Abstract
Tyrosine kinase oncogenes such as p210BCR-ABL activate multiple signal pathways. As a result, it is difficult to infer the functional relevance of a pathway acting alone or in cooperation with another. One or 2 second-tier kinases represented in the p21ras and phosphatidylinositol-3-kinase (PI-3-kinase) pathways (activated RafCAAX and gag-akt, respectively) were expressed in parental H7 interleukin-3 (IL-3)–dependent myeloid cells. IL-3–dependent cells served, independently, as recipients of p210BCR-ABL, which activated p21ras and PI-3-kinase pathways, including raf/erk and akt, respectively, en route to transformation. By contrast, neither RafCAAX nor gag-akt when expressed in parental cells in isolation produced factor-independent cells. On the other hand, H7 cells expressing both RafCAAX and gag-akt (H7gag-akt/RafCAAX) were transformed. Such transformation in H7gag-akt/RafCAAX was accomplished in the absence of active versions of Shc or cbl, and there was no evidence of Stat activity and only modest amounts of bcl-xL, a Stat5 transcriptional target protein, all of which characterized the cells transformed by BCR-ABL. However, H7gag-akt/RafCAAX cells and H7BCR-ABL cells cultured in the absence of IL-3 shared strikingly increased p65 nuclear factor κB (NFκB) activity. Treatment of cells with a specific NFκB inhibitor, parthenolide, led to loss of NFκB activity and down-regulation of antiapoptotic c-IAP2. In cells with only gag-akt/RafCAAX, this was sufficient to allow polyADP ribosyltransferase (PARP)–degradative apoptosis, but in cells with p210BCR-ABL, apoptosis was blocked, possibly by a Stat5/bcl-xL–dependent mechanism. Therefore, one hematopoietic antiapoptotic program, among others, available to certain tyrosine kinase oncogenes involves a cooperative response between raf/erk and akt, unambiguous components of p21ras and PI-3-kinase pathways, to induce p65 NFκB and c-IAP2.
Introduction
Considerable efforts have been devoted to unraveling the mechanism of leukemic transformation by the p210 (or p185) BCR-ABL product of the Philadelphia chromosome translocation in chronic myelogenous leukemia, as well as the more commonly occurring tyrosine kinase oncogene of acute myeloid leukemia, rendered by internal tandem duplication (ITD) mutation of the Flt3 receptor. In the case of p210BCR-ABL, at least 3 major independent signaling pathways are simultaneously active, and these bear upon additional subsidiary pathways as well.1-19 Activity of the independent pathways initiated by p21ras, phosphatidylinositol-3-kinase (PI-3-kinase [via tyrosine kinase–dependent p85 adaptor interactions]20-22), and one exerted on behalf of Stat5 has been observed in cells with the BCR-ABL protein.
Precise analysis of the individual contribution(s) of these independent signal pathways, such as elicited by p210BCR-ABL, toward the transformed phenotype of hematopoietic cells is quite complicated. In part, this arises because of tyrosine kinase–dependent downstream signal pathway cross-talk mediated via adaptor proteins. We wished to focus on the potential for collaboration of signals emanating from p21ras and from PI-3-kinase in hematopoietic transformation. To further restrict the potential for cross-talk in analyzing the collaboration of main components of these pathways (p21ras [raf/erk] and PI-3-kinase [akt]), we used membrane-targeted active versions of raf (RafCAAX) and akt (gag-akt) to reconstruct pathways mediated by p210BCR-ABL in the absence of confounding adaptor-mediated cross-talk. By the combined expression of both of these active pathways in interleukin-3 (IL-3)–dependent hematopoietic cells, but not when either was present alone, hematopoietic factor–dependent cell lines were transformed to growth factor independence and achieved continuous but slow proliferation, and apoptosis was retarded. These effects were linked to the activity of p65 NFκB, which was previously identified as an attribute of p210BCR-ABL–transformed cells.23 The present studies link NFκB activity and antiapoptosis to the cooperative function of raf/erk and akt within hematopoietic cells that lacked any additional interactions characteristic of an archetype tyrosine kinase oncogene, p210BCR-ABL, known to be responsible for upstream elicitation of branched signaling.
Materials and methods
Genes and vectors
The vector pGD210 containing a human p210BCR-ABL cDNA and a neomycin-resistance phosphotransferase gene was a gift of David Baltimore, PhD.24 A vector containing gag-modified bovine akt cDNA within pSG5α was obtained from Dr B. M. Th. Burgering.25 A minicassette containing these sequences plus SV40 early promoter and polyadenylation site was removed bySalI digestion, and the insert was subcloned into the pBabpuro mammalian expression vector. For transfection into cells, the vector was linearized (BstXI) or the LTR-LTR contained fragment was released (KpnI). A myc-tagged, Raf cDNA with C-terminal CAAX box, previously described,26 was removed from pBabpuro (EcoRI) and recloned into pLXSN, which encodes the neomycin-resistance phosphotransferase gene. For transfection, the vector was first linearized with BamHI. In addition to the above, a mammalian expression vector, pRCcyclinD1, incorporating the cyclin D1 cDNA and also encoding a neocassette, was a gift of Dr David Beach.
Cells and cell culture
The growth factor–dependent murine myeloid cell line H7 and its maintenance in medium with a source of IL-3 (WEHI-3 conditioned medium; WEHI-3 CM) have been described previously.27 These cells were transformed with the cDNA for human p210BCR-ABL in the vector pGD210, which also contains a neomycin-resistance cassette.1 24 H7 cells were also transfected with pLXSN RafCAAX and were maintained in McCoy's medium supplemented with 10% fetal calf serum (FCS) and 10% WEHI-3 CM plus G418, 500 to 1000 μg/mL. These cells, H7RafCAAX, were incapable of growth in the absence of IL-3. Independently, H7 cells were transfected with pBabgag-akt and were maintained in McCoy's medium supplemented with the same 10% FCS/10% WEHI-3 CM plus additional puromycin, 5 to 10 μg/mL. These cells, H7gag-akt, were also incapable of growth in the absence of IL-3. The transformed cell lines, H7gag-akt/RafCAAX1 and H7gag-akt/RafCAAX2, were derived, respectively, from transfection of H7pBabgag-akt with pLXSN-RafCAAX and from transfection of H7pLXSNRafCAAX with pBabgag-akt. These cells were maintained in McCoy's medium supplemented with 10% FCS plus G418 at 500 μg/mL and puromycin at 5 μg/mL.
Where appropriate, cells were treated with various concentrations of parthenolide, a sesquiterpene lactone–specific inhibitor of NFκB, which prevents inhibitor of kappa B (IKB) degradation and binding/release of p65 NFκB.28 Viable cell recovery, apoptosis assay, and nuclear extracts were performed at intervals of 4, 16, and 24 hours.
Cell lysates
Immunoblotting
Erk kinase assay
Cellular proteins, 250 μg/reaction, were diluted to 1 μg/mL in phosphotyrosine lysis buffer and subjected to immunoprecipitation with 5 μg antimouse ERK antibody (Santa Cruz Biotechnology, Santa Cruz, CA) along with protein G PLUS agarose (Oncogene Science, Uniondale, NY) by gentle rocking at 4°C. The agarose beads were washed twice with lysis buffer and then once with kinase buffer (30 mM Tris HCl, pH 8, 20 mM MnCl2, 2 mM MgCl2, 10 μM ATP). The pellet was resuspended in 30 μL kinase buffer along with 7.5 μg myelin basic protein (MBP; Sigma, St Louis, MO) and 37 kBq (1 μCi) γ32P-ATP.30 The kinase reaction was allowed to proceed at 37°C for 30 minutes, after which the reaction was stopped by addition of Laemmli sample buffer. The reaction contents were boiled and subjected to electrophoresis on 15% SDS-polyacrylamide gels. Phosphorylated proteins were visualized by autoradiography of the dried slab gels.
