Abstract
Chromosomal translocations involving the platelet-derived growth factor β receptor (PDGFβR) gene have been reported in some patients with chronic myelomonocytic leukemia (CMML). The resultant fusion proteins have constitutive PDGFβR tyrosine kinase activity, but the partner genes previously reported(tel, Huntingtin interacting protein 1[HIP-1], H4/D10S170) have poorly understood roles in the oncogenic activity of the fusion proteins. A novel PDGFβR fusion protein has been characterized in a patient with CMML and an acquired t(5;17)(q33;p13). Southern blot analysis on patient leukemia cells demonstrated involvement of the PDGFβR gene. Using 5′ rapid amplification of complementary DNA ends–polymerase chain reaction (RACE-PCR) on patient RNA, rabaptin-5 was identified as a novel partner fused in-frame to thePDGFβR gene. The new fusion protein includes more than 85% of the native Rabaptin-5 fused to the transmembrane and intracellular tyrosine kinase domains of the PDGFβR. Transduction with a retroviral vector expressing rabaptin-5/PDGFβRtransformed the hematopoietic cell line Ba/F3 to growth factor independence and caused a fatal myeloproliferative disease in mice. Rabaptin-5 is a well-studied protein shown to be an essential and rate-limiting component of early endosomal fusion through interaction with the Ras family GTPases Rab5 and Rab4. The fusion protein includes 3 of 4 coiled-coil domains (involved in homodimerization of native rabaptin-5), 2 caspase-3 cleavage sites, and a binding site for the tumor suppressor gene tuberin (tuberous sclerosis complex-2). Early endosomal transport is critical in regulation of various growth factor receptors, through ligand-induced clathrin-mediated endocytosis, and thus this new fusion protein links together 2 important pathways of growth regulation.
Introduction
Constitutively activated tyrosine kinases are increasingly being recognized as fusion oncoproteins in hematologic malignancies, the best known example being bcr-abl in chronic myelogenous leukemia (CML) associated with the Philadelphia chromosome.1 More recently fusion proteins have been described involving the platelet-derived growth factor β receptor (PDGFβR) in patients with chronic myelomonocytic leukemia (CMML)2-4 and acute myelogenous leukemia5 and the fibroblast growth factor receptor-1 (FGFR1) in a stem cell myeloproliferative disorder.6-8 An emerging pattern of similarity between these fusion proteins has been noted. The 3′ fusion gene contains the tyrosine kinase domain, whereas the 5′ gene has an oligomerization domain, necessary for activating the tyrosine kinase. However, it is also becoming clear that both fusion partners also confer their own unique properties that might explain the more subtle differences that exist between the transforming properties of these different fusion oncogenes. The 3 different bcr-abl fusion oncoproteins, p190bcr-abl, p210bcr-abl, and p230bcr-abl, are associated with different hematologic malignancies. The p190bcr-abl is associated with acute lymphoblastic leukemia, p210bcr-ablwith CML, and p230bcr-abl with a rare leukemia, chronic neutrophilic leukemia.1 The only difference between these fusion oncogenes is the size of the 5′ bcr partner gene, demonstrating the importance of the 5′ partner in determining the transformed phenotype.
The tyrosine kinases in the abl, PDGFβR, and FGFR1 fusion oncoproteins are constitutively activated6,7,9-11 and thus are able to activate downstream growth stimulatory or antiapoptotic pathways. Although this indicates that disruption of the normal regulation of the involved tyrosine kinase is critical to the transforming properties, less is known about whether disruption of the normal function of the 5′ partner gene might also have an important role. The fusion oncoprotein promyelocytic leukemia-retinoic acid receptor-α (PML-RARα), seen in acute promyelocytic leukemia, interferes with both PML12 and RARα pathways,13 thus, acting as a double dominant-negative oncoprotein. Little is known about the normal role of the 5′ fusion partners in the various abl, PDGFβR, and FGFR1 fusion oncoproteins, limiting the ability to study the effects of the fusion proteins on the normal pathways of the involved 5′ fusion partner.
Here we describe cloning of a new PDGFβR fusion protein from a patient with CMML and show that this novel fusion oncogene can transform hematopoietic cells. The 3′ PDGFβR, including the split tyrosine kinase domain, is fused in-frame to Rabaptin-5, an important regulator of early endosomal transport through interaction with the small Ras-related GTPases, Rab4 and Rab5.14,15 The fusion protein incorporates 85% of native Rabaptin-5, including 3 of 4 coiled-coil domains (involved in homodimerization of native Rabaptin-5),15 2 caspase-3 cleavage sites,16and a binding site for the tumor suppressor gene tuberin(tuberous sclerosis complex-2).17 Early endosomal transport is critical in regulation of various growth factor receptors, through ligand-induced clathrin-mediated endocytosis18; thus, this new fusion protein links together 2 important pathways of growth regulation.
Patient and methods
Patient
The patient is a 29-year-old man who presented with anemia (hemoglobin, 8.9 g/dL), mild thrombocytopenia (platelet count, 135 × 103/μL), and leukocytosis (white blood cell count, 59.2 × 103/μL) composed primarily of neutrophils and bands (66%) and monocytes (20%). His bone marrow was markedly hypercellular, with a myeloid-to-erythroid (M/E) ratio of 8 to 10:1, and a left shift in granulocyte maturation. Megakaryocytes were decreased in number and were dysplastic. Granulocytic dyspoiesis was noted, and 10% promonocytes were counted. The marrow morphology was consistent with the diagnosis of CMML by World Health Organization criteria.19 After he gave informed consent, he was enrolled in a clinical research study approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute.
Cytogenetics
The G-banded karyotype analysis was performed on bone marrow and peripheral blood using standard methods.20 Metaphase chromosomes were obtained from 24- and 48-hour unstimulated cultures of fresh heparinized bone marrow aspirates and from 72-hour phytohemagglutinin (PHA)-stimulated, methotrexate-synchronized cultures of heparinized blood. G-banding was done using Wright stain. Twenty-three metaphase cells from marrow, and 20 from blood, were fully analyzed at 400 to 550 and 550 to 850 band levels of resolution, respectively. Multiple digital images were captured and karyotyped using a Cytovision (Applied Imaging, Santa Clara, CA) system. Karyotypes were designated according to the International System for Human Cytogenetic Nomenclature (ISCN 1995).21
Metaphase fluorescence in situ hybridization (FISH) was also performed on marrow. Fresh slides were made and G-banded, and images were captured as described above. The slides were then destained and dehydrated in an ethanol series (70%, 85%, 100%), immersed in 70% formamide/2 × standard sodium citrate (SSC) for 5 minutes, and hybridized with either LSI CSF1R SpectrumOrange/D5S721, D5S23 SpectrumGreen, or LSI p53 SpectrumOrange probes (VYSIS, Downers Grove, IL). After 24 hours of incubation at 37°C, the slides were washed in 50% formamide/2 × SSC at 45°C. The signals were visualized using a 4′,6-diamidino-2-phenylindole-2HCl (DAPI) counterstain and a Zeiss fluorescence microscope with a Chroma filter set that includes DAPI, fluorescein isothiocyanate (FITC), and rhodamine filters. Digital images were recaptured on Cytovision.
