Abstract
Cytarabine (ara-C) requires activation into its triphosphorylated form, ara-CTP, to exert cytotoxic activity. Cytoplasmic 5′-nucleotidase (5NT) dephosphorylates ara-CMP, a key intermediate, preventing accumulation of ara-CTP and may reduce cellular sensitivity to the cytotoxic activity of ara-C. To determine whether the level of expression of 5NT is correlated with clinical outcome in patients with acute myeloid leukemia (AML) treated with ara-C, this study analyzed the levels of messenger RNA expression of high Km 5NT by real-time polymerase chain reaction at diagnosis in blast cells of 108 patients with AML. High Km 5NT was expressed at diagnosis in the blast cells of 54% of patients. In univariate analysis, (1) patients whose blast cells contained high levels (values greater than the median value for total population) of high Km 5NT at diagnosis had significantly shorter disease-free survival (DFS) than patients with low levels of high Km 5NT (11 months versus 17.5 months, P = .02) and (2) high levels of high Km 5NT also predicted significantly shorter overall survival (15.7 months versus 39 months, P = .01) in young patients (≤ 57 years; median value for the entire population). In a multivariate analysis taking into account age, karyotype risk, and other factors found to have prognostic significance in univariate analysis, (1) high Km 5NT expression was an independent prognostic factor for DFS and (2) high levels of high Km 5NT also predicted significantly shorter overall survival in young patients. These results demonstrate that the expression of high levels of high Km 5NT in blast cells is correlated with outcome in patients with AML.
Introduction
First-line chemotherapy treatment in patients with acute myeloid leukemia (AML) usually consists of a combination of cytarabine (1-β-D-arabinofuranosyl-cytosine; ara-C) and an anthracycline.1 These regimens induce complete response (CR) rates in 65% to 80% of newly diagnosed patients with AML. However, clinical outcome is unsatisfactory, as most of the patients who achieve a complete remission will relapse within 2 years from diagnosis, often with resistant disease and poor response to subsequent therapy.2 3 Insufficient activity of first-line chemotherapy therefore remains a major obstacle in the effective treatment of patients with AML. Understanding the factors that contribute to the emergence of chemoresistant leukemic cells is essential to improve outcome in patients with AML.
Resistance to anthracyclines is associated with increased expression and activity of the multidrug-resistance phenotype associated with the overexpression of transmembrane efflux pumps such as P-glycoprotein, the multidrug resistance-associated protein, and the lung resistance-related protein.4-6 However, a significant number of patients whose blasts do not express these proteins will not reach CR or relapse.7 8 This finding suggests that other mechanisms, possibly related to anthracycline or ara-C resistance, are involved.
Standard-dose ara-C enters cells mainly by nucleoside transporter carriers, in particular the human equilibrative nucleoside transporter 1 (hENT1).9 When administered at high doses, ara-C penetrates cells predominantly by passive diffusion. Inside the cell, ara-C is phosphorylated to ara-CMP by the activating enzyme deoxycytidine kinase (dCK).10 Ara-CMP is further activated by other kinases to ara-CTP.11,12 Ara-C cytotoxicity results from the inhibition of DNA synthesis by incorporation of its intracellular metabolite ara-CTP in the deoxycitidine triphosphate (dCTP) sites of the DNA molecule. Efficient intracellular activation into ara-CTP is essential to obtain the cytotoxic response observed after ara-C treatment.13-15 Ara-C catabolism can result from rapid deamination by cytidine deaminase (CDD) to the nontoxic metabolite ara-U.16 Cytoplasmic 5′-nucleotidase (5NT) activities oppose that of dCK by dephosphorylating ara-CMP, thereby preventing the production of the active form.17 18
In vitro data suggest that modifications of ara-C transport and metabolism processes affect intracellular ara-CTP concentration, inducing drug resistance.19-21 Deficiency of hENT1, dCK, and overexpression of CDD or high Km 5NT have all been reported to be associated with resistance to ara-C in different cell lines.22-25 However, the clinical significance of high Km 5NT expression in patients with AML treated with ara-C has not yet been established.
To determine the clinical significance of high Km 5NT expression levels in patients with AML, we have analyzed by quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR) the messenger RNA (mRNA) levels of high Km 5NT at diagnosis in 108 patients with AML treated with ara-C–containing regimens. These parameters were correlated with pretreatment characteristics and patient outcome. We report that the presence of high levels (values greater than the median high Km 5NT value for the total population) of expression of high Km 5NT mRNA transcripts in leukemic blasts at diagnosis is correlated with poor outcome in patients with AML.
Patients and methods
Patients
This study was performed retrospectively on samples obtained in 108 patients with AML for whom samples were available at diagnosis. Patients were treated in the Hematology Departments of Edouard Herriot and Saint Louis Hospitals in France. The diagnosis of leukemia was made by standard cytologic and histochemical examination of bone marrow smears using French-American-British (FAB) criteria. Approval was obtained from Lyon Protocol Review Board. Informed consent was provided according to the Declaration of Helsinki.
Treatment
All patients received induction therapy with ara-C–containing regimens. Most of the patients (83%) received therapy that contained ara-C (100-200 mg/m2/d intravenously [IV] on days 1 through 7) in combination with an anthracycline with or without etoposide, 6-thioguanine, or granulocyte colony-stimulating factor. Among those patients, 12 received a second induction course with high-dose ara-C (3 g/m2/12 hours on days 1 to 4). Other patients (14%) received a combination of an anthracycline and ara-C at a dose of 500 mg/m2/d IV on days 1 to 3 and on days 8 to 10. Two patients received standard-dose ara-C plus hydroxyurea, and 1 patient received high-dose ara-C (2 g/m2/d on days 1 through 4) and idarubicin (10 mg/m2/d IV on days 3 through 5). As postremission therapy, 57 patients received ara-C at doses ranging from 100 to 200 mg/m2/d (54%), 500 mg/m2/d (14%), or 3 g/m2/d (32%) combined with other agents. Twenty-one patients received cyclophosphamide and total body irradiation with autologous bone marrow transplantation (ABMT) as part of consolidation or relapse treatment.
Leukemic cells
All biological samples were obtained at diagnosis before initiation of therapy. Leukemic cells were obtained from bone marrow or peripheral blood in 63% and 37% of cases, respectively. Cell samples were collected in heparinized flasks, and mononuclear cells were isolated by Ficoll-Hypaque sedimentation. The median percentage of blast cells in the samples was 80% (range: 20%-100%).
Quantitative real-time RT-PCR
The level of mRNA expression of high Km 5NT, (accession No. D38524), was assessed by quantitative real-time PCR. Cellular RNA was extracted by using Xtract All (Eurobio, Les Ulis, France), complementary DNA (cDNA) was synthesized by using 1 μg RNA per reaction in the presence of Murine Leukaemia Virus reverse transcriptase (Life Technologies, Cergy, France). The resulting cDNA was used for quantitative real-time PCR and analyzed with an Applied Biosystem 5700 detection system (Applied Biosystems, Foster City, CA). Quantification of mRNA was performed by using the Applied Biosystem 18S Ribosomal (18S Ribo) Control Kit (Applied Biosystems). Validation assays demonstrated that all patients amplified 18S Ribosomal RNA with equal efficiency (data not shown). The PCR products of high Km 5NT were then produced using these cDNA dilutions. As control, we used a cell line expressing high Km 5NT: K562/Gem.24 Briefly, cDNA (5 μL) was mixed with Taqman Universal PCR Master Mix (Applied Biosystems), primers (300 nM each) and probes (130 nM) in a total volume of 25μL. These reactions were prepared in duplicate. The primer and probe sequences for the high Km 5NT were as follows: for, 5′-ACCTGCTGTATTACCCTTTCAGCTA; rev, 5′-GCTCCACCGTTGATTCATGA; and probe, 5′-FAM-CTCTTCAGGGCTGCCCATGTC TTGA-TAMRA. Cycling conditions were initial denaturation for 480 seconds at 95°C, followed by 40 cycles of denaturation for 15 seconds at 95°C, annealing/extension at 60°C for 45 seconds.
The data were expressed as Ct, which is the PCR cycle number at which the accumulated fluorescent signal in each reaction crosses a threshold above background. Mean Ct values were calculated for each duplicate. Mean Ct values were then normalized to the expression level in reference to a 18S Ribosomal RNA and high Km 5NT control (K562/Gem cells) by subtracting sample mean Ct to K/Gem mean Ct: ΔCt = sample mean Ct − K/Gem mean Ct. The results were then expressed as 2−(ΔCt). For each sample, a ratio between high Km 5NT 2−(ΔCt) values and 18S Ribosomal 2−(ΔCt)values were calculated and considered as the final amount of high Km 5NT mRNA (arbitrary quantitative PCR units).
Statistical analyses
PCR results were considered as dichotomic variables (high versus low levels) for statistical analyses. For high Km 5NT, values were considered high levels when they were greater than the median value in the entire population. Age, white blood cell (WBC) counts, blast percentages, platelet counts, and hemoglobin levels were considered as continuous nonnormal numerical variables. Comparison between parameters was based on the Fisher exact test (dichotomic variables) or nonparametric Mann-Whitney U test (continuous variables).