Akt kinase assay
Cellular proteins, 200 μg, were immunoprecipitated with 2.5 μg anti–protein kinase B (PKB) (Upstate Biotechnology, Lake Placid, NY) and 15 μL protein G PLUS agarose (Oncogene Science) after gentle rocking at 4°C for 3 hours. After pelleting at 2500 rpm for 5 minutes, the beads were washed twice with lysis buffer, once with H2O, and once with PKB kinase buffer, while maintaining the beads on ice at all times. Finally, the beads were resuspended in 25 μL PKB kinase buffer (20 mM HEPES, 10 mM MgCl2, 10 mM MnCl2) and exposed to substrate, histone H2B (Roche Biologicals, Indianapolis, IN), 2.5 μg/reaction, in a final volume of 30 μL along with 32P-ATP, 92.5 kBq (2.5 μCi), in a final concentration of cold/radioactive ATP, 1.25 μM/2.5 μM. The reaction was allowed to proceed at room temperature for 30 minutes with occasional agitation. The reaction was then stopped with SDS-PAGE sample buffer, boiled, subjected to electrophoresis on 15% polyacrylamide, and autoradiographed.
Gel mobility shift analysis
Cell nuclear protein lysates, 5 to 15 μg, prepared by the above protocol were placed in the electrophoretic mobility shift assay with oligonucleotide probe for NFκB site (Promega Biotech, Madison, WI), using methods described previously.30
Antibodies
The following antibodies were used for supershifting (gel mobility shift assays) or immunoblotting of proteins Western transferred onto nitrocellulose filters after SDS-PAGE: anti–Akt-1 (clone D-17), anti-Erk1 (691) (sc-94), anti-Raf C-terminal portion (clone C-12), anti-Raf N-terminal portion (sc-134) and Raf internal part (clone H-71), anti–c-myc (clone 9E10), anti-cbl (sc-170), anti–Stat5α (sc-1081), anti–NFκB p50 (sc-7178), anti–c-Rel (sc-272), and anti–Rel-B (sc-226) (Santa Cruz Biotechnology). Anti–phospho-Tyr (clone 4G10), anti-Shc (#06-203), anti-Akt/PKB (#06-558), and anti–NFκB p65 (#06-409) were from Upstate Biotechnology. Antirabbit glyceraldehyde-3-phosphate dehydrogenase (GAPDH; H86504) was from Biodesign International (Kennebunk, ME). Rabbit anti–polyADP ribosyltransferase (PARP) antibody was from Enzyme Systems Products (Livermore, CA). Horseradish peroxidase–conjugated antimouse IgG (sc-2005), antirabbit IgG (sc-2005), and antigoat IgG (sc-2020) were from Santa Cruz Biotechnology. Horseradish peroxidase–conjugated rabbit anti–sheep IgG (#6134328) was from Rockland (Gilbertsville, PA). Fluorescein isothiocyanate–labeled annexin V protein was from Becton Dickinson/Pharmingen (Thousand Oaks, CA).
Results
Expression of gag-akt and/or RafCAAX within IL-3–dependent cell line H7 leads to selective augmentation of enzymatic activity in that pathway targeted by the transfected enzyme
Stable transfections into parental H7 cells were accomplished by electroporation followed by selection of cells cultured in liquid phase, in the presence of the relevant antibiotic for which resistance was encoded by the vector used. First, H7 cells were transfected with pBabgag-akt, whose stable genomic incorporation and expression were enforced by selection of transfectants in the presence of puromycin, 5 μg/mL. Expression was verified by immunoblotting with an akt antibody (see below). Next, either H7 cells or H7gag-akt cells were transfected with pRafCAAX/LXSN, and transfectants were selected in the presence of G418, 500 μg/mL, or in the presence of puromycin and G418, respectively. H7 cells were also transfected with pGD210BCR-ABL.30
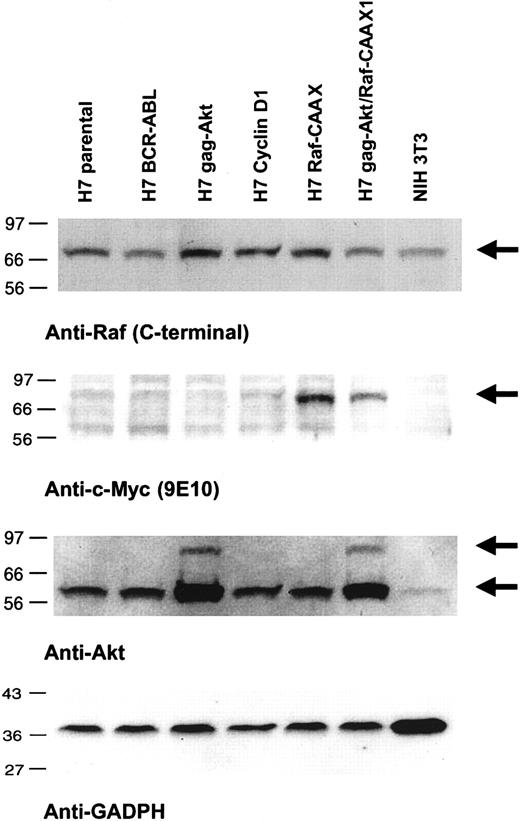
Lysates of the respective cell lines were first immunoblotted for the detection of akt and gag-akt (Figure 1). The gag-akt fusion species of 96 kd was observed only in lysates of those cells transfected with the vector pBabgag-akt, and it was observed that the amount of this species immunoreactive with the anti–akt antibody appeared to represent a significantly smaller fraction than the endogenous akt species of 66 kd (Figure 1). In fact, the amount of the 96-kd gag-akt species was variably represented from different lysates of the same cell line, indicating its highly unstable nature in lysates (Figure 1). Notably, however, it was repeatedly observed in these cell lines transfected with pBabgag-akt and expressing variable amounts of the 96-kd fusion protein that the amount of the presumed endogenous 66-kd akt species was significantly increased 2- to 3-fold as compared with equal amounts of lysates from the nontransfected cells (Figure 1). This was confirmed by reprobing the blots with antibody for a constitutively expressed protein as loading control, GAPDH (Figure 1). Thus, we were led to conclude that gag-akt is initially placed in the membrane of cells containing the construct encoding its expression, where it is capable of phosphorylation by the PDK activating kinases, and it is subsequently also observed in cell lysates as an immunoreactive cleaved species with the same apparent molecular weight as endogenous akt. This interpretation was further supported by immunoprecipitation experiments (data not shown).
Expression of RafCAAX, gag-akt, or both in IL-3–dependent H7 cells.
Lysates of the indicated cell lines were subjected to SDS-PAGE and immunoblotting as noted.
Expression of RafCAAX, gag-akt, or both in IL-3–dependent H7 cells.
Lysates of the indicated cell lines were subjected to SDS-PAGE and immunoblotting as noted.
We next immunoblotted lysates for detection of RafCAAX, a task that was facilitated by the presence of an N-terminal myc tag encoded in the vector (Figure 1). Cell lysates were immunoblotted with 9E10 monoclonal antibody, raised specifically against the myc tag epitope. Both H7RafCAAX and H7gag-akt/RafCAAX expressed the 72-kd RafCAAX with the myc tag (Figure 1). Interestingly, when 9E10 immunoprecipitates were probed with an antibody against the N-terminal domain of c-raf, but not with a C-terminal antibody, selective detection of the 72-kd Raf epitope was observed in these same lysates (data not shown). This is because the C-terminal epitope of endogenous c-raf is blocked by the CAAX box in pRafCAAX/LXSN, resulting in failure of detection of the C-terminal epitope (Figure 1). Thus, taken in total, we had cell lines expressing either RafCAAX or gag-akt, or both these species, and the relative equality of expression of these respective proteins, when comparing the cell lines transfected with both vectors versus those transfected with only a single species, was fortified by reprobing blots for 2 different protein loading controls, using GAPDH and C-terminal (endogenous) c-raf antibodies (Figure 1).