Southern blotting
For Southern blotting on patient material, 1.5 × 107 mononuclear bone marrow cells were isolated by Ficoll sedimentation from fresh bone marrow. Peripheral blood mononuclear cells from a healthy volunteer were used as a control. After overnight incubation in proteinase K, genomic DNA was phenol-chloroform extracted using standard methods.22 DNA (10 μg) from the patient and control were restriction enzyme digested overnight at 37°C with either BamHI (Promega, Madison, WI), EcoRI (Promega, WI), HindIII (Boehringer Mannheim, Mannheim, Germany), or PvuII (Boehringer Mannheim). After electrophoretic separation on a 1% agarose gel and overnight transfer to HYbond N nylon membrane (Amersham, Arlington Heights, IL), the membrane was probed with a 1.1-kb (HindIII-XhoI fragment) genomic PDGFβR probe23 (generously provided by Dr Gary Gilliland [Boston, MA]), using standard Southern blot technique.22The probe was 32P-labeled using Prime-It RmT random primer labeling kit (Stratagene, La Jolla, CA). The membrane was then exposed to a Storm 860 PhosporImager (Molecular Dynamics, Sunnyvale, CA). For Southern blotting on mouse splenic cells, spleens were gently crushed through a nylon mesh and DNA was extracted as described above. DNA was digested with BamHI before electrophoresis and blotting. A 300-bp polymerase chain reaction (PCR)–generated probe against enhanced green fluorescence protein (eGFP) was used to probe the membrane.
Molecular cloning of breakpoint
The t(5;17) breakpoint was molecularly characterized using a 5′/3′ RACE (rapid amplification of complementary DNA [cDNA] ends) kit (Roche Molecular Biochemicals, Indianapolis, IN) as described initially by Frohman.24 Briefly, total messenger RNA (mRNA) from patient peripheral blood mononuclear cells (Ficoll separated) and normal control bone marrow stroma was extracted using RNA-STAT (Tel-Test, Friendswood, TX) according to the manufacturer's protocol. Then mRNA from patient peripheral blood mononuclear cells was extracted using QuickPrep Micro mRNA purification kit (Amersham Pharmacia Biotech, Piscataway, NJ). Total RNA and mRNA were reverse transcribed using AMV reverse transcriptase and reverse PDGFβR primer 2369PR (5′-TAGATGGGTCCTCCTTTGGTG-3′).5 After cDNA purification (High Pure PCR purification kit, Roche Molecular Biochemicals), a 5′ poly-A tail was appended using terminal transferase. The tailed cDNA was amplified using Expand High Fidelity polymerase (Roche Molecular Biochemicals) using the following PCR conditions: 94°C for 2 minutes, 94°C for 15 seconds, annealing at 58°C for 30 seconds, elongation for 40 seconds, cycle elongation of 20 second for each cycle after 10 cycles (for a total of 35 cycles) using the forward Qo-T16V RACE primer (5′-GACCACGCGTATCGATGTCGACT(16)V-3′) and a reverse PDGFβR primer PDGFβR-Po (5′-GTAACGTGGCTTCTTCTGCCA-3′).5The diluted first-round PCR product (1:20) was reamplified in a nested PCR reaction with the same conditions as the first round using a forward Qi RACE primer (5′-GACCACGCGTATCGATGTCGAC-3′) and a reverse PDGFβR primer PDGFβR-Pi (5′-TGAGGATGAGAAGGGAGATGATGG-3′).5 The nested PCR products from both patient and healthy control were TA-cloned into pCR2.1-TOPO vector (TOPOΠ TA Cloning Kit, Invitrogen, Carlsbad, CA) and vector inserts from selected colonies were sequenced using an automated sequencer. The DNA sequences were analyzed by the BLAST algorithm (http://www.ncbi.nlm.nih.gov/BLAST/).
Reverse transcription–PCR of fusion breakpoints or native Rabaptin-5 and PDGFβR
Nested PCR primers for both potential breakpoints (5′ Rabaptin-5/PDGFβR 3′ and 5′ PDGFβR/Rabaptin-5 3′) were designed. Patient, negative control total RNA (1 μg), and negative control water were reverse transcribed using MuLV Reverse Transcriptase and Random Hexamers primers (Perkin-Elmer, Norwalk, CT). The cDNA was amplified using Taq DNA polymerase (Perkin-Elmer) in a nested PCR using the following cycle conditions for both outer and inner cycle: 95°C for 2 minutes followed by 35 cycles of 95°C for 1 minute, 60°C for 1.5 minutes, 72°C for 2 minutes, and final extension of 72°C for 8 minutes. Primers for amplifying the 5′Rabaptin-5/PDGFβR 3′ breakpoint were: RP2151F (5′-AAGCACAGCCTGCATGTGTC-3′), RP2574R (5′-GGTCCACGTAGATGTACTCA-3′) (outer) and RP2269F (5′-CAGCAGACCACGTAGAAGAA-3′), RP2433R (5′-CTGAGATCACCACCACCTTA-3′) (inner). The 5′ PDGFβR/Rabaptin-5 3′ primers were: PR1684F (5′-AGCCGAACATCATCTGGTCT-3′), PR2106R (5′-GCTGTTCAACGGTAGCCTTA-3′) (outer) and PR1785F (5′-GAGACTAACGTGACGTACTG-3′), PR2034R (5′-GACTCTCAAGC- TGTTGAGAC-3′) (inner). The same PCR conditions were used to amplify the native genes,PDGFβR or rabaptin-5. Primers PR1684F, RP2574R (outer) and PR1785F, RP2433R (inner) were used for PDGFβR. Primers RP2151F, PR2106R (outer) and RP2269F, PR2034R (inner) were used for rabaptin-5.