Overall survival (OS) and disease-free survival (DFS) curves were calculated from the date of diagnosis until the date of disease progression, death, or last follow-up according to the Kaplan-Meier method and compared by the log-rank test. Differences were considered significant if the P value was ≤ .05. Variables studied in univariate analysis included high Km 5NT expression levels, sex, age, FAB subtype, WBC count, percentage of blasts, karyotype, platelet counts, hemoglobin levels, clinical symptoms and signs (sepsis, fever of noninfectious origin, lymphadenopathy, hemorrhage, enlarged liver or spleen, and involvement of the central nervous system), treatment response, number of cycles, and consolidation or relapse treatment with ABMT. Prognostic factors of significant value in univariate analysis were entered in a multivariate analysis by using the Cox proportional hazards model for covariate analysis of censored data on survival.
Results
Patient population and treatment outcome
The patients in this cohort had a median age of 57 years (range: 19-89). Fifty-two percent of the patients were female. At diagnosis, 34% of patients had sepsis. Fever of noninfectious origin was present in 24% of the patients, lymphadenopathy in 23%, hemorrhage in 22%, enlarged liver and spleen in 8%, respectively, and involvement of the central nervous system in 2%.
According to FAB criteria the majority of patients had M1 (20%), M2 (18%), M4 (18%), or M5 (33%) morphology. The other patients had M3 (4%), M6 (4%), or M7 (2%) subtypes. One patient had myelodysplastic syndrome (1%). The median and range values for WBC, percentage of blasts in peripheral blood, percentage of blasts in bone marrow, hemoglobin levels, and platelet count levels are listed in Table1. Cytogenetic data were available for 90 patients: 17 patients were categorized as having a low-risk karyotype (16 inversion or t(8;21) translocation), 41 patients had an intermediate-risk karyotype (normal karyotype), and 32 patients had a high-risk karyotype (other karyotypes).
The CR rate after induction therapy was 78%. CR was attained after one cycle in 88% of the patients with CR, and 2 cycles in 12% of the patients. Seventy-two percent of patients attaining CR relapsed. With a median follow-up of 14.2 months, the overall death rate was 72%. The median DFS and OS values of the entire group were 11.5 (range: 1.1-87.2) and 14.4 months (range: 0.03-120.8), respectively.
Expression of high Km 5NT in blast cells at diagnosis by quantitative RT-PCR
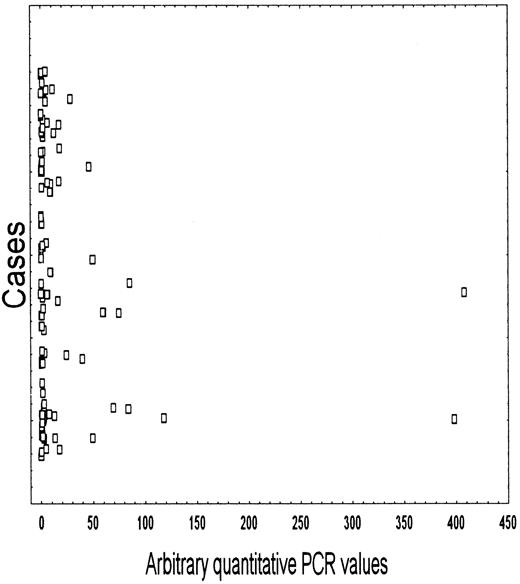
The mean and median values of high Km 5NT were 17.1 ± 57 and 1.9 (range: 0.01-408), respectively. Samples were considered as expressing high levels of 5NT mRNA when they were greater than the median value in the entire population. According to this division, 54% of patients were considered as having high levels of high Km 5NT mRNA. The distribution of 5NT values is shown in Figure1.
Pattern of mRNA expression and distribution of high Km 5NT in patients with AML at diagnosis.
Pattern of mRNA expression and distribution of high Km 5NT in patients with AML at diagnosis.
High levels of high Km 5NT expression were significantly correlated with relapse (57.5% with low 5NT values versus 82% in patients with high 5NT values, P = .02). Univariate analyses did not reveal correlations between high Km 5NT levels and the other clinical, laboratory, or treatment characteristics, other than lower than median values of hemoglobin (data not shown).
Relationship between clinical and laboratory variables and clinical outcome
In univariate analysis, age older than 57 years (P = .02), high-risk karyotype (P = .002), lower than median platelet counts (P = .02), and lack of ABMT consolidation therapy (P = .01) were related with a shorter DFS. Likewise, age older than 57 years (P = .001), high-risk karyotype (P = .008), lack of response to induction chemotherapy (P = .00001), and lack of ABMT (P = .04) were related to a shorter OS.