Next, kinase assays were performed to examine activity for signaling within the pathways targeted by the exogenous proteins expressed by the vectors. First, we performed akt immune complex kinase assays to distinguish the akt activity between cells transfected with the gag-akt vector versus those transfected with the RafCAAX vector, where, by comparison, one would expect to observe augmented akt activity in the former. In this experiment, NIH 3T3 cells passaged in regular medium versus the same cells acutely stimulated with epidermal growth factor (EGF), a strong akt agonist, served as controls (Figure2).
Abundant kinase activity of akt is conferred to H7 cells by gag-akt, but not RafCAAX expression.
Lysates of NIH 3T3 cells, treated or not with EGF, and lysates of H7RafCAAX cells or H7gag-akt/RafCAAX cells, both taken from their normal growth medium, were immunoprecipitated with akt antibody or control antibody (mock), and kinase assays were performed.
Abundant kinase activity of akt is conferred to H7 cells by gag-akt, but not RafCAAX expression.
Lysates of NIH 3T3 cells, treated or not with EGF, and lysates of H7RafCAAX cells or H7gag-akt/RafCAAX cells, both taken from their normal growth medium, were immunoprecipitated with akt antibody or control antibody (mock), and kinase assays were performed.
Unstimulated NIH 3T3 cells had no discernable akt activity, whereas EGF stimulated abundant activity of akt for the substrate H2B within NIH 3T3 cells (Figure 2). Similarly, H7RafCAAX cells in regular passage medium in nonexponential growth had little measurable akt kinase activity, but the H7gag-akt/RafCAAX cells had strong activity of the kinase for H2B substrate (Figure 2). A control lane in which H7gag-akt/RafCAAX lysate was treated with nonimmune immunoglobulin lacked the activity seen in the specific immunoprecipitate (Figure 2). In other experiments, we studied the kinase activity of akt in IL-3–dependent cells acutely stimulated with WEHI-3 CM, and we observed that 3- to 5-fold increases in akt activity could be stimulated by treatment (data not shown). In addition, we compared the akt activity ratios between BCR-ABL.A54 cells versus H7 parental cells starved of IL-3, versus H7gag-akt cells similarly starved, and we observed comparable activity of akt within cells with p210BCR-ABL and those with membrane-targeted, constitutively active akt, both of which were 2- to 3-fold greater, respectively, than a background level of activity in the starved H7 parental cells (data not shown).
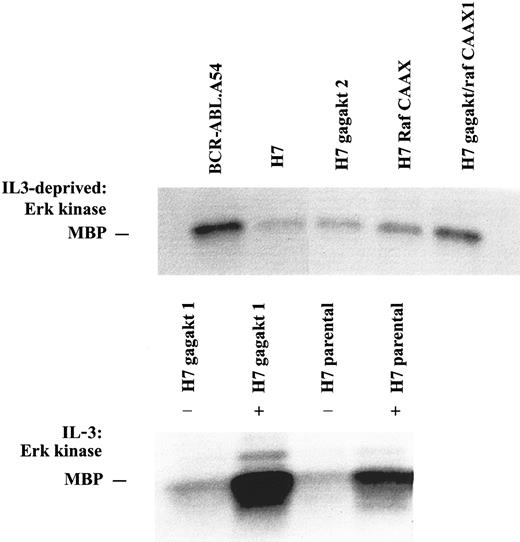
We next conducted a complementary analysis of activity in the raf/erk pathway using a well-characterized erk kinase assay with MBP substrate (Figure 3). First, a series of the cells with or without RafCAAX or gag-akt were depleted of growth factors, and lysates were prepared and immunoprecipitated with anti-erk, followed by washing and immune complex kinase assay using MBP substrate (Figure 3). BCR-ABL.A54 cells had abundant erk activity, whereas H7 parental cells starved of IL-3 had little activity and H7gag-akt cells similarly starved also lacked kinase activity of erk (Figure 3). By contrast, there was greater erk activity apparent in the H7RafCAAX cells, and even more kinase activity of erk, comparable to that observed in BCR-ABL.A54 cells, was observed in H7gag-akt/RafCAAX lysates (Figure3). We also observed strong stimulation of erk kinase activity by IL-3 stimulation of the parental cells and H7gag-akt cells, and the higher basal levels of erk activity seen in the presence of IL-3 in medium supporting factor-dependent cells were verified by blotting lysates with anti–phospo-erk antibodies (Figure 3 and data not shown).
Abundant kinase activity of erk observed in cells with p210BCR-ABL or RafCAAX is independent of the presence of IL-3, whereas erk activity is induced in other cells by IL-3.
Lysates of the cells noted, taken either from IL-3–deprived or IL-3–stimulated cultures as noted, were immunoprecipitated with erk antibody, and the precipitate was subjected to immune complex kinase assay with MBP.
Abundant kinase activity of erk observed in cells with p210BCR-ABL or RafCAAX is independent of the presence of IL-3, whereas erk activity is induced in other cells by IL-3.
Lysates of the cells noted, taken either from IL-3–deprived or IL-3–stimulated cultures as noted, were immunoprecipitated with erk antibody, and the precipitate was subjected to immune complex kinase assay with MBP.
No evidence was found for adaptor protein (shc, cbl) or Stat5 involvement in cells transformed by combined expression of gag-akt and Raf-CAAX
We conducted a formal analysis within the H7gag-akt/RafCAAX cells for the possible existence of activated forms of adaptors implicated in p21ras and PI-3-kinase/akt activation initiated by tyrosine kinase oncogenes or growth factor receptors. For this purpose, we used BCR-ABL.A54 cells and IL-3–starved H7 parental cells as positive and negative controls, respectively. This was done to support selectivity of downstream signaling by the transfected membrane-active kinases and to better prove functional independence of the cooperative activity of gag-akt and RafCAAX toward perpetuation of a factor-independent state of growth.
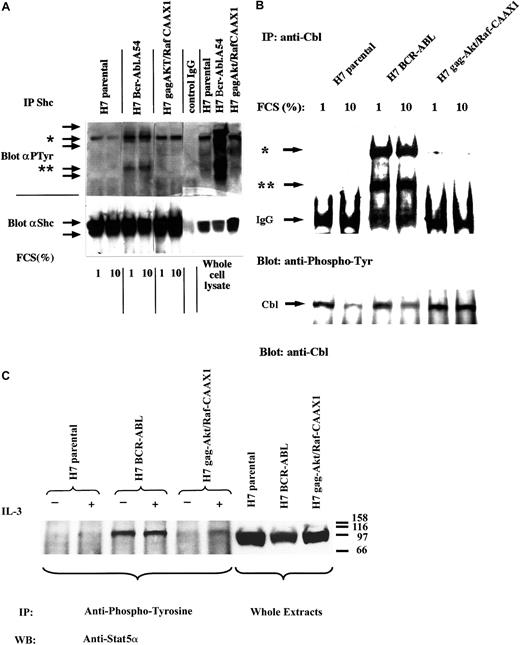
First, whole-cell lysates of starved cells were immunoblotted with antiphosphotyrosine to examine for possible constitutive tyrosine signaling within H7gag-akt/RafCAAX cells that might belie autonomous function of the membrane-active downstream kinases (Figure4A, right panel). As compared with BCR-ABL.A54, which displayed prominent phosphotyrosine bands at 210, 110, 52, and 35 kd, parental H7 cells and H7gag-akt/RafCAAX cell lysates displayed only a prominent 145-kd band, putative SHIP (Figure4A, right panel). Next, cell lysates were immunoprecipitated with anti-Shc and then immunoblotted with antiphosphotyrosine to look for an “active bait” Shc species that could participate in recruitment of Grb-2 or PI-3-kinase (Figure 4A). In BCR-ABL.A54 cell lysates, there was abundant tyrosine-phosphorylated Shc that was immunoprecipitated, whereas in the H7gag-akt/RafCAAX and in the H7 parental cell lysates, no tyrosine-phosphorylated Shc was observed despite quantitatively equal immunoprecipitation of total Shc protein in each lysate, as revealed by reprobing the blot with anti-Shc (Figure 4A, bottom). In addition, anti-Shc immunoprecipitates from the BCR-ABL.A54 cell lysates coprecipitated a tyrosine-phosphorylated 145-kd species, putative SHIP (Figure 4A). Similarly coprecipitated with Shc from the H7 parental and H7gag-akt/RafCAAX lysates was the same tyrosine-phosphorylated putative SHIP species, which is known to be bound, when it is tyrosine phosphorylated, by Shc PTB domain (Figure 4A).