Construction of a Rabaptin-5/PDGFβR retroviral expression vector
To generate the full-length Rabaptin-5/PDGFβR expression plasmid, Rabaptin-5 cDNA sequences were excised from a plasmid (provided by Dr Marino Zerial, Heidelberg, Germany) and PDGFβR cDNA sequences from a tel/PDGFβR retroviral vector in the MSCV retroviral backbone containing both the tel/PDGFβR and eGFP cDNA with an IRES25 (provided by Dr Gary Gilliland, Boston, MA). The tel-PDGFβR sequence was flanked by EcoRI restrictions sites. To generate a unique 5′ restriction site, the MSCV plasmid was partially digested with EcoRI, generating a singly cut plasmid, and a oligonucleotide linker containing aSwaI site and EcoRI sticky ends was ligated into the plasmid. The cDNA generated from patient bone marrow cells was amplified using reverse transcription (RT)–PCR to generate a 603-bp fragment spanning the Rabaptin-5/PDGFβR breakpoint. The breakpoint region included unique SacII (in the PDGFβRportion of the gene) and SapI (in the rabaptin-5portion of the gene) restriction enzyme sites. Furthermore, anotherSacII site was generated at the 5′ end of the PCR product using a tailed PCR primer. The amplified breakpoint product wasSacII digested and ligated into the SacII site of tel/PFGDβR. After ligation, the breakpoint sequence and orientation were confirmed by sequencing. To generate the full-length Rabaptin-5/PDGFβR construct, the full-length Rabaptin-5 plasmid was digested using SpeI (upstream of the 5′ end and Kozak consensus sequence) and BamHI (downstream of theSapI site). The resulting 2.5-kb fragment was blunt-ended using Pfu DNA polymerase (PCR Polishing Kit, Stratagene) and subsequently digested with SapI. This fragment containing a 5′ blunt end and 3′ SapI site was ligated into the MSCV plasmid containing the tel-PDGFβR with the Rabaptin-5/PDGFβR breakpoint after digestion with SapI and SwaI (blunt end cutting enzyme). Correct ligation of all ligation sites was confirmed by sequencing. We also generated a mutantrabaptin-5/PDGFβR containing a Lys>Arg mutation in amino acid 635 of the PDGFβR. This mutant renders the tyrosine kinase domain of the PDGFβR inactive.9 The Lys635Arg tel/PDGFβR plasmid (provided by Gary Gilliland, Boston, MA) was SacII/BamH1 digested and the resulting 884-bp fragment ligated into the same restriction sites of the Rabaptin-5/PDGFβR MSCV plasmid.
Transformation of Ba/F3 cells
Bosc-23 cells were transfected with the MSCV plasmids using calcium phosphate precipitation.26 Retroviral supernatant was collected 48 hours after transfection, filtered (Millex HV, 45 μm) and 1 × 106 Ba/F3 cells incubated per 1 mL supernatant with 10 μg/mL polybrene. Twenty-four hours later cells were placed in fresh RPMI media, with 10% fetal calf serum (FCS) and interleukin-3 (IL-3) (1 ng/mL). Then, 48 hours after retroviral infection the cells were flow sorted and GFP+ cells were collected and expanded in RPMI media with 10% FCS and 10% WEHI-conditioned media, as a source of IL-3.
Murine bone marrow transplant
Six days before harvesting BALB/c bone marrow cells, 5-fluorouracil (Fluka, Milwaukee, WI), 150 mg/kg, was administered by intraperitoneal injection. Bone marrow was harvested by flushing femurs and tibia and 9 × 106 bone marrow cells were plated on RetroNectin-coated plates (100 mm; Takara, Japan) in 10 mL Dulbecco modified Eagle medium (DMEM) containing 10% FCS, murine IL-3 (10 ng/mL), human-IL-6 (50 ng/mL), human-Flt-3 (100 ng/mL), and rat stem cell factor (SCF; 100 ng/mL) for 48 hours. At 48 and 72 hours, 5 mL media was replaced with retroviral supernatant from transiently transfected Bosc-23 cells (as described above) supplemented with the same cytokines as during the 48-hour prestimulation. At 96 hours the cells were collected and 1 × 106 cells were injected into the tail vein of lethally irradiated BALB/c mice (radiation dose: 450 cGy twice, 6 hours apart). Two weeks after transplantation blood counts were monitored biweekly by retro-orbital phlebotomy. Premorbid diseased animals were killed by CO2 asphyxiation.
Western blotting
Ba/F3 cells were washed in phosphate-buffered saline (PBS) and suspended in lysis buffer containing complete protease inhibitor cocktail (Boehringer Mannheim). After addition of sodium dodecyl sulfate (SDS) sample buffer and boiling, the samples were run on 8% SDS-polyacrylamide gels, transferred to a nitrocellulose membrane, and immunoblotted using a rabbit anti-PDGFβR antibody (P-20, C-terminal; 1:2000; Santa Cruz Biotechnology, Santa Cruz, CA), followed by a secondary antirabbit horseradish peroxidase (HRP)–conjugated antibody (1:20 000). Bound antibodies were detected with SuperSignal (Pierce, Rockford IL) enhanced luminol and oxidizing reagent as specified by the manufacturer.
Results
A novel translocation in a patient with CMML involving thePDGFβR gene
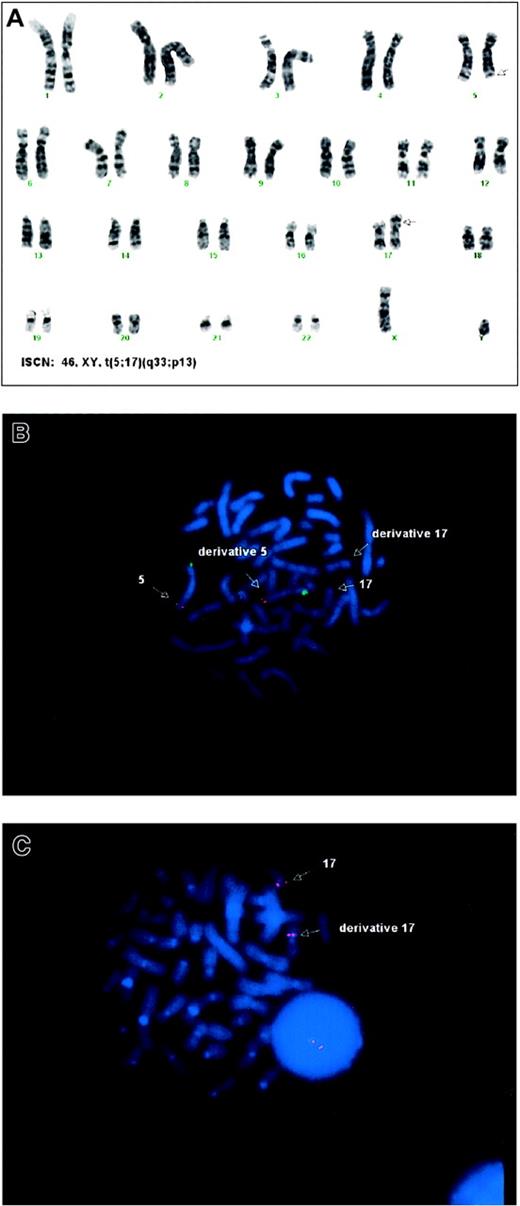
Cytogenetic analysis revealed that 23 of 23 marrow metaphase cells had a 46,XY,t(5;17)(q33;p13) karyotype (Figure1A). This same translocation was also found in 20 additional marrow metaphase spreads that were screened for chromosomes 5 and 17. Analysis of PHA-stimulated peripheral blood lymphocytes revealed a normal 46,XY male karyotype in 20 of 20 metaphase cells (data not shown), confirming that t(5;17) was a novel acquired rearrangement in the patient's leukemic clone. Metaphase FISH demonstrated the chromosomal breakpoints to be just distal (telomeric) to the CSF1R/3′ PDGFβR loci on 5q (Figure 1B), and also telomeric to p53 on 17p (Figure 1C).
