of surface molecules such as CD16, 2B4, CD94, and NKG2D. However, most (16 of 18) of the patients analyzed displayed a rather homogeneous weak expression of the NKp46 receptor. Moreover, in all instances the low expression of NKp46 was paralleled by low expression of NKp30 and NKp44. Cells characterized by this surface phenotype were previously described in healthy individuals as NK cells with NCRdullphenotype.7 It is of note, however, that in healthy donors the NCRdull phenotype is found in a subset of NK cells and only in rare cases (< 1 of 20) corresponds to the majority of NK cells. Thus, it appears that in patients with AML the NCRdull phenotype is significantly more represented than in healthy individuals. The remaining 2 patients with AML expressed an NCR surface density that was comparable to the NCRbrightphenotype observed in most healthy individuals. Moreover, in line with previous reports on NK cells derived from healthy individuals,7 the expression of NKG2A was confined to AML-NK cells expressing the NCRbright phenotype.
Phenotypic analysis of NK cells from patients with acute leukemia shows abnormal NCRdull expression.
NK cells were cultured as described in “Materials and methods” and then analyzed by flow cytometry for expression of the indicated molecules. Blank areas correspond to isotype-matched negative controls. The first row corresponds to the analysis of NK cells from a representative healthy donor, whereas the 4 other rows show the phenotype of NK cells obtained from patients with AML (NK-AML cells) at diagnosis (before chemotherapy). All phenotypes were controlled at least 3 times during the culture period (from 2 to 7 weeks), and no major changes in NK phenotypes were observed. The 4 examples of NK-AML are representative of the 18 patients analyzed.
Phenotypic analysis of NK cells from patients with acute leukemia shows abnormal NCRdull expression.
NK cells were cultured as described in “Materials and methods” and then analyzed by flow cytometry for expression of the indicated molecules. Blank areas correspond to isotype-matched negative controls. The first row corresponds to the analysis of NK cells from a representative healthy donor, whereas the 4 other rows show the phenotype of NK cells obtained from patients with AML (NK-AML cells) at diagnosis (before chemotherapy). All phenotypes were controlled at least 3 times during the culture period (from 2 to 7 weeks), and no major changes in NK phenotypes were observed. The 4 examples of NK-AML are representative of the 18 patients analyzed.
Analysis of the cytolytic activity mediated by AML-NK cells
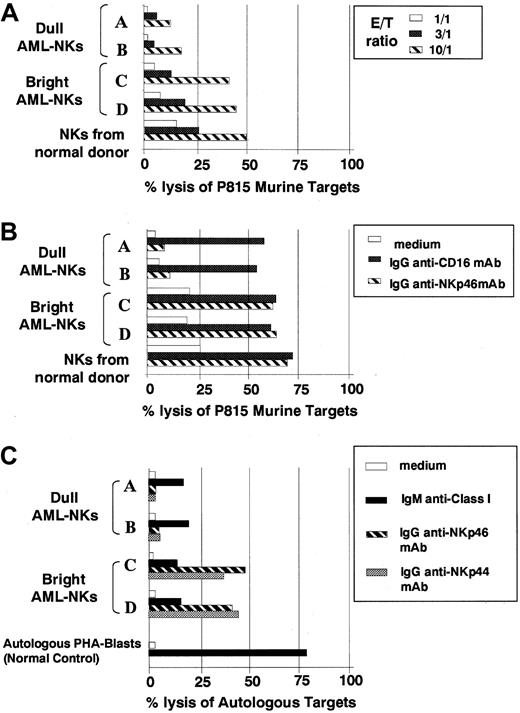
Previous studies demonstrated that the ability of human NK cells to kill murine targets correlates with the surface density of the NKp46 receptor.4 7 Indeed, in healthy individuals, killing of murine target cells such as the (FcγR+) P815 mastocytoma cell line is mainly confined to NK cells expressing the NCRbright phenotype, and mAb-mediated blocking of the NKp46 receptor is sufficient to abrogate this cytolytic effect.