Absence of confounding cross-talk signaling in cells transformed by gag-akt/RafCAAX.
(A) Cell lysates derived from IL-3–deprived H7 parental cells, from H7 BCR-ABL.A54 cells in the absence of IL-3, and H7gag-akt/RafCAAX cells, also in the absence of IL-3, were subjected to SDS-PAGE and antiphosphotyrosine immunoblotting (right panel indicates whole-cell lysate) or, additionally, aliquots of lysates were subjected to immunoprecipitation with anti-Shc and then processed for SDS-PAGE and antiphosphotyrosine immunoblotting (left side). Afterward, the blot was stripped and reprobed with anti-Shc. Asterisk (*) indicates putative SHIP band, whereas (**) indicates Shc. (B) Aliquots of the aforementioned cell lysates derived after IL-3 starvation in the presence of 1% FCS or 10% FCS were immunoprecipitated with anti-Cbl, then immunoblotted with antiphosphotyrosine. Top arrow (*) demonstrates pp120 cbl; bottom arrow (**) demonstrates putative ppShc. Next, the blot was stripped and then reprobed with anti-Cbl. (C) The aforementioned cell lines were starved of IL-3 or had IL-3 (WEHI-3 CM) added for 1 hour, and then cell lysates were made and were either used as whole-cell extracts (right panel) or were immunoprecipitated with antiphosphotyrosine. The precipitates and the whole-cell lysates were subjected to SDS-PAGE and immunoblotting for Stat5α. There was no increase by IL-3 treatment of tyrosine-phosphorylated Stat5α in cells with p210BCR-ABL. In H7gag-akt/RafCAAX1 cells, tyrosine phosphorylation of Stat5 was seen at this time point (60 minutes). For H7 parental cells, a shorter time course (5-30 minutes) of IL-3 treatment (not shown) revealed tyrosine phosphorylation of Stat5α.
Absence of confounding cross-talk signaling in cells transformed by gag-akt/RafCAAX.
(A) Cell lysates derived from IL-3–deprived H7 parental cells, from H7 BCR-ABL.A54 cells in the absence of IL-3, and H7gag-akt/RafCAAX cells, also in the absence of IL-3, were subjected to SDS-PAGE and antiphosphotyrosine immunoblotting (right panel indicates whole-cell lysate) or, additionally, aliquots of lysates were subjected to immunoprecipitation with anti-Shc and then processed for SDS-PAGE and antiphosphotyrosine immunoblotting (left side). Afterward, the blot was stripped and reprobed with anti-Shc. Asterisk (*) indicates putative SHIP band, whereas (**) indicates Shc. (B) Aliquots of the aforementioned cell lysates derived after IL-3 starvation in the presence of 1% FCS or 10% FCS were immunoprecipitated with anti-Cbl, then immunoblotted with antiphosphotyrosine. Top arrow (*) demonstrates pp120 cbl; bottom arrow (**) demonstrates putative ppShc. Next, the blot was stripped and then reprobed with anti-Cbl. (C) The aforementioned cell lines were starved of IL-3 or had IL-3 (WEHI-3 CM) added for 1 hour, and then cell lysates were made and were either used as whole-cell extracts (right panel) or were immunoprecipitated with antiphosphotyrosine. The precipitates and the whole-cell lysates were subjected to SDS-PAGE and immunoblotting for Stat5α. There was no increase by IL-3 treatment of tyrosine-phosphorylated Stat5α in cells with p210BCR-ABL. In H7gag-akt/RafCAAX1 cells, tyrosine phosphorylation of Stat5 was seen at this time point (60 minutes). For H7 parental cells, a shorter time course (5-30 minutes) of IL-3 treatment (not shown) revealed tyrosine phosphorylation of Stat5α.
Next, lysates of the above cell lines were immunoprecipitated with anti-cbl that targets an adaptor, when tyrosine phosphorylated, implicated in PI-3-kinase activation by binding to p85 SH2s (Figure 4B). In fact, anti-cbl immunoprecipitates of BCR-ABL.A54 cells contained abundant tyrosine-phosphorylated cbl and a coprecipitating Ptyr 49-kd species, putative Shc (Figure 4B). By contrast, there was little tyrosine-phosphorylated cbl detected in the anti-cbl immunoprecipitates of lysates from H7 parental cells or H7gag-akt/RafCAAX, despite comparable efficiency of immunoprecipitation (Figure 4B).
We also confirmed the absence of tyrosine-phosphorylated, active Stat5 in lysates of H7gag-akt/RafCAAX cells grown in the absence of IL-3 (Figure 4C). Unlike BCR-ABL.A54 cell lysates, which contained abundant tyrosine-phosphorylated Stat5a detectable in the absence of IL-3, H7gag-akt/RafCAAX contained active Stat5 only upon IL-3 stimulation (Figure 4C). In this experiment, IL-3–starved H7 parental cells similarly lacked active Stat5, and stimulation of phospho-Stat5 by IL-3 was not seen at the relatively late time point used (60 minutes); however, another experiment demonstrated stimulation of Stat5 phosphorylation at 5 to 30 minutes (data not shown).
Thus, taken in total, cells expressing both gag-akt and RafCAAX were capable of growth in the absence of IL-3 (see below), and this state of competence for growth was accomplished in the absence of any discernable stimulus, in the form of phosphotyrosine-initiated, adaptor-mediated parallel signaling, from above the level of these second-tier kinases. We next established the parameters for growth of cells containing one or both of these kinases as compared with the fully transformed BCR-ABL.A54 cells.
Competence for factor-independent growth of H7gag-akt/RafCAAX cells differs from that of cells with p210BCR-ABL in degree, but common functional end points are summoned, including NFκB in particular
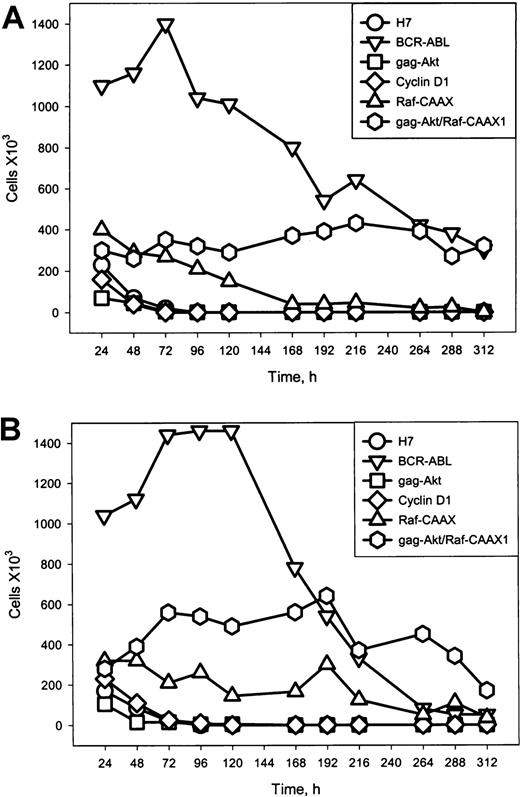
Implication of cooperative activity by raf/erk and akt in the transformed phenotype of cells containing p210BCR-ABL was sought first by documenting growth characteristics of cells expressing gag-akt, RafCAAX, or both, versus the parental H7 cells on the one hand and versus BCR-ABL.A54 cells (Figure 5).