Cytogenetic analyses.
(A) G-banded karyotype from bone marrow showing the t(5;17)(q33;p13). (B) Metaphase FISH demonstrating that the LSI CSF1R SpectrumOrange/D5S721, D5S23 SpectrumGreen (VYSIS) dual-color probes are present on the normal and derivative chromosomes 5. (C) Metaphase FISH demonstrating the LSI p53 SpectrumOrange (VYSIS) probe is present on both the normal and derivative chromosomes 17.
Cytogenetic analyses.
(A) G-banded karyotype from bone marrow showing the t(5;17)(q33;p13). (B) Metaphase FISH demonstrating that the LSI CSF1R SpectrumOrange/D5S721, D5S23 SpectrumGreen (VYSIS) dual-color probes are present on the normal and derivative chromosomes 5. (C) Metaphase FISH demonstrating the LSI p53 SpectrumOrange (VYSIS) probe is present on both the normal and derivative chromosomes 17.
Southern blot analysis on patient DNA from bone marrow cells using a genomic PDGFβR probe revealed rearrangement of thePDGFβR gene in all restriction enzyme digests tested (Figure 2). As shown, the control DNA from normal bone marrow stroma had a single band in all lanes, whereas the patient sample had 2 bands, one of the same size as seen in the healthy control and another band that was not seen in the control sample, indicating translocation of part of the PDGFβRgene. This confirms involvement of the PDGFβR gene in the t(5;17)(q33;p13).
Translocation of
PDGFβR gene in patient-derived genomic DNA.Patient and normal control genomic DNA was restriction enzyme digested (BamHI, EcoRI, HindIII, andPvuII), electrophoretically separated, and transferred to a nitrocellulose membrane. Southern blotting was performed using a 1.1-kb (HindIII-XhoI fragment) genomic PDGFβR probe (see “Patient and methods”). Arrows indicate patient-specific bands, showing translocation involving the PDGFβRgene.
Translocation of
PDGFβR gene in patient-derived genomic DNA.Patient and normal control genomic DNA was restriction enzyme digested (BamHI, EcoRI, HindIII, andPvuII), electrophoretically separated, and transferred to a nitrocellulose membrane. Southern blotting was performed using a 1.1-kb (HindIII-XhoI fragment) genomic PDGFβR probe (see “Patient and methods”). Arrows indicate patient-specific bands, showing translocation involving the PDGFβRgene.
Cloning of the t(5;17)(q33;p13) breakpoints
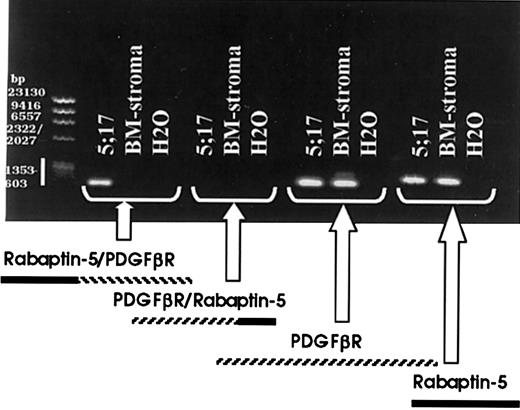
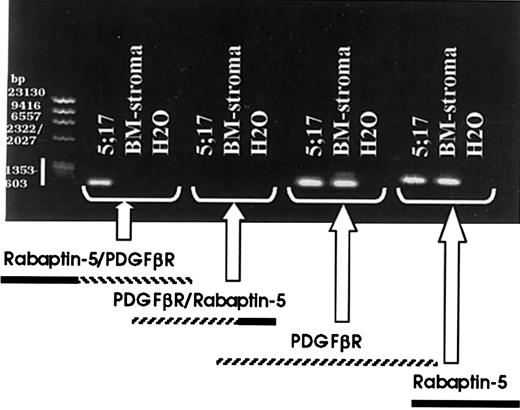
To identify the fusion partner of the translocatedPDGFβR, 5′RACE-PCR (Figure3A) was performed. Briefly, patient and normal marrow stroma control mRNA were reverse transcribed and a 5′poly-A tail was added. cDNA was amplified, using nested PCR with forward RACE primers and reverse PDGFβR-specific primers. The normal stroma mRNA resulted in a strong band (Figure 3B) and subsequent cloning and sequencing confirmed amplification of the nativePDGFβR. Use of 5′RACE on patient-derived total mRNA and polyA mRNA failed to reveal an amplified band, but instead showed an amplification smear (Figure 3B). The PCR amplification product from both the poly-A and total mRNA samples was TA-cloned and 24 isolated colonies were subsequently purified and sequenced. One of 24 colonies contained a 454- nucleotide insert with a partial PDGFβRsequence fused to a novel gene partner. None of the other 23 colonies contained a partial PDGFβR sequence. BLAST search on the 454-nucleotide insert revealed the 3′ PDGFβR sequence to be fused to rabaptin-5. Previous physical mapping has localized rabaptin-5 to band 17p13 near, but telomeric top53. Our FISH analysis showing p53 is centromeric to the breakpoint on 17p (Figure 1C) supports the finding thatPDGFβR is fused to rabaptin-5. Analysis of the breakpoint sequence revealed an in-frame fusion (Figure 3C). RT-PCR using primers spanning the breakpoint on patient and normal bone marrow stroma mRNA revealed patient-specific expression of the 5′ Rabaptin-5/PDGFβR 3′ (Figure 4), confirming the 5′RACE-PCR results. The reciprocal fusion construct, that is, 5′ PDGFβR/Rabaptin-5 3′, was not detected but both native PDGFβR and Rabaptin-5 mRNA were expressed in patient and normal bone marrow stroma (Figure 4).
