Abstract
The nature of the cells that seed the thymus of an irradiated recipient after intravenous (IV) transfer of bone marrow (BM) cells was investigated using 2 approaches. First, direct entry of a small number of donor BM cells into the thymus was tracked using a Ly-5 marker. Second, secondary IV transfer of the seeded thymus cells into a secondary recipient was used as an assay for precursor activity. A range of cell types was found to enter the recipient thymus initially, including B-lineage cells and myeloid cells, but T precursors were undetectable by flow cytometry over the first few days. Although all cells initially entering the thymus proliferated, no sustained thymus reconstitution was seen until day 4, when recognizable T-lineage precursors began to appear. The secondary transfer assays revealed the presence of lymphoid precursors in the recipient thymus, including T, NKT, NK, and B precursor activity, with a notable early burst of B-lineage generative capacity. There was no evidence of sustained myeloid precursor or multipotent stem cell activity, even though these were seen if BM cells were injected directly into the recipient thymus rather than introduced into the bloodstream. It is concluded that even though many cell types may initially enter an irradiated thymus, the thymus acts as a sieve, allowing lymphoid precursors, but not multipotent stem cells, to seed the environmental niches that permit selected precursor cell development and thymus reconstitution.
Introduction
The thymus is the essential organ for T-cell development.1 In adult mice the production of thymocytes is dependent on a continuing input of precursors from bone marrow (BM).2-5 After seeding into the thymus, these precursors proliferate and differentiate along the pathways that lead to the production of mature T lymphocytes within the thymic environment, after which they migrate to peripheral lymphoid tissues.6 Our previous studies on the earliest detectable intrathymic precursor cells in the adult mouse thymus demonstrated that they have a surface antigenic phenotype generally similar to that of the BM hematopoietic stem cells (HSC) but that they differ from HSC in expressing Sca-27-9 and a moderate level of CD4.8,10Furthermore, when this precursor population was transferred intravenously (IV) into irradiated recipients, all lymphoid cells (T cells, B cells, and NK cells), but no myeloid cells (erythroid, macrophage, granulocytes) could be generated.9 This indicated that the earliest detectable intrathymic precursor population has a developmental potential restricted to the lymphoid lineage and that it differs from the multipotent HSC in BM. However, whether these early intrathymic lymphoid precursors represent the cells that directly seed thymus from BM is unclear. Searching for cells with the same surface phenotype and developmental potential in adult mouse BM has failed to reveal the same precursor population,11suggesting that the early intrathymic lymphoid precursors are not themselves the original thymus-seeding cells. Although studies from this and other laboratories showed that the most primitive BM plural potent HSC were unlikely to be the direct precursors for thymocytes,12,13 it remains unclear whether the thymus-seeding precursors are multipotent or lineage restricted. Because the number of BM-derived precursors that effectively seed a restricted number of thymus niches may be extremely small,7 their detection and analysis may be beyond the limits of direct cell isolation techniques.
To address this issue, we studied the thymus-seeding cells from adult mouse BM for their hematopoietic lineage developmental potential using a 2-step cell transfer system. BM cells from C57BL/6 (Ly-5.2) mice were transferred intravenously into irradiated Ly-5.1 recipients, and the phenotype of cells that entered the thymus of each recipient was examined. Then the developmental potential of these thymus-seeding cells was examined by transferring thymocytes from the first recipients into a group of secondary Ly-5.1 recipients; this was the crucial expansion step to reveal their precursor potential. The hematopoietic lineage of the progeny of these thymus-seeding precursors in the secondary recipients was then analyzed. We found that the primary thymus was initially seeded by cells of mixed lineage but that only lymphoid-lineage progeny could be detected in the secondary recipients. These findings suggested that various types of cells initially enter the irradiated thymus, but those with extended precursor function have a lymphoid-restricted potential and are not multipotent HSC.
Materials and methods
Mice
C57BL/6 (Ly-5.2) mice (4-6 weeks old) were used as BM donors in the reconstitution assays. C57BL/6 Ly-5.1-Pep3b mice (8-12 weeks old) were used as recipients. The mice were bred and maintained at the animal facility of The Walter and Eliza Hall Institute under specific pathogen-free conditions.
Antibodies
Antibodies used for immunomagnetic bead depletion were anti-CD8 (clone 53-6.7), anti-CD3 (clone KT3-1.1), and anti-B220 (clone RA3-6B2). Antibodies used for immunofluorescence staining were anti–Thy-1.2 (clone 30H-12) used as Texas Red and Cy3 conjugates; anti–Ly-5.2 (clone AL1-4A2) used as fluorescein isothiocyanate (FITC) or biotin conjugates; anti-B220 (clone RA3-6B2) used as a phycoerythrin (PE) conjugate; anti–Mac-1 (clone M1/70.15) and anti–Gr-1 (clone RB6-8C5) used as Cy5 conjugates; anti-NK1.1 (clone DX5) used as a biotin conjugate; anti-CD3 (clone KT3-1.1) used as a Texas Red conjugate; anti–c-kit (clone 2B8) used as an allophycocyanin (APC) conjugate; anti-CD25 (clone PC61) used as an FITC or Cy3 conjugate; and anti-CD19 (clone ID3) used as an FITC conjugate. Fluorescence reagents used for second-stage staining were PE-avidin (PE-Av), Texas Red-avidin (TR-Av), and Alexa 594-avidin.
Immunofluorescence staining and flow cytometry analysis
The cells, harvested from different tissues, were first incubated with 50 μg normal rat immunoglobulin and anti–mouse immunoglobulin FcRγ antibody (clone 2.4G2) before the addition of the appropriate combination of the above-mentioned fluorescence-conjugated antibodies against hematopoietic lineage markers and other cell surface markers of interest. Four fluorescent color flow cytometry analyses were performed on FACStar Plus (Becton Dickinson, San Jose, CA). Dead cells were excluded based on their low forward light scatter and propidium iodide (PI) staining. Files of 50 000 to 100 000 cells were usually collected.
Bone marrow cell transfer
BM cells were harvested by flushing the femoral and tibial bone shafts with cold mouse tonicity balanced salt solution (BSS) containing 3% fetal calf serum. Red cells and dead cells were removed by density centrifugation through a Nycodenz (Nycomed Pharma AS, Oslo, Norway) medium at a density of 1.091 g/cm3. To remove T-lineage cells and mature surface Ig+ B cells, BM cells were coated with anti-CD8 and anti-CD3. Then the coated cells were removed using magnetic beads (Paesel and Lorei, Hanau, Germany) coated with goat anti–rat IgG, which is cross-reactive with mouse immunoglobulin. In some experiments, anti-B220 antibody was added to deplete all B-lineage cells. BM cells (20-30 × 106) prepared as above were transferred intravenously into each lethally irradiated (2 doses of 5.5 Gy γ-irradiation with a 3-hour interval) Ly-5.1 recipient mouse.
Labeling of bone marrow cells with 5,6-carboxy-succinimidyl-fluorescein-ester
To examine cell division of the thymus-seeding cells after BM transfer, BM cells prepared as above were labeled with a fluorescent dye, 5,6-carboxy-succinimidyl-fluorescein-ester (CFSE; Molecular Probes, Eugene, OR) before IV transfer.14 15 Two microliters CFSE stock solution (5 mM in dimethyl sulfoxide) was incubated with 10 × 106/mL BM cells for 10 minutes at 37°C. Cells were washed immediately after incubation, then injected intravenously into irradiated Ly-5.1 recipient mice.
Analysis of the phenotype of the thymus-seeding cells after bone marrow transfer
Ly-5.1 recipient mice received lethal irradiation followed by IV injection of 20-30 × 106 C57BL/6 (Ly-5.2) BM cells depleted of T cells and mature B cells. At 1 to 7 days after transfer, the thymuses were harvested from these recipients, and cell suspensions were prepared. Donor-derived cells were marked by staining the cells with antibody to Ly-5.2. Fluorescence-conjugated mouse IgG3was used for the isotype-matched control staining. Cells were also stained for some precursor and lineage markers, including c-kit, CD25, CD19, B220, and Mac-1. Surface phenotypes of the Ly-5.2+ donor-derived cells were then determined by gating during flow cytometry analysis.
Secondary cell transfer
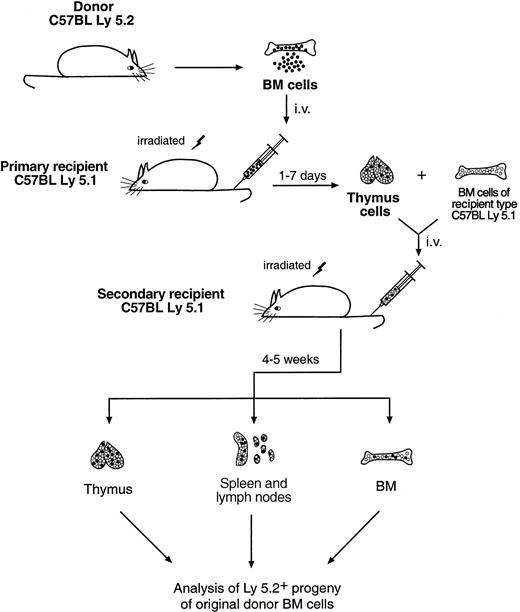
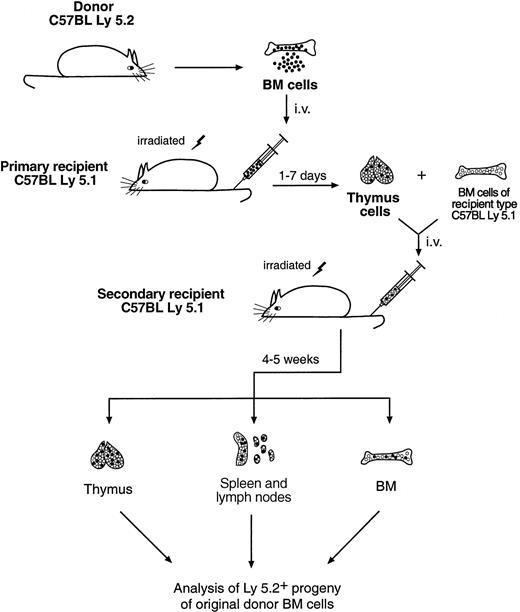
To determine the developmental potential of the thymus-seeding cells, thymocytes from the first Ly-5.1 recipients (8-10 mice per group), which had received 20-30 × 106 C57BL/6 depleted BM cells, were harvested at 1 to 7 days after BM transfer. These thymocytes, containing the thymus-seeding cells and their immediate progeny, were then injected intravenously into 2 to 3 lethally irradiated secondary Ly-5.1 recipients. Recipient-type (Ly-5.1) unfractionated BM cells (5 × 104) were coinjected to ensure survival. The experimental scheme is shown in Figure1.
Scheme of the secondary cell transfer experiment.
For details, see “Materials and methods.”
Scheme of the secondary cell transfer experiment.
For details, see “Materials and methods.”
Analysis of hematopoietic lineage reconstitution by the thymus-seeding precursors
The developmental potential of the thymus-seeding precursors was determined by analyzing the capacity for hematopoietic lineage reconstitution in the secondary recipients. Suspensions of thymuses, pooled lymph nodes and spleen, and BM from the secondary Ly-5.1 recipients were prepared 4 to 5 weeks after IV transfer of thymus cells of the primary recipients (Figure 1). Progeny of the initial thymus-seeding cells were revealed by the expression of Ly-5.2. As above, the hematopoietic lineage of these progeny was determined by flow cytometry analysis of their expression of lineage markers, including Thy-1, B220, Mac-1, Gr-1, NK1.1, CD3, and CD19.
Intrathymic injection
In some experiments, BM cells were injected directly into the thymuses of primary recipients using a procedure described elsewhere.16 17 Briefly, 2.5 × 106 T-cell– and B-cell–depleted BM cells were suspended in 10 μL BSS and injected into one lobe of the thymus of sublethally irradiated (750 Gy) recipients. Thymocytes from the recipients were harvested at different time points and transferred intravenously to secondary recipients for further precursor activity assays, as described above.
Results
Kinetics of cell entrance into thymus after transfer of BM cells
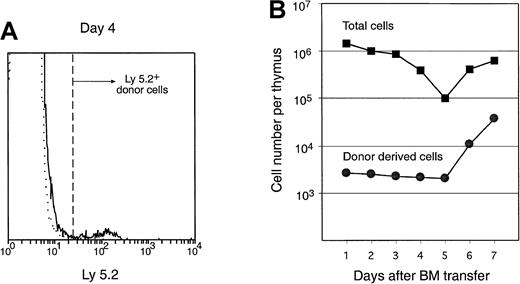
The entry of cells into the thymus was monitored by counting the number of donor-derived Ly-5.2+ cells at various times after IV BM cell transfer to irradiated Ly-5.1 recipient mice. To study the entry of BM precursors rather than that of recirculating mature lymphocytes, T-cell– and mature B-cell–depleted BM cells were transferred. At 1 to 7 days after transfer, thymus cells of the recipients were stained with FITC–anti–Ly-5.2 and analyzed on a flow cytometer. The average number of donor cells per thymus was calculated by multiplying the total number of cells per thymus by the proportion of Ly-5.2+ cells. As shown in Figure2, the total number of cells per thymus dropped rapidly from day 1 to day 5 after irradiation and BM transfer because of irradiation-induced cell death. The entry of donor cells into the thymus was very low; roughly 1 per 1000 transferred BM cells entered each thymus at day 1. Nevertheless, this low number could be counted, gated, and analyzed further. The number of donor-derived cells in the thymus was maintained at a constant level from day 1 to day 5, suggesting either that the cells entering the thymus at day 1 were simply maintained, without further seeding or proliferation, or that there was a balance between limited cell proliferation and continuing input of cells versus cell death over this period. Five days after BM transfer, both the total thymus cell number and the donor-derived cell number increased rapidly. This was probably because both the residual irradiation-surviving recipient thymocytes and the thymus-seeding cells began to proliferate. Alternatively, this could have been because the thymus was seeded by a second wave derived from both the transferred BM cells and the recipient radiation-surviving BM cells.
Number of total cells and donor-derived cells in the recipient thymus after IV BM transfer.
T-cell– and mature B-cell–depleted C57BL/6 BM cells (25 × 106) were injected intravenously into irradiated Ly-5.1 recipients. Thymocytes of the recipients were harvested at indicated days after BM transfer, stained with anti–donor type Ly-5.2 antibody, and analyzed by flow cytometry. Donor-derived cells were revealed by gating for Ly-5.2+ cells. An example of Ly-5.2 staining on the recipient thymocytes 4 days after primary BM transfer is shown by the solid line, and the isotype-matched background staining is shown by the dotted line in panel A. Number of total thymocytes per thymus (closed squares) was counted, and number of donor-derived cells (closed circles) was calculated from the proportion of Ly-5.2+ cells, as shown in panel B.
Number of total cells and donor-derived cells in the recipient thymus after IV BM transfer.
T-cell– and mature B-cell–depleted C57BL/6 BM cells (25 × 106) were injected intravenously into irradiated Ly-5.1 recipients. Thymocytes of the recipients were harvested at indicated days after BM transfer, stained with anti–donor type Ly-5.2 antibody, and analyzed by flow cytometry. Donor-derived cells were revealed by gating for Ly-5.2+ cells. An example of Ly-5.2 staining on the recipient thymocytes 4 days after primary BM transfer is shown by the solid line, and the isotype-matched background staining is shown by the dotted line in panel A. Number of total thymocytes per thymus (closed squares) was counted, and number of donor-derived cells (closed circles) was calculated from the proportion of Ly-5.2+ cells, as shown in panel B.
Do the initial BM-derived cells in the thymus divide?
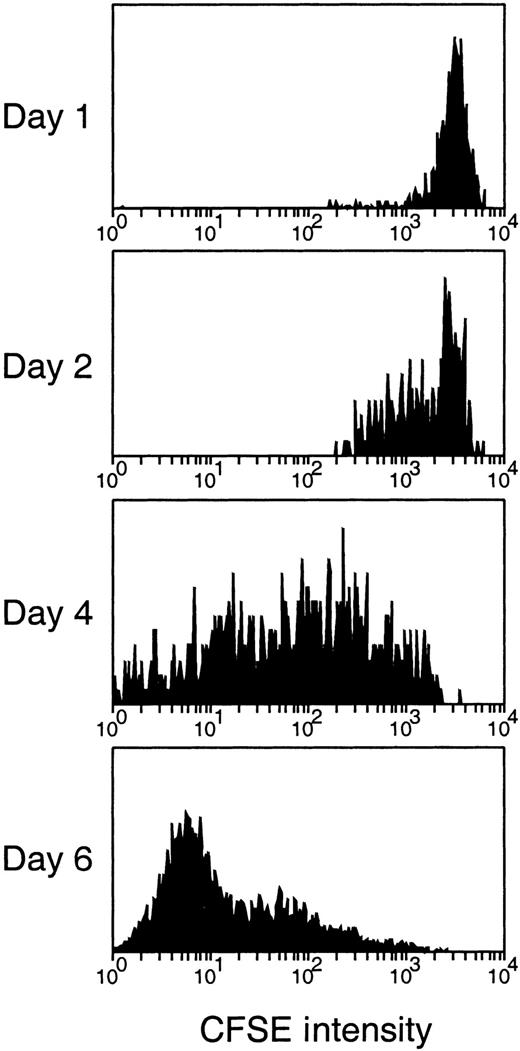
The relatively constant number of donor BM-derived cells in the recipient thymus from day 1 to day 5 after transfer was a surprise. This posed the question of whether the initially seeding cells were quiescent for the first 5 days of seeding or whether there was a dynamic balance of cell division, cell entry, and cell death over this period. To determine whether the donor-derived cells in the thymus proliferated, donor BM cells were first labeled with the persisting dye, CFSE, whose fluorescence intensity halves with each division.14 Because BM represented a heterogeneous population of cells, the fluorescence intensity of initial labeling was broader than with lymphocytes, and division produced a spread of fluorescence rather than discrete peaks. Nevertheless, as shown in Figure 3, there was a progressive loss of CFSE fluorescence from the donor BM-derived cells in the thymus over the first 6 days, indicating that most of the cells that entered the thymus and persisted had undergone division.
Detection of cell division of the thymus-seeding cells after IV transfer of CFSE-labeled BM cells.
CFSE fluorescence intensity of the thymus-seeding cells (Ly-5.2+) was measured at the time indicated after BM transfer. Note that because BM represented a heterogeneous population of cells, the fluorescence intensity of initial labeling obtained was broader than with lymphocytes, and the division produced a wide spread of fluorescence rather than discrete peaks.
Detection of cell division of the thymus-seeding cells after IV transfer of CFSE-labeled BM cells.
CFSE fluorescence intensity of the thymus-seeding cells (Ly-5.2+) was measured at the time indicated after BM transfer. Note that because BM represented a heterogeneous population of cells, the fluorescence intensity of initial labeling obtained was broader than with lymphocytes, and the division produced a wide spread of fluorescence rather than discrete peaks.
T precursors among donor BM-derived cells in the thymus
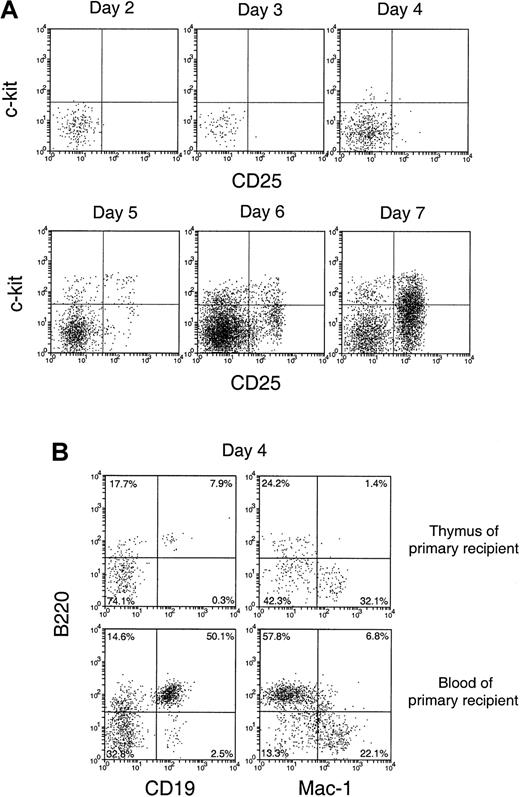
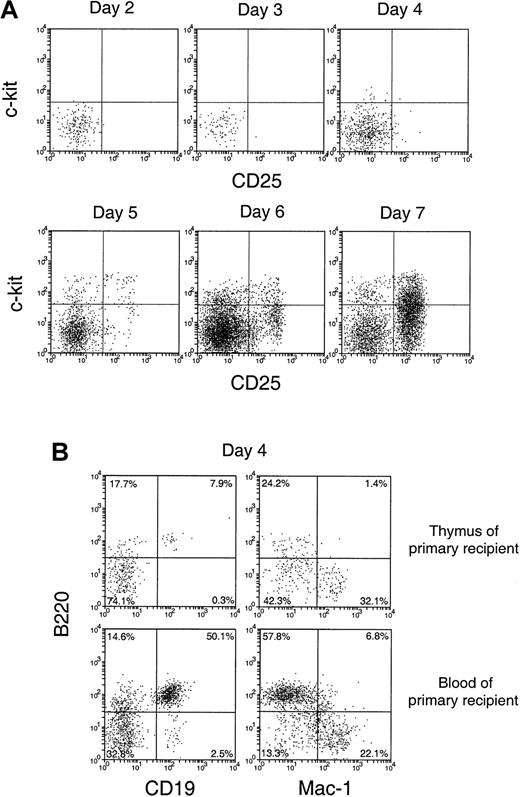
To characterize the cells that entered the thymus and their immediate division products, cell suspensions from the recipient thymus were stained in 3 fluorescent colors, using anti–Ly-5.2 to select the few donor BM-derived cells and 2 other monoclonal antibodies to determine surface phenotype. In the first studies, we looked for the appearance of cells representing recognizable steps in T-cell development. Almost all the donor-derived cells in the thymus from days 2 to 7 after BM transfer were CD4−8− (data not shown). Accordingly, we looked for markers of earlier T precursor cells, using CD25 and c-kit.18 19 As shown in Figure 4A, the donor-derived cells in the thymus were mainly CD25−c-kit− up to 3 days after BM transfer. At day 4, a small number of CD25−c-kit+ cells, a phenotype of the earliest detectable intrathymic T precursors, emerged. This was followed by the emergence of cells with the phenotype of pro-T cells (CD25+c-kit+) at day 5, then of pre-T cells (CD25+c-kit−) at day 6. By day 7, cells with the phenotypes of pre-T cells (CD25+c-kit−), together with more mature T precursors (CD25−c-kit−), became the major subpopulations of donor-derived cells with a distribution similar to that of CD3−CD4−CD8− T-precursor populations in the normal thymus.
Phenotype of the small number of donor-derived cells in the recipient thymus after BM transfer.
C57BL/6 (Ly-5.2) T-cell– and mature B-cell–depleted BM cells (25 × 106) were injected intravenously into lethally irradiated Ly-5.1 recipient mice. Thymocytes were harvested from Ly-5.1 recipient mice at indicated days after transfer, stained for Ly-5.2 plus other markers, and analyzed by flow cytometry. (A) Expression of c-kit and CD25 on donor-derived cells. (B) Expression of B220, CD19, and Mac-1 on donor-derived cells.
Phenotype of the small number of donor-derived cells in the recipient thymus after BM transfer.
C57BL/6 (Ly-5.2) T-cell– and mature B-cell–depleted BM cells (25 × 106) were injected intravenously into lethally irradiated Ly-5.1 recipient mice. Thymocytes were harvested from Ly-5.1 recipient mice at indicated days after transfer, stained for Ly-5.2 plus other markers, and analyzed by flow cytometry. (A) Expression of c-kit and CD25 on donor-derived cells. (B) Expression of B220, CD19, and Mac-1 on donor-derived cells.
Non–T-lineage cells among the donor BM-derived thymus cells
It was unclear whether the donor-derived CD25−c-kit− cells found in the recipient thymus 2 to 6 days after BM transfer were all T-lineage cells or whether other lineages were involved. Accordingly, the expression of other lineage markers, including B220, CD19, and Mac-1, was examined. As shown in Figure 4B (upper panels), at day 3 a high proportion (approximately 60%) of these thymus-seeding cells was either B220+ or Mac-1+. Similar proportions of B220+ and Mac-1+ cells were also found among the donor-derived CD25−c-kit−cells up to day 6 (data not shown). Although both B-lineage cells and myeloid cells were found, at this stage it was unclear whether the thymus had been seeded by precursors of these lineages or whether mature forms had entered the thymus directly.
Although the levels of donor BM-derived cells detected in the primary recipient thymus were low at 1 to 5 days after BM transfer, these donor-derived cells were unlikely to be the leukocytes contained in the thymic vasculature. If this were the case, similar proportions of the different lineages should have been found in the blood and in the thymuses of primary recipients. When the lineage composition of the donor-derived cells in these 2 sites of the primary recipients were compared, different proportions of lymphoid and myeloid cells among the donor-derived cells were revealed. As shown in Figure 4B (upper panels), among the donor-derived cells in the recipient thymus, 8% were B220+CD19+ and 32% were Mac-1+. In contrast, among the donor-derived cells in the recipient blood, more than 50% were B220+CD19+, and 22% were Mac-1+(Figure 4B, lower panels). This indicated that, though it could not be excluded that a small proportion of donor-derived cells came from the leukocytes contained in the thymic vasculature, most of the donor-derived cells detected in each primary recipient thymus were actually from the thymus itself.
Secondary transfer assay for the precursor function of thymus-seeding cells
The extremely small number of donor BM-derived cells in the thymus 1 to 6 days after transfer made further direct analysis by flow cytometry difficult. It was important to distinguish entry into the thymus of a few mature or near-mature cells with limited developmental potential from entry of true thymus-seeding precursors with a full capacity for thymus regeneration. Accordingly, a functional test was devised to focus attention on the latter precursors and to determine their lineage potential, based on a transfer into secondary recipients (Figure 1). At various times after the primary transfer of Ly-5.2+ BM cells, the Ly-5.1 recipient thymus was removed and a cell suspension was prepared, and this was transferred intravenously into lethally irradiated Ly-5.1 secondary recipients. At a fixed 4 to 5 weeks after this secondary transfer, the Ly-5.2+ progeny of the transferred cells (and of the original Ly-5.2+ BM) were analyzed in the secondary recipient thymus, the pooled spleen plus lymph nodes, and the BM. Despite the small numbers of these donor BM-derived Ly-5.2+progeny, with proper isotype-matched background control staining these donor-derived cells could be gated, counted, and further analyzed as shown in Table 1 and Figures5, 6, and7. The cell lineage of these progeny was determined initially by staining for Thy-1.2 (T cells), for B220 (B cells), and for Mac-1 and Gr-1 (myeloid cells), as in Figures 5to 7. To verify that the lymphoid identification detected normal T-cell and B-cell progeny, additional staining for CD3 (T cells) and CD19 (B cells) was used (Figure 8). Furthermore, to check for the potential to develop NK and NKT cells, staining for NK1.1 and CD3 was combined; the phenotypes NK1.1+CD3− and NK1.1+CD3+ represented NK cells and NKT cells, respectively,20 as shown in Figure9. Under these conditions, the expected behavior of T-lineage–restricted precursors would first seed then reseed only into the thymus, with production only of T cells. The expected behavior of lymphoid-restricted precursors would be initial seeding in the thymus, then restricted production of B cells and NK cells, but not myeloid cells in BM and peripheral sites, together with T cells in the thymus. The feature of multipotent progenitors such as HSC would be initial seeding in the thymus and then efficient production of all lineages in other sites. Relatively mature precursor cells with limited proliferation potential would not be expected to give detectable progeny in this secondary transfer system.
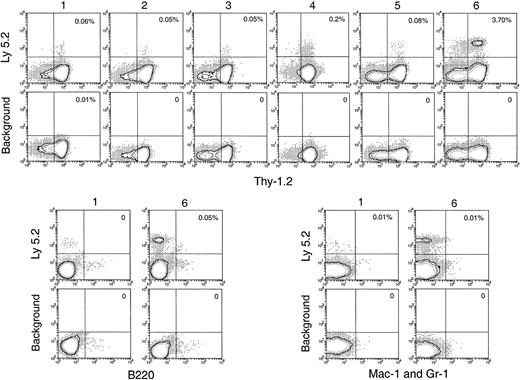
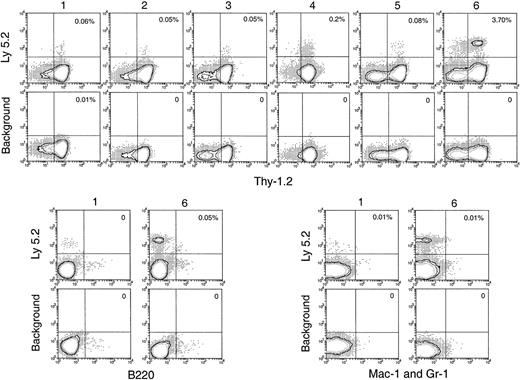
Thymus reconstitution in secondary recipients after transfer of primary recipient thymus cells.
T-cell– and mature B-cell–depleted BM cells from C57BL/6 (Ly-5.2) mice were injected intravenously into lethally irradiated primary Ly-5.1 recipient mice. At various times after primary transfer, thymocytes were harvested from the primary Ly-5.1 recipients and injected intravenously into lethally irradiated secondary Ly-5.1 recipient mice, along with the recipient type Ly-5.1 BM cells to ensure recipient survival. Four weeks after reconstitution, donor-derived cells in the thymuses of the secondary recipients were analyzed by flow cytometry. Although few progeny of the original BM cells were present, they could be selected as Ly-5.2+ cells and characterized by other markers. Background stainings, in which either a fluorescence-conjugated, isotype-matched control was used or the anti–Ly-5.2 antibody was omitted, gave almost no (less than 0.01%) positive staining. Percentage of donor-derived cells that expressed each indicated lineage marker in the secondary recipient is shown in the upper-right quadrant of each plot. Results shown in this figure are representative of 3 such experiments for each time point that gave similar results. Each experiment included 2 to 3 recipient mice.
Thymus reconstitution in secondary recipients after transfer of primary recipient thymus cells.
T-cell– and mature B-cell–depleted BM cells from C57BL/6 (Ly-5.2) mice were injected intravenously into lethally irradiated primary Ly-5.1 recipient mice. At various times after primary transfer, thymocytes were harvested from the primary Ly-5.1 recipients and injected intravenously into lethally irradiated secondary Ly-5.1 recipient mice, along with the recipient type Ly-5.1 BM cells to ensure recipient survival. Four weeks after reconstitution, donor-derived cells in the thymuses of the secondary recipients were analyzed by flow cytometry. Although few progeny of the original BM cells were present, they could be selected as Ly-5.2+ cells and characterized by other markers. Background stainings, in which either a fluorescence-conjugated, isotype-matched control was used or the anti–Ly-5.2 antibody was omitted, gave almost no (less than 0.01%) positive staining. Percentage of donor-derived cells that expressed each indicated lineage marker in the secondary recipient is shown in the upper-right quadrant of each plot. Results shown in this figure are representative of 3 such experiments for each time point that gave similar results. Each experiment included 2 to 3 recipient mice.
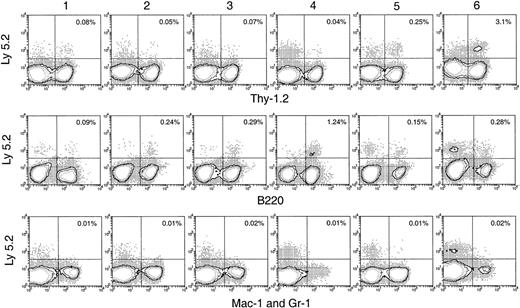
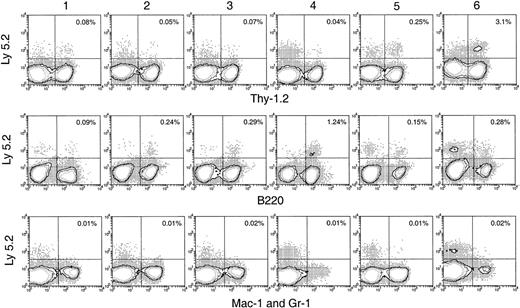
Hematopoietic lineage reconstitution in the spleen and lymph nodes of secondary recipients after transfer of primary recipient thymus cells.
Experimental procedures are the same as described in Figure 5. Isotype-matched background staining, which was similar to that in Figure 5, is not shown in this figure. Results shown in this figure are representatives of 3 similar experiments for each time point that gave similar results. Each experiment included 2 to 3 recipient mice.
Hematopoietic lineage reconstitution in the spleen and lymph nodes of secondary recipients after transfer of primary recipient thymus cells.
Experimental procedures are the same as described in Figure 5. Isotype-matched background staining, which was similar to that in Figure 5, is not shown in this figure. Results shown in this figure are representatives of 3 similar experiments for each time point that gave similar results. Each experiment included 2 to 3 recipient mice.
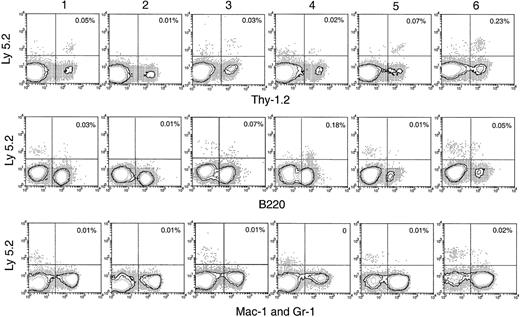
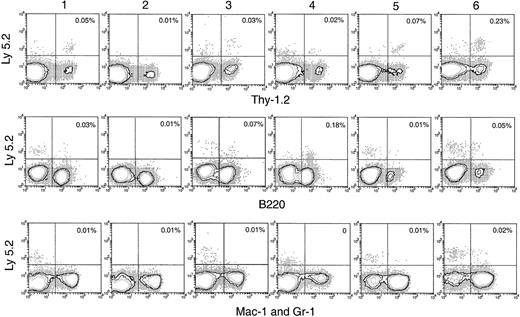
Hematopoietic lineage reconstitution in the BM of secondary recipients after transfer of primary recipient thymus cells.
Experimental procedures are the same as described in Figure 5. Isotype-matched background staining, which gave no nonspecific staining for Ly-5.2, is not shown in this figure.
Hematopoietic lineage reconstitution in the BM of secondary recipients after transfer of primary recipient thymus cells.
Experimental procedures are the same as described in Figure 5. Isotype-matched background staining, which gave no nonspecific staining for Ly-5.2, is not shown in this figure.
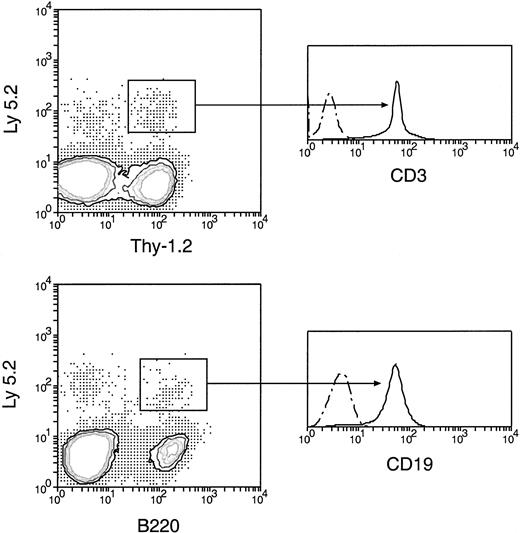
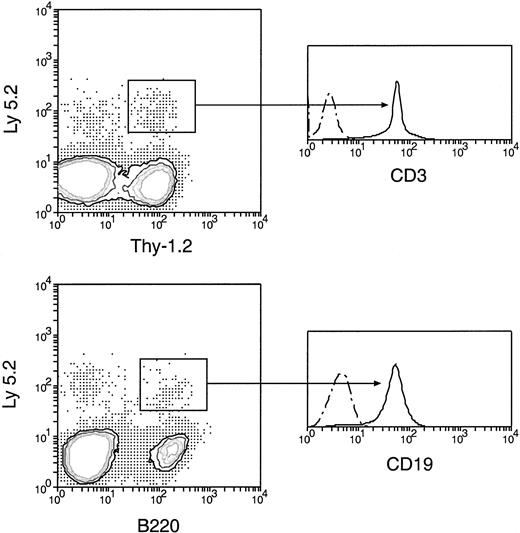
Normal T-and B-lineage cells of original donor BM origin were produced by secondary transfer of cells from the primary recipient thymus.
Cells from the thymus of the primary recipients were harvested 5 days after the original BM transfer, then injected intravenously into secondary recipients. Cells from the spleen and lymph nodes of secondary recipients 4 weeks after secondary transfer were then analyzed, as in Figure 5. The original BM-derived Ly-5.2+Thy-1.2+ and Ly-5.2+B220+ cells were further examined for the expression of CD3 and CD19, respectively. All original BM-derived Thy-1.2+ cells were also CD3+, and all the original BM-derived B220+ cells were also CD19+. Dots and dashes represent background staining.
Normal T-and B-lineage cells of original donor BM origin were produced by secondary transfer of cells from the primary recipient thymus.
Cells from the thymus of the primary recipients were harvested 5 days after the original BM transfer, then injected intravenously into secondary recipients. Cells from the spleen and lymph nodes of secondary recipients 4 weeks after secondary transfer were then analyzed, as in Figure 5. The original BM-derived Ly-5.2+Thy-1.2+ and Ly-5.2+B220+ cells were further examined for the expression of CD3 and CD19, respectively. All original BM-derived Thy-1.2+ cells were also CD3+, and all the original BM-derived B220+ cells were also CD19+. Dots and dashes represent background staining.
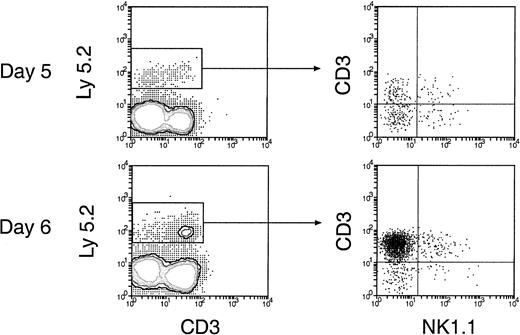
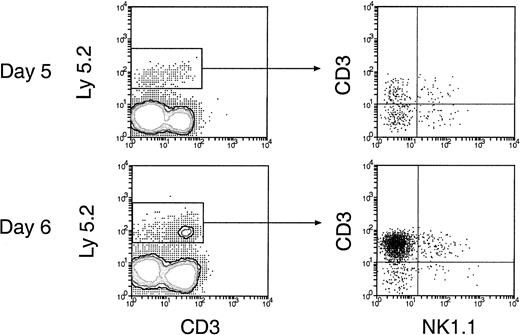
Reconstitution of NK and NKT cells in the spleen and lymph nodes of secondary recipients.
Thymus cells from the primary recipients 5 and 6 days after initial BM transfer were injected intravenously into secondary recipients and were then analyzed 4 weeks later. The procedures for cell transfer are the same as described in Figure. 5. Donor-derived NK and NKT cells were revealed by staining cells for Ly-5.2, together with CD3 and NK1.1. Expression of NK1.1 and CD3 on the donor BM-derived cells, gated as Ly-5.2+ cells, is shown.
Reconstitution of NK and NKT cells in the spleen and lymph nodes of secondary recipients.
Thymus cells from the primary recipients 5 and 6 days after initial BM transfer were injected intravenously into secondary recipients and were then analyzed 4 weeks later. The procedures for cell transfer are the same as described in Figure. 5. Donor-derived NK and NKT cells were revealed by staining cells for Ly-5.2, together with CD3 and NK1.1. Expression of NK1.1 and CD3 on the donor BM-derived cells, gated as Ly-5.2+ cells, is shown.
Thymus reconstitution after secondary transfer
T-lineage cells of donor origin (Ly-5.2+Thy-1.2+) were detected in the thymuses of the secondary receipts 4 weeks after secondary transfer (Figure 5). The numbers obtained on secondary transfer were small from day 1 to day 5 after primary transfer (Table 1), suggesting there had been little expansion of thymus-seeding T precursors over this period. However, an increased number of T-lineage progeny were obtained on secondary transfer of thymus cells 6 days after primary transfer (Table 1). Interestingly, this corresponded to the increase in the total number of donor BM-derived cells and the total number of cells with a T-precursor phenotype in the thymuses of the primary recipients (Figures 2B, 4A). This indicated that thymus-seeding precursors had, as expected, T-precursor potential and that extensive expansion of T precursors occurred 6 days after seeding. An important point was that, in contrast to the primary recipients, no progeny cells of the B or myeloid lineage were seen in the secondary recipient thymus (Figure 5).
No myeloid cell reconstitution in spleen, lymph node, and BM after secondary transfer
BM and spleen would be considered more favorable environments for B- and myeloid-cell development; hence, the presence of progeny cells in these organs would be a more sensitive test for any precursors of these lineages that seeded the original thymus. Thus, it was important that no significant number of myeloid cell progeny (Ly-5.2+Mac-1+ or Ly-5.2+Gr-1+) was found in any of these tissues after secondary transfer (Figures 6, 7). This suggested that the myeloid cells seen in the thymus after primary transfer were themselves mature or were the progeny of relatively late cells of the myeloid lineage rather than of true myeloid precursors or multipotent stem cells.
Lymphoid cell reconstitution in spleen, lymph node, and BM after secondary transfer
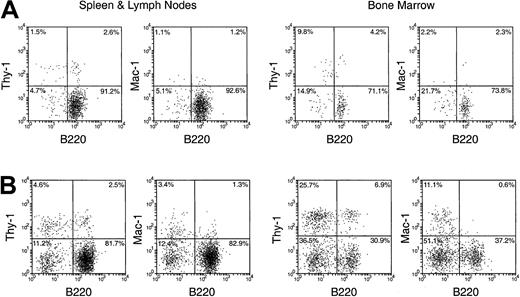
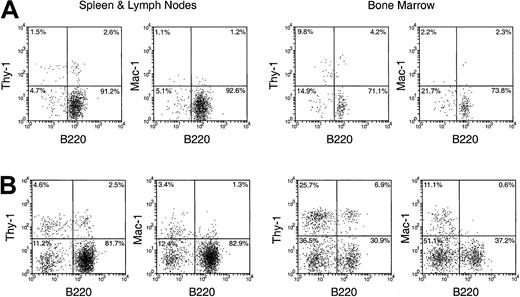
In the spleen, lymph nodes, and BM of the secondary recipients, progeny T cells (Ly-5.2+Thy-1.2+) and B cells (Ly-5.2+B220+) could be detected at nearly all times after primary transfer, as shown in Figures 6 and 7. These were normal T and B cells, being CD3+ and CD19+respectively (Figure 8). However, the relative proportion of T or B cells changed markedly with time after transfer. At early time points (1-4 days after primary transfer), the proportion of T-cell progeny was lower than that of B-cell progeny. The percentage and the absolute number of T-lineage progeny increased significantly 5 to 6 days after primary transfer, in line with the thymus reconstruction in the secondary recipients and the increase in cells with a T-precursor phenotype in the primary recipient thymus. In contrast, B-lymphocyte progeny were predominant at earlier times after primary transfer, with the highest absolute numbers produced by secondary transfer of thymus cells 4 days after primary transfer (Table 1) and a decline in the proportion as the T-cell progeny increased at days 5 and 6 (Figure 6). This suggested that the production of B-lineage progeny from thymus cells transferred at early times could originate from a few B-lineage–committed precursors that seeded the primary recipient thymus, along with the earlier precursors for the T lymphocyte. Alternatively, some of the B220+ donor-derived cells detected in the secondary recipients may be the progeny of B-lineage–committed precursors contained in the thymic vasculature of the primary recipients. To exclude the possibility that the low level of donor-derived cells detected in the secondary recipients were actually progeny of the precursors contained within the blood vessels in the thymus of primary recipients, we compared the progeny in secondary recipients, transferred with either thymus cells or blood cells from the primary recipient, 4 days after BM transfer. As shown in Figure 10 in the spleen plus lymph nodes and in the BM of secondary recipients, the lineage composition of the progeny derived from the thymus cells and from the blood cells of the primary recipients were different. The progeny of thymus cells from the primary recipients were predominantly B220+ lymphoid cells in spleen and lymph nodes (approximately 93%) and in BM (approximately 75%) of the secondary recipient (Figure 10A). A small number of Thy-1+ and few, if any, Mac-1+donor-derived cells were detected (Figure 10A). In contrast, the progeny of blood cells from the primary recipients included all lymphoid and myeloid lineages in the spleen and lymph nodes (approximately 83% B220+, 5% Thy-1+, and 4% Mac-1+) and in BM (37% B220+, 26% Thy-1+, and 12% Mac-1+) of the secondary recipients (Figure 10B). The differences in lineage composition between the progeny of thymus cells and of blood cells of the primary recipients indicated that most, if not all, progeny detected in the secondary recipients transferred with primary recipient thymus cells were the products of thymus-seeding precursors. Furthermore, as shown in Figure 9, a small number of Ly-5.2+ progeny cells with phenotype NK1.1+CD3− and NK1.1+CD3+ was detected, indicating that the cells seeding the thymus of the primary recipients had the potential to form NK cells and NKT cells, respectively.
Comparison of the hematopoietic progeny in the secondary recipients 4 weeks after IV transfer of cells from thymus and from blood of the primary recipients.
T-cell– and mature B-cell–depleted BM cells from C57BL/6 (Ly-5.2) mice were injected intravenously into lethally irradiated primary Ly-5.1 recipient mice. Four days after primary transfer, thymus cells and blood cells were harvested from the primary Ly-5.1 recipients and injected intravenously, respectively, into lethally irradiated secondary Ly-5.1 recipients as described in Figure 5. Four weeks after reconstitution, donor-derived cells in the spleen plus lymph nodes and in the BM of the secondary recipients were analyzed by flow cytometry using the procedures described in “Materials and methods.” Stainings for lineage markers on the Ly-5.2+ donor-derived cells in the secondary recipients transferred with the thymus cells of primary recipients are shown in panel A. Stainings for lineage markers on the Ly-5.2+ donor-derived cells in the secondary recipients transferred with the blood of primary recipients are shown in panel B.
Comparison of the hematopoietic progeny in the secondary recipients 4 weeks after IV transfer of cells from thymus and from blood of the primary recipients.
T-cell– and mature B-cell–depleted BM cells from C57BL/6 (Ly-5.2) mice were injected intravenously into lethally irradiated primary Ly-5.1 recipient mice. Four days after primary transfer, thymus cells and blood cells were harvested from the primary Ly-5.1 recipients and injected intravenously, respectively, into lethally irradiated secondary Ly-5.1 recipients as described in Figure 5. Four weeks after reconstitution, donor-derived cells in the spleen plus lymph nodes and in the BM of the secondary recipients were analyzed by flow cytometry using the procedures described in “Materials and methods.” Stainings for lineage markers on the Ly-5.2+ donor-derived cells in the secondary recipients transferred with the thymus cells of primary recipients are shown in panel A. Stainings for lineage markers on the Ly-5.2+ donor-derived cells in the secondary recipients transferred with the blood of primary recipients are shown in panel B.
Lymphoid and myeloid cells can be generated in secondary recipients if BM cells are injected directly into the thymus of the primary recipient
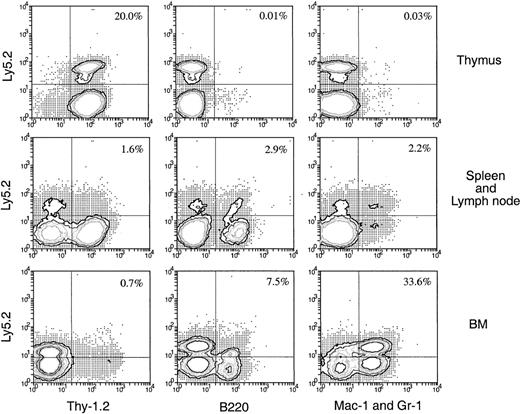
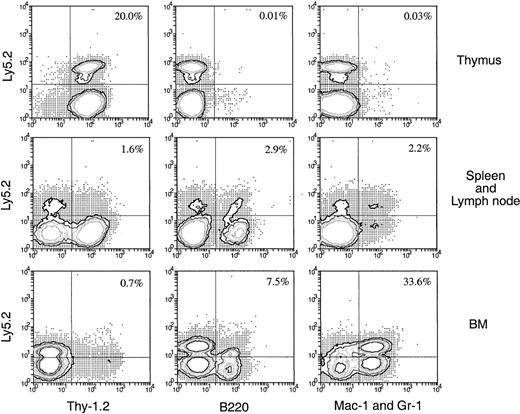
The precursor cells that enter the thymus after IV injection of BM appear to be lymphoid restricted, suggesting that the thymus acts as a filter excluding multipotent or early myeloid precursors. An alternative explanation would be that the multipotent stem cells enter the thymus but are unable to survive or develop, or they enter but become instantly lymphoid restricted. To test this, Ly-5.2+BM cells were injected directly into the thymuses of the primary Ly-5.1+ recipients rather than being introduced intravenously 3 days later, and the thymus cells were harvested and transferred intravenously into secondary recipients. In this case, though 10-fold fewer BM cells (2.5 × 106) were injected into each thymus, the number of donor-derived cells detected in each primary recipient thymus was approximately 10-fold higher (2.8 × 104) than that found in the primary recipient thymus (2.5 × 103) 3 days after IV transfer of 25 × 106 BM cells (data not shown). Therefore, each secondary recipient in this experiment received 10-fold more original donor BM-derived cells. Four weeks after IV transfer of thymocytes from the primary recipients, the Ly-5.2+ progeny in various organs were assessed for lineage markers. As shown in Figure11, significant numbers of donor-derived cells were detected in various organs of the secondary recipients. The frequency of these donor-derived cells was much higher (13-fold in spleen and lymph nodes and 400-fold in BM) than the expected 10-fold increase in frequency detected in the secondary recipients that received thymus cells from the intravenously injected mice (Figures 6, 7). Among the donor-derived cells, both lymphoid and myeloid progeny could be detected, with a much higher proportion of myeloid (33.6%) than lymphoid (8.2%) progeny detected in BM. Thus, if multipotent HSC are introduced artificially into the thymus, they survive and expand for at least 3 days, retaining the possibility of forming myeloid cells in the appropriate environment.
Hematopoietic lineage reconstitution in the secondary Ly-5.1 recipients when the primary recipients received direct intrathymic injection of BM cells.
Thymus cells from primary Ly-5.1 recipients were harvested 3 days after direct intrathymic injection of T-cell– and mature B-cell–depleted BM cells from C57BL/6 (Ly-5.2) mice, then were transferred intravenously to secondary Ly-5.1 recipients. Cells from the thymus, spleen plus lymph nodes, and BM of the secondary recipients were analyzed 4 weeks after the secondary transfer, as in Figure 5. The result shown in this figure is a representative of 2 such experiments that gave similar results. Each experiment included 2 to 3 recipient mice.
Hematopoietic lineage reconstitution in the secondary Ly-5.1 recipients when the primary recipients received direct intrathymic injection of BM cells.
Thymus cells from primary Ly-5.1 recipients were harvested 3 days after direct intrathymic injection of T-cell– and mature B-cell–depleted BM cells from C57BL/6 (Ly-5.2) mice, then were transferred intravenously to secondary Ly-5.1 recipients. Cells from the thymus, spleen plus lymph nodes, and BM of the secondary recipients were analyzed 4 weeks after the secondary transfer, as in Figure 5. The result shown in this figure is a representative of 2 such experiments that gave similar results. Each experiment included 2 to 3 recipient mice.
Discussion
In this study, the cells entering the thymus after IV transfer of BM cells into irradiated recipients were analyzed in 2 distinct steps. The first step was direct flow cytometry analysis using the Ly-5.2 marker to select the few donor BM-derived thymus cells. Despite the small proportion of such cells, they could be gated and analyzed by other markers. The second step was a 2-way assay for the presence of functional precursor cells, and it involved a secondary IV transfer of the thymus cells from the primary recipients into freshly irradiated secondary recipients (Figure 1). Again, despite the small number of donor-derived progeny, they could be detected and analyzed. The isotype-matched controls gave clean background staining in all these assays (Figures 2A, 5), which helped to exclude the possibility that the small number of donor-derived cells detected in these experiments represented nonspecific background staining. This double transfer assay enabled us to distinguish hematopoietic cells that entered the thymus without further development from true precursor cells that effectively seeded the organ.
Donor BM-derived cells could be detected in the thymus as early as 1 day after the initial transfer, albeit at very low levels. It was evident that a mixture of cell types entered the thymus at early times (1-4 days) after transfer. More than half of those cells bore markers of the myeloid (Mac-1+) and B-cell (B220+) lineages (Figure 4B). In contrast, markers of the T-cell lineage were absent at early times after transfer. Even markers of early T precursors (c-kit and CD25) took several days to be detectable, indicating that only a small proportion of the cells entering the thymus were the T precursors that would provide eventual effective seeding and thymus reconstruction. This fits with the earlier evidence of Lepault and Weissman4 that many more cells entered the irradiated thymus than ever produced effective seeding and reconstitution. A limited number of environmental niches for effective seeding would be one explanation for this finding. An alternative explanation for the mixed cell types of donor-derived cells detected in the primary recipient thymus is that they could represent the few cells contained in the thymic vasculature. However, our observations of differences in lineage composition of donor-derived cells in the thymus and in the blood of the primary recipients (Figure 4B) support the argument that most donor-derived cells detected in the thymus of the primary recipients were actually from the thymus itself.
Almost all the BM-derived cells that entered the thymus and survived several days went on to divide soon after transfer, as shown by the CFSE labeling data (Figure 3). Yet there was only a slight increase in the total number of donor-derived cells in the thymus for 5 days, and there was no detectable expansion of early T precursors until day 4. Most early proliferation was presumably balanced by cell death or exit, and much of it was of non–T-lineage cells. Expansion in the total number of BM donor-derived cells in the thymus began after day 5 (Figure 2). It was coincident with an increase in the proportion of donor-derived cells with the surface phenotype expected of early T precursors (Figure 4A) and with an increase in T-precursor activity as detected functionally by the double transfer assay.
In view of the presence of donor-derived myeloid cells in the thymus at early times after BM transfer, it was important to determine whether early myeloid precursor cells or multipotent HSC had seeded the thymus. Because direct intrathymic injection of T- and B-lineage–depleted BM cells did allow the persistence of HSC and myeloid precursors in the thymus and these could readily be revealed by our secondary transfer assay (Figure 11), it was clear that any entry of HSC or early myeloid precursors by the normal seeding route should be detectable. This agrees with earlier work by Spangrude and Scollay7 showing that the thymus is an adequate environment for myeloid cell development if HSCs are artificially introduced by intrathymic injection. However, despite this favorable thymic environment, no donor-derived myeloid precursor activity was detected among the recipient thymus cells after IV transfer of BM, which was evidenced by the lack of detection of myeloid progeny in the secondary recipients.
The lack of detectable levels of myeloid progeny in the secondary recipients receiving thymus cells of the primary intravenously BM-injected mice was probably not due to the frequency of myeloid progeny below the level of detection in these assays. On the contrary, lineage composition analysis of the Ly-5.2+ progeny showed that in the spleen and lymph nodes of secondary recipients of thymus cells from intrathymically injected mice (Figure 11), the ratio of lymphoid (4.5%) to myeloid (2.2%) Ly-5.2+ progeny was approximately 2:1. Accordingly, if the nature of the donor-derived cells detected in the primary recipient thymus in these 2 cell transfer systems is the same, a similar ratio of lymphoid versus myeloid progeny should be detected in the spleen and lymph nodes of the secondary recipients of thymus cells from intravenously injected mice. If this was the case, approximately 0.18% of myeloid progeny should be detected in the spleen and lymph nodes of these secondary recipients, because in total 0.36% lymphoid progeny was detected (Figure 6). This estimated frequency (0.18%) was well above the detection threshold (0.05% or greater) in these assays and so should be readily detectable. However, few, if any, myeloid progeny (approximately 0.02%) were detected in the spleen and lymph nodes of these secondary recipients (Figure 6). More strikingly, in the BM of the secondary recipients transferred with thymus cells from intrathymically injected mice, 4-fold more myeloid (33.6%) than lymphoid (8.2%) Ly-5.2+ progeny were detected (Figure 11). In contrast, in the BM of the secondary recipients transferred with thymus cells from intravenously injected mice, only lymphoid (0.1%) but not myeloid progeny could be detected (Figure 7). Furthermore, the frequency of donor-derived cells in the secondary recipients of the IV transfer experiment was much higher than the expected 10-fold detected in the secondary recipients of IV transfer experiments.
The most likely explanation for the above observations is that when BM cells were directly injected into the primary recipient thymus, some of the multipotent HSCs were introduced into the thymus. These HSCs could survive in the thymic environment, and they expanded more efficiently than the lineage-restricted progenitors and retained the potential to generate progeny of all hematopoietic lineages in the secondary recipients. Thymus-seeding precursors detected in the primary recipient thymus after IV transfer of BM had limited expansion potential and gave rise to only lymphoid progeny.
In contrast to the almost undetectable levels of myeloid progeny, T lymphocytes, NKT cells, NK cells, and B lymphocytes could all be detected in the spleen and lymph nodes of secondary recipients after IV transfer of thymus cells from the primary recipients of BM cells (Figures 6, 9). Clearly, the thymus was receptive to seeding by lymphoid precursor cells, not just by T-restricted precursors. As expected, the T-precursor activity within the primary recipient thymus expanded markedly as the number of cells with the surface markers of early T precursors increased. Results of the secondary transfer experiments would most simply be explained by the entry into the thymus of a common lymphoid-restricted precursor,21 but we cannot at this stage exclude the entry of separate T and B precursors.
Overall, these results indicate that despite the initial entry of many cell types into the organ, the thymus acts as a sieve and selects the precursor cells to seed the appropriate niches and to undergo extended development. Even though many different hematopoietic cells are made available in the bloodstream by IV injection of BM cells, only lymphoid precursors, and not early myeloid precursors or multipotent stem cells, provide efficient seeding and regeneration of an irradiation-depleted thymus.
We thank Mrs A. D'Amico for her excellent technical assistance and Dr F. Battye, D. Kaminaris, V. Lapatis, and J. Parker for their assistance with flow cytometry analysis.
Supported by the National Health and Medical Research Council, Australia. S.M. is supported by a Kansai Medical University fellowship. L.W. is a clinical investigator of the Cancer Research Institute (New York).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Li Wu, The Walter and Eliza Hall Institute of Medical Research, PO Royal Melbourne Hospital, Victoria 3050, Australia; e-mail: wu@wehi.edu.au