Growth characteristics of cells expressing gag-akt, RafCAAX, or both, versus the parental H7 cells and versus BCR-ABL.A54 cells.
p210BCR-ABL confers rapid growth of H7 cells in the absence of IL-3, and in medium supplemented with low serum (1% FCS; A top) and in 10% FCS (B; bottom), whereas the combination of gag-akt/RafCAAX, but neither gag-akt nor RafCAAX alone, allows for slower but continuous cell growth. Cells were washed with phosphate-buffered saline and plated at 1 × 106 viable cells/mL, and viability counting (trypan blue) was performed at 24-hour intervals. Note that cells with BCR-ABL rapidly overgrow the supplied medium after reaching plateau viable cell concentrations at 1.5 to 2 × 106 viable cells/mL. These cells subsequently die off after overgrowth. Also, cell lines other than those expressing RafCAAX or coexpressing gag-akt/RafCAAX die off. Slow, continuous cell growth is observed in H7gag-akt/RafCAAX1 in the presence of 10% FCS (B), but viability of these cells is maintained at a stable level in 1% FCS (A). H7RafCAAX cells maintain viability for a prolonged period, but are not capable of proliferation.
Growth characteristics of cells expressing gag-akt, RafCAAX, or both, versus the parental H7 cells and versus BCR-ABL.A54 cells.
p210BCR-ABL confers rapid growth of H7 cells in the absence of IL-3, and in medium supplemented with low serum (1% FCS; A top) and in 10% FCS (B; bottom), whereas the combination of gag-akt/RafCAAX, but neither gag-akt nor RafCAAX alone, allows for slower but continuous cell growth. Cells were washed with phosphate-buffered saline and plated at 1 × 106 viable cells/mL, and viability counting (trypan blue) was performed at 24-hour intervals. Note that cells with BCR-ABL rapidly overgrow the supplied medium after reaching plateau viable cell concentrations at 1.5 to 2 × 106 viable cells/mL. These cells subsequently die off after overgrowth. Also, cell lines other than those expressing RafCAAX or coexpressing gag-akt/RafCAAX die off. Slow, continuous cell growth is observed in H7gag-akt/RafCAAX1 in the presence of 10% FCS (B), but viability of these cells is maintained at a stable level in 1% FCS (A). H7RafCAAX cells maintain viability for a prolonged period, but are not capable of proliferation.
Cells transformed by p210 BCR-ABL doubled frequently and achieved very high culture density of more than 1.5 million cells/mL (Figure 5). By contrast, after IL-3 deprivation in either low serum or in 10% serum, all of the following cell lines rapidly died off: H7 parental, H7gagakt, and H7 (ectopic) cyclin D1–expressing (control) cells. On the other hand, H7RafCAAX cells persisted for 4 to 5 days in 1% FCS before dying off entirely, but they persisted somewhat longer in 10% FCS. However, H7gagakt/RafCAAX cells, removed from IL-3 and serum, maintained their viable cell numbers indefinitely, and in 10% FCS they grew continuously (Figure 5). Inhibition of apoptosis in H7 cells with RafCAAX or gag-akt/RafCAAX was confirmed by analysis of the subG0 DNA content of propidium iodide–loaded cells by fluorescence-activated cell sorter analysis (data not shown).
As might be predicted from these results of proliferation, expression of the nuclear c-myc gene, whose transcripts were detected on Northern blot (and normalized for a constitutively expressed control gene, GAPDH), was not so strong in H7gagakt/RafCAAX cells as in p210BCR-ABL–transformed cells (data not shown). Nor was expression of cyclin D1 protein as great in cells with H7gag-akt/RafCAAX as in p210BCR-ABL (data not shown). Thus, cell cycle–dependent proteins were understimulated in H7 cells transformed with the combination of gag-akt/RafCAAX compared with BCR-ABL. We also observed the ability of the combination of gag-akt/RafCAAX to result in factor-independent slow proliferation of the FDC-P1 cell line (V. Gelfanov, H. S. Boswell, unpublished observations, 2000). The uniform ability of this oncogene (akt/raf) combination to mediate a hypoproliferative transformed state in different cell lines suggested that some other function rather than excessive cell-cycle transit alone would be foremost in the transforming program.
A recently appreciated target for downstream signal integration, known to contribute to both inhibition of apoptosis and proliferation induction, is NFκB. We therefore examined the hypothesis that synergism between raf and akt signaling leads to NFκB activity augmentation. In fact, p210BCR-ABL has been noted to selectively activate a p65/p50 transcriptionally active species of NFκB not characteristic of cytokine-dependent cell lines.23
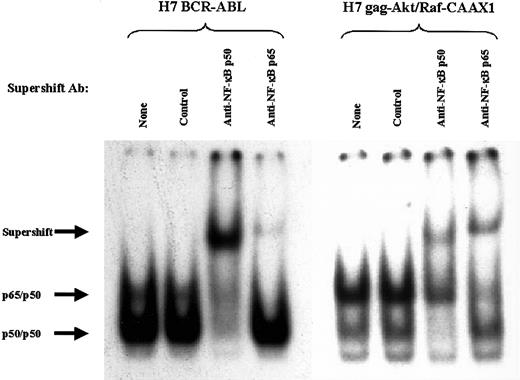
First, gel mobility shift analysis was performed to search for the previously observed p65 heterodimeric species of NFκB within extracts of cells with p210BCR-ABL (Figure6, left panel). Indeed, 2 specific complexes were seen with extracts of BCR-ABL.A54 cells bound to the NFκB oligonucleotide probe (Figure 6, left panel). The composition of the 2 complexes was addressed with a supershift protocol (Figure 6). A major quantitative supershift of the faster migrating putative p50 homodimer occurred with anti-p50 antibody but not with anti-p65 antibody (Figure 6). By contrast, anti-p65 antibody caused supershifting of the slower migrating complex consisting of NFκB heterodimer, without affecting the p50/p50 species of BCR-ABL.A54 cells (Figure 6, left panel).
Supershift analysis of the composition of NFκB complexes in the extracts of H7 BCR-ABL.A54 cells and H7gag-akt/RafCAAX1 cells.
Use of antibodies against relB (control), p65, and p50 reveals the composition of the 2 complexes shared in common by the above cell types. Arrows indicate position of supershift.
Supershift analysis of the composition of NFκB complexes in the extracts of H7 BCR-ABL.A54 cells and H7gag-akt/RafCAAX1 cells.
Use of antibodies against relB (control), p65, and p50 reveals the composition of the 2 complexes shared in common by the above cell types. Arrows indicate position of supershift.
Nuclear protein extract from H7gag-akt/RafCAAX cells, when placed in gel mobility shift assay alongside that described above, showed the same 2 separately migrating complexes bound to NFκB oligonucleotide probe (Figure 6, right panel). Indeed, the relative abundance of the slower mobility p65 heterodimeric species, which was supershifted by both anti-p65 and anti-p50 antibodies (partial effect on this complex by anti-p50), was even greater in these extracts than in those from BCR-ABL.A54 cells (Figure 6, right panel). The faster mobility complex, composed of p50 species as assessed by supershifting, was less abundant than in BCR-ABL.A54 cell extract (Figure 6). For further confirmation of the synergistic contribution of gag-akt and RafCAAX to the observed p65 NFκB activity, another stably transfected H7gag-akt/RafCAAX cell line was derived by transfection of the 2 vectors in reverse chronology (see “Materials and methods”). Extracts from these cells, H7gag-akt/RafCAAX2, showed the same overabundance of the p65 heterodimeric NFκB species in gel mobility shift assay (data not shown; see below).