Molecular identification of the t(5;17)(q33;p13) fusion gene using 5′RACE-PCR.
(A) A schematic showing the 5′RACE method used to identify the breakpoint. Patient total RNA and mRNA and normal marrow stroma total RNA was reverse transcribed. A 5′ poly-A tail was appended on the resulting cDNA. Using a nested PCR the PDGFβR native or fusion gene was amplified using reverse PDGFβR primers (PDGFβR-Po [outer]/PDGFβR-Pi [inner]) and forward RACE primers (tailed poly-T Qo-T(17)N and Qi [inner]; see “Patient and methods”). (B) After electrophoretic separation on 1% agarose gel a distinct band was seen in the nested PCR product from normal bone marrow stroma (arrow), whereas a smear was seen in PCR products derived from either patient total RNA or mRNA (box). TA cloning and sequencing of the bone marrow–derived band (arrow) revealed the native PDGFβR. The PCR smear (box) was TA cloned. (C) After screening and sequencing 24 TA-cloned colonies derived from the patients PCR product, a blast search in GenBank revealed a 454-nucleotide insert in a single colony with an in-frame fusion between rabaptin-5 and thePDGFβR. Shown is part of the sequence over the breakpoint (rabaptin-5 sequence in bold).
Molecular identification of the t(5;17)(q33;p13) fusion gene using 5′RACE-PCR.
(A) A schematic showing the 5′RACE method used to identify the breakpoint. Patient total RNA and mRNA and normal marrow stroma total RNA was reverse transcribed. A 5′ poly-A tail was appended on the resulting cDNA. Using a nested PCR the PDGFβR native or fusion gene was amplified using reverse PDGFβR primers (PDGFβR-Po [outer]/PDGFβR-Pi [inner]) and forward RACE primers (tailed poly-T Qo-T(17)N and Qi [inner]; see “Patient and methods”). (B) After electrophoretic separation on 1% agarose gel a distinct band was seen in the nested PCR product from normal bone marrow stroma (arrow), whereas a smear was seen in PCR products derived from either patient total RNA or mRNA (box). TA cloning and sequencing of the bone marrow–derived band (arrow) revealed the native PDGFβR. The PCR smear (box) was TA cloned. (C) After screening and sequencing 24 TA-cloned colonies derived from the patients PCR product, a blast search in GenBank revealed a 454-nucleotide insert in a single colony with an in-frame fusion between rabaptin-5 and thePDGFβR. Shown is part of the sequence over the breakpoint (rabaptin-5 sequence in bold).
RT-PCR identifies the patient specific 5′ Rabaptin-5/PDGFβR 3′ fusion breakpoint, but not the reciprocal breakpoint.
RT-PCR was carried out on patient myeloid cell total RNA and normal donor bone marrow stroma total RNA, using primers to identify the 2 potential breakpoint products, 5′ Rabaptin-5/PDGFβR 3′ and 5′ PDGFβR/Rabaptin-5 3′, as well as the 2 involved native gene products.
RT-PCR identifies the patient specific 5′ Rabaptin-5/PDGFβR 3′ fusion breakpoint, but not the reciprocal breakpoint.
RT-PCR was carried out on patient myeloid cell total RNA and normal donor bone marrow stroma total RNA, using primers to identify the 2 potential breakpoint products, 5′ Rabaptin-5/PDGFβR 3′ and 5′ PDGFβR/Rabaptin-5 3′, as well as the 2 involved native gene products.
Rabaptin-5/PDGFβR, a novel CMML fusion protein
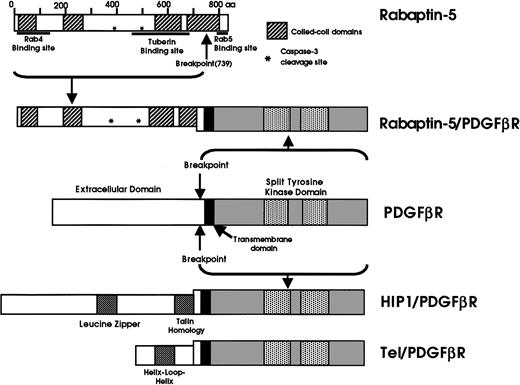
Rabaptin-5 is a well-characterized protein playing a critical role in the regulation of endocytosis through regulation of the small Ras-family GTPases Rab4 and Rab5.14,15 It is an 862-amino acid, 100-kd coiled-coil protein, localized mainly in the cytosol, but is recruited to early endosomes through GTP-dependent interaction with Rab5.14 The native protein includes 4 coiled-coil domains mediating homodimerization, an N-terminal Rab4 binding site, a C-terminal Rab5 binding site,15 a binding site for the tumor suppressor tuberin (tuberous-sclerosis complex-2),17and 2 caspase-3 cleavage sites16 (Figure5). The breakpoint in the novel Rabaptin-5/PDGFβR fusion protein occurs at amino acid residue 739, fusing 85% of the native Rabaptin-5 to the transmembrane and intracellular portion of the PDGFβR. The novel fusion protein has 1318 amino acids and a predicted molecular weight of 150 kd. It includes 3 and one-half of the 4 coiled-coil domains, the Rab4 and tuberous sclerosis binding sites, and the caspase-3 cleavage sites, but lacks the Rab5 binding site (Figure 5).
A schematic showing the novel fusion oncoprotein, Rabaptin-5/PDGFβR.
The important defined functional protein domains and the breakpoints from the 2 involved proteins, Rabaptin-5 and PDGFβR are shown. The previously well-characterized fusion proteins, tel/PDGFβR and HIP1/PDGFβR, are shown for comparison.
A schematic showing the novel fusion oncoprotein, Rabaptin-5/PDGFβR.
The important defined functional protein domains and the breakpoints from the 2 involved proteins, Rabaptin-5 and PDGFβR are shown. The previously well-characterized fusion proteins, tel/PDGFβR and HIP1/PDGFβR, are shown for comparison.