Correlation between high Km 5NT levels and clinical outcome
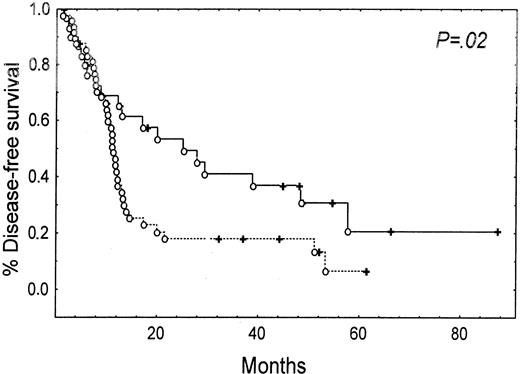
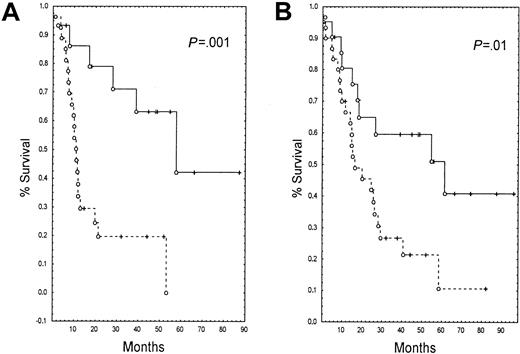
Patients whose blasts expressed high levels of high Km 5NT had a shorter DFS (11 months versus 17.5 months, P = .02) (Figure 2) than patients with lower than median levels. High levels of expression of 5NT were not correlated with OS in the entire population (data not shown). However, in younger patients (age < 57 years, 52 patients), high levels of high Km 5NT were correlated with a shorter DFS (11 months versus 41.8 months, P = .001) (Figure3A) and shorter OS (15.7 months versus 39 months, P = .01) (Figure 3B).
Kaplan-Meier estimate of disease-free survival (DFS) and expression of high Km 5NT at diagnosis.
(--) indicates patients who express 5NT; (—), patients who did not express 5NT.
Kaplan-Meier estimate of disease-free survival (DFS) and expression of high Km 5NT at diagnosis.
(--) indicates patients who express 5NT; (—), patients who did not express 5NT.
Kaplan-Meier estimate of survival parameters and expression of 5NT at diagnosis in young patients (< 57 years, median for the entire population).
(--) indicates patients who express 5NT; (—), patients who did not express 5NT. (A) Disease-free survival (DFS), (B) overall survival (OS).
Kaplan-Meier estimate of survival parameters and expression of 5NT at diagnosis in young patients (< 57 years, median for the entire population).
(--) indicates patients who express 5NT; (—), patients who did not express 5NT. (A) Disease-free survival (DFS), (B) overall survival (OS).
A multivariate analysis of the total population (Table2) identified poor-risk karyotype, elevated high Km 5NT levels, and low platelet counts as factors significantly associated with shorter DFS, whereas only response to induction chemotherapy and age were significantly associated with OS. In the younger patients, only high Km 5NT levels and karyotype were independent prognostic factors for DFS, whereas only high Km 5NT was correlated with OS (Table 2).
Correlation between high Km 5NT levels, ara-C doses, and response to treatment
We then determined whether the level of expression of high Km 5NT had an effect on the response to induction therapy. For each patient, we calculated the ara-C dose received during induction therapy, postremission therapy, or the total ara-C dose received during first-line treatment. There were no differences between ara-C doses received during the induction course or first-line treatment between patients expressing low levels or high levels of high Km 5NT (data not shown).
The patient population was divided into 3 groups according to the ara-C dose received during induction therapy: a low-dose group that received ≤ 0.7 g (lower quartile value for the entire population), an intermediate dose group that received > 0.7 to ≤ 3 g, and a high-dose group that received > 3 g (upper quartile value for the entire population). No correlations were observed between response to induction chemotherapy, high Km 5NT levels, and the dose of ara-C received in the entire population. However, we observed that in patients expressing low levels of high Km 5NT, CR was attained in only 44% of low-dose patients, whereas 79% of intermediate-dose patients and 84% of high-dose patients obtained a CR (P = .07). This finding was not observed in patients expressing high levels of high Km 5NT (P = .56).
A similar analysis was performed by dividing the patient population according to the total ara-C dose received during first-line treatment: a low-dose group that received < 1.4 g (lower quartile), an intermediate-dose group that received > 1.4 to ≤ 7 g; and a high-dose group receiving > 7 g (upper quartile). Patients in the low-dose group had a lower chance of attaining CR (53%) than patients in the intermediatedose (93%) and high-dose (88%) groups (P = .03). This finding was not observed in patients expressing high levels of high Km 5NT (P = .21).