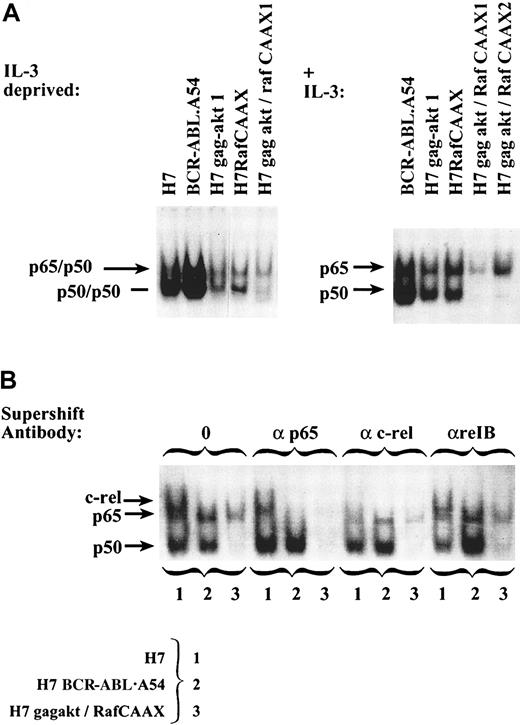
Assessment of the relative abundance of the 2 aforementioned complexes in the cell lines taken after IL-3 deprivation or, alternatively, from their normal culture medium containing 10% FCS (BCR-ABL.A54 cells and H7gag-akt/RafCAAX cells cultured without IL-3 required) showed the lowest ratio of the putative p65 heterodimeric species/p50 homodimeric species in the H7 parental cells (Figure7A, left). The highest such ratio was observed in H7gag-akt/RafCAAX1 and H7gag-akt/RafCAAX2 cells (Figure7A). Next in rank order was the relative p65 NFκB content within the BCR-ABL.A54 cell extract, containing roughly equal intensity of the p65 heterodimeric species with the p50/p50 species, then the 2 cell lines with gag-akt only and RafCAAX only, respectively, which had slightly less of a band-shift species with the mobility of the p65 heterodimer compared with p50 homodimer (Figure 7A). During the performance of these comparisons, it was noted that the band-shift species associated with H7 parental cells was not only of lower relative intensity, but also had very slightly retarded mobility compared with the heterodimeric p65 NFκB complex of the cells with p210BCR-ABL or gag-akt/RafCAAX. We therefore specifically addressed its identity in another supershift experiment using, in addition to antibodies against p65 and rel B, an antibody against c-rel (Figure 7B). In fact, the slower mobility complex characteristic of H7 parental cells was specifically and quantitatively supershifted and neutralized by antibody to c-rel, whereas only p65 antibody was active against the slightly faster mobility complex of Bcr-Abl.A54 cells or H7gag-akt/RafCAAX (Figure 7B). We also examined whether the state of the growth medium affected the abundance of the different complexes that characterized H7 parental cells versus BCR-ABL.A54 cells, and noted a slight induction of the c-rel complex upon addition of WEHI-3 CM (IL-3); however, no effect on abundance of p65-containing complex was seen upon IL-3 treatment of H7 parental cells (data not shown).
Comparison of the composition of NFκB DNA-binding activity present in IL-3–dependent parental H7 cells versus derivative cell lines with p210Bcr-Abl, gag-akt, RafCAAX, or both gag-akt/RafCAAX demonstrates unique presence of c-rel in IL-3–dependent H7 cells.
(A) On the left, extracts were made from cells deprived of IL-3 and then these extracts were placed into a gel mobility shift experiment with an NFκB oligonucleotide probe labeled with 32P-ATP, and then the resulting gel was dried and autoradiographed. On the right, extracts of the cells taken from usual growth medium, including IL-3 only for H7 parental cells, H7gag-akt, and H7RafCAAX cells (no IL-3 was added to BCR-ABL.A54 or H7gag-akt/RafCAAX cells), were subjected to gel mobility shift. Arrows on left note the mobility of complexes composed of p50 homodimers, heterodimers of p65, or complexes of c-rel. (B) Extracts of cells deprived of IL-3 were placed into a gel mobility shift experiment with NFκB oligonucleotide, and the composition of complexes was established by supershift protocol using antibodies to c-rel, p65, and rel B (negative control). Exclusive appearance of c-rel was noted in H7 parental cell extracts and exclusive appearance of p65 in extracts of H7 BCR-ABL.A54 and H7gag-akt/RafCAAX cells. Supershifted complexes rose to the top of the gel (not pictured). Note that a control gel shift for constitutive complex of CREB (not shown) demonstrated that the H7gag-akt/RafCAAX extract was slightly underloaded.
Comparison of the composition of NFκB DNA-binding activity present in IL-3–dependent parental H7 cells versus derivative cell lines with p210Bcr-Abl, gag-akt, RafCAAX, or both gag-akt/RafCAAX demonstrates unique presence of c-rel in IL-3–dependent H7 cells.
(A) On the left, extracts were made from cells deprived of IL-3 and then these extracts were placed into a gel mobility shift experiment with an NFκB oligonucleotide probe labeled with 32P-ATP, and then the resulting gel was dried and autoradiographed. On the right, extracts of the cells taken from usual growth medium, including IL-3 only for H7 parental cells, H7gag-akt, and H7RafCAAX cells (no IL-3 was added to BCR-ABL.A54 or H7gag-akt/RafCAAX cells), were subjected to gel mobility shift. Arrows on left note the mobility of complexes composed of p50 homodimers, heterodimers of p65, or complexes of c-rel. (B) Extracts of cells deprived of IL-3 were placed into a gel mobility shift experiment with NFκB oligonucleotide, and the composition of complexes was established by supershift protocol using antibodies to c-rel, p65, and rel B (negative control). Exclusive appearance of c-rel was noted in H7 parental cell extracts and exclusive appearance of p65 in extracts of H7 BCR-ABL.A54 and H7gag-akt/RafCAAX cells. Supershifted complexes rose to the top of the gel (not pictured). Note that a control gel shift for constitutive complex of CREB (not shown) demonstrated that the H7gag-akt/RafCAAX extract was slightly underloaded.
Inhibition of NFκB and c-IAP2 expression was associated with apoptosis in H7gag-akt/RafCAAX cells lacking active Stat5 and with limiting Bcl-xL, but apoptosis was blunted in BCR-ABL.A54 cells containing Stat5 and Bcl-xL despite inhibition of c-IAP2 expression
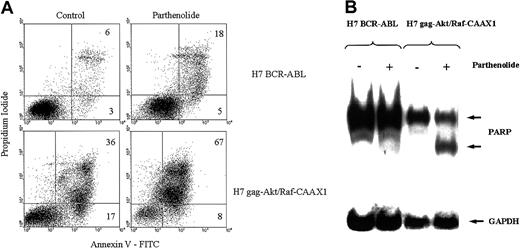
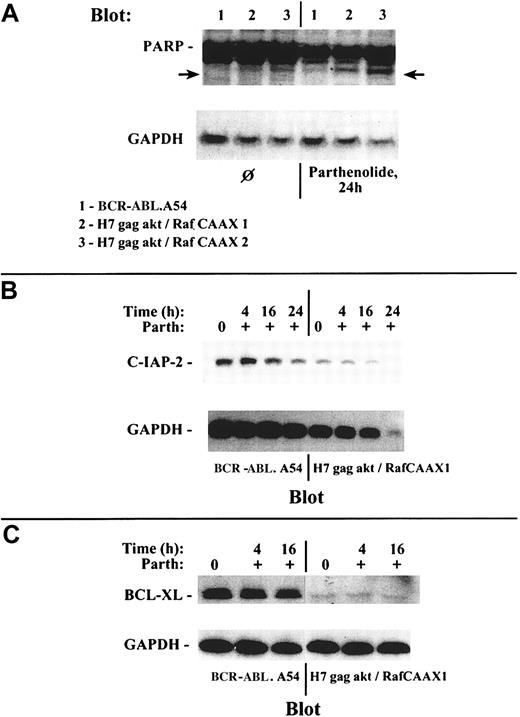
Having isolated akt and raf/erk pathways to NFκB from other pathways downstream from p210BCR-ABL, it was of interest to compare the consequences of loss of functional NFκB expression in cells with Stat5 and NFκB (BCR-ABL.A54) versus cells with only NFκB (H7gag-akt/RafCAAX). We used a specific inhibitor of NFκB, parthenolide, a sesquiterpene lactone,28 in preliminary experiments to study the consequences of specific NFκB inhibition and performed control assays of dose- and time-dependent viable cell recovery and corresponding inhibition of NFκB by gel shift. We found that there was a relation between parthenolide dose and inhibition of viable cell recovery, with a requirement for approximately 17 to 24 hours for cell killing to occur at all doses of parthenolide up to 10 μM (IC50 approximately 5 μM; data not shown). At a parthenolide concentration of 10 μM, loss of viable cell recovery of H7gag-akt/RafCAAX1 was preceded by significant inhibition of specific NFκB binding at 4 hours (greater than 75% inhibition of binding to specific NFκB oligo by gel shift in both H7gag-akt/RafCAAX1 and also BCR-ABL.A54; data not shown). However, doses of parthenolide up to 40 μM for 24 hours did not reduce the viability of BCR-ABL.A54 beyond background, whereas H7gag-akt/RafCAAX1 cells were reduced by 85%. In addition to viability counting, 2 measures of apoptosis were determined: annexin V staining for exteriorized phosphatidylserine and cleavage of PARP, a caspase-3 target (Figure8). By both of these measures, BCR-ABL.A54 cells were refractory to the parthenolide treatment, which resulted in dramatic apoptosis in H7gag-akt/RafCAAX cells (Figure 8).
Parthenolide, a specific NFκB inhibitor, produces apoptosis in H7gag-akt/RafCAAX cells, but is inactive in this regard in the sister cells transformed by p210BCR-ABL.
The aforementioned cells were treated or not with parthenolide, 10 μM, for 24 hours, and then apoptosis was measured by flow cytometric analysis of annexin V binding and propidium iodide uptake (A) or by analysis of PARP cleavage on immunoblot (B). In (A), apoptotic cells are those in the upper right quadrant with dual staining with propidium iodide and annexin V. In (B), the 85-kd degradation product of caspase-cleaved whole PARP (118 kd) is observed after parthenolide treatment of H7gag-akt/RafCAAX.
Parthenolide, a specific NFκB inhibitor, produces apoptosis in H7gag-akt/RafCAAX cells, but is inactive in this regard in the sister cells transformed by p210BCR-ABL.
The aforementioned cells were treated or not with parthenolide, 10 μM, for 24 hours, and then apoptosis was measured by flow cytometric analysis of annexin V binding and propidium iodide uptake (A) or by analysis of PARP cleavage on immunoblot (B). In (A), apoptotic cells are those in the upper right quadrant with dual staining with propidium iodide and annexin V. In (B), the 85-kd degradation product of caspase-cleaved whole PARP (118 kd) is observed after parthenolide treatment of H7gag-akt/RafCAAX.
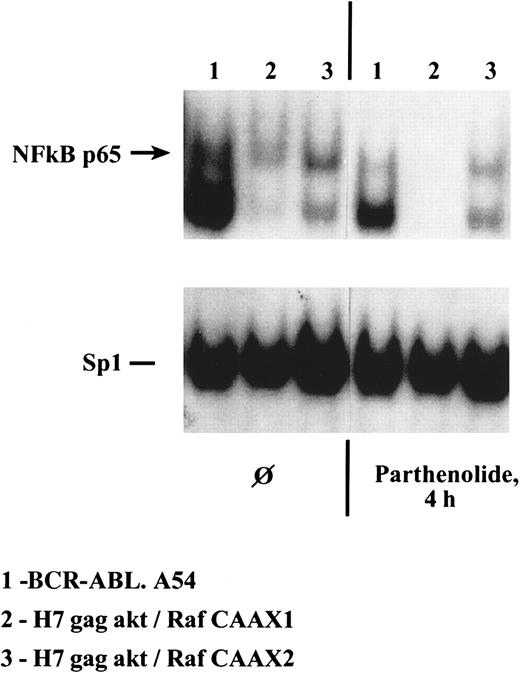
Simultaneous assay of specific caspase-mediated apoptosis (cleavage of PARP) and measurement by immunoblotting of c-IAP2 (an NFκB target that inhibits caspase-3 activation by caspase 9) and bcl-xL (a Stat5 target) were performed on the lysates of cells whose NFκB and Sp1 transcription factor binding capacity were also studied. Both H7gag-akt/RafCAAX1 and BCR-ABL.A54 cells treated with parthenolide had marked inhibition of NFκB binding at 4 hours without any obvious effect on Sp1 binding in a gel shift experiment, and no inhibition of viability had yet taken place (Figure 9and data not shown). In these cells, inhibition of c-IAP2 expression was apparent later, at 16 to 24 hours, and PARP cleavage occurred only in H7gag-akt/RafCAAX cells but not in BCR-ABL.A54 (Figure10). Further, BCR-ABL.A54 cells expressed abundant bcl-xL protein, in keeping with its origination by Stat5-dependent transcriptional processes.15 31 On the other hand, H7gag-akt/RafCAAX cells expressed modest amounts of bcl-xL, and this content was not further diminished by parthenolide's inhibition of NFκB (Figure 10).
Capacity for generation of p65 NFκB within H7 cells containing either p210BCR-ABL or gag-akt/RafCAAX is severely inhibited by treatment of cells with parthenolide for 4 hours, whereas Sp1 transcription factor binding activity is unaffected.
Lysates were made of the noted cells treated for 4 hours with parthenolide, 40 μM, and then gel shifts of equal quantities of protein were performed with a radiolabeled NFκB oligonucleotide probe.
Capacity for generation of p65 NFκB within H7 cells containing either p210BCR-ABL or gag-akt/RafCAAX is severely inhibited by treatment of cells with parthenolide for 4 hours, whereas Sp1 transcription factor binding activity is unaffected.
Lysates were made of the noted cells treated for 4 hours with parthenolide, 40 μM, and then gel shifts of equal quantities of protein were performed with a radiolabeled NFκB oligonucleotide probe.
Multiparameter analysis demonstrates differing effect of NFκB inhibition and resultant c-IAP2 depletion toward induced PARP cleavage as a manifestation of apoptosis within resistant BCR-ABL A54 cells with abundant bcl-xL versus sensitive H7gag-akt/RafCAAX cells with minimal bcl-xL expression.
(A) Lysates were prepared from the noted cell types either without prior treatment or after treatment for 24 hours with parthenolide, and then they were immunoblotted with anti-PARP. Cells transformed by gag-akt/RafCAAX showed induced PARP degradation to generate an 85-kd PARP species, but those transformed by p210BCR-ABL were refractory to this treatment and retained their unmodified 116-kd PARP species intact. (B) Treatment of BCR-ABL.A54 cells and of H7gag-akt/RafCAAX cells with parthenolide was performed, and at various time intervals, lysates were prepared for immunoblotting of c-IAP2 expression. Loss of c-IAP2 expression was noted to have occurred in BCR-ABL.A54 cells by 16 hours (27% reduction by densitometry), and the loss was more extensive at 24 hours (56% reduction). In the case of H7gag-akt/RafCAAX cells, the inhibition of c-IAP2 expression by parthenolide was quite marked at 16 hours (64% reduction) and inhibition was total at 24 hours; in addition, the latter cells yielded lower total protein (controlled for by GAPDH expression), which was devoid of c-IAP2 associated with extensive apoptosis. (C) Lysates prepared from BCR-ABL.A54 cells or H7gag-akt/RafCAAX1 cells either untreated or treated for the indicated periods with parthenolide were immunoblotted for bcl-xL. There was high expression of bcl-xL in BCR-ABL.A54 cells and very little bcl-xL in H7gag-akt/RafCAAX cells. Neither cell type evidenced inhibition of bcl-xL expression by parthenolide treatment.
Multiparameter analysis demonstrates differing effect of NFκB inhibition and resultant c-IAP2 depletion toward induced PARP cleavage as a manifestation of apoptosis within resistant BCR-ABL A54 cells with abundant bcl-xL versus sensitive H7gag-akt/RafCAAX cells with minimal bcl-xL expression.
