Abstract
Ferritin, the iron-storing molecule, is made by the assembly of various proportions of 2 different H and L subunits into a 24-mer protein shell. These heteropolymers have distinct physicochemical properties, owing to the ferroxidase activity of the H subunit, which is necessary for iron uptake by the ferritin molecule, and the ability of the L subunit to facilitate iron core formation inside the protein shell. It has previously been shown that H ferritin is indispensable for normal development, since inactivation of the H ferritin gene by homologous recombination in mice is lethal at an early stage during embryonic development. Here the phenotypic analysis of the mice heterozygous for the H ferritin gene (Fth+/− mice) is reported, and differences in gene regulation between the 2 subunits are shown. The heterozygousFth+/− mice were healthy and fertile and did not present any apparent abnormalities. Although they had iron-overloaded spleens at the adult stage, this is identical to what is observed in normal Fth+/+ mice. However, these heterozygous mice had slightly elevated tissue L ferritin content and 7- to 10-fold more L ferritin in the serum than normal mice, but their serum iron remained unchanged. H ferritin synthesis from the remaining allele was not up-regulated. This probably results from subtle changes in the intracellular labile iron pool, which would stimulate L ferritin but not H ferritin synthesis. These results raise the possibility that reduced H ferritin expression might be responsible for unexplained human cases of hyperferritinemia in the absence of iron overload where the hereditary hyperferritinemia-cataract syndrome has been excluded.
Introduction
Iron is essential to all living organisms. However, it is poorly soluble at physiological pH and reacts with oxygen, catalyzing the formation of potentially toxic reactive oxygen species. Therefore, living organisms have developed ferritin, a highly specialized molecule that can sequester iron in a nontoxic and readily available form. Ferritins are made of 24 subunits assembled into a protein shell that delimits an internal cavity where iron can accumulate in large amounts.1 Mammalian ferritins are heteropolymers of 2 subunit types, the H and the L chains. A third subunit, the G subunit, is also found in serum but is thought to derive from the L subunit through glycosylation during the secretion process.2 Although the mechanism of serum ferritin production is not fully understood, serum ferritin levels are a good index of tissue iron stores. H and L ferritin subunits are encoded by 2 separate genes that are under specific transcriptional regulations. The L gene has very little tissue-specific regulations whereas multiple conditions activate H ferritin gene transcription,3including cell differentiation, changes in the cell proliferation status, oncogenes, cytokines, and heme. Iron does not change the transcription rate of the H gene whereas it stimulates transcription of the L gene, at least in the liver.4 However, the translation of both H and L ferritin messenger RNAs (mRNAs) is regulated by iron, through interactions between the iron regulatory element (IRE) motif present in the 5′ noncoding region of these mRNAs and cytoplasmic iron sensors, called iron regulatory proteins (IRPs).5 In the absence of iron, IRP1 and IRP2 have a high RNA-binding affinity and their interaction with IRE motifs repress ferritin mRNA translation and ferritin synthesis. Increase in the intracellular iron pool leads to a change in IRP1 conformation6 or degradation of IRP27 and subsequent translation of ferritin mRNAs. The translation of both H and L ferritin mRNAs is generally considered to be activated by iron, although tissue-iron overload progressively leads to the preferential synthesis of L-rich isoferritins. This is usually accompanied by a progressive increase in serum ferritin levels, and serum ferritin determinations are widely used in clinics and have become part of the routine assessment of body iron stores. It has become increasingly evident that several clinical conditions can be associated with elevated serum ferritin levels in the absence of iron overload, although the origin of these hyperferritinemias is not fully understood, with the exception of the hereditary hyperferritinemia-cataract syndrome. In this pathological condition, a point mutation in the IRE of the L ferritin gene impairs the negative feedback regulation that normally operates on ferritin synthesis in conditions of low iron entry into the cells.8-10 This results in inappropriate L ferritin synthesis in most tissues and increased serum ferritin levels. The abnormal accumulation of iron-free L ferritin in the lens is probably responsible for the onset of cataract, by a mechanism that is not yet known. Besides cataracts, the patients do not present any signs of abnormal iron metabolism, suggesting that the L ferritin homopolymers that accumulate in the various tissues are probably nonfunctional and do not interfere with the normal cell iron metabolism. Measurements of iron uptake by immortalized lymphoblastoid cells from patients with hyperferritinemia-cataract syndrome have indeed confirmed this hypothesis.11 Although ferritin has long been considered to be the protein solely implicated in the constitution of iron stores, it has now become evident that ferritin function extends well beyond iron storage. The broad range of ferritin functions results from the formation of functionally distinct heteropolymers, with different subunit composition. The H subunit contains a ferroxidase center that catalyzes Fe(II) oxidation, whereas the L subunit has no catalytic activity but facilitates nucleation and mineralization of the iron core.12 Experimental cellular models in which ferritin has been overexpressed by transfection have shown that the H subunit has an active role in chelating the intracellular iron pool.13,14H ferritin–mediated depletion of the intracellular iron pool offers a better protection of the cells against oxidative stress14,15 and also impairs the cell proliferation.16 Our observation that inactivation of the H ferritin gene by homologous recombination in the mouse is lethal during embryonic development has highlighted the lack of functional redundancy between the 2 subunits.17 Homozygous mutants for the H ferritin gene (Fth) die in utero between 3.5 and 9.5 days of gestation, suggesting that the complete absence of H ferritin subunits is incompatible with life. On the other hand, the heterozygous Fth+/− mice are perfectly healthy and have no obvious phenotype. However, here we report that these mice have increased serum ferritin levels in the absence of iron overload, suggesting that a 2-fold reduction in the amount of H subunit is sufficient to stimulate L ferritin synthesis without stimulating H ferritin synthesis from the remaining allele. Therefore, H ferritin deficiency represents a situation that can lead to isolated hyperferritinemia in the absence of iron overload.
Materials and methods
Animals
Fth+/− mice of mixed C57Bl/6 × 129SV genetic background were maintained under normal housing conditions and were regularly intercrossed. To induce parenteral iron loading, 10 mg iron dextran (Sigma-Aldrich, St Quentin, France) was injected subcutaneously 3 times, at 5-day intervals, and mice were killed 5 days after the final injections.
Histology
Tissues were isolated from Fth+/− mice and their control Fth+/+ littermates and fixed in 3.5% fomaldehyde for 3 to 5 hours. Fixed tissues were then subjected to routine histological processing, and the sections were stained with Perls Prussian blue for the detection of tissue iron.
Electron microscopy
For electron microscopy, tissues were cut into 1-μL blocks and immediately fixed in 2.5% glutaraldehyde-buffered solution (phosphate-buffered saline, pH 7.4) for 2 hours at +4°C. After washing in PBS, blocks were fixed for 2 hours in 1% buffered osmium tetroxide solution, dehydrated in graded series of ethanol, and embedded in epoxy resin. Semithin sections stained with toluidine blue were made on each block for orientation. Ultrathin sections stained with uranyl acetate and lead citrate were examined with a Jeol 1010 electron microscope (Tokyo, Japan). To identify electron-dense iron-containing granules, counterstaining was omitted in some cases.
Tissue homogenization
Mice tissues were collected, weighed, minced, and dissolved in 10 mL lysis buffer (20 mM Tris-HCl, pH 7.4, 1 mM sodium azide, 1 mM phenylmethyl sulfonyl fluoride, 10 μM leupeptin, 1 μM pepstatin, 1 mM benzamidin) per gram of wet tissue. Tissue homogenizer or sonication was used to disrupt the cells. Debris was precipitated by centrifugation at 10 000 rpm for 10 minutes. All the steps were performed at +4°C. Supernatants were use to determine iron and ferritin contents or, in some cases, to analyze IRE/IRP interactions. Protein concentration was determined with BCA reagent (Pierce) with the use of bovine serum albumin (BSA) as standard.
Measurement of tissue iron
Iron concentration was determined by atomic absorption spectrometry on a Spectra-A 4440 (Varian, Palo Alto, CA) as previously described.18
Enzyme-linked immunosorbent assay for tissue H and L ferritin subunit determination
Ferritin concentration was determined by enzyme-linked immunosorbent assays (ELISAs) specific for the H and L chains as previously described.18 Microtiter plates were coated with 1 μg polyclonal antibody specific for mouse H or L-chain ferritin. Soluble mouse-tissue homogenates or standard ferritins were diluted in PBST-BSA (50 mM sodium phosphate, pH 7.4, 150 mM NaCl, 0.05% vol/vol Tween-20, 1% BSA), and added to the plates. The presence of ferritin was revealed by incubation with the same antibody labeled with horseradish peroxidase. Peroxidase activity was developed with o-phenylenediamine dihydrochloride (Sigma). Standard ferritins consisted of recombinant mouse H or L ferritin subunits.
Measurement of serum iron and ferritinemia
We punctured 0.5 to 1 mL blood at the time of killing through the abdominal aorta onto dry tubes. Serum was collected after coagulation and centrifugation. Serum iron levels were determined by spectrophotometry with the FlexFer kit (Dabe Behring, Newark, DE). Serum L ferritin was measured by means of the the L-subunit–specific ELISA described above, with a minor modification. When necessary, serum dilutions were performed by means of a commercial diluant provided with the kit for human serum ferritin assays (Dabe Behring), instead of the PBST-BSA solution used for tissue extracts.
RNAse protection assay
Total RNA from mouse tissues was isolated by means of RNAplus (Q.Biogene, Illkirch, France). For quantification of L ferritin mRNA, a genomic fragment containing exon 1 from the mouse L ferritin gene and 60 base pairs of promoter region were used to generate a 190-base antisense RNA probe, using SP6 polymerase in the presence of [32P]–uridine 5′triphosphate. The rat glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe was synthesized by means of T7 RNA polymerase after digestion of a pBluescript GAPDH plasmid by PvuII and StyI. Unprotected riboprobe was 184 bases long with a 164-base protected fragment. We hybridized 5 μg total RNA with 5 × 104cpm of each probe in 80% formamide-hybridization buffer overnight at 55°C. Following RNase A + T1 and proteinase K digestion, the protected fragments were separated on a denaturing 8% polyacrylamide gel. Radioactivity associated with the bands was quantified by means of an Instantimager (Packard Instruments, Meriden, CT).
RNA gel shift assays of IRP activity
Mouse tissue extracts were prepared as described above and diluted at 1 μg/μL in Tris buffer before use. IRE-IRP interactions were measured as previously described,10 by incubating a [32P]-labeled IRE-Fth mRNA probe (3 × 104 cpm at 104 cpm/ng), transcribed in vitro from pIL2CAT (kindly provided by Dr M. Hentze, Heidelberg, Germany) with 5 μg cytoplasmic extracts. After 15 minutes' incubation at room temperature, 5 mg/mL heparin was added for another 10 minutes. IRE-protein complexes were run on a 4% nondenaturing polyacrylamide gel. In parallel experiments, extracts were treated with 2% β-mercaptoethanol (βME) prior to the addition of the IRE probe to allow full expression of IRE binding activity. The IRE/IRP complexes were quantified with an Instantimager.
Statistical analysis
Statistical significance was evaluated by means of the unpaired Student t test with the Welch modification for comparison between 2 means. Tissue ferritin increase with age was analyzed by linear regression, and slopes were considered to be statistically different at P < .05. Serum ferritin increase with age was analyzed by nonlinear regression. GraphPad Prism software (GraphPad Software, San Diego, CA) was used for statistical evaluation.
Results
Progressive iron loading of the spleen in both normal and heterozygous Fth knockout mice
In a previous study, we showed that a complete defect in H ferritin subunit is lethal at an early stage during fetal life, between 3.5 and 9.5 days of embryonic development. We also showed that mice with a single disrupted Fth allele are phenotypically indistinguishable from their control littermates. They are healthy and fertile, do not present any gross tissue abnormality, and have normal hematological parameters in both blood and bone marrow.17
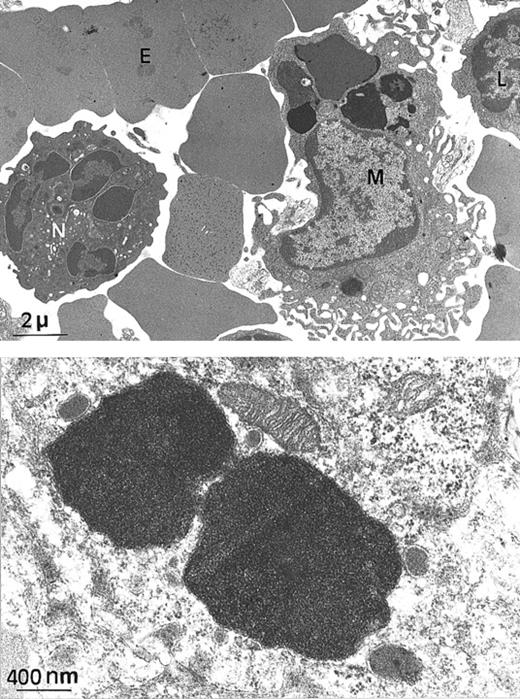
Since H ferritin has been shown to control the size of the labile iron pool (LIP) and to affect the IRE-IRP interactions, we explored the possibility that the Fth+/− mice, although apparently normal, might have unbalanced iron homeostasis. We intercrossed Fth+/− mice and analyzed heterozygous Fth knockout mice and their control littermates at several ages up to 45 weeks of age. These mice were of mixed C57Bl/6 × 129SV genetic background. We performed Perls staining on various tissues and quantitative measurements of tissue iron by atomic absorption spectroscopy. The amount of iron that accumulated in the liver, spleen, and heart of bothFth+/+ and Fth+/− mice increased with age during the first 3 or 4 months of postnatal development but did not noticeably differ between controls and heterozygous mice (Table 1). However, there was important variability in tissue iron among individual mice of the same age and same genotype. This might be due to C57BL/6 and 129SV iron-related genes segregating in the F2 mice. It is noteworthy that in mice, the spleen appears as a major site of iron storage since iron accumulation is readily detectable by Perls blue staining (Figure 1C, E-F), whereas it is not in the liver (Figure 1A). Ultrastructural analysis of a 25-week-old spleen shows that iron accumulates only in macrophages (Figure2A). Higher magnification of the electron-dense particles shows the typical microcrystalline array of iron deposits, surrounded by membranes (Figure 2B). There was no visible cell damage in these iron-loaded macrophages in either genotype (not shown). We also assessed the ability of the knockout mice to face an iron challenge by 3 subcutaneous injections of iron dextran over a 2-week period (30 mg total iron) into 45-week-old mice. Surprisingly, this induced massive iron loading of the liver, whereas iron deposits did not change much in the spleens (Table 1). Both genotypes developed similar iron overload. These results highlight some unusual features of iron homeostasis in mice, especially a progressive accumulation of iron in spleen macrophages, which is not modified by reduced H ferritin content.
Iron stain in spleen and liver in wild-type and heterozygous
Fth+/− mice. Perls Prussian blue staining of liver (panel A) or spleen (panels B-F) from wild-type (panels A-C) or heterozygous (panels D-F) animals. Animals were analyzed at 6 weeks (panels B,D), 10 weeks (panel E), or 25 weeks (panels A,C,F) after birth. In both genotypes, there is a progressive iron loading of the spleen red pulp, with very few blue deposits in the white pulp, whereas there is no stainable iron in the liver (× 100).
Iron stain in spleen and liver in wild-type and heterozygous
Fth+/− mice. Perls Prussian blue staining of liver (panel A) or spleen (panels B-F) from wild-type (panels A-C) or heterozygous (panels D-F) animals. Animals were analyzed at 6 weeks (panels B,D), 10 weeks (panel E), or 25 weeks (panels A,C,F) after birth. In both genotypes, there is a progressive iron loading of the spleen red pulp, with very few blue deposits in the white pulp, whereas there is no stainable iron in the liver (× 100).
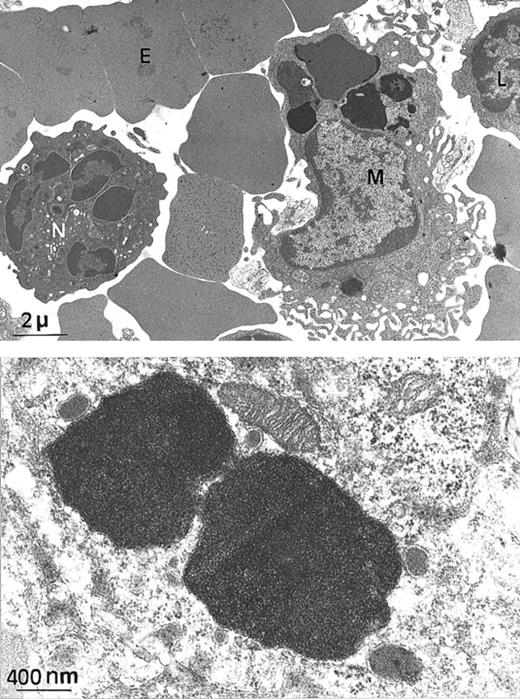
Electron microscopy of spleen from a
Fth+/− mouse. (A) At this low magnification, several spleen cells are visible, such as macrophage (M), lymphocyte (L), neutrophil (N), and erythrocyte (E). Arrows indicate electron-dense intracytoplasmic vesicles likely to contain iron (× 4000). (B) Higher magnification of 2 macrophage intracytoplasmic vesicles, showing typical paracrystalline arrangements of iron deposits (no counterstaining, × 20 000).
Electron microscopy of spleen from a
Fth+/− mouse. (A) At this low magnification, several spleen cells are visible, such as macrophage (M), lymphocyte (L), neutrophil (N), and erythrocyte (E). Arrows indicate electron-dense intracytoplasmic vesicles likely to contain iron (× 4000). (B) Higher magnification of 2 macrophage intracytoplasmic vesicles, showing typical paracrystalline arrangements of iron deposits (no counterstaining, × 20 000).
Up-regulation of L ferritin synthesis inFth+/−mouse organs
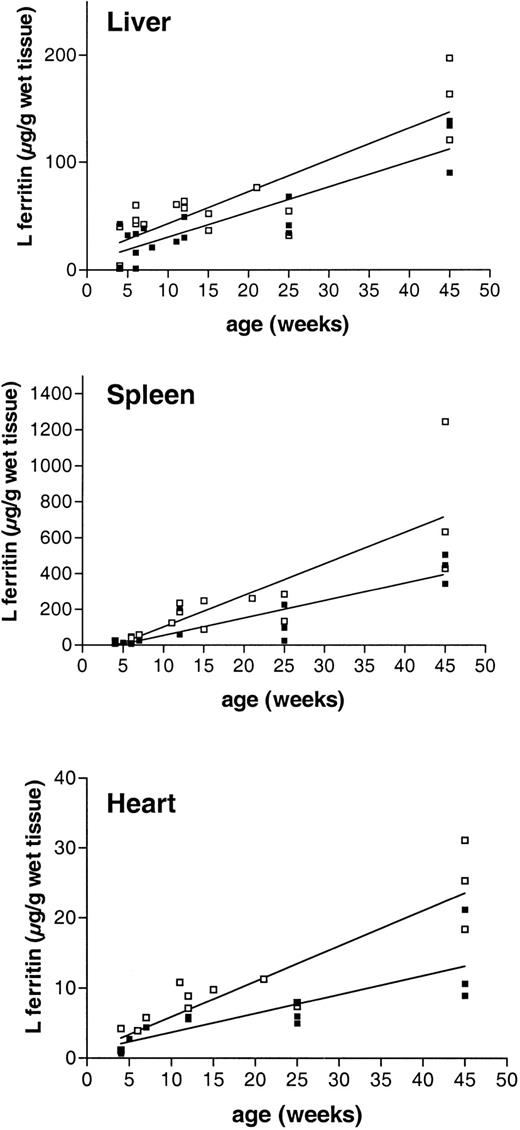
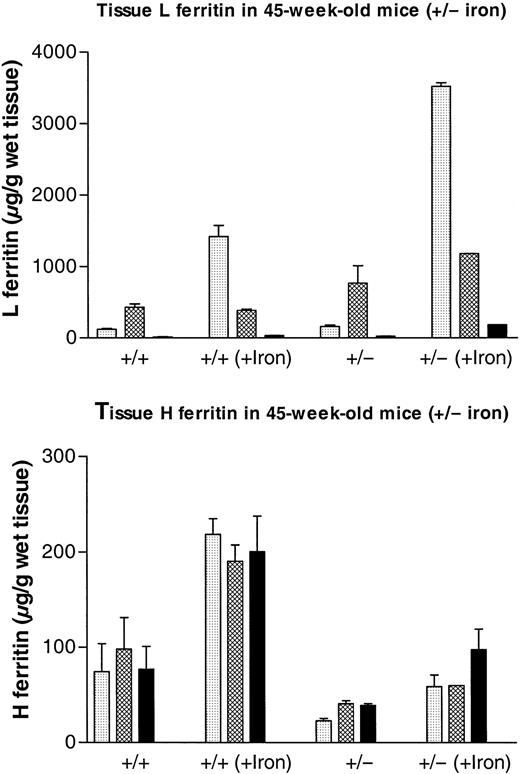
We have previously shown that inactivation of one Fthallele does not lead to up-regulation of the remaining allele. Indeed, measurements of tissue H ferritin content by ELISA by means of subunit-specific antibodies show that there is at least a 2-fold reduction in the amount of H subunit accumulating in the various organs of the Fth+/− mice (Table2). Tissue L ferritin content increased progressively in the postnatal period and up to 45 weeks of age. Furthermore, in the organs that have been tested, namely liver, spleen, and heart, the trends in the data are consistent with a 1.5- to 2-fold higher L ferritin content in the heterozygous mice as compared with their control littermates (Figure 3, Table 2), although the differences were not statistically significant. The mean ± SD calculated on 10 to 15 animals of each genotype show important individual variability (Table 2), but this is due mostly to the progressive increase in tissue L ferritin content with age. Following iron dextran injections, the difference in L ferritin content between Fth+/+ andFth+/− mice became statistically significant (Figure 4A), both in the spleen (P = .0006) and in the liver (P = .006). In iron-loaded animals, L ferritin was 2.5-fold higher in the liver of heterozygous knockout mice as compared with their control littermates and 3-fold higher in the spleen. In the heart, the increase in L ferritin content following iron dextran injections was also higher in H ferritin–deficient mice. Stimulation of H ferritin synthesis following iron injections was moderate (2-fold), identical in all the organs and in both genotypes (Figure 4B). Therefore, it appears that a partial defect in tissue H ferritin synthesis leads to a moderate, nonstatistically significant increase in L ferritin synthesis, which becomes statistically significant following iron injections. These combined modifications result in markedly different H-to-L subunit ratios (Table 2), which are likely to affect ferritin function in iron homeostasis. In contrast, H ferritin synthesized by the remaining allele is not up-regulated and injections of iron stimulate H ferritin synthesis only moderately.
Tissue L ferritin accumulation with age in
Fth+/+ andFth+/− mice.Fth+/− mice (■) and their control littermates (▪) were killed at different times after birth and up to 45 weeks. Liver, spleen, and heart were excised, and L ferritin contents were measured by ELISA with the use of polyclonal subunit-specific anti–mouse ferritin antibodies. The results are expressed as micrograms of recombinant mouse L subunit per gram of wet tissue. The linear regression is shown for both genotypes.
Tissue L ferritin accumulation with age in
Fth+/+ andFth+/− mice.Fth+/− mice (■) and their control littermates (▪) were killed at different times after birth and up to 45 weeks. Liver, spleen, and heart were excised, and L ferritin contents were measured by ELISA with the use of polyclonal subunit-specific anti–mouse ferritin antibodies. The results are expressed as micrograms of recombinant mouse L subunit per gram of wet tissue. The linear regression is shown for both genotypes.
Effect of iron loading on H and L ferritin accumulation in liver, spleen, and heart from
Fth+/+ andFth+/− mice. ThreeFth+/− mice and 3 control littermates received 3 subcutaneous injections of iron dextran (total iron, 30 mg) at 5-day intervals. Mice were killed 5 days after the final injection, together with 3 noninjected animals of each genotype. Both H-type and L-type ferritins were assayed in liver ( ), spleen (▩), and heart (▪) by means of subunit-specific ELISA. Results are expressed as micrograms of recombinant mouse L or recombinant mouse H ferritin per gram of wet weight and are the mean ± SD of 3 mice. Iron contents for the same organs are shown in Table 1. P values for Fth+/+(iron) vs Fth+/− (iron): P = .006 (liver); P = .0006 (spleen);P = .001 (heart).
), spleen (▩), and heart (▪) by means of subunit-specific ELISA. Results are expressed as micrograms of recombinant mouse L or recombinant mouse H ferritin per gram of wet weight and are the mean ± SD of 3 mice. Iron contents for the same organs are shown in Table 1. P values for Fth+/+(iron) vs Fth+/− (iron): P = .006 (liver); P = .0006 (spleen);P = .001 (heart).
Effect of iron loading on H and L ferritin accumulation in liver, spleen, and heart from
Fth+/+ andFth+/− mice. ThreeFth+/− mice and 3 control littermates received 3 subcutaneous injections of iron dextran (total iron, 30 mg) at 5-day intervals. Mice were killed 5 days after the final injection, together with 3 noninjected animals of each genotype. Both H-type and L-type ferritins were assayed in liver ( ), spleen (▩), and heart (▪) by means of subunit-specific ELISA. Results are expressed as micrograms of recombinant mouse L or recombinant mouse H ferritin per gram of wet weight and are the mean ± SD of 3 mice. Iron contents for the same organs are shown in Table 1. P values for Fth+/+(iron) vs Fth+/− (iron): P = .006 (liver); P = .0006 (spleen);P = .001 (heart).
), spleen (▩), and heart (▪) by means of subunit-specific ELISA. Results are expressed as micrograms of recombinant mouse L or recombinant mouse H ferritin per gram of wet weight and are the mean ± SD of 3 mice. Iron contents for the same organs are shown in Table 1. P values for Fth+/+(iron) vs Fth+/− (iron): P = .006 (liver); P = .0006 (spleen);P = .001 (heart).
L ferritin mRNA is not modified in Fth+/−organs
The moderate increase in tissue L ferritin content observed in the heterozygous mice could result from transcriptional activation of the gene. To test this possibility, we quantified L ferritin mRNA by RNAse protection assay. Tissue mRNAs from normal and heterozygous mice were cohybridized to a mouse L ferritin and a rat GAPDH probe. Intensities of the protected fragments were quantified by means of an Instantimager, and results are expressed as the L ferritin–to–GAPDH ratio. Figure 5 shows the results of a typical experiment. The ratio, calculated from results obtained for 2 independent experiments performed on 6 mice of each genotype, show that there is no difference in L ferritin mRNA betweenFth+/− andFth+/+ mice.
Quantification of liver and spleen L ferritin mRNA by RNAse protection assay.
Five micrograms of total RNA extracted from mouse tissues was hybridized with 5 × 104 cpm of a GAPDH probe and 5 × 104 cpm of an L ferritin probe. (A) Autoradiogram of a typical experiment. The protected fragments for L ferritin mRNA (LFt) and for GAPDH are shown for the liver and spleen of 2Fth+/+ and 2 Fth+/−mice. (B) Intensities of the protected fragments were quantified by Instantimager. Results are the mean of 2 independent experiments performed on 6 mice of each genotype. They are expressed as the ratio of radioactivity associated with each protected fragment. M indicates DNA markers. Sizes are indicated in bases.
Quantification of liver and spleen L ferritin mRNA by RNAse protection assay.
Five micrograms of total RNA extracted from mouse tissues was hybridized with 5 × 104 cpm of a GAPDH probe and 5 × 104 cpm of an L ferritin probe. (A) Autoradiogram of a typical experiment. The protected fragments for L ferritin mRNA (LFt) and for GAPDH are shown for the liver and spleen of 2Fth+/+ and 2 Fth+/−mice. (B) Intensities of the protected fragments were quantified by Instantimager. Results are the mean of 2 independent experiments performed on 6 mice of each genotype. They are expressed as the ratio of radioactivity associated with each protected fragment. M indicates DNA markers. Sizes are indicated in bases.
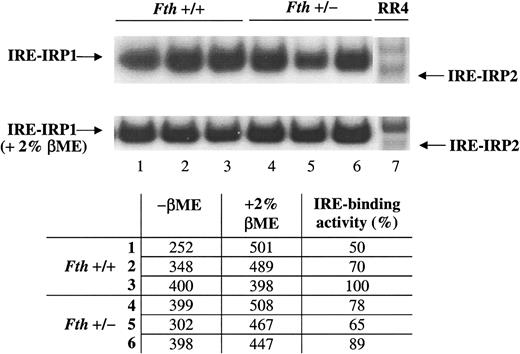
IRE-binding activity of IRP1 is not modified inFth+/−organs
H ferritin has been shown to control the size of the LIP. It is possible to assume that a half-normal level of H ferritin content in the heterozygous knockout mice could result in increased LIP and inactivation of the IRE-binding activity of IRPs. This would in turn stimulates L ferritin synthesis. To test this hypothesis, we performed gel retardation assays using an IRE probe and cytoplasmic extracts of tissues from normal and heterozygous knockout mice. In liver of mice from both genotypes, IRP1 exhibited a high IRE-binding activity and there was only a weak stimulation after 2% βME treatment of the cytoplasmic extracts (Figure 6). No IRP2-binding activity could be detected in the liver or in the spleen (not shown). Quantification of the radioactivity associated with the IRP1-IRE complexes, before and after 2% βME treatment, by means of an Instantimager showed that on average, 70% of IRP1 is in the apo-form possessing an IRE-binding activity, in both control and Fth+/− mice. In the spleen, only 50% of the IRP1 has an IRE-binding activity (not shown), but there again, no difference is observed between the 2 genotypes. It is possible that half of the normal amount of H ferritin results only in subtle differences in the regulatory iron pool and in IRE-binding affinity of IRP1, which are not possible to detect by gel retardation assays.
Gel shift assay of IRE-IRP interactions in livers from
Fth+/+ andFth+/− mice. ThreeFth+/+ (1-3) and 3Fth+/− (4-6) mice were killed and cytoplasmic extracts were prepared from their livers. Equal amounts of proteins were analyzed for IRE binding in the presence or absence of 2% βME, by means of a sense human H ferritin IRE probe. Extracts from RR4, a mouse microglial cell line, were used to control for the position of IRE-IRP1 and IRE-IRP2 complexes. Radioactivity associated with the IRE-IRP1 complex was quantified by means of an Instantimager. IRP1 activity was expressed as a percentage of the value obtained after 2% βME treatment of the cytoplasmic extracts, which allows one to estimate the total IRE bonding capacity of IRP1.
Gel shift assay of IRE-IRP interactions in livers from
Fth+/+ andFth+/− mice. ThreeFth+/+ (1-3) and 3Fth+/− (4-6) mice were killed and cytoplasmic extracts were prepared from their livers. Equal amounts of proteins were analyzed for IRE binding in the presence or absence of 2% βME, by means of a sense human H ferritin IRE probe. Extracts from RR4, a mouse microglial cell line, were used to control for the position of IRE-IRP1 and IRE-IRP2 complexes. Radioactivity associated with the IRE-IRP1 complex was quantified by means of an Instantimager. IRP1 activity was expressed as a percentage of the value obtained after 2% βME treatment of the cytoplasmic extracts, which allows one to estimate the total IRE bonding capacity of IRP1.
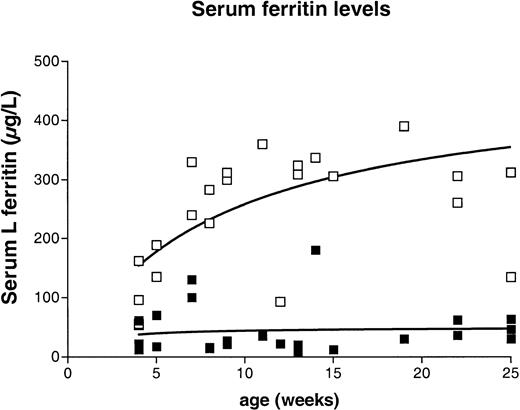
The increase in tissue L ferritin results in hyperferritinemia in Fth+/−mice
The results presented above indicate that inactivation of the H ferritin gene results in increased L ferritin content in several tissues that is not due to increased iron deposition. We wanted to test the effect of this unexpected discrepancy between tissue ferritin and iron stores on the usual serum parameters of iron status. We measured serum iron by spectrophotometry and serum L ferritin using the mouse L-subunit–specific ELISA. As expected from the tissue iron measurements, we found that mice lacking one Fth allele had serum iron values identical to those of control mice (10 ± 3 μM). There was, however, a progressive increase in serum L ferritin content with age, and the values were markedly elevated in the heterozygous knockout mice as compared with control mice (Figure7). Normal values ranged from 12 to 200 μg/L, with a mean of 44 μg/L whereas the values obtained for the age-matched knockout mice were between 54 and 510 μg/L, with a mean of 255 μg/L. This shows that a reduced H ferritin content is sufficient to increase L ferritin in several organs, resulting in markedly elevated serum ferritin levels in the absence of iron overload.
Serum ferritin levels in
Fth+/+ andFth+/− mice between 5 and 25 weeks of age. Serum ferritin levels were measured by the same ELISA that was used to measure tissue L ferritin content, with slight modifications (see “Materials and methods”). Results are expressed as micrograms of recombinant L ferritin per milliliter. The nonlinear regression curve is shown for both genotypes. The mean value was 43.6 ± 8.5 μg/L for the controls and 255 ± 25 μg/L for age-matched heterozygous animals. The P value calculated by the unpaired t test with Welch correction wasP = .0001. ▪, Fth+/+; ■,Fth+/−.
Serum ferritin levels in
Fth+/+ andFth+/− mice between 5 and 25 weeks of age. Serum ferritin levels were measured by the same ELISA that was used to measure tissue L ferritin content, with slight modifications (see “Materials and methods”). Results are expressed as micrograms of recombinant L ferritin per milliliter. The nonlinear regression curve is shown for both genotypes. The mean value was 43.6 ± 8.5 μg/L for the controls and 255 ± 25 μg/L for age-matched heterozygous animals. The P value calculated by the unpaired t test with Welch correction wasP = .0001. ▪, Fth+/+; ■,Fth+/−.
Discussion
We previously reported that a complete defect in Fthexpression is lethal at an early stage during embryonic development, demonstrating that H ferritin is indispensable for normal development.17 In this paper, we show that a 2-fold reduction in the H ferritin content in tissues is sufficient to increase serum ferritin levels without changing tissue iron distribution. This shows that fine tuning between the 2 subunits is required for proper control of ferritin synthesis, irrespective of iron homeostasis.
It has been shown that H and L subunits cooperate to facilitate iron oxidation and storage by the ferritin molecule, through the ferroxidase center of the H subunit, which stimulates iron uptake by the molecule, and the nucleation center of the L subunit, which facilitates iron core formation.12 It is likely that in the complete absence of H subunit, L ferritin homopolymers are not competent in iron chelation and storing and that cell division and differentiation are totally impaired in the absence of iron stores. A minimum of 1 or 2 H subunits in ferritin polymers is thought to be sufficient to allow formation of ferritin molecules, which have the capacity to sequester iron.19 However, from our data, we can infer that the overall subunit composition of tissue ferritin is a key element in the control of ferritin synthesis. The L-to-H subunit ratio is 3- to 4-fold higher in the Fth+/− mice as compared with their control Fth+/+ littermates, resulting from a combination of haplo-insufficency at the H locus and activated L ferritin synthesis. Surprisingly, this has no effect on iron homeostasis. Tissue iron load is not modified in any of the organs tested (liver, spleen, and heart), suggesting that intestinal iron absorption remains normally regulated. Although it has been proposed that ferritin regulates iron transfer from the apical to the basolateral side of enterocytes,20 our data do not support a role for ferritin subunit composition in this process.
Tissue iron stores in mice progressively increase in the post-natal period up to 25 weeks of age. Surprisingly, in adult mice, the spleen appears as the major site of iron storage and contains 4- to 5-fold more iron than the liver. This spleen iron is probably partially associated with hemosiderin, since the iron–to–L ferritin ratio is different in spleen and liver, being 37μg iron to 47μg L ferritin in the liver vs 205 μg iron to 144μg L ferritin in the spleen. Electron micrograph studies revealed that this iron is present almost exclusively in macrophages and is located within vesicular structures. Microcrystalline arrays of iron deposits are seen in lysosomelike structures and have the typical appearance of iron deposits seen in the liver of hemochromatotic patients.21 This iron accumulation in the spleen is probably strain-specific and could be related to the presence of a different allele at a locus that governs iron recycling following red blood cell destruction in macrophages. Abnormal iron loading of the spleen has been observed in ceruloplasmin knockout mice. In that case, the targeting was obtained in Swiss-Webster mice, which do not accumulate iron in their spleens, and spleen iron overload developed only following absence of ceruloplasmin expression.22 It is possible that various levels of ceruloplasmin expression in the different strains is responsible for incomplete iron recycling from macrophages. However, subunit composition of the ferritin molecule is not a major regulator of this process, since spleen iron levels were very similar inFth+/+ and Fth+/− mice. This retention of iron in the spleen is probably independent of the rate of intestinal iron absorption. Increased iron absorption in the gut of HFE knockout mice results in liver iron overload and diminished spleen iron content.23 Furthermore, the high accumulation of iron in the spleen is wiped out by inactivation of the heme oxygenase 1 gene, which is the major actor of macrophage heme degradation and iron recycling, suggesting that spleen iron accumulates following destruction of red cell heme in macrophages.24
Tissue L ferritin was moderately elevated in mice with a mutatedFth allele as compared with their control littermates. However, tissue iron loads and serum iron values did not differ between the 2 types of mice at any age, even following repeated iron injections.
Although this increased L ferritin content was not statistically significant, it was observed in 3 different organs and at all ages between 5 and 25 weeks after birth. Furthermore, this higher L ferritin content in Fth+/− mice was exacerbated by repeated iron injections, suggesting that it results directly from a modified intracellular iron homeostasis. This observation could result from transcriptional activation of the L ferritin gene in response to H ferritin haplo-insufficiency, since underlying transcription rates of ferritin genes governs ferritin synthesis.25,26 However, we have not been able to show differences in L ferritin mRNA content between Fth+/+ andFth+/− mice. The increase in tissue L ferritin content observed in the Fth+/− mice is suggestive of a translation activation of L ferritin synthesis, without concomitant activation of the remaining H ferritin allele. Indeed, H ferritin content remains 2-fold lower in knockout mice as compared with their control littermates. On the other hand, exogenous iron is able to stimulate H ferritin synthesis 2-fold in both types of mice (Figure 4). This would suggest that increased L ferritin synthesis in the heterozygous knockout mice would not be a direct effect of iron. Despite extensive studies on IRE and IRP, little is known about ferritin expression in tissues. Both H and L ferritin mRNAs contain an IRE motif in their 5′ noncoding region. These are highly similar, and the only notable difference concerns a nucleotide at position 6 of the consensus CAGUGX loop sequence, where a C is present in the H ferritin IRE and a U in the L ferritin IRE.27 This position is very tolerant toward base changes, and both sequences retain a high binding affinity for the IRPs.28 The H ferritin IRE has been extensively used in experimental systems and can confer translational regulation by iron to a reporter gene mRNA.29-31 Iron supplementation of cell cultures or iron injections into rats have also been shown to stimulate synthesis of both subunits.3 It is conceivable that in tissues, H ferritin mRNA might not be stimulated with the same efficacy as the L ferritin mRNA or, alternatively, that an additional level of regulation at the subunit level prevents accumulation of the H subunit. Taken together with data on H ferritin–transfected cells,13-15 our data demonstrate that artificial modification of the H-subunit content through gene targeting or transfection results in altered regulation of L ferritin synthesis.
The most striking phenotype observed in theFth+/− mice was a progressive increase in serum L ferritin levels. At 25 weeks of age, Fth+/−mice have serum ferritin levels around 300 μg/L as compared with 20 μg/L in Fth+/+ mice. It is intriguing that in mice carrying a mutated Fth allele, there is an apparent discrepancy between the elevation of L ferritin in the tissue and in the serum. The L ferritin assay we have used in this paper is based on subunit-specific antibodies that have been raised against the recombinant L subunit, and the same recombinant protein is used for calibration. Serum ferritin present in these mice is therefore antigenically related to tissue L ferritin. The origin of serum ferritin is not yet fully elucidated. It has been proposed that it is a secreted form of the L subunit, on the basis of the observation that it is iron-poor and partially glycosylated, which is contrary to tissue ferritin. Both tissue and serum ferritins are likely to derive from the same gene, although some authors have suggested that serum ferritin is encoded by a different gene from tissue ferritin.32 The recent identification of the human hereditary hyperferritinemia cataract syndrome is strongly in favor of a unique gene encoding both forms. In this syndrome, both tissue and serum L ferritin synthesis are elevated, as a result of point mutation/deletion in the IRE of the L ferritin mRNA produced by the gene present on chromosome 19q13.4-qter.33 These mutated IREs have lower affinity binding to IRPs.34 Consequently, L ferritin mRNA is actively translated irrespective of iron movements, and ferritin synthesis becomes constitutive.
The hyperferritinemia observed in the Fth+/−mice in the absence of abnormal iron overload suggests that reduced H ferritin expression might be a cause of isolated hyperferritinemia in humans. The inclusion of serum ferritin determination in clinical assessment of body iron status has highlighted the fact that patients older than 50 years with elevated serum ferritin levels and no other symptoms of iron overload are frequently found.33 It is conceivable that these patients have a reduced tissue H ferritin content resulting in hyperferritinemia. The H ferritin gene expression is highly regulated at the transcriptional levels, and several abnormal metabolic conditions (hyperthyroidy, atherosclerosis) have been shown to activate Fth gene transcription.3Alternatively, polymorphisms at the H ferritin locus could be responsible for a low expressed allele and reduced H ferritin gene expression, and this hypothesis is currently being tested.
The authors wish to thank Alain Morau for technical assistance with the electron microscopy studies and Bernard Lardeux for providing the GAPDH RNA probe.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Carole Beaumont, INSERM U409, Faculté Xavier Bichat, BP 416, 16 Rue Henri Huchard, 75870 Paris cedex 18, France; e-mail: beaumont@bichat.inserm.fr.



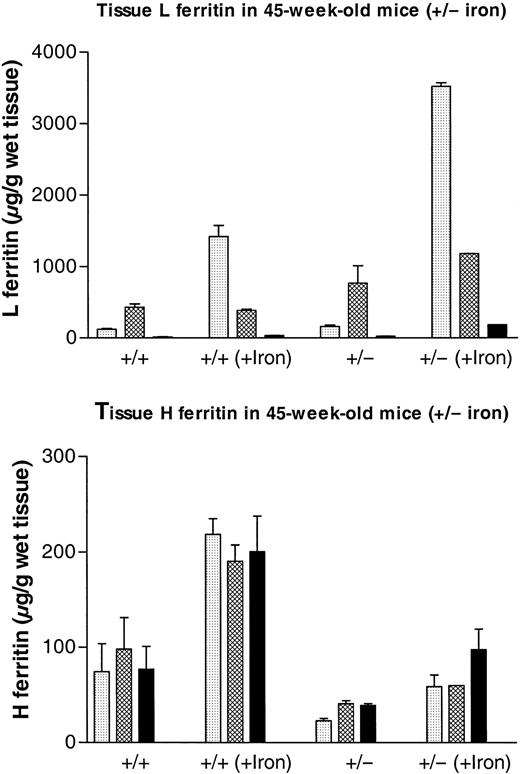





 ), spleen (▩), and heart (▪) by means of subunit-specific ELISA. Results are expressed as micrograms of recombinant mouse L or recombinant mouse H ferritin per gram of wet weight and are the mean ± SD of 3 mice. Iron contents for the same organs are shown in Table
), spleen (▩), and heart (▪) by means of subunit-specific ELISA. Results are expressed as micrograms of recombinant mouse L or recombinant mouse H ferritin per gram of wet weight and are the mean ± SD of 3 mice. Iron contents for the same organs are shown in Table