AML-NK cells were analyzed for spontaneous cytolytic activity against P815 target cells (Figure 2A). In line with results obtained in healthy donors, NK cells derived from AML patients characterized by NCRdull phenotype displayed poor cytolytic activity against these target cells. On the contrary, NK cells derived from AML patients characterized by NCRbrightphenotype displayed a cytolytic activity comparable in magnitude to that of NCRbright NK cells derived from healthy donors.
Cytolytic activity of NK cells derived from patients with acute leukemia.
Cultured AML-NK cells at diagnosis were tested, in comparison with control NKs from a healthy donor, for cytotoxicity against P815 murine target (A,B). (A) The spontaneous NK-mediated cytotoxicity was evaluated at different effector-to-target ratios (10:1, stripped bars; 3:1, black bars; 1:1, white bars). (B) NK cells were analyzed in redirected killing experiments either in the absence (white bars) or in the presence of IgG1 mAb directed to anti-CD16 (black bars) or anti-NKp46 (stripped bars) mAbs, at an effector-to-target (E/T) ratio 3:1. (C) The same 4 representative AML-NK cells were assessed for cytolytic activity against autologous AML blasts either in the absence (white bars) or presence (black bars) of an anti-HLA class I mAb (of IgM isotype) at an E/T ratio of 10:1. The same effector and target cells were also tested in the presence of anti-NKp46 (striped bars) or anti-NKp44 (shaded bars) of IgG1 isotype in a redirected killing assay in which the mAb is substituting the natural ligands for the receptors. The normal control is represented by a polyclonal NK cell population derived from a healthy individual that has been assessed for cytotoxicity against autologous PHA blasts. These panels are representative of 3 different experiments, and similar results were obtained with NK cells from 8 additional AML patients.
Cytolytic activity of NK cells derived from patients with acute leukemia.
Cultured AML-NK cells at diagnosis were tested, in comparison with control NKs from a healthy donor, for cytotoxicity against P815 murine target (A,B). (A) The spontaneous NK-mediated cytotoxicity was evaluated at different effector-to-target ratios (10:1, stripped bars; 3:1, black bars; 1:1, white bars). (B) NK cells were analyzed in redirected killing experiments either in the absence (white bars) or in the presence of IgG1 mAb directed to anti-CD16 (black bars) or anti-NKp46 (stripped bars) mAbs, at an effector-to-target (E/T) ratio 3:1. (C) The same 4 representative AML-NK cells were assessed for cytolytic activity against autologous AML blasts either in the absence (white bars) or presence (black bars) of an anti-HLA class I mAb (of IgM isotype) at an E/T ratio of 10:1. The same effector and target cells were also tested in the presence of anti-NKp46 (striped bars) or anti-NKp44 (shaded bars) of IgG1 isotype in a redirected killing assay in which the mAb is substituting the natural ligands for the receptors. The normal control is represented by a polyclonal NK cell population derived from a healthy individual that has been assessed for cytotoxicity against autologous PHA blasts. These panels are representative of 3 different experiments, and similar results were obtained with NK cells from 8 additional AML patients.
AML-NK cells were also evaluated against P815 in a redirected killing assay in the presence of anti-CD16 or anti-NKp46 mAbs (Figure 2B). In agreement with previous data7 obtained with NK cells from healthy individuals, anti-CD16 mAb induced a comparable enhancement of cytotoxicity both in NCRdull and NCRbrightAML-NK cells. However, the enhancement of cytotoxicity induced by anti-NKp46 mAb was confined to NCRbright AML-NK cells, whereas only little effects were observed in NCRdull AML-NK cells.
Altogether these results suggest that AML-NK cells expressing different NCR surface densities display functional properties comparable to the corresponding NCRdull and NCRbright NK cells derived from healthy individuals.
Analysis of the cytolytic activity mediated by AML-NK cells against autologous leukemic target cells
AML-NK cells from patients displaying NCRdull or NCRbright phenotype were analyzed for cytolytic activity against autologous leukemic cells. These target cells, as determined by the use of mAb directed to a monomorphic determinant of HLA class I, expressed HLA class I molecules, although, in most instances, the level of fluorescence was slightly lower as compared with B-lymphocyte cell lines (B-LCLs) derived from the same individuals (not shown). These findings suggested that in these patients the expression of HLA class I may, at least in part, protect leukemic cells from NK-mediated cytotoxicity. As predicted by their surface phenotype, NCRdull AML-NK cells displayed poor spontaneous cytotoxicity against both autologous (Figure 2C) and allogeneic (not shown) leukemic cells. Moreover, the low levels of cytolytic activity were only moderately (from 3%-4% to 15%-18% lysis) incremented in the presence of anti-HLA class I mAbs, indicating that the poor cytolytic effect was not simply consequent to inhibitory signals generated by KIR/HLA class I interactions (Figure 2C).
In line with their ability to kill murine P815 target cells, AML-NK cells characterized by the NCRbright phenotype displayed strong cytolytic activity against allogeneic leukemic blasts derived from NCRdull patients (not shown). Surprisingly, however, these NCRbright AML-NK cells were poorly cytolytic against autologous leukemic cells even in the presence of anti-HLA class I mAbs (Figure 2C). Moreover, an NK cell population derived from a healthy individual (used as a control) was able to kill autologous PHA blasts in the presence of anti-HLA class I mAb. Thus, AML-NK cells, independent from their NCR phenotype, are characterized by the inability to kill autologous AML blasts. In the same set of experiments we also evaluated whether AML-NK cells derived from NCRbright patients were able to kill autologous AML blasts under conditions in which the need for NCR ligands is substituted by anti-NCR mAbs. Thus, we performed a redirected killing assay in which different AML-NK cell populations were assessed for cytolytic activity against autologous (FcγR+) AML blasts in the absence or in the presence of IgG1 mAbs specific for NKp46 or NKp44 molecules. It can be seen (Figure 2C) that AML-NKbrightcells efficiently killed autologous AML blasts in the presence of either anti-NKp46 or anti-NKp44 mAb. However, AML-NKdullcells, although responsive to anti-CD16 mAb, gave poor responses in the presence of anti-NCR mAb.
Susceptibility of AML blasts to killing mediated by normal allogeneic NK cells
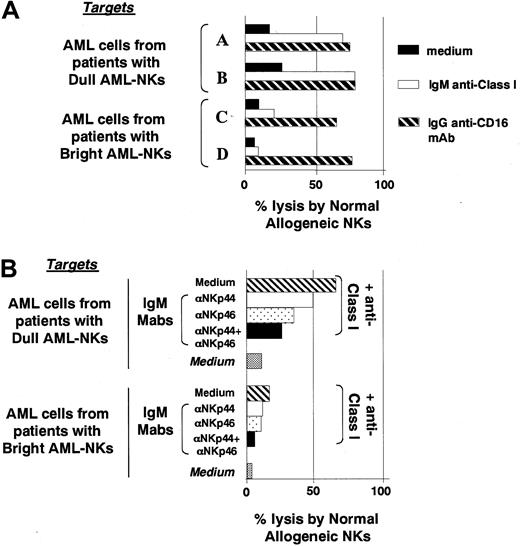
Altogether, the above results suggested that the low levels of cytolytic activity displayed by AML-NK cells against the autologous leukemia may reflect the existence of insufficient NCR/ligand interactions. In NCR[dull] AML-NK cells this defect may be easily explained by the low number of NCR molecules at the NK cells surface. On the contrary, in the case of AML-NK cells characterized by an NCRbright phenotype, the inability to kill autologous leukemic blasts may be due to an insufficient expression of NCR ligands on leukemic blasts.