Rabaptin-5/PDGFβR transforms hematopoietic cells and causes a myeloproliferative disease in mice
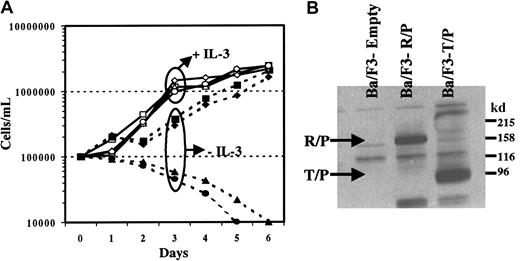
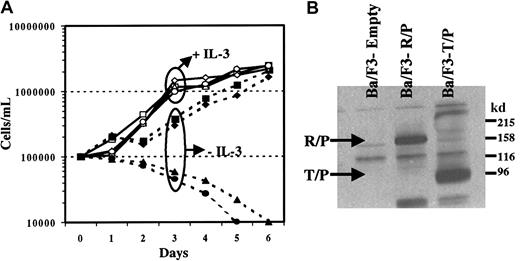
To test the transforming capabilities of Rabaptin-5/PDGFβR, we generated a bicistronic MSCV-based retroviral plasmid containing Rabaptin-5/PDGFβR, as well as eGFP. We also generated a kinase-inactive Rabaptin-5/PDGFβR mutant, carrying a single amino acid substitution in the active tyrosine kinase domain of the PDGFβR portion of fusion protein. These constructs were compared to the previously described fusion oncogene, tel/PDGFβR, and an empty vector carrying only eGFP. The IL-3–dependent murine hematopoietic cell line Ba/F3 was infected with these retroviral vectors. As shown (Figure 6A), cells infected with the 4 different vectors all had a similar growth curve in the presence of IL-3. When IL-3 was not present, the cells infected with the MSCV empty vector (eGFP alone) and the kinase-inactive mutant of Rabaptin-5/PDGFβR died. This growth pattern in the absence or presence of IL-3 was identical to what was seen in Ba/F3 cells without retroviral infection (data not shown). In contrast, cells infected with either Rabaptin-5/PDGFβR or tel/PDGFβR were IL-3 independent (Figure 6A). The Rabaptin-5/PDGFβR and tel/PDGFβR transduced cells had a similar growth rate without IL-3. The expression of the fusion genes was confirmed by Western blotting (Figure 6B). The IL-3 independence of these cells suggests a transforming property of the novel PDGFβR fusion oncogene. The lack of effects of the kinase-inactive mutant confirms the importance of the kinase domain, suggesting that the novel fusion protein transforms cell lines through constitutive activation of the tyrosine kinase domain, similar to other tyrosine kinase fusion oncogenes.
Rabaptin-5/PDGFβR transforms Ba/F3 cells to growth factor independence.
(A) Ba/F3 cells were infected with bicistronic MSCV retroviral vectors containing eGFP alone (empty virus, circle) or eGFP along with Rabaptin-5/PDGFβR (box), tel/PDGFβR (diamond), or the kinase-inactive mutant of Rabaptin-5/PDGFβR (triangle). After infection, cells were flow sorted for eGFP positivity, expanded, and subsequently incubated in media with (open symbols) or without (closed symbols) IL-3, 1 ng/mL. The data represent the mean of 3 independent cultures. (B) Presence of expressed fusion protein in Ba/F3 cell lines transformed with either Rabaptin-5/PDGFβR (R/P) or tel/PDGFβR (T/P). Membranes were immunoblotted using an anti-PDGFβR (C-terminal) antibody.
Rabaptin-5/PDGFβR transforms Ba/F3 cells to growth factor independence.
(A) Ba/F3 cells were infected with bicistronic MSCV retroviral vectors containing eGFP alone (empty virus, circle) or eGFP along with Rabaptin-5/PDGFβR (box), tel/PDGFβR (diamond), or the kinase-inactive mutant of Rabaptin-5/PDGFβR (triangle). After infection, cells were flow sorted for eGFP positivity, expanded, and subsequently incubated in media with (open symbols) or without (closed symbols) IL-3, 1 ng/mL. The data represent the mean of 3 independent cultures. (B) Presence of expressed fusion protein in Ba/F3 cell lines transformed with either Rabaptin-5/PDGFβR (R/P) or tel/PDGFβR (T/P). Membranes were immunoblotted using an anti-PDGFβR (C-terminal) antibody.
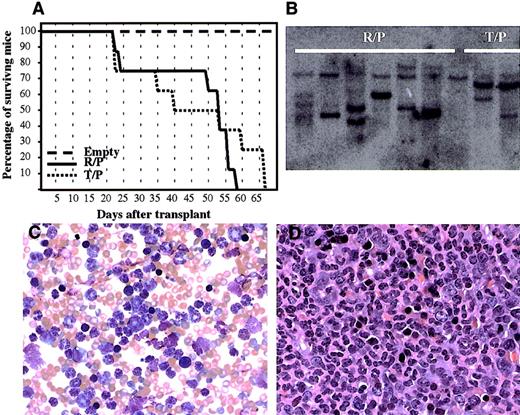
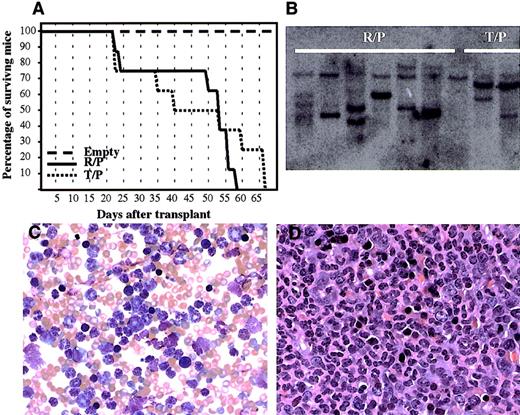
To confirm the transforming property of rabaptin-5/PDGFβR,we introduced the novel fusion gene, as well astel/PDGFβR, into murine bone marrow cells using retroviral transduction, and transplanted these marrow cells into lethally irradiated BALB/c mice. Mice receiving bone marrow transplants transduced with rabaptin-5/PDGFβR ortel/PDGFβR developed a rapidly fatal myeloproliferative disorder (Figure 7A). Serial blood counts revealed a maximal white cell count varying between 104.6 and 393 K/μL (mean 254.9 K/μL) in rabaptin-5/PDGFβR mice compared to 178.2 to 507.3 K/μL (mean 314.3 K/μL) in thetel/PDGFβR mice. Mice given transplants with the MSCV empty vector all survived and had normal white cell counts (mean 10.4 K/μL). All animals receiving transplants withrabaptin-5/PDGFβR or tel/PDGFβR had developed markedly elevated white cell counts by 4 weeks after transplantation. The peripheral blood smears of animals receiving transplants ofrabaptin-5-PDGFβR showed predominantly mature granulocytes (> 95%) (Figure 7C), and this was confirmed by flow cytometry: more than 95% of peripheral blood leukocytes were Gr-1(bright)/Mac1(dull) positive, CD3/B220/c-kit negative. This hematologic picture was indistinguishable from the disease seen when the animals received cells transduced with the tel-PDGFβR vector. This myeloproliferative syndrome was also similar to the clinical characteristics of the index patient. Autopsies on the leukemic mice showed marked hepatosplenomegaly, with granulocytic infiltration into spleen (Figure7D), liver, and lungs. There was no lymphadenopathy or thymic hyperplasia noted. Southern blot analysis on splenic DNA from the leukemic mice, using a vector-specific probe (to evaluate proviral integration), revealed 2 to 5 distinct bands, suggesting at least oligoclonal expansion of transformed hematopoietic cells (Figure 7B). In 2 of 8 animals receiving transplants withrabaptin-5/PDGFβR, and 3 of 8 animals receiving transplants with tel/PDGFβR, there was a significant drop in the peripheral blood leukocyte count before death. This was associated with severe anemia and absence of erythroid maturation in the bone marrow, but no evidence of blastic transformation in the peripheral blood or bone marrow. Furthermore, there was no evidence of thymic hyperplasia or lymphadenopathy in these animals. Further in vivo studies are required to delineate this progression from a predominantly myeloproliferative state toward a less well-defined hypoproliferative state.
Rabaptin-5/PDGFβR retrovirally transduced into murine bone marrow causes a fatal myeloproliferative disease.
(A) Survival of lethally irradiated mice receiving bone marrow transplants transduced with empty plasmid, Rabaptin-5/PDGFβR (R/P), or tel-PDGFβR (T/P) containing retroviruses. (B) Southern blot of splenic genomic DNA using an eGFP probe demonstrating proviral integration with several bands detected in samples from 6 mice receiving transplants with Rabaptin-5/PDGFβR (R/P) and 3 mice with tel/PDGFβR. (C) A representative peripheral blood smear from a mouse with the rabaptin-5/PDGFβR-mediated myeloproliferative disease (× 400) revealing marked leukocytosis with predominantly mature granulocytes. (D) Formalin-fixed, hematoxylin and eosin-stained spleen sections from a mouse with Rabaptin-5/PDGFβR-mediated myeloproliferative disease (× 400) revealing near total replacement of normal spleen architecture by mature granulocytes.
Rabaptin-5/PDGFβR retrovirally transduced into murine bone marrow causes a fatal myeloproliferative disease.
(A) Survival of lethally irradiated mice receiving bone marrow transplants transduced with empty plasmid, Rabaptin-5/PDGFβR (R/P), or tel-PDGFβR (T/P) containing retroviruses. (B) Southern blot of splenic genomic DNA using an eGFP probe demonstrating proviral integration with several bands detected in samples from 6 mice receiving transplants with Rabaptin-5/PDGFβR (R/P) and 3 mice with tel/PDGFβR. (C) A representative peripheral blood smear from a mouse with the rabaptin-5/PDGFβR-mediated myeloproliferative disease (× 400) revealing marked leukocytosis with predominantly mature granulocytes. (D) Formalin-fixed, hematoxylin and eosin-stained spleen sections from a mouse with Rabaptin-5/PDGFβR-mediated myeloproliferative disease (× 400) revealing near total replacement of normal spleen architecture by mature granulocytes.
Discussion
Fusion oncogenes generated as a consequence of reciprocal chromosomal translocations are commonly seen in hematologic malignancies. The consequence is invariably disruption of key regulatory pathways involved in cell growth or survival. Fusion oncoproteins in myeloproliferative disorders commonly involve deregulated protein tyrosine kinases such as abl, PDGFβR, and FGFR1.1,2,8 Here we describe a novel PDGFβRfusion oncogene, rabaptin-5/PDGFβR. Rabaptin-5 is a well-characterized protein with known functions. The 3 priorPDGFβR fusion partners, tel,2HIP-1,4 and H4/D10S1703have poorly defined cellular functions and their role in oncogenesis is not fully understood. Rabaptin-5 has been shown to be an essential and rate-limiting component of early endosomal fusion through interaction with the Ras family GTPases Rab5 and Rab4. The fusion protein in our patient includes 3 of 4 coiled-coil domains (involved in homodimerization of native rabaptin-5), 2 caspase-3 cleavage sites, and a binding site for the tumor suppressor gene tuberin(tuberous sclerosis complex-2).
Clathrin-mediated endocytosis is a process by which cells internalize selected components of the plasma membrane, thus clearing receptor-bound hormones and growth factors, internalizing channels, and transporters and recycling synaptic vesicles.18 The net result of clathrin-mediated endocytosis with regard to mitogenic signaling is attenuation, through the down-regulation of surface signaling receptors.27
Rab proteins are small GTPases that regulate vesicular transport in endocytosis and exocytosis pathways, where they temporally and spatially coordinate the vesicular transport process. To date more than 40 distinct Rab proteins have been identified; each is believed to be associated with a particular organelle or pathway. Twelve Rab proteins have been localized to the endocytic pathway, including the Rabaptin-5 interacting proteins, Rab4 and Rab5. They are both present in early endosomes, Rab4 in early and recycling endosomes and Rab5 in clathrin-coated vesicles and early endosomes.28 The Rab proteins are tightly regulated by accessory proteins that modulate Rab protein activity by controlling membrane association, nucleotide binding, and hydrolysis.29 Rabaptin-5 is a critical accessory protein for Rab5 and Rab4. Rabaptin-5 exists as a homodimer (mediated by coiled-coil domains) in the cell cytosol,15and is recruited to early endosomes by Rab5 in a GTP-dependent manner.14 There, Rabaptin-5 stabilizes Rab5 in a GTP-bound active form by down-regulating GTP hydrolysis.30 Once in the early endosomes, Rabaptin-5, through its interaction with Rab5, is an essential and rate-limiting component of the endocytic process required for homotypic fusion between early endosomes and heterotypic fusion between clathrin-coated vesicles and early endosomes.14 31
There is increasing evidence that disruption of the endocytosis process might have a role in malignant transformation.27,32Mutations in internalization domains of both epidermal growth factor receptors and granulocyte colony-stimulating factor receptors (found in patients with Kostman syndrome developing acute myelogenous leukemia) have been shown to transform cell lines.33,34 Further evidence for a role of disruption in endocytotic pathways in hematologic malignancies is the presence of other endocytosis-related proteins in chromosomal translocations. AF-1p(Eps15)35 and EEN (SH3p8)36are both fused as C-terminal partners to MLL, a gene commonly involved in a variety of hematologic malignancies. TheCALM (AP180) gene has been found as an N-terminal fusion partner to the AF10 gene both in the human cell line U93737 and in patients with hematologic malignancies.38 Finally, the tumor suppressor tuberin (tuberous sclerosis complex-2) is a Rab5 GTPase activating (GAP) protein, and interacts directly with Rabaptin-5.17 The tumor suppressor activity of tuberin is encoded by functional domains in the C-terminus that contains the GAP activity.39
The previously described PDGFβR fusion oncogenes,tel/PDGFβR, HIP-1/PDGFβR, andH4-D10S170/PDGFβR have common themes, including the same breakpoint in the PDGFβR gene and constitutive tyrosine activation due to an oligomerization domain in the 5′ fusion partner.3,9,10 Apart from the oligomerization domains, little is known about the transforming properties of the 5′ fusion partner. Detailed mutational analysis of the HIP-1/PDGFβRhave revealed that constitutive activation of the tyrosine kinase domain is a critical element in the transforming property.10 Tyrosine kinase activation is closely correlated with oligomerization of the fusion product. InHIP-1/PDGFβR, the talin homology region adjacent to the breakpoint mediates the oligomerization. Interestingly, there wereHIP-1 deletion mutants with both intact oligomerization and kinase activity that lacked the transforming property, indicating that regions in HIP-1 other than the talin homology oligomerization domain might be critical in the transformation.10
In summary, we have cloned a novel PDGFβR fusion oncogene and provide evidence for its leukemogenic properties. The new fusion partner, rabaptin-5, has coiled-coil domains that would be predicted to oligomerize the fusion protein, and thus activate the tyrosine kinase domains of the PDGFβR. The extensive prior characterization and known functions of Rabaptin-5 provide a unique opportunity to study the role of the 5′ partner in a PDGFβR fusion protein. Furthermore, the newly described fusion protein links together 2 proteins with critical roles in growth regulation, the protein tyrosine kinase PDGFβR, and Rabaptin-5, an important molecule in the process of growth factor receptor down-regulation.
We are grateful to Gary Gilliland and Ema Anastasiadou (Boston, MA) for providing tel-PDGFβR plasmids and the PDGFβR genomic probe, Dr Marino Zerial (Heidelberg, Germany) for contributing a Rabaptin-5 plasmid, and Dr David Bodine (Bethesda, MD) for providing Bosc-23 cells.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Magnus K. Magnusson, Hematology Branch, National Heart, Lung, and Blood Institute, Bldg 10, Rm 7C103, 9000 Rockville Pike, Bethesda, MD 20892; e-mail: magnussm@nhlbi.nih.gov.


![Fig. 3. Molecular identification of the t(5;17)(q33;p13) fusion gene using 5′RACE-PCR. / (A) A schematic showing the 5′RACE method used to identify the breakpoint. Patient total RNA and mRNA and normal marrow stroma total RNA was reverse transcribed. A 5′ poly-A tail was appended on the resulting cDNA. Using a nested PCR the PDGFβR native or fusion gene was amplified using reverse PDGFβR primers (PDGFβR-Po [outer]/PDGFβR-Pi [inner]) and forward RACE primers (tailed poly-T Qo-T(17)N and Qi [inner]; see “Patient and methods”). (B) After electrophoretic separation on 1% agarose gel a distinct band was seen in the nested PCR product from normal bone marrow stroma (arrow), whereas a smear was seen in PCR products derived from either patient total RNA or mRNA (box). TA cloning and sequencing of the bone marrow–derived band (arrow) revealed the native PDGFβR. The PCR smear (box) was TA cloned. (C) After screening and sequencing 24 TA-cloned colonies derived from the patients PCR product, a blast search in GenBank revealed a 454-nucleotide insert in a single colony with an in-frame fusion between rabaptin-5 and thePDGFβR. Shown is part of the sequence over the breakpoint (rabaptin-5 sequence in bold).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/8/10.1182_blood.v98.8.2518/6/m_h82011660003.jpeg?Expires=1765969927&Signature=DhH6I0E4LNCCMafxwJSiuTNntTa~Of5ghR9BTT78QmsecKa-XrwDw9dF~LbAxQzUWfOnWwLJRXMvBSNV42lBjsBSVYDsRc4D0bTBJuDD9ZysLYXXn9vyvwaDBhIe0Vs~N3YQJMfbrLcfLLBzFunayCXE-xWWpcyWRDyFJxKTonajC2~M~pXfnNQVNkzNn4iwBkQwc6wdZc4J9yXGxX-UXyM3ZjGU2cwKe-psV3Aya2o~HW2R6VHSOSSwepfJ68x2mesj5QD7~RnaSOhpntFGONCze0ybmpzNlfctWqDJx1lU8A66e7ZgILdZsEubzdq9GpHzMba3MMoC0M-HHb0ezg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)




![Fig. 3. Molecular identification of the t(5;17)(q33;p13) fusion gene using 5′RACE-PCR. / (A) A schematic showing the 5′RACE method used to identify the breakpoint. Patient total RNA and mRNA and normal marrow stroma total RNA was reverse transcribed. A 5′ poly-A tail was appended on the resulting cDNA. Using a nested PCR the PDGFβR native or fusion gene was amplified using reverse PDGFβR primers (PDGFβR-Po [outer]/PDGFβR-Pi [inner]) and forward RACE primers (tailed poly-T Qo-T(17)N and Qi [inner]; see “Patient and methods”). (B) After electrophoretic separation on 1% agarose gel a distinct band was seen in the nested PCR product from normal bone marrow stroma (arrow), whereas a smear was seen in PCR products derived from either patient total RNA or mRNA (box). TA cloning and sequencing of the bone marrow–derived band (arrow) revealed the native PDGFβR. The PCR smear (box) was TA cloned. (C) After screening and sequencing 24 TA-cloned colonies derived from the patients PCR product, a blast search in GenBank revealed a 454-nucleotide insert in a single colony with an in-frame fusion between rabaptin-5 and thePDGFβR. Shown is part of the sequence over the breakpoint (rabaptin-5 sequence in bold).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/8/10.1182_blood.v98.8.2518/6/m_h82011660003.jpeg?Expires=1766312186&Signature=u8S2538ZvOVX~4PG5wIKq7cVIaV5wNcYWvwXEq-Id920U7pn2p1lHYTq~O-LNG3W531LGEhs3c9glmfrxoPU2DiLzgP12zf7xsHI8RN4vPIlnnfzy1tHtr0e~MKFFdEZO91tEVpYKVMinOIlZ0el~DAyUwRM~qliJBlJnWJruyMqSoLuG2nn92fjQZO2xAl7pebVC0R3HVTw9sUzsrx1Pil2~Nq~i~Yzs9nCvZp~4FxYsWbUtGjiC4ez~lfrS0SCm9jUNXuBapC6LFunW8awY9UUTQxVWfRSPQ5BlARuIU9YHda1Vtwfy0BC5kaqF~3NE1IiwqHzgjuUUKGq2fKn3w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)