Discussion
Our data show that high levels of high Km 5NT mRNA in leukemic blasts at diagnosis are independently correlated with a shorter DFS and with a shorter OS in younger patients. We also observed that the CR rates were correlated with the dose of ara-C received in patients with low levels of high Km 5NT but not in patients with high levels of high Km 5NT. Although these results require confirmation on a large series of patients, these results warrant exploration of 5NT modulation strategies, particularly in patients with high levels of expression.
The 5NT family comprises various membrane-bound, cytosolic, and mitochondrial proteins. These enzymes participate, along with nucleoside kinases, in the regulation of deoxyribonucleotide metabolism18 by catalyzing the hydrolytic cleavage of 5′-ribonucleotides and 5′-deoxyribonucleotides to nucleosides and phosphates. CD73, or ecto-5NT, is the best-characterized form of 5NT.26,27 It is bound to the plasma membrane by a glycosyl phosphatidylinositol anchor. Cloning of CD73 (accession No. NM002526)28 confirmed that this enzyme is involved in the hydrolysis of extracellular adenosine monophosphate (AMP).29,30 CD73 is frequently expressed in acute leukemias and is required for the uptake of extracellular nucleotides because the highly polar nucleotides do not permeate the plasma membrane, whereas nucleosides can be transported by nucleoside transport proteins.26 31
Several cytosolic activities have been cloned and characterized.18,32,33 The high Km 5NT (also designated as cN-II; accession No. D38524) studied in this patient population32,34-36 was the first cytoplasmic 5NT cloned.33 5′-3′-Nucleotidase (dNT-1; also described as PN-II37; accession No. AF154829) is a cytosolic nucleotidase that possesses higher affinity for the 5′-phosphates of deoxyribonucleosides and the 2′- and 3′-phosphates of either ribonucleosides or deoxyribonucleosides.18,38AMP-selective cytosolic 5′-nucleotidase (cN-I; accession No. AJ131243) has been implicated in adenosine formation during adenosine triphosphate breakdown.39 Rampazzo et al40recently described a mitochondrial 5NT, designated dNT-2 (accession No.AF210652), that specifically dephosphorylates the 5′- and 2′3′)-phosphates of uracil and thymine deoxyribonucleotides. dNT-2 may be involved in the protection of mitochondrial DNA replication from an excess of dTTP.40
Other studies have identified the clinical relevance of the level of nucleotidase activity as a cause of resistance to purine analogues. High levels of 5NT enzyme activity have been correlated to a worse clinical response and/or outcome in acute lymphocytic leukemia (ALL), chronic lymphocytic leukemia (CLL), and hairy cell leukemia (HCL). In ALL, 5NT-positive patients have a higher relapse rate and thus a poorer prognosis than 5NT-negative patients.41 5NT activity was also associated with a lower rate of CR to 6-mercatopurine.42,43 In patients with B-CLL or HCL treated with 2-chlorodeoxyadenosine, pretreatment levels of measured 5NT activity were significantly lower in responders than in nonresponding patients.44 45
To our knowledge, this is the first time that high levels of expression of high Km 5NT, as determined by quantitative PCR, is related to outcome in patients with AML. The possible prognostic value of the other 5NTs clearly requires to be evaluated in this setting. Although we have not unequivocally shown that high levels of expression of 5NT are directly involved in resistance to ara-C, the alternative hypothesis that high Km levels reflect disease aggressivity is not supported by our data. Furthermore, among the group of patients expressing low levels of high km 5NT at diagnosis, low-dose ara-C during induction or first-line treatment yielded a lower CR rate than intermediate or higher ara-C doses. This finding was not observed in patients expressing high levels of high Km 5NT. The respective roles of induction and postremission doses of ara-C remain to be determined.
In short, high levels of expression of the high Km 5NT independently identify a poor prognosis group of patients with AML. Modulation of high Km 5NT activity warrants investigation as a means of increasing the efficacy of induction therapy in patients with AML with high levels of expression in their blast cells.
Supported in part by the Ligue Contre le Cancer du Rhône, the Fondation de France (Fondation contre la Leucémie) PHRC 1996, and the National Cancer Institute of Canada. C. G. is a recipient of the Michel Clavel grant.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Carlos M. Galmarini, Unité INSERM 453-Laboratoire de Cytologie Analytique, Faculté de Médécine Rockefeller, 8, avenue Rockefeller, 69373, Lyon CEDEX 08, France; e-mail: fgalma@rockefeller.univ-lyon1.fr.