To verify this possibility we analyzed the different leukemic cells for susceptibility to killing by NK cells derived from unrelated healthy individuals. A representative experiment is shown in Figure3A in which the cytolytic activity against leukemic blasts was evaluated in the absence or in the presence of anti-HLA class I mAb (to prevent KIR/HLA interactions). It is evident that under these conditions, unlike autologous AML-NK cells (Figure 2C), normal NCRbright allogeneic NK cells displayed strong cytolytic activity against leukemic cells derived from NCRdull patients. On the contrary, the same normal NK cells displayed poor cytotoxicity against leukemic cells derived from NCRbright patients even in the presence of anti-HLA class I mAb. In line with data presented in Figure 2C, AML blasts (FcγR+) derived from NCRbright patients displayed a high susceptibility to killing when used as target cells in redirected killing experiments performed in the presence of anti-CD16 mAb (Figure 3A). This finding (together with data of Figure 2C) ruled out the possibility that in the case of AML blasts derived from NCRbright patients the low susceptibility to lysis may be due to an intrinsic resistance of leukemic cells to NK-mediated killing. Altogether, these experiments indicate that leukemic blasts derived from NCRbright patients are resistant to lysis mediated not only by autologous (Figure 2C) but also by normal allogeneic NCRbright NK cells (Figure 3A).
Killing of AML blasts by normal allogeneic NK cells.
(A) The lysis of AML blasts by normal allogeneic NCRbrightNK cells was evaluated either in the absence (black bars) or in the presence of anti-HLA class I mAb of IgM isotype (white bars) or of anti-CD16 mAb of IgG1 isotype (striped bars). The latter was used to induce redirected killing against AML blasts that are FcγR+. (B) The cytolytic activity mediated by a normal allogeneic NK cell population was evaluated in the presence of anti-NCR mAbs (of IgM isotype) as indicated. In blocking experiments the anti-NCR mAbs used were in all instances of IgM isotype to avoid redirected killing effects because of the presence of FcγR on leukemia target cells. These experiments were performed at an E/T ratio of 10:1.
Killing of AML blasts by normal allogeneic NK cells.
(A) The lysis of AML blasts by normal allogeneic NCRbrightNK cells was evaluated either in the absence (black bars) or in the presence of anti-HLA class I mAb of IgM isotype (white bars) or of anti-CD16 mAb of IgG1 isotype (striped bars). The latter was used to induce redirected killing against AML blasts that are FcγR+. (B) The cytolytic activity mediated by a normal allogeneic NK cell population was evaluated in the presence of anti-NCR mAbs (of IgM isotype) as indicated. In blocking experiments the anti-NCR mAbs used were in all instances of IgM isotype to avoid redirected killing effects because of the presence of FcγR on leukemia target cells. These experiments were performed at an E/T ratio of 10:1.
To directly evaluate the involvement of NCRs in AML killing by allogeneic NCRbright normal NKs, these cells were assessed for cytolytic activity either in the absence or in the presence of mAbs specific for NCRs. In this case, the mAbs used to block the NCR-mediated recognition of AML target cells were of IgM isotype. This isotype was necessary to avoid redirected killing effects against the FcγR+ AML blasts. A representative experiment is shown in Figure 3B. It can be seen that mAb-mediated masking of NKp46 and/or NKp44 induced a significant inhibition of the cytolytic activity against leukemic cells from a patient with the NCRdullNK-AML. Similar results were obtained in 8 other AMLs with NCRdull NK-AMLs. Thus, lysis of AML blasts by normal NK cells appears to be highly dependent on NCR function.
NK-mediated killing of B-LCLs derived from patients with AML
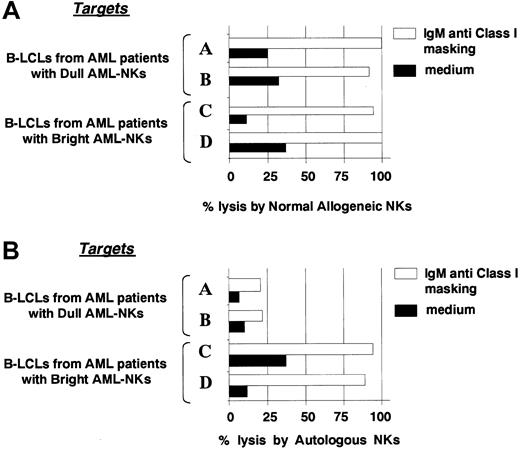
Further studies were performed by using as target cells B-LCLs derived from the various AML patients. These cells were first analyzed for susceptibility to killing by NK cells derived from healthy donors either in the absence or in the presence of anti-HLA class I mAb. As shown in Figure 4A in the presence of anti-HLA class I mAb, all B-LCLs were susceptible to killing mediated by normal NK cells. Moreover, in line with previous reports4 killing could be inhibited by the use of anti-NCR mAbs (not shown). These results suggest that, unlike leukemic cells, B-LCLs derived from all the AML patients analyzed normally express ligand(s) able to activate NCR functions. This finding was confirmed by experiments in which B-LCLs were analyzed for susceptibility to killing by autologous AML-NK cells (in the presence of anti-HLA class I mAb). As shown in the representative experiment reported in Figure 4B, AML-NK cells displaying the NCRdull phenotype were poorly cytolytic against autologous B-LCLs, whereas those characterized by the NCRbright phenotype displayed strong cytotoxicity.
Killing of B-LCLs derived from leukemic patients by normal allogeneic or autologous NK cells.
(A) The same normal NK cell population reported in Figure 3 was assessed for cytotoxicity against 4 different B-LCLs derived from the same 4 representative patients. (B) B-LCLs were assessed for susceptibility to killing by autologous AML-NK cells. In both cases the cytolytic assays were performed either in the absence (black bars) or in the presence (white bars) of anti-HLA class I mAb. The E/T ratio was 12:1. This experiment is representative of 3 independent assays.
Killing of B-LCLs derived from leukemic patients by normal allogeneic or autologous NK cells.
(A) The same normal NK cell population reported in Figure 3 was assessed for cytotoxicity against 4 different B-LCLs derived from the same 4 representative patients. (B) B-LCLs were assessed for susceptibility to killing by autologous AML-NK cells. In both cases the cytolytic assays were performed either in the absence (black bars) or in the presence (white bars) of anti-HLA class I mAb. The E/T ratio was 12:1. This experiment is representative of 3 independent assays.
Discussion
In the present study we show that leukemic cells derived from patients with AML are generally poorly susceptible to lysis mediated by autologous NK cells and that this defect is likely due to insufficient numbers of NCR/ligand(s) interactions. In most of the patients analyzed, this defect is consequent to the low (dull) expression of NCR, ie, the receptors that are crucial for target cell recognition and for induction of NK-mediated cytotoxicity.4 6 In the remaining patients the poor NK-mediated cytotoxicity against autologous leukemia blasts appears to be the result of a mechanism of tumor escape based on a defective expression of surface ligands by leukemic cells.
In most instances no dramatic down-regulation of HLA class I expression has been detected in the AML blasts analyzed. However, it is well established that transformed cells may selectively down-regulate only one or few alleles and that, in this case, the use of anti-HLA class I mAb specific for monomorphic determinants may not be sufficient to reveal a partial HLA class I–deficiency.3,5,19 Thus, it is possible that at least some of the leukemic blasts analyzed are carrying a HLA class I–defective phenotype. If this is the case, in principle AML blasts should become susceptible to cytotoxicity mediated by NK cells expressing the relevant HLA-specific inhibitory receptor.1,2 However, our present data suggest that the protective role of HLA class I may be incremented by additional mechanisms that are based on defective NCR/ligand(s) interactions. Indeed, in most cases our functional analysis of AML-NK cells against leukemic blasts has been performed both in the absence and in the presence of anti-HLA class I mAb. The disruption of the interaction between HLA class I and the inhibitory receptors expressed at the AML-NK cell surface should allow these cells to kill autologous leukemic blasts.1,2 Because our results indicate that killing of autologous AML blasts cannot significantly be incremented in the presence of anti-HLA class I mAb, we may conclude that the mechanism of protection of AML blasts from autologous AML-NK cells is not simply based on “inhibition of lysis.” Rather, our present data suggest an additional mechanism that renders AML-NK cells unable to attack autologous leukemia is based on “lack of NK cell triggering.” The latter mechanism, however, is likely to be relevant especially in those leukemias that have first lost HLA class I expression. A possible approach to the analysis of HLA class I down-regulation in patients with AML is provided by the study of NK clones expressing inhibitory surface receptors for one or another HLA class I molecules. Along this line we observed that in most instances NK clones derived from healthy donors and expressing CD94/NKG2A as the unique HLA class I–specific inhibitory receptor were capable of killing AML blasts. Moreover, their cytolytic activity could not be significantly increased by anti-HLA class I mAb (not shown). The same NK clones were unable to kill target cell transfectants expressing appropriate HLA class I ligands (used as controls), and, in this case, lysis could be reverted by anti-HLA class I mAb. This finding suggests that the surface density of the “nonclassical” HLA-E molecules may be, at least in part, decreased in the various AMLs analyzed possibly consequent to down-regulation of one or more “classical” HLA class I molecules. In this context we also observed that a NK clone expressing the p58.1 (KIRD2L1) receptor (but lacking other inhibitory receptors) was able to kill at least 2 AMLs expressing the appropriate HLA-C alleles (not shown). However, the same AML blasts appeared to express sufficient amounts of other HLA class I alleles as suggested by the observation that NK clones expressing other inhibitory receptors (p70/KIR3DL1 or p140/KIR3DL2) were unable to kill AML blasts expressing the appropriate HLA class I ligands. Importantly, in this case, lysis could be reverted by anti-HLA class I mAbs. These data would indicate that one or more (but not all) HLA class I alleles may be defective in the AML blasts of the patients analyzed. This in turn would allow NK cells expressing adequate amounts of NCR to kill AML blasts because of insufficient interactions between HLA class I and their inhibitory receptors. Remarkably, in most (16 of 18) patients analyzed in this study the lack of NK cell triggering was consequent to the dull expression of NCR. Indeed, normal NK cells were able to kill leukemic blasts derived from this group of patients, whereas autologous NK cells characterized by the NCRdull phenotype were not. Previous studies indicated that in normal NK cells the NCRdull phenotype is stable and is not modified on in vitro culture.7
However, no information is available so far on the mechanisms regulating the level of NCR expression in NK cells and whether the NCR surface densities may be under the control of the microenvironment by cell-to-cell contacts and/or cytokine secretion. In this context, we performed cocultures of NCRbright AML-NK with leukemic cells from NCRdull patients; no substantial changes in NCR surface density could be detected in 4 independent experiments (data not shown). Identical results were obtained by using supernatants from cultured leukemias (data not shown). These experiments suggest that leukemic blasts are not directly involved in NCR down-regulation by cell-to-cell contact or by the release of soluble factors (not shown). Moreover, we excluded the leukemic origin of AML-NKs by performing interphase nuclei fluorescent in situ hybridization on cytospin preparations of NKs from 3 patients selected for the presence of trisomy 8 in their blast cells at diagnosis. In the 3 patients with trisomy 8, all AML-NK cells presented with 2 fluorescent signals, thus excluding the presence of trisomy 8 (data not shown).
The down-regulation of the TCR-associated CD3-ζ chains is one of the mechanisms leading to abnormal immune responses in hematologic malignancies, including chronic lymphocytic leukemia,35myeloid malignancies,36 and Hodgkin disease37or in other cancers.38,39 Importantly, CD3-ζ chains are involved in the transduction of activating signals also in NK cells. In particular CD3-ζ chains associate with different triggering receptors, including CD16, NKp30, and NKp46.4 Thus, in principle, a defective expression of CD3-ζ chain in AML patients could be responsible of the low expression of NCR at the NK cell surface. However, previous studies have demonstrated that in cell transfectants the expression of NKp308 and NKp46,40 unlike CD16, is independent from CD3-ζ. Moreover, NCRdull AML-NK cells express normal amounts of CD16 that, on mAb-mediated cross-linking in redirected killing assay, induced an optimal enhancement of cytolytic activity.7These data also exclude the possibility that NCRdull AML-NK cells may be characterized by a deficiency in perforin and/or granzime. A further hypothesis could be that NCRdull AML-NKs represent NK cells at an immature stage. This hypothesis is unlikely because these cells, at variance with immature NK cells, express CD16 and KIR molecules. A recent report indicated that in NK cells displaying the NCRdull phenotype the cytolytic activity may be induced by NKG2D.11 However, an important role for this receptor in the mechanisms of killing of AML blasts appears unlikely. Indeed, we found that, unlike anti-NCR, anti-NKG2D mAb could not modify killing of leukemic blasts by normal NK cells (not shown). Moreover, AML blasts did not express the NKG2D ligand MICA, as demonstrated by the use of a specific mAb11 or messenger RNA expression by reverse transcriptase–polymerase chain reaction analysis (not shown). Finally, AML blasts are unlikely to express other NKG2D ligands such as ULBPs because these molecules have been detected in cells of epithelial but not of hematopoietic origin.13
A minor group of the patients analyzed in this study was characterized by AML-NK cells that expressed adequate amounts of NCRbright molecules. This characterization allowed these NK cells to kill allogeneic leukemic cells derived from NCRdull patients (not shown) as well as autologous B-LCLs and murine P815 target cells (Figures 4 and 2). On the contrary, such NCRbright AML-NK cells were unable to kill autologous leukemic blasts. This finding may suggest that these tumor cells have evolved a mechanism of escape from NK-cell mediated recognition based on the down-regulation of target cell ligands. This finding is further supported by the fact that NK cells derived from normal individuals also did not display cytotoxicity against leukemic blasts from this group of patients. A possible interpretation of these findings is that leukemic cells express inadequate amounts of NCR-specific ligands. In this context, although NCRs appear to represent the main NK receptors involved in killing of AML blasts, the nature of their specific ligands remains to be identified.4 An alternative possibility could be that these leukemic cells are not killed because of the lack of molecules involved in cell adhesion. In this context, however, the surface expression of LFA3 and ICAM-1 was comparable to that of leukemic cells derived from patients characterized by NCRdull NK cells (data not shown).
In conclusion, we have identified in patients with AML 2 possible mechanisms of tumor escape from NK cell–mediated recognition. The first mechanism, found in a small proportion of the patients analyzed, appears to be based on the in vivo selection of leukemic cells lacking the ligands involved in NK cell triggering. The second mechanism, found in the majority of cases, is based on the down-regulation of NCRs, ie, the main receptors involved in NK-mediated recognition and killing of tumor targets. In this case, because of the low NCR surface density on AML-NK cells, there would be no need for a selection of the leukemic blasts toward a NCR ligand(s)–deficient phenotype.
We thank F. Mallet, M. Illiano, and J. Viscardi for excellent technical assistance and T. Baffi for secretarial assistance.
Supported by Groupement Entreprise Français Lutte Cancer, Association pour la Recherche contre le Cancer, Ligue contre le Cancer de Bastia, Fédération Nationale des Centres de Lutte Contre le Cancer, Fondation Contre la Leucémie, European Association for Cancer Research, Etablissement Français des Greffes, Fondation pour la Recherche Médicale, Société Française contre le Cancer, Associazione Italiana per la Ricerca sul Cancro (A.I.R.C.), Istituto Superiore di Sanità (I.S.S.), and Ministero dell'Università e della Ricerca Scientifica e Tecnologica (M.U.R.S.T.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Régis T. Costello, Unitéd'Immunologie des Tumeurs & Département d'Hématologie, Institut Paoli-Calmettes, Université de la Méditerranée, Marseille, France; e-mail:regis.costello@free.fr.