(A) Lysates were prepared from the noted cell types either without prior treatment or after treatment for 24 hours with parthenolide, and then they were immunoblotted with anti-PARP. Cells transformed by gag-akt/RafCAAX showed induced PARP degradation to generate an 85-kd PARP species, but those transformed by p210BCR-ABL were refractory to this treatment and retained their unmodified 116-kd PARP species intact. (B) Treatment of BCR-ABL.A54 cells and of H7gag-akt/RafCAAX cells with parthenolide was performed, and at various time intervals, lysates were prepared for immunoblotting of c-IAP2 expression. Loss of c-IAP2 expression was noted to have occurred in BCR-ABL.A54 cells by 16 hours (27% reduction by densitometry), and the loss was more extensive at 24 hours (56% reduction). In the case of H7gag-akt/RafCAAX cells, the inhibition of c-IAP2 expression by parthenolide was quite marked at 16 hours (64% reduction) and inhibition was total at 24 hours; in addition, the latter cells yielded lower total protein (controlled for by GAPDH expression), which was devoid of c-IAP2 associated with extensive apoptosis. (C) Lysates prepared from BCR-ABL.A54 cells or H7gag-akt/RafCAAX1 cells either untreated or treated for the indicated periods with parthenolide were immunoblotted for bcl-xL. There was high expression of bcl-xL in BCR-ABL.A54 cells and very little bcl-xL in H7gag-akt/RafCAAX cells. Neither cell type evidenced inhibition of bcl-xL expression by parthenolide treatment.
Discussion
An extensive descriptive analysis of the mechanism for hematopoietic transformation by representative leukemic tyrosine kinase oncogenes p210BCR-ABL, mutant (ITD) Flt3, and TelPDGF has been presented. Simultaneous activity of multiple upstream and downstream effectors has been described, including, respectively, active p21ras, raf, PI-3-kinase, and akt, then followed downstream by c-myc, cyclin D1, c-jun/AP-1, Stat5, and, more recently, NFκB.1-19,30-39 However, attempts at isolation of individual pathways and their effector end points have been only partially successful. For example, the property of redundancy for adaptor protein interaction encoded within different BCR-ABL protein modules has hampered attempts at attribution of specific hematopoietic developmental functions to one BCR-ABL domain, one pathway, or one downstream effector.40-43 Recently, however, one pathway emanating from p210BCR-ABL and ending on Stat5 was isolated for analysis in the same parental cells as those cells that also, independently, bear p210BCR-ABL, and in which dominant-negative (DN) inhibition of the Stat5 signaling molecule had been examined previously.31 Indeed, a constitutively activated Stat5 could transform IL-3–dependent BaF3 parental cells to a lesser degree than could p210BCR-ABL, in keeping with the partial inhibitory effect of DN Stat5 on p210BCR-ABL–mediated proliferation and viability.31 This was interpreted to reflect involvement of other pathways in the program of hematopoietic transformation by p210BCR-ABL, in addition to Stat5. However, it was recently reported that p210BCR-ABL is at least capable of transforming bone marrow cells from Stat5a/b-deficient cells in vitro and in vivo.44
Left to be fully addressed, then, is the question of what is the minimal cooperation needed among other major pathways identified in the cell transformed by tyrosine kinase oncogenes such as p210BCR-ABL to achieve independence from hematopoietic growth factor(s) for antiapoptosis, rendered by a defined target protein. It was with this goal in mind that we coexpressed active versions of second-tier kinases in the p21ras and PI-3-kinase pathways within the same parental cells that were also independently engineered to express p210BCR-ABL; in the latter situation, we and others have noted activity of p21ras and PI-3-kinase.1,8-11,45 46 Ultimately, we discovered that raf and akt expressed together in cells, each in an active form, was a relatively pure cooperative stimulus to p65 NFκB activation.
In fact, we observed that coexpression of gag-akt and RafCAAX rendered IL-3–independent proliferation to H7 factor-dependent parental cells, and this state was accomplished in the absence of confounding signaling from upstream tyrosine kinases or their subservient adaptor signal amplifiers. This state was also independent of the downstream transcriptional mediator Stat5, previously demonstrated to contribute functionally to p210BCR-ABL transformation.12-15 31 In addition, our study found that the end point effectors cyclin D1 and c-myc (data not shown) were not fully reconstituted to the level elicited by p210BCR-ABL even upon cooperation of gag-akt with RafCAAX. Apparently as a direct result, the proliferative rate of H7gag-akt/RafCAAX cells was significantly blunted compared with those H7 cells with p210BCR-ABL.
However, our study has more important implications in the clarity it provides for viewing the potential antiapoptotic programs downstream from certain tyrosine kinase oncogenes such as p210BCR-ABL. In fact, the absence of significant Stat5 and bcl-xL activity in H7gag-akt/RafCAAX cells allows one to view a parallel antiapoptotic pathway in isolation. We demonstrated here that such a pathway at a minimum includes the cooperation of raf/erk and akt to elicit p65 NFκB, which is responsible for c-IAP2 induction, thus blocking activation of the caspase 9–dependent apoptosis cascade. c-IAP2 may not be acting alone as an antiapoptotic effector of NFκB because TRAFs 1 and 2 and the A1 genes are also known to use NFκB for their transcription.47-49 On the other hand, it is known that the raf/erk pathway acting through p90rsk, and also akt and raf acting individually, have the capacity to phosphorylate the proapoptotic Bad protein for its inactivation (Bad Ser112 by akt, Bad Ser136 by mitochondrial raf, and Bad Ser112 by rsk).18,19 50However, our attempts to demonstrate Bad protein expression in our cells yielded negative results (data not shown).
One may ask how cooperative interaction of raf/erk and akt activates NFκB in the context of hematopoietic cells. Recently, 2 groups have reported that akt was a major, if not the sole, contributor to NFκB activation by cytokines in mesenchymal cells.51,52 On the other hand, 2 other investigators noted a requirement for collaboration of other signals with akt for stimulating NFκB functional activity in other contexts.53,54 In one of those studies, the role of akt was deemed to be in transactivation augmentation, not nuclear translocation, which was attributed to other functions (eg, Raf or ral activity) of a mutant H-Ras oncogene stimulus.53 In the other study, akt needed to cooperate with phorbol ester stimulation (a known pKC-dependent raf activator55) for NFκB stimulation.54
Finally, our study also distinguished the major species of NFκB that characterize H7 parental cells under the influence of an IL-3 signal versus those same cells with p210BCR-ABL or with gag-akt/RafCAAX (Figure 7). In the latter 2 cases, p65 NFκB was abundant, yet this species was substituted in IL-3–dependent parental cells by c-rel (Figure 7). Interestingly, there are significant differences in the capacity for p65 NFκB versus c-rel to transactivate certain promoters with canonical and/or variant NFκB sites, including the human immunodeficiency virus type-1 LTR, IL-2 receptor-α promoter, IKBα promoter, and the IgG1 (γ1 heavy chain) gene.56,57 All of the above promoters are repressed by c-rel/p50 heterodimers and are transcriptionally activated by p65/p50 NFκB.56 57
In summary, the present report focuses on the functional effect of cooperation between 2 pathways (raf/erk and akt) known to operate downstream from p210BCR-ABL (as a possibly representative tyrosine kinase oncogene). The study demonstrates that the combination of active versions of raf and akt can transform hematopoietic cells by an antiapoptotic and hypoproliferative effect through NFκB, acting in the relative absence of Stat5.
We acknowledge the gift of reagents by Drs Albert S. Baldwin, Jr, B. M. Th. Burgering, and D. Beach, as well as assistance from Dr Nikkil Patel and helpful discussions from Elizabeth Smith.
Supported in part by a Merit Review Award from the Department of Veterans Affairs and by the Leukemia and Lymphoma Society (formerly Leukemia Society of America) (to H.S.B.) and by American Cancer Society grant RPG-00-122-01-TBE (to H.N.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
H. Scott Boswell, Division of Hematology/Oncology, Indiana University Medical Center, Rm 202, R4 Building, 1044 West Walnut St, Indianapolis, IN 46202; e-mail: hboswell@iupui.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal