Abstract
Although the mature neutrophil is one of the better characterized mammalian cell types, the mechanisms of myeloid differentiation are incompletely understood at the molecular level. A mouse promyelocytic cell line (MPRO), derived from murine bone marrow cells and arrested developmentally by a dominant-negative retinoic acid receptor, morphologically differentiates to mature neutrophils in the presence of 10 μM retinoic acid. An extensive catalog was prepared of the gene expression changes that occur during morphologic maturation. To do this, 3′-end differential display, oligonucleotide chip array hybridization, and 2-dimensional protein electrophoresis were used. A large number of genes whose mRNA levels are modulated during differentiation of MPRO cells were identified. The results suggest the involvement of several transcription regulatory factors not previously implicated in this process, but they also emphasize the importance of events other than the production of new transcription factors. Furthermore, gene expression patterns were compared at the level of mRNA and protein, and the correlation between 2 parameters was studied.
Introduction
Studies of normal myeloid maturation from many laboratories have identified genes that may play critical roles in myeloid differentiation.1-4 Current studies suggest that these events are dependent on a cascade of molecular changes that involve complex modulation of mRNA transcription. Furthermore, studies of acute leukemia have suggested that the disease arises from the accumulation of myeloid precursors arrested at early stages of differentiation and associated, in many cases, with chromosomal rearrangements that alter the structure of specific transcription factors.5 Nevertheless, the molecular events underlying the production of mature myeloid cells are not well understood and appear to use interacting pathways and networks, the elucidation of which requires an extensive description of the molecular components available to the myeloid cell.
An extensive body of information is accumulating with respect to gene expression profiles of mammalian cells. However, much of the information available in public databases has been accumulated by the use of techniques such as single oligonucleotide chips or cDNA arrays that measure fewer than 6000 of potentially 30 000 to 120 000 transcripts. The more limited range of analyses reported by the serial analysis of gene expression (SAGE)6,7 technique accurately estimates changes in levels of the more abundant mRNAs but requires extensive redundant analyses to measure changes in the patterns of expression of scarce mRNAs. We have used a modified polymerase chain reaction (PCR)–based cDNA differential display (DD) method in which single restriction fragments derived from the 3′ end of cDNAs are separated on a sequencing gel.8 9 Bands from the gel can be identified initially by sequencing, but then comparison of patterns from different samples can be made without further sequencing. This sensitive and reproducible method detects, in principle, most cDNAs regardless of whether they are represented in existing databases.
Systematic analysis of the function of genes can also be performed at the protein level. This approach has the advantage of being closest to function, because proteins perform most of the reactions necessary for the cell. The most common method of proteome analysis is the combination of 2-dimensional gel electrophoresis (2DE) to separate and visualize protein and mass spectrometry (MS) for protein identification.10 Several such analyses of yeast and of normal or malignant mammalian cells have been performed. To date, however, there have been few studies in which both mRNA and protein have been compared by applying analyses to the same samples. The studies of Anderson11 and Gygi12 showed that there is not a good correlation between mRNA and protein levels, in yeast or human liver cells. However, other analyses disagree with this conclusion (Greenbaum et al, manuscript submitted, and Futcher et al14). Furthermore, global correlations between changes in mRNA and protein levels have not been examined during the execution of any developmental program.
The MPRO cell line was derived by transduction of a dominant-negative retinoic acid receptor construct into normal mouse bone marrow cells. It is a granulocyte-macrophage colony-stimulating factor (GM-CSF)–dependent line arrested at a promyelocytic stage of development.15,16 After treatment with all-trans retinoic acid (ATRA) most of the cells acquire the morphology of mature neutrophils and begin to produce neutrophil lactoferrin and gelatinase, 2 proteins characteristic of neutrophil secondary granules.17 As such, it offers a valuable model for studying neutrophil differentiation in vitro.
We now report the analysis of mRNA expression changes during the process of MPRO cell maturation to neutrophils and compare the results with a limited analysis of cellular protein composition. mRNA expression changes were studied by combining the use of oligonucleotide arrays and DD. A database (dbMC) with comprehensive genomic information for myeloid differentiation program was constructed (accessible at http://www.bioinfo.mbb.yale.edu/expression/neutrophil). We have grouped the changes in mRNA levels of a large number of genes into 6 patterns, with implications for the genetic program of myeloid differentiation.
We also compared 2-dimensional high-resolution gel electrophoretograms from control cells and cells differentiated for 72 hours in the presence of ATRA. Fifty protein spots whose relative intensity changed prominently during differentiation were examined by mass spectrometry. The results suggest a poor correlation between mRNA expression and protein abundance, indicating that it may be difficult to extrapolate directly from individual mRNA changes to corresponding ones in protein levels (as estimated from 2DE).
Materials and methods
Cell lines
MPRO cells and HM-5 cells provided by Dr Schickwann Tsai (Fred Hutchinson Cancer Research Center, Seattle, WA)15 were used throughout the study. The cells proliferated continuously as a GM-CSF–dependent cell line at 37°C in Iscoves modified Dulbecco medium (Gibco BRL, Grand Island, NY) supplemented with 5% to 10% fetal calf serum (Gibco BRL) and 10% HM-5–conditioned medium as a source of GM-CSF. Morphologic differentiation of the blocked MPRO promyelocytes was induced by treatment with 10 μM ATRA (Sigma, St Louis, MO). Controls were cultured in the absence of ATRA but with the same volume of vehicle (ethanol).
RNA isolation and differential display
After exposure to 10 μM ATRA for 0, 24, 48, or 72 hours, total cellular RNA was isolated from MPRO cells using TRIzol reagent (Life Technologies, Gaithersburg, MD). cDNA was then synthesized using a T-7 Sal-Oligo d(T) 32 primer as described previously.8,18 The double-stranded cDNA was digested with 1 of 9 different restriction enzymes (ApaI, BglII, BamHI,EagI, EcoRI, HindIII, XbaI,KpnI, and SphI) and ligated to Y-shaped adaptors with a complementary overhang. DNA fragments were then amplified by PCR as described previously.8,18 PCR products were separated on a sequencing gel of 6% polyacrylamide with 7 M urea. The gel was dried and exposed to x-ray film. Genes from differential display gels, whose maximum intensity changes equaled 2+ on a scale of 1+ to 8+, were recorded as significantly changed.19 Individual DNA bands were recovered from the gels, amplified by PCR, and sequenced.
Oligonucleotide chip analysis of RNA samples
Ten micrograms total RNA from each sample (0, 24, 48, or 72 hours) was used to prepare cDNA. This cDNA was transcribed with T7 RNA polymerase to prepare a fluorescently labeled probe.20,21Each sample was hybridized to mouse array chip (Mu11K Array; Affymetrix, Santa Clara, CA) containing oligonucleotide probe sets corresponding to approximately 7000 known genes or ESTs represented by UniGene clusters.22 cDNAs were considered present if their probe set results were rated as such by the GeneChip software (Affymetrix) and if the average difference (AD) between perfect match and mismatch probe pairs was not less 100 U. If a gene was represented by more than one array probe set, the average of all probe sets for the gene was taken. Genes with AD values between 100 and 200 were considered unchanged because of their low expression levels. Those genes with AD values equal to or more than 200 U at one time point were further studied by rescaling, threshold, and normalization methods described in the MIT Center for Genome Research Web site.13 A value of 20 was assigned to any gene with an AD below 20 at some time point.
Bioinformatics and database development
All the sequences or gene fragments were searched using Blast against GenBank and TIGR gene indices. A database of genes or ESTs whose expression levels changed during myeloid differentiation was constructed containing information for each band or gene. This included GenBank matches, Locus Link or Unigene clusters, expression patterns, tissue distribution, synonym(s) protein name, gene name(s), notations of possible functions, poly A signal and sequence quality, and hyperlinks to the database searches, sequence trace files, and related references. All gene data were then gathered into a cluster file. Supplementary information is available athttp://bioinfo.mbb.yale.edu/expression/neutrophil.
Classification and analysis of DNA fragments
Sequences from differential display analyses were classified as representing known genes, ESTs, genomic sequences, or novel genes as described.19 23 Known genes from both differential display and arrays were clustered into 27 functional categories and searched against SWISS-PROT (http://www.expasy.cbr.nrc.ca/cgi-bin/sprot-search-ful) or PIR (http://www.pir.georgetown.edu/). Information such as function, subcellular location, family and superfamily classification, map position, similarity, synonym(s) protein name, gene name(s), and so on was recorded in a variety of databases.
Northern blot analysis
Thirty micrograms total cellular RNA per lane from time-course MPRO cells were loaded onto 1.2% formaldehyde-agarose gels, then transferred to Hybond-N+ membranes (Amersham Pharmacia Biotech, Uppsala, Sweden). After standard prehybridization, membranes were hybridized overnight at 65°C with radiolabeled cDNA probes (ordered from Research Genetics according to their dbEST Image ID). Membranes were washed at a final stringency of 60°C in 0.1 × SSC.
Immobilized pH gradient 2-dimensional gel electrophoresis and mass spectrometry
Induced MPRO cells collected at 0 and 72 hours were lysed with lysis buffer (540 mg urea, 20 mg dithiothreitol, 20 μL Pharmalyte [3-10], 1.4 mg phenylmethylsulfonyl fluoride, 1 μg each aprotinin, leupeptin, pepstatin A, and antipain 50 μg TLCK, and 100 μg TPCK/1 mL). We applied 100 μL each MPRO cell lysate (2.5 × 106cells/100 μL) to immobilized pH gradient (IPG) strips (pH 3-10 L; Amersham Pharmacia Biotech), and IPG electrophoresis was conducted for 16 hours (20 100 Vh) using an Immobiline Drystrip Kit (Amersham Pharmacia Biotech). Electrophoresis in the second dimension was carried out in a 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel with the Laemmli-SDS continuous system in a Protean II xi 2-D cell (Bio-Rad) run at 40 mA constant current for 4.5 hours. Proteins were detected by Brilliant Blue G–colloidal staining.24Protein spots were excised from the gel and digested with trypsin. ACTH clip (average [M+H] 2466.70) and bradykinin (average [M+H] 1061.23) were used for calibration of peptide masses. One microliter sample digest was mixed with 1.0 μL α-cyano-4-hydroxy cinnamic acid (4.5 mg/mL in 50% CH3CN, 0.05% TFA) matrix solution and 1 μL calibrants (100 fmol) each. The spectra of the peptides were acquired in reflector/delayed extraction mode on a Voyager-DE STR mass spectrometer (Perseptive Biosystems, Foster City, CA). Peptides were identified using the ProFound search engine.39
Results
Differentiation of MPRO cells
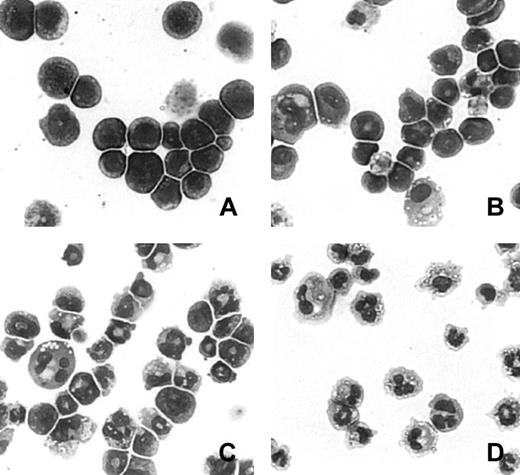
Figure 1 illustrates the morphologic changes in an MPRO cell population representative of those used for RNA expression analysis. Undifferentiated MPRO cells resembled promyelocytes under the light microscope (Figure 1A). After induction with ATRA for 24 hours, the cells morphologically differentiated into metamyelocytes (Figure 1B). At 48 hours, the cells further developed into metamyelocytes and band neutrophils (Figure 1C). At 72 hours, nearly 100% of MPRO cells became mature neutrophils (Figure 1D).
Morphology of MPRO cells during differentiation.
MPRO cells were induced as described in “Materials and methods,” concentrated by cytospin, and Wright-Giemsa stained. (A) Uninduced MPRO cells. (B) MPRO cells induced with ATRA for 24 hours. (C) MPRO cells induced with ATRA for 48 hours. (D) MPRO cells induced with ATRA for 72 hours.
Morphology of MPRO cells during differentiation.
MPRO cells were induced as described in “Materials and methods,” concentrated by cytospin, and Wright-Giemsa stained. (A) Uninduced MPRO cells. (B) MPRO cells induced with ATRA for 24 hours. (C) MPRO cells induced with ATRA for 48 hours. (D) MPRO cells induced with ATRA for 72 hours.
Identification of mRNAs by differential display assay
MPRO cellular mRNA was analyzed at 0, 24, 48, and 72 hours after ATRA treatment. Nine restriction enzymes were used in a 3′-end DD approach. During MPRO differentiation, 1109 fragments corresponding to 837 transcripts were found to change substantially in expression levels (Figure 2). These represented approximately 279 known genes, 112 ESTs, and 59 putative new genes, each with a perfect or fair polyadenylation signal at an appropriate distance from the oligo-dT priming site. The gene information detected by DD was collected in database dbMCd.
Distribution of genes obtained by DD assay.
MPRO cell mRNA was analyzed at 0, 24, 48, and 72 hours after ATRA treatment; 1109 fragments corresponding to 837 transcripts were found to change substantially in expression levels. The total 837 transcripts were classified into 6 categories according to the bioinformatic analysis. Percentages show the gene distributions in these 6 categories. Information for each transcript was collected in database dbMCd.
Distribution of genes obtained by DD assay.
MPRO cell mRNA was analyzed at 0, 24, 48, and 72 hours after ATRA treatment; 1109 fragments corresponding to 837 transcripts were found to change substantially in expression levels. The total 837 transcripts were classified into 6 categories according to the bioinformatic analysis. Percentages show the gene distributions in these 6 categories. Information for each transcript was collected in database dbMCd.
Identification of mRNAs by oligonucleotide chip assay
We used an oligonucleotide chip containing 13 179 probe sets corresponding to approximately 7000 murine genes to analyze patterns of mRNA expression in the same RNA samples used for DD. The information obtained by oligonucleotide arrays was collected in the database dbMCa.
We clustered the genes by their similarity to idealized expression patterns. For instance, the expression pattern of an ideal gene that is overexpressed (high) at time 0 and underexpressed (low) at 24, 48, and 72 hours, would be high-low-low-low (HLLL). Overall we have (24 - 2) idealized patterns excluding HHHH and LLLL. Pearson correlation was used as the measure of similarity of each gene expression pattern,x = (x1,x2,x3,x4) to each of the 14 idealized patternsy = (y1,y2,y3,y4). The 4 entries of x and y corresponded to the 4-dimensional gene expression levels at 0, 24, 48, and 72 hours, respectively. Each gene was assigned to a cluster labeled by the idealized pattern that had the maximal correlation with that gene. We selected only genes that hybridized well compared with the background (considered “present” by GeneChip software) and had maximal AD amplitude greater than 200 U in at least 1 of the 4 stages. We further tabulated the 14 patterns according to whether the gene expression changed at early (0-hour), intermediate (24- and 48-hour), and late (72-hour) time points and whether gene expression monotonically increased (up-regulated), monotonically decreased (down-regulated), or was not monotonic (transient). Table 1 shows 8 clusters of 104 genes that had significant changes of mRNA levels, arranged according to the temporal stage and the monotonic/transient changes of expression levels.
Genes differently regulated during the different stages of mouse promyelocytic cell line differentiation process
| Category . | Timing . | ||
|---|---|---|---|
| Early . | Middle . | Late . | |
| Up-regulation | LHHH (n = 10) | LLHH (n = 6) | LLLH (n = 13) |
| Mad P2rx1Itgb2 II1r2 Lcn2 Itpr5 Cebpb H2-D Etohi6 Zyx | Piral Cybb Pfc Pira5 Cd53Ifngr2 | Il1a Csflr Ii CtslS100a8 L-CCR CtssAldo1 Rac2 Fpr1Ctsd Ubb Ptmb4 | |
| Down-regulation | HLLL (n = 11) | HHLL (n = 1) | HHHL (n = 37) |
| Tcrg-V4 Ly64 Ctsg Spi2-1 Mcpt8 Myc Myb Tlr4 Npm1 Erh Hsp60 | Mpo | Actx Irf2 EL2 Rpl19 Actb Ly6e Atf1 Hist2 Psma2 Gnas Zfp36 Il4ra LtbrShfdg1 Max Rps8 Csf2rbl Slpi Tctex1 Tpi Btf3Cntf Gys3 Slc10a1 Ctsb Sepp1 Rtn3 Ccnb2 S100a9 Cf11 Hist5-2ax Rela Copa Gstm1 Gnb2-rs1 Grn RPL8 | |
| Transient | LLHL (n = 9) | ||
| SellKlf2 Pira6 Pirb Lst1 Ltf Sema4d Stat6Mmp9 | |||
| LHHL (n = 17) | |||
| CebpaLyzs Fcgr3 Arf5 Lamp1 Stat3 Csf2ra Osi Actg Sfpi1 Gpx3 Ptprc Prtn3 Irf1 Rps6ka1 Ltb4r Myln | |||
| Category . | Timing . | ||
|---|---|---|---|
| Early . | Middle . | Late . | |
| Up-regulation | LHHH (n = 10) | LLHH (n = 6) | LLLH (n = 13) |
| Mad P2rx1Itgb2 II1r2 Lcn2 Itpr5 Cebpb H2-D Etohi6 Zyx | Piral Cybb Pfc Pira5 Cd53Ifngr2 | Il1a Csflr Ii CtslS100a8 L-CCR CtssAldo1 Rac2 Fpr1Ctsd Ubb Ptmb4 | |
| Down-regulation | HLLL (n = 11) | HHLL (n = 1) | HHHL (n = 37) |
| Tcrg-V4 Ly64 Ctsg Spi2-1 Mcpt8 Myc Myb Tlr4 Npm1 Erh Hsp60 | Mpo | Actx Irf2 EL2 Rpl19 Actb Ly6e Atf1 Hist2 Psma2 Gnas Zfp36 Il4ra LtbrShfdg1 Max Rps8 Csf2rbl Slpi Tctex1 Tpi Btf3Cntf Gys3 Slc10a1 Ctsb Sepp1 Rtn3 Ccnb2 S100a9 Cf11 Hist5-2ax Rela Copa Gstm1 Gnb2-rs1 Grn RPL8 | |
| Transient | LLHL (n = 9) | ||
| SellKlf2 Pira6 Pirb Lst1 Ltf Sema4d Stat6Mmp9 | |||
| LHHL (n = 17) | |||
| CebpaLyzs Fcgr3 Arf5 Lamp1 Stat3 Csf2ra Osi Actg Sfpi1 Gpx3 Ptprc Prtn3 Irf1 Rps6ka1 Ltb4r Myln | |||
Arrays of Affymetrix Mu11k containing 13103 probe sets corresponding to 12002 GenBank accessions were used for hybridization. Arrays were hybridized with streptavidin-phycoerythrin (Molecular Probes) biotin-labeled RNA and scanned. Intensity for each feature of the array was captured using Genechip software (Affymetrix), and a single raw expression level for each gene was derived from the 20 probe pairs representing each gene using a trimmed mean algorithm. For each gene, an AD of 24-, 48-, and 72-hour samples was calibrated by dividing the slope of the linear regression line for a graph with the x-axis the AD of 0-hour probe sets and the y-axis the AD of the respective time point (24, 48, or 72 hours). A threshold of 20 U was assigned to any gene with a calculated expression level below 20 because discrimination of expression below this level could not be performed with confidence.38 Each gene expression profile was categorized as described in Tables 3, 4, and 5. For the 4 time points, the minimum AD of the relatively higher group (MIN-H) was divided by the maximum AD of the relatively low group (MAX-L), and those genes whose MIN-H/MAX-L greater than 2 were selected as meaningfully regulated. Genes were sorted in descending order based on the MIN-H/MAX-L. Genes in boldface are those whose expression level was in the top 20% (ie, maximum AD of 4 time points greater than 3000), and genes in italics are those in the bottom 20% (ie, maximum AD of 4 time points less than 300). The differentiation period was grouped into 3 stages: early (0-hour), middle (24-hour and 48-hour), and late (72-hour) stages.
AD indicates average difference; gene symbols are expanded in an at the end of this article.
Principal component analysis determined whether we could comprehensively present multidimensional data (4-dimensional in our case) in a simple 2-dimensional graph. First, we found the 4 principal components, which were the axes of the most compact 4-dimensional ellipsoid that encompassed the 4-dimensional cloud of data. Each axis was a different linear combination of the original 4 variables. Then we verified that the first 2 principal components (the first 2 largest axes of the ellipsoid) captured most (95.2%) of the variation of the data. Therefore, the data could be faithfully projected (with a minor loss of information) into a 2-dimensional graph, with the 2 largest principal components as the x- and y-axes. As shown in Figure3, genes tend to coalesce in clusters, according to their labels determined by their similarity to an ideal expression pattern. In summary, a genomic (global) picture of the distribution of genes according to their similarity to predetermined idealized multidimensional expression patterns is concisely displayed in a 2-dimensional graph.
Gene clusters in the first 2 principal component spaces.
Principal component analysis allowed us to present the multidimensional data (in this case, 4-dimensional data of each gene expression pattern) in a simple 2-dimensional graph. We derived the 4 principal components, which are a linear combination of the standardized expression intensities (zero mean and unit variance) at 0, 24, 48, and 72 hours. The first 2 principal components captured most of the variation of the data (approximately 85%). Therefore, the data can be displayed (with a minor loss of information) in a 2-dimensional graph. The first and second principal components, c1 and c2, are given by the linear combinations c1 = 0.747 · n1 − 0.11 · n2 − 0.656 · n3 + 0 · n4 andc2 = 0.278 · n1 + 0.353 · n2 + 0.233 · n3 − 0.863 · n4, where n1, n2, n3, and n4 are the rescaled and standardized expression levels at 0, 24, 48, and 72 hours, respectively. The axes legends c1 and c2 stand for the first 2 principal components. In this paper we used the Pearson correlation to measure the similarity of each gene with the idealized expression patterns, as opposed to the Euclidean distance we used in a previous work,19 because clusters were better separated using this measure. In both cases, we presented the data in the 2-dimensional space of the lowest principal components. The data had a tendency to be circularly distributed when we used the Pearson correlation as a distance measure.
Gene clusters in the first 2 principal component spaces.
Principal component analysis allowed us to present the multidimensional data (in this case, 4-dimensional data of each gene expression pattern) in a simple 2-dimensional graph. We derived the 4 principal components, which are a linear combination of the standardized expression intensities (zero mean and unit variance) at 0, 24, 48, and 72 hours. The first 2 principal components captured most of the variation of the data (approximately 85%). Therefore, the data can be displayed (with a minor loss of information) in a 2-dimensional graph. The first and second principal components, c1 and c2, are given by the linear combinations c1 = 0.747 · n1 − 0.11 · n2 − 0.656 · n3 + 0 · n4 andc2 = 0.278 · n1 + 0.353 · n2 + 0.233 · n3 − 0.863 · n4, where n1, n2, n3, and n4 are the rescaled and standardized expression levels at 0, 24, 48, and 72 hours, respectively. The axes legends c1 and c2 stand for the first 2 principal components. In this paper we used the Pearson correlation to measure the similarity of each gene with the idealized expression patterns, as opposed to the Euclidean distance we used in a previous work,19 because clusters were better separated using this measure. In both cases, we presented the data in the 2-dimensional space of the lowest principal components. The data had a tendency to be circularly distributed when we used the Pearson correlation as a distance measure.
Correlation between array and DD analyses
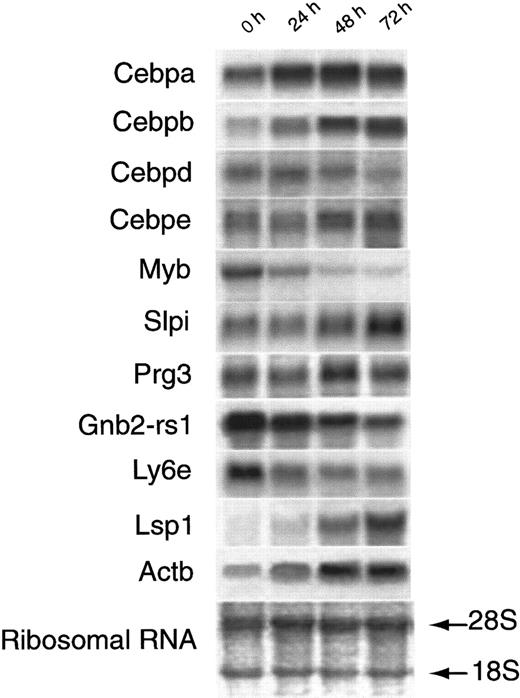
We have previously demonstrated a correlation coefficient of 0.93 between visual estimates of changes in band intensity on DD and Phosphorimager System (Molecular Dynamics, Sunnyvale, CA) estimates of band intensity and a correlation coefficient of 0.88 between hybridization intensity changes of mRNA on Northern blot analyses and changes in band intensity on DD.19 In a few cases there were clear discrepancies in the pattern of expression of a gene, as estimated by DD and by oligonucleotide chip analysis. We chose the 6 most extreme cases and examined the levels of mRNA change for these genes by Northern blot analysis (Figure4). In 5 cases, the Northern blot results agreed with the results of the DD analysis, whereas the results of Gnb2-rs1 disagreed with the oligonucleotide array but duplicate bands from DD showed a relatively high level of expression in the 0 time sample that did not correlate with the Northern blot (Table2). One possible explanation for these findings was the change in the relative use of different polyadenylation sites after the addition of ATRA to the MPRO cells.
Northern blot analysis of selected mRNAs.
Equivalent amounts of RNA from MPRO cells induced by ATRA at different time points (0 hour, 24 hours, 48 hours, and 72 hours) were resolved by formaldehyde-agarose gel electrophoresis, stained to verify the amount of loading. Eleven genes were separately probed on the RNA filters. The gene symbol of each probe was listed at the left of a related Northern blot result. Detailed information on these 11 probes was listed in Table 5. One of the RNA-blotted membrane photographs is shown with methylene blue–stained 28S and 18S RNA subunits demonstrating the quality and quantity of RNA loaded in individual lanes.
Northern blot analysis of selected mRNAs.
Equivalent amounts of RNA from MPRO cells induced by ATRA at different time points (0 hour, 24 hours, 48 hours, and 72 hours) were resolved by formaldehyde-agarose gel electrophoresis, stained to verify the amount of loading. Eleven genes were separately probed on the RNA filters. The gene symbol of each probe was listed at the left of a related Northern blot result. Detailed information on these 11 probes was listed in Table 5. One of the RNA-blotted membrane photographs is shown with methylene blue–stained 28S and 18S RNA subunits demonstrating the quality and quantity of RNA loaded in individual lanes.
Expression patterns of genes detected by Northern blot analysis
| Gene symbol . | Gene accession . | AD value by array . | Intensity by DD . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h . | 24 h . | 48 h . | 72 h . | 0 h . | 24 h . | 48 h . | 72 h . | ||
| Cebpa | M62362 | 33 | 212 | 182 | 44 | — | — | — | — |
| Cebpb | X62600 | 390 | 1248 | 1380 | 1903 | — | — | — | — |
| Cebpd | X61800 | 157 | 262 | 168 | 430 | — | — | — | — |
| Cebpe | — | — | — | — | — | — | — | — | — |
| Myb | M12848 | 892 | 356 | 230 | 435 | — | — | — | — |
| Slpi | U73004 | 617 | 501 | 783 | 402 | 1 | 2 | 3 | 3 |
| Prg3 | W45834 | 153 | 259 | 339 | 345 | 5 | 1 | 1 | 2 |
| Gnb2-rs1 | X75313 | 4231 | 3623 | 3215 | 3403 | 4 | 4 | 1 | 1 |
| Ly6e | U04268 | 3061 | 5391 | 2844 | 1282 | 3 | 2 | 1 | 1 |
| Lsp1 | M90316 | 65 | 376 | 840 | 28 | 2 | 3 | 5 | 6 |
| Actb | X03765 | 3095 | 3588 | 3976 | 2434 | 1 | 2 | 3 | 2 |
| Gene symbol . | Gene accession . | AD value by array . | Intensity by DD . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 h . | 24 h . | 48 h . | 72 h . | 0 h . | 24 h . | 48 h . | 72 h . | ||
| Cebpa | M62362 | 33 | 212 | 182 | 44 | — | — | — | — |
| Cebpb | X62600 | 390 | 1248 | 1380 | 1903 | — | — | — | — |
| Cebpd | X61800 | 157 | 262 | 168 | 430 | — | — | — | — |
| Cebpe | — | — | — | — | — | — | — | — | — |
| Myb | M12848 | 892 | 356 | 230 | 435 | — | — | — | — |
| Slpi | U73004 | 617 | 501 | 783 | 402 | 1 | 2 | 3 | 3 |
| Prg3 | W45834 | 153 | 259 | 339 | 345 | 5 | 1 | 1 | 2 |
| Gnb2-rs1 | X75313 | 4231 | 3623 | 3215 | 3403 | 4 | 4 | 1 | 1 |
| Ly6e | U04268 | 3061 | 5391 | 2844 | 1282 | 3 | 2 | 1 | 1 |
| Lsp1 | M90316 | 65 | 376 | 840 | 28 | 2 | 3 | 5 | 6 |
| Actb | X03765 | 3095 | 3588 | 3976 | 2434 | 1 | 2 | 3 | 2 |
Gene symbol and gene accession refer to National Center for Biotechnology Information databases and, in particular, to Locus Link. AD value is the average difference in the value of hybridization intensity between the set of perfectly matched oligonucleotides and the set of mismatched oligonucleotide in the oligonucleotide array. Band intensities from DD were semiquantified on a scale from 1 (+) to 8 (++++++++). These estimates are shown as boldface numbers in this table.19 Both AD value and intensity of genes were studied at 4 time points corresponding to MPRO cells induced for the indicated times.
DD indicates differential display; MPRO, mouse promyelocytic cell line; for gene symbols, see the at the end of this article.
Constructing a database for mRNA level changes during myeloid differentiation
Based on the data obtained above, an in-house database (dbMC) was constructed that included 2 subdatabases, dbMCd and dbMCa, for collecting gene information from DD or oligonucleotide arrays, respectively. Each entry in dbMC is accompanied by a so-called executive summary. The linkage between dbMCd and dbMCa was established by UniGene ID and cluster ID. dbMC contains the temporal expression patterns of genes during the MPRO cell differentiation process, including not only products represented in public databases but also novel transcripts.
Analysis of gene expression patterns during MPRO differentiation
Many of the genes identified in this study were found in myeloid cells or were implicated in myeloid development for the first time. We detected 8 cytokines25 and chemokines whose mRNA levels changed more than 5-fold by arrays and 2-fold by DD during the maturation of MPRO cells (see our Web site,http://bioinfo.mbb.yale.edu/expression/neutrophil). Among these were 2 members of the CC chemokine family. Interleukin-1α (IL-1α) was up-regulated at the late stage of differentiation (LLLH pattern, Table 1).
mRNA for approximately 52 receptors was detected by one or the other method. A number of the receptors known to be present on mature neutrophils showed late induction of mRNA, and their levels of induction were high, indicating that the expression of these products is a prominent event late in neutrophil maturation (Table3). Rarely was mRNA for receptors down-regulated, consistent with myeloid maturation being accompanied by increasing responsiveness of the cell to a variety of external stimuli.
Receptors expressed during myeloid differentiation process
| Maximal fold change . | Gene symbol . | Gene accession . | AD value by array . | |||
|---|---|---|---|---|---|---|
| 0 h . | 24 h . | 48 h . | 72 h . | |||
| Less than 2 | ||||||
| Bzrp | D21207 | 641 | 658 | 881 | 887 | |
| Cmkar4 | X99581 | 508 | 447 | 378 | 684 | |
| Crry | M34173 | 433 | 384 | 506 | 506 | |
| Csf2rb1 | M34397 | 318 | 345 | 410 | 241 | |
| Htr5a | Z18278 | 188 | 272 | 273 | 339 | |
| M6pr | X64068 | 536 | 409 | 408 | 649 | |
| MPPIR | AA116789 | 232 | 84 | 63 | 381 | |
| TCRGB | M26053 | 165 | 212 | 244 | 299 | |
| Tnfrsfla | M59377 | 0 | 1 | 1 | 1 | |
| 2 or more, less than 3 | ||||||
| Cmkbr1 | U28404 | 221 | 244 | 504 | 638 | |
| Crhr | X72305 | 121 | 200 | 250 | 355 | |
| Csf2ra | M85078 | 171 | 372 | 402 | 254 | |
| Ebi3 | AF013114 | 187 | 270 | 428 | 148 | |
| Grid1 | D10171 | 128 | 164 | 150 | 257 | |
| Ifngr | J05265 | 141 | 263 | 327 | 251 | |
| Il2rg | U21795 | 205 | 184 | 231 | 477 | |
| Ldlr | X64414 | 1399 | 1653 | 1665 | 3968 | |
| P40-8 | J02870 | 849 | 677 | 381 | 640 | |
| Plaur | X62701 | 312 | 443 | 476 | 734 | |
| Rarg | M34476 | 102 | 113 | 114 | 218 | |
| Srb1 | U37799 | 126 | 232 | 132 | 258 | |
| 3 or more, less than 4 | ||||||
| Cr2 | M29281 | 83 | 138 | 243 | 77 | |
| Csf2rb2 | M29855 | 209 | 249 | 437 | 111 | |
| Fcer1g | J05020 | 2398 | 2766 | 3365 | 8751 | |
| Fcgr2b | X04648 | 1703 | 1652 | 1431 | 4605 | |
| Ifngr2 | U69599 | 1 | 2 | 2 | 3 | |
| 4 or more, less than 5 | ||||||
| Nr4a1 | X16995 | 96 | 188 | 202 | 401 | |
| 5 or more | ||||||
| I11r2 | X59769 | 482 | 1796 | 2872 | 3818 | |
| C5r1 | L05630 | 185 | 434 | 808 | 1078 | |
| Drd2 | X55674 | 0 | 0 | 0 | 219 | |
| Fcgr3 | M14215 | 1 | 1 | 1 | 2 | |
| Fpr1 | L22181 | 0 | 89 | 141 | 671 | |
| GCR | AA240711 | 2 | 0 | 0 | 0 | |
| L-CCR | AA034646 | 48 | 175 | 314 | 2056 | |
| NMDARGB | AAB20211 | 2 | 2 | 0 | 0 | |
| P2rx1 | X84896 | 79 | 346 | 530 | 744 | |
| Pira1 | U96682 | 0 | 43 | 172 | 378 | |
| Pira5 | U96686 | 274 | 391 | 954 | 1874 | |
| Pira6 | U96687 | 122 | 635 | 2014 | 1716 | |
| Pirb | U96689 | 191 | 445 | 966 | 747 | |
| Sell | M25324 | 46 | 104 | 570 | 20 | |
| Tcrg-V4 | M54996 | 1650 | 78 | 65 | 315 | |
| Maximal fold change . | Gene symbol . | Gene accession . | AD value by array . | |||
|---|---|---|---|---|---|---|
| 0 h . | 24 h . | 48 h . | 72 h . | |||
| Less than 2 | ||||||
| Bzrp | D21207 | 641 | 658 | 881 | 887 | |
| Cmkar4 | X99581 | 508 | 447 | 378 | 684 | |
| Crry | M34173 | 433 | 384 | 506 | 506 | |
| Csf2rb1 | M34397 | 318 | 345 | 410 | 241 | |
| Htr5a | Z18278 | 188 | 272 | 273 | 339 | |
| M6pr | X64068 | 536 | 409 | 408 | 649 | |
| MPPIR | AA116789 | 232 | 84 | 63 | 381 | |
| TCRGB | M26053 | 165 | 212 | 244 | 299 | |
| Tnfrsfla | M59377 | 0 | 1 | 1 | 1 | |
| 2 or more, less than 3 | ||||||
| Cmkbr1 | U28404 | 221 | 244 | 504 | 638 | |
| Crhr | X72305 | 121 | 200 | 250 | 355 | |
| Csf2ra | M85078 | 171 | 372 | 402 | 254 | |
| Ebi3 | AF013114 | 187 | 270 | 428 | 148 | |
| Grid1 | D10171 | 128 | 164 | 150 | 257 | |
| Ifngr | J05265 | 141 | 263 | 327 | 251 | |
| Il2rg | U21795 | 205 | 184 | 231 | 477 | |
| Ldlr | X64414 | 1399 | 1653 | 1665 | 3968 | |
| P40-8 | J02870 | 849 | 677 | 381 | 640 | |
| Plaur | X62701 | 312 | 443 | 476 | 734 | |
| Rarg | M34476 | 102 | 113 | 114 | 218 | |
| Srb1 | U37799 | 126 | 232 | 132 | 258 | |
| 3 or more, less than 4 | ||||||
| Cr2 | M29281 | 83 | 138 | 243 | 77 | |
| Csf2rb2 | M29855 | 209 | 249 | 437 | 111 | |
| Fcer1g | J05020 | 2398 | 2766 | 3365 | 8751 | |
| Fcgr2b | X04648 | 1703 | 1652 | 1431 | 4605 | |
| Ifngr2 | U69599 | 1 | 2 | 2 | 3 | |
| 4 or more, less than 5 | ||||||
| Nr4a1 | X16995 | 96 | 188 | 202 | 401 | |
| 5 or more | ||||||
| I11r2 | X59769 | 482 | 1796 | 2872 | 3818 | |
| C5r1 | L05630 | 185 | 434 | 808 | 1078 | |
| Drd2 | X55674 | 0 | 0 | 0 | 219 | |
| Fcgr3 | M14215 | 1 | 1 | 1 | 2 | |
| Fpr1 | L22181 | 0 | 89 | 141 | 671 | |
| GCR | AA240711 | 2 | 0 | 0 | 0 | |
| L-CCR | AA034646 | 48 | 175 | 314 | 2056 | |
| NMDARGB | AAB20211 | 2 | 2 | 0 | 0 | |
| P2rx1 | X84896 | 79 | 346 | 530 | 744 | |
| Pira1 | U96682 | 0 | 43 | 172 | 378 | |
| Pira5 | U96686 | 274 | 391 | 954 | 1874 | |
| Pira6 | U96687 | 122 | 635 | 2014 | 1716 | |
| Pirb | U96689 | 191 | 445 | 966 | 747 | |
| Sell | M25324 | 46 | 104 | 570 | 20 | |
| Tcrg-V4 | M54996 | 1650 | 78 | 65 | 315 | |
Receptors are identified as present whose maximal AD values were more than or equal to 200 U in this study. Genes were sorted by their expression patterns as follows: first by the average difference value, then by the difference between minimum and maximum AD for the 4 time points, and last by the alphabetical order of gene symbols. Genes were ordered according to the maximal fold change of AD values. Abbreviations of gene names are taken from gene symbols listed in the Locus Link portion of the National Center for Biotechnology Information database where available. Numbers in bold denote those gene expression patterns obtained by differential display rather than by oligonucleotide array assays. The other information is presented as in the legend to Table 2.
AD indicates average difference; gene symbols are expanded in an at the end of this article.
Expression of mRNA for granule proteins
Neutrophils contain several types of granules that develop at different stages of myeloid maturation.3,17 26 Levels of mRNAs encoding secondary granule proteins, such as lactoferrin, increased as the cells matured (Table 4). The level of mRNA for Mmp9, reported as a tertiary granule protein, increased markedly between 24 and 48 hours after the induction of differentiation, whereas mRNAs for secondary granule proteins either increased less markedly or showed a maximum increase by 24 hours. mRNAs for several primary granule constituents, such as myeloperoxidase and cathepsin G, were present in unstimulated cells and decreased as the cells matured. There was a discrepancy in the measurements of proteoglycan mRNA by DD and oligonucleotide chips, but Northern blots showed that it reached a peak at 48 hours and then declined (Figure 4). Cathepsin D is reported as a primary granule protein, but its pattern of mRNA expression more closely resembled that of secondary granule constituents. In addition to known granule components, mRNAs for several other cathepsins were up-regulated during myeloid differentiation, in parallel with or later than the tertiary granule protein mRNAs.
Granule constituents expressed during mouse promyelocytic cell line cell differentiation
| Granule constituent . | Gene symbol . | Gene accession . | AD value by array . | |||
|---|---|---|---|---|---|---|
| 0 h . | 24 h . | 48 h . | 72 h . | |||
| Azurophil (primary) granules | ||||||
| Man2c1 | AA161860 | 178 | 134 | 99 | 164 | |
| Ctsb | M65270 | 442 | 480 | 595 | 389 | |
| Ctsd | X52886 | 214 | 1087 | 1828 | 2784 | |
| Ctsg | M96801 | 1509 | 405 | 46 | 286 | |
| E12 | U04962 | 658 | 1273 | 843 | 157 | |
| E1a2 | AA689016 | 47 | 159 | 134 | 163 | |
| Gus-s | M63836 | 544 | 226 | 266 | 254 | |
| Lyzs | M21050 | 0 | 1 | 1 | 3 | |
| Mcpt8 | X78545 | 831 | 268 | 66 | 491 | |
| Mpo | X15378 | 3788 | 3009 | 776 | 692 | |
| Prg | X16133 | 2621 | 2653 | 2920 | 9859 | |
| Possible granule proteins | ||||||
| Ctsc | AA144887 | 252 | 194 | 342 | 576 | |
| Ctse | X97399 | 1 | 3 | 4 | 5 | |
| Ctsh | U06119 | 45 | 124 | 195 | 156 | |
| Ctsl | X06086 | 16 | 11 | 31 | 237 | |
| Ctss | AA089333 | 12 | 9 | 88 | 463 | |
| Specific secondary granules | ||||||
| Cpa3 | J05118 | 621 | 270 | 90 | 801 | |
| Cd36l2 | AB008553 | 113 | 93 | 157 | 187 | |
| Cnlp | X94353 | 80 | 479 | 704 | 626 | |
| Cybb | U43384 | 8 | 24 | 91 | 128 | |
| Ear2 | — | 0 | 1 | 1 | 2 | |
| Fpr1 | L22181 | 178 | 220 | 235 | 846 | |
| Itgb2 | X14951 | 0 | 2 | 4 | 2 | |
| Lcn2 | W13166 | 916 | 3513 | 3931 | 6036 | |
| Ltf | J03298 | 19 | 162 | 333 | 138 | |
| MBP | W45834 | 5 | 1 | 1 | 2 | |
| Mmp13 | X66473 | 44 | 43 | 72 | 178 | |
| Ngp | L37297 | 2661 | 4782 | 2311 | 6912 | |
| Tertiary granules | ||||||
| Mmp9 | Z27231 | 0 | 1 | 2 | 2 | |
| Granule constituent . | Gene symbol . | Gene accession . | AD value by array . | |||
|---|---|---|---|---|---|---|
| 0 h . | 24 h . | 48 h . | 72 h . | |||
| Azurophil (primary) granules | ||||||
| Man2c1 | AA161860 | 178 | 134 | 99 | 164 | |
| Ctsb | M65270 | 442 | 480 | 595 | 389 | |
| Ctsd | X52886 | 214 | 1087 | 1828 | 2784 | |
| Ctsg | M96801 | 1509 | 405 | 46 | 286 | |
| E12 | U04962 | 658 | 1273 | 843 | 157 | |
| E1a2 | AA689016 | 47 | 159 | 134 | 163 | |
| Gus-s | M63836 | 544 | 226 | 266 | 254 | |
| Lyzs | M21050 | 0 | 1 | 1 | 3 | |
| Mcpt8 | X78545 | 831 | 268 | 66 | 491 | |
| Mpo | X15378 | 3788 | 3009 | 776 | 692 | |
| Prg | X16133 | 2621 | 2653 | 2920 | 9859 | |
| Possible granule proteins | ||||||
| Ctsc | AA144887 | 252 | 194 | 342 | 576 | |
| Ctse | X97399 | 1 | 3 | 4 | 5 | |
| Ctsh | U06119 | 45 | 124 | 195 | 156 | |
| Ctsl | X06086 | 16 | 11 | 31 | 237 | |
| Ctss | AA089333 | 12 | 9 | 88 | 463 | |
| Specific secondary granules | ||||||
| Cpa3 | J05118 | 621 | 270 | 90 | 801 | |
| Cd36l2 | AB008553 | 113 | 93 | 157 | 187 | |
| Cnlp | X94353 | 80 | 479 | 704 | 626 | |
| Cybb | U43384 | 8 | 24 | 91 | 128 | |
| Ear2 | — | 0 | 1 | 1 | 2 | |
| Fpr1 | L22181 | 178 | 220 | 235 | 846 | |
| Itgb2 | X14951 | 0 | 2 | 4 | 2 | |
| Lcn2 | W13166 | 916 | 3513 | 3931 | 6036 | |
| Ltf | J03298 | 19 | 162 | 333 | 138 | |
| MBP | W45834 | 5 | 1 | 1 | 2 | |
| Mmp13 | X66473 | 44 | 43 | 72 | 178 | |
| Ngp | L37297 | 2661 | 4782 | 2311 | 6912 | |
| Tertiary granules | ||||||
| Mmp9 | Z27231 | 0 | 1 | 2 | 2 | |
Shown are the possible granule protein cDNAs represented on the oligionucleotide arrays, sorted by their expression patterns as follows: first by the average difference AD value, then by the granule types, and last by the alphabetical order of gene symbols. Data are presented as described in the legend to Table 3.
AD indicates average difference; gene symbols are expanded in an at the end of this article.
mRNAs for transcription factors
Transcription factor genes, including several identified at the sites of consistent chromosome rearrangements in acute myeloid leukemia, have been implicated in normal myeloid differentiation and in the expression of neutrophil proteins.2,5 27 However comprehensive information concerning the expression of these transcription factors during myeloid development is not readily available. Therefore, we compared gene names and identifiers in our databases to those of the transcription factor database Transfac (http://www.transfac.gbf-braunschweig.de/TRANSFAC) and determined which factors contained in this database were present at detectable levels in MPRO cell mRNA, using Affymetrix software for the criteria for inclusion of mRNAs from approximately 200 murine transcription factors probe sets on the oligonucleotide chip. Of these, 54 were expressed and 13 showed changes of 3-fold or more in chip signal (Table5).
Transcription modulators presented during myeloid differentiation
| Maximal fold change . | Gene symbol . | Gene accession . | AD value by array . | |||
|---|---|---|---|---|---|---|
| 0 h . | 24 h . | 48 h . | 72 h . | |||
| Less than 2-fold | ||||||
| Zfp11-6 | AB020542 | 2630 | 2989 | 2795 | 2515 | |
| Btf3 | W13502 | 3 | 3 | 2 | 1 | |
| Gata2 | AB000096 | 562 | 770 | 472 | 730 | |
| Hmgi | J04179 | 337 | 348 | 177 | 232 | |
| Idb1 | M31885 | 455 | 787 | 721 | 637 | |
| Max | M63903 | 256 | 224 | 312 | 172 | |
| Nfatc2 | AA560093 | 2313 | 3218 | 2396 | 2542 | |
| Pm1 | U33626 | 173 | 281 | 329 | 306 | |
| Rarg | M34476 | 102 | 113 | 114 | 218 | |
| Rela | M61909 | 297 | 260 | 304 | 244 | |
| Sox15 | W53527 | 419 | 461 | 484 | 837 | |
| Ybx1 | M62867 | 643 | 489 | 472 | 496 | |
| Zfp162 | Y12838 | 671 | 734 | 720 | 992 | |
| 2 or more, less than 3 | ||||||
| Cebpd | X61800 | 157 | 262 | 168 | 430 | |
| Idb2 | M69293 | 244 | 210 | 310 | 604 | |
| Jund1 | W29356 | 1274 | 2002 | 1434 | 3085 | |
| Lyl1 | X57687 | 399 | 342 | 347 | 891 | |
| Nfe2 | L09600 | 458 | 743 | 1042 | 505 | |
| Nfkb1 | L28117 | 953 | 2044 | 1876 | 2034 | |
| Pbx1 | AF020196 | 611 | 303 | 345 | 212 | |
| sfpi1 | A34693 | 375 | 784 | 991 | 529 | |
| Tif1b | U67303 | 673 | 659 | 420 | 863 | |
| Trp53 | P10361 | 259 | 149 | 125 | 361 | |
| Usf2 | U12283 | 129 | 185 | 285 | 192 | |
| Ybx3 | L35549 | 96 | 169 | 210 | 119 | |
| Zfp216 | AA510137 | 82 | 151 | 204 | 106 | |
| 3 or more, less than 4 | ||||||
| Irf1 | M21065 | 85 | 207 | 278 | 198 | |
| Klf2 | U25096 | 62 | 86 | 246 | 77 | |
| Myb | M12848 | 892 | 356 | 230 | 435 | |
| Stat3 | AA396029 | 484 | 1057 | 1012 | 290 | |
| Tfdp1 | Q08639 | 307 | 560 | 505 | 1093 | |
| 4 or more, less than 5 | ||||||
| Cebpb | X62600 | 390 | 1248 | 1380 | 1903 | |
| Stra14 | Y07836 | 223 | 383 | 510 | 936 | |
| 5 or more | ||||||
| Cebpa | M62362 | 33 | 212 | 182 | 44 | |
| Grg | X73359 | 99 | 565 | 916 | 1005 | |
| Mad | X83106 | 0 | 111 | 167 | 327 | |
| Myc | L00039 | 314 | 112 | 62 | 173 | |
| Etohi6 | W89667 | 169 | 386 | 313 | 1003 | |
| TBX1 | AA542220 | 0 | 0 | 1 | 2 | |
| Maximal fold change . | Gene symbol . | Gene accession . | AD value by array . | |||
|---|---|---|---|---|---|---|
| 0 h . | 24 h . | 48 h . | 72 h . | |||
| Less than 2-fold | ||||||
| Zfp11-6 | AB020542 | 2630 | 2989 | 2795 | 2515 | |
| Btf3 | W13502 | 3 | 3 | 2 | 1 | |
| Gata2 | AB000096 | 562 | 770 | 472 | 730 | |
| Hmgi | J04179 | 337 | 348 | 177 | 232 | |
| Idb1 | M31885 | 455 | 787 | 721 | 637 | |
| Max | M63903 | 256 | 224 | 312 | 172 | |
| Nfatc2 | AA560093 | 2313 | 3218 | 2396 | 2542 | |
| Pm1 | U33626 | 173 | 281 | 329 | 306 | |
| Rarg | M34476 | 102 | 113 | 114 | 218 | |
| Rela | M61909 | 297 | 260 | 304 | 244 | |
| Sox15 | W53527 | 419 | 461 | 484 | 837 | |
| Ybx1 | M62867 | 643 | 489 | 472 | 496 | |
| Zfp162 | Y12838 | 671 | 734 | 720 | 992 | |
| 2 or more, less than 3 | ||||||
| Cebpd | X61800 | 157 | 262 | 168 | 430 | |
| Idb2 | M69293 | 244 | 210 | 310 | 604 | |
| Jund1 | W29356 | 1274 | 2002 | 1434 | 3085 | |
| Lyl1 | X57687 | 399 | 342 | 347 | 891 | |
| Nfe2 | L09600 | 458 | 743 | 1042 | 505 | |
| Nfkb1 | L28117 | 953 | 2044 | 1876 | 2034 | |
| Pbx1 | AF020196 | 611 | 303 | 345 | 212 | |
| sfpi1 | A34693 | 375 | 784 | 991 | 529 | |
| Tif1b | U67303 | 673 | 659 | 420 | 863 | |
| Trp53 | P10361 | 259 | 149 | 125 | 361 | |
| Usf2 | U12283 | 129 | 185 | 285 | 192 | |
| Ybx3 | L35549 | 96 | 169 | 210 | 119 | |
| Zfp216 | AA510137 | 82 | 151 | 204 | 106 | |
| 3 or more, less than 4 | ||||||
| Irf1 | M21065 | 85 | 207 | 278 | 198 | |
| Klf2 | U25096 | 62 | 86 | 246 | 77 | |
| Myb | M12848 | 892 | 356 | 230 | 435 | |
| Stat3 | AA396029 | 484 | 1057 | 1012 | 290 | |
| Tfdp1 | Q08639 | 307 | 560 | 505 | 1093 | |
| 4 or more, less than 5 | ||||||
| Cebpb | X62600 | 390 | 1248 | 1380 | 1903 | |
| Stra14 | Y07836 | 223 | 383 | 510 | 936 | |
| 5 or more | ||||||
| Cebpa | M62362 | 33 | 212 | 182 | 44 | |
| Grg | X73359 | 99 | 565 | 916 | 1005 | |
| Mad | X83106 | 0 | 111 | 167 | 327 | |
| Myc | L00039 | 314 | 112 | 62 | 173 | |
| Etohi6 | W89667 | 169 | 386 | 313 | 1003 | |
| TBX1 | AA542220 | 0 | 0 | 1 | 2 | |
Shown are the transcription factors identified as present by the oligonucleotide array analysis whose maximal AD between perfect match and mismatch oligonucleotide sets was greater than or equal to 200 U in this study. Data are presented as described in the legend to Table 3.
AD indicates average difference; gene symbols are expanded in an at the end of this article.
The changes in certain transcription factors, such as the moderate down-regulation of myb and myc and the up-regulation of the Max dimerization protein MAD, were consistent with the shift of the cells from a proliferative to a differentiated state.28 Some changes are more difficult to explain, such as the up-regulation of DP1, a partner for E2f factors in the regulation of S-phase genes, and the mild up-regulation of theId genes, commonly associated with an inhibition of differentiation by competition with bHLH transcriptional activators.29
The C/EBP family has been extensively studied with respect to myeloid differentiation.2 30 Absolute levels of the C/EBP α and δ mRNAs were low, probably at the borderline of significance for the oligonucleotide chip assay, whereas the level of C/EBP β appeared higher. In addition, there were discrepancies between the chip estimates and the mRNA levels observed by Northern blotting with specific probes for these genes. In particular, the latter method, more sensitive and specific, showed that C/EBP α began to decline in the most mature cells, whereas C/EBP δ mRNA declined progressively beginning at 24 hours after the onset of differentiation.
C/EBP ε is a more recently cloned C/EBP family member. Previous studies indicated it is expressed in a large array of human leukemia cell lines blocked at various stages of differentiation and that it is up-regulated during granulocytic differentiation.31 A C/EBP ε probe was not included in the oligonucleotide chips, and this mRNA was not detected by DD. Therefore, we examined the C/EBP ε expression patterns by quantitative PCR and Northern blot analysis (Figure 4). C/EBP ε exon 1 was PCR amplified from MPRO RNAs using primers RY48 (AGCCCCCGACACCCTTGATGA) and RY49 (TGGCACACTGCGGGCAGACAG).32 The results showed that C/EBP ε is expressed throughout myeloid differentiation, with expression levels increased moderately in the later stages.
We detected a number of other transcription factors that are broadly expressed or that have been reported in other studies of hematopoiesis (Table 5). Some of the factors that were most strongly induced during differentiation have been studied in other contexts but not previously implicated in hematopoiesis, such as a mammalian homologue to theDrosophila enhancer of split gene, a transcriptional silencer. The mammalian gene is expressed at relatively high levels as measured by the oligonucleotide chip and is a candidate for mediation of the silencing of growth-related genes in the maturing neutrophil. Another candidate transcriptional silencer, Tif1b, may serve as a corepressor for the KRAB domain family of zinc finger transcription factors and also may mediate binding of the heterochromatin protein HP1 to DNA.33
There were 26 transcription factors whose mRNAs showed no significant changes by oligonucleotide chip analysis and were not identified as differentially regulated genes by differential display assays. PU.1, a factor necessary for the production of neutrophils and the expression of several neutrophil genes,34 showed less than a 3-fold increase in mRNA, below the threshold for a significant change. Other candidate hematopoietic transcription factors, such as PEBP1aB2 (AML1), GATA-1, and SP-2, were represented on the oligonucleotide chips, but their mRNA levels were so low that they were reported as absent in this study. The possibility that small changes in the levels or ratios of some transcription factors could produce marked changes in transcription potentially limits the ability of data generated by present methods to explain transcriptional changes during differentiation.
Protein expression patterns of MPRO cells during ATRA induction
We visually compared the 2DE patterns from MPRO cells at the same time points used for mRNA analysis. In most cases the peptides identified for a given protein were derived from regions along the entire length of the protein, indicating the observed products were not the result of proteolytic degradation. These data must be considered with several caveats: membrane and other hydrophobic proteins and very basic proteins are not well displayed by the standard 2DE approach, and proteins present at low levels will be missed.35 In addition, to simplify MS analysis, we used a Coomassie dye stain rather than silver to visualize proteins, and this decreased the sensitivity of detection of minor proteins. The MS method we used was sufficiently sensitive to identify proteins that could barely be visualized by colloidal blue staining. However, a limitation of the method for the mouse is that the current database lacks predicted amino acid sequences for a substantial fraction of murine genes. In addition, very small proteins give only a few peptides, making statistically confident identification difficult.
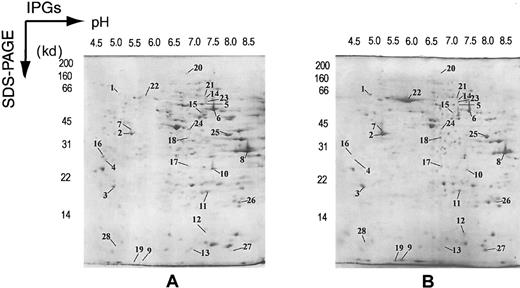
Figure 5 shows the analytical colloidal blue–stained 2DE IPG reference maps of differentiated MPRO cells. Expression patterns of more than 500 protein spots were detected and observed through the entire series of gels. Protein spots could easily be cross-matched to each other, indicating the reproducibility of the method. As marked on the gel pictures (Figure 5), 50 proteins with a wide range of molecular weights (1 to 200 kd), isoelectric points (4 to 9), and abundances were subjected to MS protein identification. The results are presented in Table 6.
2DE electrophoretograms of MPRO cells.
MPRO cell lysate (2.5 × 106 cell/sample) was loaded for 2DE analysis. Gels were stained with brilliant blue G–colloidal dye. (A) 2DE map of uninduced MPRO cell (0 hour). (B) 2DE map of matured MPRO cells (72 hours). Protein spots marked in the maps were considered differentially expressed and were subjected to MS analysis. The resultant protein information is listed in Table 6.
2DE electrophoretograms of MPRO cells.
MPRO cell lysate (2.5 × 106 cell/sample) was loaded for 2DE analysis. Gels were stained with brilliant blue G–colloidal dye. (A) 2DE map of uninduced MPRO cell (0 hour). (B) 2DE map of matured MPRO cells (72 hours). Protein spots marked in the maps were considered differentially expressed and were subjected to MS analysis. The resultant protein information is listed in Table 6.
Correlation of expression patterns between mRNA level and protein level
| Spot . | Protein definition . | Gi number . | Predicted value . | Percentage (%) . | 2DE pattern . | cDNA expression pattern . | Ag . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| kd . | pI . | 0 h . | 72 h . | 0 h . | 72 h . | |||||
| 1 | GRP 78 | 2506545 | 72.4 | 5.1 | 1 | 3 | 1321 | 1043.3 | N | |
| 2 | Actin, gamma, cytoplasmic | 6752954 | 41.77 | 5.3 | 40 | 3 | 6 | 0 | 2 | Y |
| 3 | RHO GDI 2 | 2494703 | 22.83 | 4.9 | 33 | 3 | 3 | 341 | 441.6 | Y |
| 4 | Proliferating cell nuclear antigen | 7242171 | 28.77 | 4.7 | 42 | 1 | 0 | 544 | 430.9 | Y |
| 5 | APS kinase | 4038346 | 69.8 | 7.1 | 24 | 2 | 1 | 43 | 50.7 | N |
| 6 | Pyruvate kinase 3 | 6755074 | 57.9 | 7.2 | 48 | 6 | 4 | 3047 | 5880.3 | N |
| 7 | Melanoma X-actin | 6671509 | 41.72 | 5.3 | 39 | 1 | 3 | 2539 | 341.3 | N |
| 8 | Glyceraldehyde-3-phosphate dehydrogenase | 6679937 | 35.79 | 8.7 | 39 | 8 | 7 | 3073 | 5742.3 | N |
| 9 | Stefin 3 | 461911 | 10.99 | 5.9 | 48 | 0 | 4 | N/A | N/A | — |
| 10 | Guanine nucleotide binding protein, beta-2, related sequence1 | 6680047 | 35.06 | 7.9 | 21 | 4 | 2 | 139 | 303.1 | N |
| 11 | Triosephosphate isomerase | 6678413 | 26.69 | 6.9 | 26 | 3 | 3 | 3312 | 2660.1 | Y |
| 12 | Testis-derived c-abl protein | 1196524 | 17.19 | 7 | 51 | 2 | 3 | 152 | 126.9 | N |
| 13 | RNA binding motif protein 3 | 7949121 | 16.59 | 6.8 | 25 | 1 | 0 | 628 | 812.4 | N |
| 14 | Collapsin response mediator | 6681019 | 62.16 | 6.4 | 36 | 2 | 0 | Absent | Absent | N |
| 15 | Lamin A | 220474 | 47.52 | 6.6 | 35 | 2 | 0 | Absent | Absent | N |
| 16 | 47-kd keratin | 52783 | 35.82 | 4.8 | 29 | 3 | 0 | Absent | Absent | N |
| 17 | sid478p | 5931565 | 31.3 | 6.7 | 30 | 1 | 2 | Absent | Absent | N |
| 18 | MHC class II H2-IA-beta-5 | 3169662 | 28.6 | 7.1 | 39 | 1 | 2 | N/A | N/A | — |
| 19 | Androgen-binding protein: subunit alpha | 739346 | 8.04 | 6.4 | 68 | 0 | 2 | Absent | Absent | N |
| 20 | Neuronal apoptosis inhibitory protein | 5932010 | 158.7 | 6 | 17 | 1 | 0 | N/A | N/A | — |
| 21 | PAD type IV | 6755018 | 74.46 | 7.2 | 21 | 1 | 3 | N/A | N/A | — |
| 22 | Human serum albumin homologoue | 3212625 | 66.45 | 5.7 | 24 | 0 | 6 | N/A | N/A | — |
| 23 | syncrip | 6576815 | 62.53 | 7.2 | 33 | 2 | 1 | N/A | N/A | — |
| 24 | Transamidinase | 1730203 | 48.22 | 7.2 | 31 | 3 | 1 | N/A | N/A | — |
| 25 | PGK crigr phosphoglycerate | 1730519 | 44.54 | 8.3 | 47 | 5 | 4 | 1088 | 1402.3 | N |
| 26 | Proliferation-associated gene A | 6754976 | 22.16 | 8.6 | 53 | 3 | 1 | N/A | N/A | — |
| 27 | Putatuve peroxisomal antioxidant enzyme | 3913065 | 17 | 7.8 | 55 | 0 | 3 | N/A | N/A | — |
| 28 | IgE chain C2 region | 2137430 | 12.1 | 5.2 | 38 | 0 | 1 | N/A | N/A | — |
| Spot . | Protein definition . | Gi number . | Predicted value . | Percentage (%) . | 2DE pattern . | cDNA expression pattern . | Ag . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| kd . | pI . | 0 h . | 72 h . | 0 h . | 72 h . | |||||
| 1 | GRP 78 | 2506545 | 72.4 | 5.1 | 1 | 3 | 1321 | 1043.3 | N | |
| 2 | Actin, gamma, cytoplasmic | 6752954 | 41.77 | 5.3 | 40 | 3 | 6 | 0 | 2 | Y |
| 3 | RHO GDI 2 | 2494703 | 22.83 | 4.9 | 33 | 3 | 3 | 341 | 441.6 | Y |
| 4 | Proliferating cell nuclear antigen | 7242171 | 28.77 | 4.7 | 42 | 1 | 0 | 544 | 430.9 | Y |
| 5 | APS kinase | 4038346 | 69.8 | 7.1 | 24 | 2 | 1 | 43 | 50.7 | N |
| 6 | Pyruvate kinase 3 | 6755074 | 57.9 | 7.2 | 48 | 6 | 4 | 3047 | 5880.3 | N |
| 7 | Melanoma X-actin | 6671509 | 41.72 | 5.3 | 39 | 1 | 3 | 2539 | 341.3 | N |
| 8 | Glyceraldehyde-3-phosphate dehydrogenase | 6679937 | 35.79 | 8.7 | 39 | 8 | 7 | 3073 | 5742.3 | N |
| 9 | Stefin 3 | 461911 | 10.99 | 5.9 | 48 | 0 | 4 | N/A | N/A | — |
| 10 | Guanine nucleotide binding protein, beta-2, related sequence1 | 6680047 | 35.06 | 7.9 | 21 | 4 | 2 | 139 | 303.1 | N |
| 11 | Triosephosphate isomerase | 6678413 | 26.69 | 6.9 | 26 | 3 | 3 | 3312 | 2660.1 | Y |
| 12 | Testis-derived c-abl protein | 1196524 | 17.19 | 7 | 51 | 2 | 3 | 152 | 126.9 | N |
| 13 | RNA binding motif protein 3 | 7949121 | 16.59 | 6.8 | 25 | 1 | 0 | 628 | 812.4 | N |
| 14 | Collapsin response mediator | 6681019 | 62.16 | 6.4 | 36 | 2 | 0 | Absent | Absent | N |
| 15 | Lamin A | 220474 | 47.52 | 6.6 | 35 | 2 | 0 | Absent | Absent | N |
| 16 | 47-kd keratin | 52783 | 35.82 | 4.8 | 29 | 3 | 0 | Absent | Absent | N |
| 17 | sid478p | 5931565 | 31.3 | 6.7 | 30 | 1 | 2 | Absent | Absent | N |
| 18 | MHC class II H2-IA-beta-5 | 3169662 | 28.6 | 7.1 | 39 | 1 | 2 | N/A | N/A | — |
| 19 | Androgen-binding protein: subunit alpha | 739346 | 8.04 | 6.4 | 68 | 0 | 2 | Absent | Absent | N |
| 20 | Neuronal apoptosis inhibitory protein | 5932010 | 158.7 | 6 | 17 | 1 | 0 | N/A | N/A | — |
| 21 | PAD type IV | 6755018 | 74.46 | 7.2 | 21 | 1 | 3 | N/A | N/A | — |
| 22 | Human serum albumin homologoue | 3212625 | 66.45 | 5.7 | 24 | 0 | 6 | N/A | N/A | — |
| 23 | syncrip | 6576815 | 62.53 | 7.2 | 33 | 2 | 1 | N/A | N/A | — |
| 24 | Transamidinase | 1730203 | 48.22 | 7.2 | 31 | 3 | 1 | N/A | N/A | — |
| 25 | PGK crigr phosphoglycerate | 1730519 | 44.54 | 8.3 | 47 | 5 | 4 | 1088 | 1402.3 | N |
| 26 | Proliferation-associated gene A | 6754976 | 22.16 | 8.6 | 53 | 3 | 1 | N/A | N/A | — |
| 27 | Putatuve peroxisomal antioxidant enzyme | 3913065 | 17 | 7.8 | 55 | 0 | 3 | N/A | N/A | — |
| 28 | IgE chain C2 region | 2137430 | 12.1 | 5.2 | 38 | 0 | 1 | N/A | N/A | — |
The proteins listed here are represented by the spots marked in the electrophoretograms shown in Figure 5.
Protein definition, Gi number, and predicted value refer to the protein name, accession number, and properties derived from the National Center for Biotechnology Information protein database. The column labeled % shows the percentage of peptides predicted from the protein sequence that were detected by mass spectroscopy. The expression level of protein spots expressed in mouse promyelocytic cell line cell induced by all-trans retinoic acid for 0 hours and 72 hours (Figure5) were scored on a scale of 1 (+) to 8 (++++++++) in the 2DE pattern column. The cDNA expression patterns of the cognate mRNAs are listed in the cDNA expression pattern column abstracted from the dbMC database. The genes not represented on the oligonucleotide arrays were marked as N/A. Ag showed the correlation of gene patterns at mRNA level or protein level.
Y indicates agreement and N discrepancy between changes in cDNA and protein spot intensity. The numbers in bold were obtained with DD. 2DE indicates 2-dimensional gel electrophoresis; IgE, immunoglobulin E; DD, differential display.
Comparing the theoretical value of the molecular weight andpI of each protein to that of the observed value, we confidently identified 28 proteins in the expected position on the gels (spots 1 to 28). Some of the other proteins with strong matches to the murine databases migrated to a somewhat unexpected pIposition. Nine spots gave clear peptide peaks on mass spectroscopy but did not match any known gene. Their identification will require amino acid sequence analysis or availability of more extensive murine databases. We searched for the expression patterns of the genes cognate to the expressed proteins in dbMC (Table 6). Nineteen genes were found in dbMC, the mRNA for 5 genes was reported as absent, and 13 genes were present during MPRO differentiation. Comparison of the expression patterns showed only 4 genes of 18 present on the oligonucleotide chips whose expression was consistent at the RNA level and protein level. None of these was on the list of the genes that were differentially expressed significantly (5-fold or greater change by array or 2-fold or greater change by DD).
Discussion
We explored the temporal patterns of gene expression during myeloid development. A database has been developed to provide a reference for later research on the molecular mechanisms underlying normal myeloid development.
The MPRO cell system morphologically mimics normal myeloid differentiation and biochemically proceeds further toward mature neutrophils than most other in vitro systems. Because the arrest in differentiation of MPRO cells growing in the absence of ATRA is not physiologic, there is a theoretical risk that gene expression in these cells is not coordinated in the way that it is in normal differentiation. It is encouraging that, for the most part, the timing of expression of genes for proteins of the various neutrophil granules is consistent with the timing of the morphologic and biochemical appearance of these granule components during normal myeloid differentiation.
The DD technique provides certain advantages for detecting and comparing mRNA levels in different samples. First, the method is, in principle, similar to competitive RT-PCR, and, with the use of stringent PCR conditions, is expected to be about as reliable. Second, display patterns are reproducible. Third, the method detects the levels not only of RNAs already represented in the database but also of unknown RNA species that may represent “new” genes. Fourth, closely related genes can be distinguished regardless of cross-hybridization, provided there are some single nucleotide differences in the 3′ end sequence. Limitations associated with this technique are that numerous gels are necessary to get complete information and that comparison of the levels of different mRNAs is only approximate because of the differential amplification of bands of different size or sequence.
Oligonucleotide chip analysis is a fast and effective means of accessing mRNA expression patterns.20 Cluster analysis of groups of samples by this approach is effective. However, the present results indicate that alternative methods of verification are desirable before the data on an unexpected change in a particular gene are definitively accepted.
To obtain the broadest range of information from the myeloid differentiation process, both differential display and oligonucleotide chip techniques were applied in the current study. As a result, 65.3% of the observed changes in mRNA levels came from the differential display method and 41.5% came from oligonucleotide chip assays.
Our data showed in general that changes in expression pattern by the 2 methods agreed qualitatively but that there was some quantitative variation. Our results indicate that DD may be a more accurate way to detect changes in levels of gene expression than the oligonucleotide chip assay. However, improvements in the types of oligonucleotides used in arrays may close this gap in the future.
The mRNAs for a limited number of transcription factors vary in a pattern correlating with that of the mRNAs for primary or secondary granule proteins. However, more detailed information is needed, and the underlying mechanisms of granule gene regulation remain unclear. The number of potential positive and negative regulatory factors found here is sufficiently small as to make it feasible to perform in vivo studies, such as chromatin immunoprecipitation.
The oligonucleotide chip used in this study focused on known genes, whereas the DD method samples all polyadenylated transcripts. The latter method generated a large number of products not associated with known genes, in part because the mouse genome is not as well represented in the database as the human genome. However, our experience with DD and human mRNAs indicates that substantial fractions of the products represented as ESTs or not represented at all in the public databases are cDNA copies from introns, hnRNA, or other RNA with internal A runs.
Approximately 59 sequences obtained from gel-display bands had significant changes in the level of expression and a sequence that did not match that for any named gene in the public databases. Of these, 38 had plausible or excellent polyA signals. This is only an approximate estimate of the number of new genes found36 because a fraction of the mRNAs for known genes still had poor polyA signals. In addition, the full 3′ untranslated region is often not known for characterized genes, and in some cases these new genes may prove to be identical to products identified by the oligonucleotide chips when more complete sequences are obtained. At the least, their presence indicates that a substantial fraction of the regulatory or functional circuitry of maturing myeloid cells remains unexplored and that valuable tools for their investigation will emerge from a combination of RNA expression studies and analysis of emerging genomic sequences.
The desired end point for the description of gene expression in a biologic system is not only the analysis of mRNA transcript levels but also the accurate measurement of protein abundance. The developments in 2DE and new MS instrumentation make it possible to accomplish this work rapidly and efficiently. In this study, we attempted to identify a number of the proteins differentially expressed between uninduced and ATRA-differentiated MPRO cells and to examine the relation between mRNA and protein expression levels for these genes representing the same state.
For protein levels based on estimated intensity of Coomassie dye staining in 2DE, there was poor correlation between changes in mRNA levels and estimated protein levels. Other groups have studied the correlation between mRNA and protein levels in yeast and liver cells.11,12,14 In the liver cell experiments,11,12 correlation coefficients of 0.4 to less than 0.5 were observed. In an extensive study in yeast,11,12 the correlation coefficient was high if the most abundant mRNAs and proteins were considered. If a handful of these products was omitted, the remaining correlation coefficient was 0.4 or less. However, one could restore some of the correlation by averaging individual data points into broad proteomic categories.37
The discrepancies between mRNA and protein levels in MPRO cells appear to be substantially larger than those observed for yeast. Possible causes for the discrepancies include translational regulation, differential expression of certain mRNAs at various stages of cell growth in vitro, post-translational protein modification that varies with the stage of maturation of the cells, and selective degradation or excretion of proteins in vivo. Furthermore, here we are focusing on a developmental time-course, whereas the yeast study concentrated on the organism in vegetative growth. New techniques, equipment, and bioinformatic analysis tools must be developed to make such systematic, global, and quantitative analyses feasible.
The initial studies of protein expression presented here provide a cautionary note for efforts to interpret cell composition and function in relation to mRNA levels. Discrepancies we observed between gene expression and protein abundance suggest that selective post-transcriptional controls may be at least as important as changes in mRNA levels in determining the protein composition of neutrophils and that they are phenomena less well explored than transcriptional control. Analysis of mRNA expression patterns is itself only a small beginning toward a genome-wide description of cellular components.
We thank Dr S. Tsai (Fred Hutchinson Cancer Research Center) for his kind gift of the MPRO cell line, Dr Fuki M. Hisama (Yale University School of Medicine) for helpful advice, and the staff at Gene Logic Inc for data and support.
Gene symbols used in tables: Actb: actin, beta, cytoplasmic; Actg: actin, gamma, cytoplasmic; Actx: melanoma X-actin; Aldo1: aldolase 1, A isoform; Arf5: ADP-ribosylation factor 5; Atf1: activating transcription factor 1; Atf2: activating transcription factor 2; Btf3: basic transcription factor 3a; Bzrp: peripheral-type benzodiazepine receptor; C5r1: complement component 5, receptor 1/G protein-coupled receptor (C5a); Ccnb2: cyclin B2; Cd36l2: CD36 antigen (collagen type I receptor, thrombospondin receptor)-like 2; Cd53: CD53 antigen; Cebpa: CCAAT/enhancer binding protein C/EBP, alpha; Cebpb: CCAAT/enhancer binding protein (C/EBP), beta; Cebpd: CCAAT/enhancer binding protein (C/EBP), delta; Cebpe: CCAAT/enhancer binding protein (C/EBP), epsilon; Cfl1: cofilin 1, nonmuscle; Cmkar4: chemokine (C-X-C) receptor 4; Cmkbr1: chemokine (C-C) receptor 1/Mip1a receptor; Cnlp: cathelin-like protein; Cntf: ciliary neurotropic factor/zinc finger protein PZF; Copa: coatomer protein complex subunit alpha; Cpa3: carboxypeptidase A3, mast cell; Cr2: complement receptor 2; Crhr: corticotropin releasing hormone receptor; Crry: complement receptor–related protein; Csf1r: CSF 1 (M-CSF) receptor/c-fms/CD115; Csf2ra: CSF 2 (GM-CSF) receptor, alpha, low-affinity/CD116; Csf2rb1: CSF 2 (GM-CSF) receptor, beta 2, low-affinity/IL 3 receptor-like protein (AIC2B)/CDw131; Csf2rb2: CSF 2 (GM-CSF) receptor, beta 2, low-affinity/IL-3 receptor (AIC2A); Ctsb: cathepsin B; Ctsc: cathepsin C; Ctsd: cathepsin D; Ctse: cathepsin E; Ctsg: cathepsin G; Ctsh: cathepsin H; Ctsl: cathepsin L; Ctss: cathepsin S; Cybb: cytochrome b-245, beta; Drd2: dopamine receptor 2; E2f1: E2F transcription factor 1; Ear2: eosinophil-associated ribonuclease 2; Ebi3: Epstein-Barr virus–induced gene 3/cytokine receptor–like molecule (EBI3); El2: Balb/c neutrophil elastase; Ela2: elastase 2; Erh: enhancer of rudimentary homolog (Drosophila); Etohi6: ethanol induced 6/sterol regulatory element binding transcription factor 1 (SREBF1) homolog; F2rl2: coagulation factor II (thrombin) receptor–like 2; Fcer1g: Fc receptor, IgE, high affinity I, gamma polypeptide; Fcgr2b: Fc receptor, IgG, low affinity IIb; Fcgr3: Fc receptor, IgG, low affinity III; Fpr1: formyl peptide receptor 1/fMLP receptor; Gabpb1: GA repeat binding protein (GABP-beta1 subunit); Gata2: GATA-binding protein 2; Gnas: guanine nucleotide binding protein, alpha stimulating; Gnb2-rs1: guanine nucleotide binding protein, beta-2, related sequence 1; Gpx3: glutathione peroxidase 3; Grg: related to Drosophila groucho gene; Grid1: glutamate receptor channel subunit delta 1; Grn: granulin; Gstm1: glutathione-S-transferase, mu 1; Gus-s: beta-glucuronidase structural; Gys3: glycogen synthase 3, brain; H2-D: histocompatibility 2, D region locus 1; Hist2: histone gene complex 2; Hist5–2ax: H2A histone family, member X; Hmgi: high mobility group protein I; Hsp60: heat shock protein, 60 kDa; Htr5a: 5-hydroxytryptamine (serotonin) receptor 5A; Idb1: inhibitor of DNA binding 1/helix-loop-helix DNA binding protein regulator (Id); Idb2: inhibitor of DNA binding 2; Ifngr: interferon gamma receptor; Ifngr2: interferon gamma receptor 2; Ii: Ia-associated invariant chain; Il1a: IL1 alpha; Il1r2: IL1 receptor, type II; Il2rg: IL2 receptor, gamma chain; Il4ra: IL4 receptor, alpha; Il10rb: IL10 receptor, beta; Il17r: IL17 receptor; Irf1: interferon regulatory factor 1; Irf2: interferon regulatory factor-2; Itgb2: integrin beta 2 (Cd18); Itpr5: inositol 1,4,5-trisphosphate receptor (type 2); Jund1: Jun proto-oncogene–related gene d1/transcription factor JUN-D; Klf2: Kruppel-like factor LKLF; L-CCR: lipopolysaccharide inducible C-C chemokine receptor–related; Lcn2: lipocalin 2; Ldlr: low density lipoprotein receptor; Lsp1: Lymphocyte–specific 1/S37/pp52; Lst1: leucocyte–specific transcript 1; Ltb4r: leukotriene B4 receptor; Ltbr: lymphotoxin-beta receptor; Ltf: lactotransferrin; Ly64: lymphocyte antigen 64; Ly6e: lymphocyte antigen 6 complex, locus E; Lyl1: lymphoblastomic leukemia/bHLH factor; Lyzs: lysozyme; M6pr: mannose-6-phosphate receptor, cation dependent; Mad: Max dimerization protein; Man2c1: mannosidase, alpha, class 2C, member 1; Max: Max protein; Maz: MYC-associated zinc finger protein (purine-binding transcription factor); MBP: eosinophil granule major basic protein precursor; Mcpt8: mast cell protease 8; Mll: myeloid/lymphoid or mixed-lineage leukemia; Mmp13: matrix metalloproteinase 13/collagenase; Mmp9: matrix metalloproteinase 9/gelatinase B; Mpo: myeloperoxidase; Myb: myeloblastosis oncogene; Mybl2: myeloblastosis oncogene-like 2; Myc: myelocytomatosis oncogene; Myln: myosin light chain, alkali, nonmuscle; Nfatc2: nuclear factor of activated T cells, cytoplasmic 2; Nfe2: nuclear factor, erythroid-derived 2, 45 kDa; Nfkb1: NF-kappa-B (p105); Ngp: neutrophilic granule protein; NMDRGB: N-methyl-D-aspartate receptor glutamate-binding chain homolog; Npm1: nucleophosmin 1; Nr4a1: nuclear receptor subfamily 4, group A, member 1; Osi: oxidative stress induced; P2rx1: purinergic receptor P2X, ligand-gated ion channel, 1; P2ry2: purinergic receptor P2Y, G-protein–coupled 2; P40–8: P40–8, functional/laminin receptor; Pbx1: pre B-cell leukemia transcription factor 1; Pfc: properdin factor, complement; Pira1: paired-Ig–like receptor A1; Pira5: paired-Ig–like receptor A5; Pira6: paired-Ig–like receptor A6; Pirb: paired-Ig–like receptor B; Plaur: urokinase plasminogen activator receptor; PMI: putative receptor protein (SP:P17152 ); Pml: promyelocytic leukemia; Prg: proteoglycan, secretory granule; Prg3: proteoglycan 3/eosinophil major basic protein 2; Prtn3: proteinase 3; Psma2: proteasome (prosome, macropain) subunit, alpha type 2; Ptmb4: prothymosin beta 4; Ptprc: protein tyrosine phosphatase, receptor type, C; Rac2: RAS-related C3 botulinum substrate 2; Rarg: retinoic acid receptor, gamma; Rela: avian reticuloendotheliosis viral (v-rel) oncogene homolog A/NF-kappa-B p65; Rpl19: ribosomal protein L19; RPL8: ribosomal protein L8; Rps6ka1: ribosomal protein S6 kinase polypeptide 1; Rps8: ribosomal protein S8; Rtn3: reticulon 3; S100a8: S100 calcium binding protein A8 (calgranulin A); S100a9: S100 calcium-binding protein A9 (calgranulin B); Sdfr2: stromal cell–derived factor receptor 2; Sell: selectin L (lymphocyte adhesion molecule 1); Sema4d: semaphorin 4D; Sepp1: selenoprotein P, plasma, 1; Sfpi1: SFFV proviral integration 1; Shfdg1: split hand/foot deleted gene 1; Slc10a1: solute carrier family 10 (sodium/bile acid cotransporter family), member 1; Slpi: secretory leukocyte protease inhibitor; Sox15: SRY-box containing gene 15; Spi2–1: serine protease inhibitor 2-1; Srb1: scavenger receptor class B1; Stat3: signal transducer and activator of transcription 3; Stat5a: signal transducer and activator of transcription 5A; Stat6: signal transducer and activator of transcription 6; Stra14: basic-helix-loop-helix protein-retinoic acid induced; Tbx1: TBX1 protein/LPS-induced TNF-alpha factor homolog; Tcrgb: T-cell–receptor germline beta-chain gene constant region; Tcrg-V4: T-cell–receptor gamma, variable 4; Tctex1: t-complex testis expressed 1; Tfdp1: transcription factor Dp 1; Tif1b: transcriptional intermediary factor 1, beta; Tlr4: toll-like receptor 4; Tnfrsf1a: TNF receptor superfamily, member 1a; Tnfrsf1b: TNF superfamily, member 1b; Tomm70a: translocase of outer mitochondrial membrane 70 (yeast) homolog A; Tpi: triosephosphate isomerase; Trp53: transformation-related protein 53; Ubb: ubiquitin B; Usf2: upstream transcription factor 2; Ybx1: Y box transcription factor; Ybx3: Y box binding protein; Zfp11–6: zinc finger protein s11–6; Zfp18: zinc finger protein 18 homolog; Zfp36: zinc finger protein 36; Zfp162: zinc finger protein 162; Zfp216: zinc finger protein 216; Zfpm1: zinc finger protein, multitype 1; Znfn1a1: zinc finger protein, subfamily 1A, 1 (Ikaros); Zyx: zyxin.
Supported by grants from the National Institutes of Health (NIH) (CA42556) and Gene Logic (A143558, DK54369, and HL63357). Z.L. is supported by NIH grant HL 63357. P.E.N. is supported by NIH grant DK 54369, grants from the Arthritis Foundation and the Charles H. Hood Foundation, and the Pierce Family Cancer Research Fund. M.G. is supported by the Keck Foundation and by NIH grant GM54160-04.
L.W. and S.Y. contributed equally to this research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Sherman M. Weissman, Department of Genetics, Boyer Center for Molecular Medicine, Yale University School of Medicine, Rm 336, 295 Congress Ave, New Haven, CT 06536-0812; e-mail: sherman.weissman@yale.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal