Abstract
Cytomegalovirus (CMV) reactivation in immunocompromised recipients of allogeneic stem cell transplantation is a cause of morbidity and mortality from viral pneumonitis. Antiviral drugs given to reactivating patients have reduced the mortality from CMV but have toxic side effects and do not always prevent late CMV disease. Cellular immunotherapy to prevent CMV disease is less toxic and could provide prolonged protection. However, a practical approach to generating sufficient quantities of CMV-specific cytotoxic T cells (CTLs) is required. This study describes a system for generating sufficient CMV-specific CTLs for adoptive immunotherapy of HLA-A*0201 bone marrow transplant recipients from 200 mL donor blood. Donor monocytes are used to generate dendritic cells (DCs) in medium with autologous plasma, interleukin 4, granulocyte-macrophage colony-stimulating factor, and CD40 ligand. The DCs are pulsed with the immunodominant HLA-A*0201–restricted CMV peptide pp65495-503, and incubated with donor T cells. These cultures are restimulated twice with peptide-pulsed lymphoblastoid cell lines (LCLs) or CD40-ligated B cells and purified with phycoerythrin (PE)–labeled pp65495-503/HLA-A*0201 tetramers by flow sorting, or with anti-PE paramagnetic beads. The pure tetramer-positive population is then rapidly expanded to obtain sufficient cells for clinical immunotherapy. The expanded CTLs are more than 80% pure, of memory phenotype, with a Tc1 cytokine profile. They efficiently kill CMV-infected fibroblasts and express the integrin VLA-4, suggesting that the CTLs could cross endothelial barriers. This technique is reproducible and could be used for generating CMV-specific CTLs to prevent CMV disease after allogeneic blood and marrow transplantation.
Introduction
Cytomegalovirus (CMV) is a ubiquitous human herpes virus present in peripheral myeloid cells of 50% to 90% of normal individuals.1 Primary infection in an immunocompetent host is usually asymptomatic, but the virus has lifelong latency. CMV reactivation after allogeneic bone marrow stem cell transplantation, when the host is immunocompromised, can cause interstitial pneumonitis in approximately one third of infected patients; it has a mortality rate of 30% to 40%, despite treatment with ganciclovir and CMV-specific immunoglobulins.2-4Prophylaxis with acyclovir or preemptive treatment with ganciclovir can be only partially effective, although the majority of patients undergo prophylaxis or are treated effectively with these agents.1,5-7 Myelosuppression from ganciclovir may lead to graft failure.1,8,9 Continued treatment with antiviral drugs after bone marrow transplantation may induce drug resistance and delay endogenous reconstitution of CMV-specific immunity in patients, resulting in late CMV disease.8 10-14 These complications are especially encountered after transplantation from partially mismatched related donors and other mismatched transplants that require T-cell depletion.
Because of the shortcomings of current treatments, there is growing interest in the adoptive transfer of CMV-specific cytotoxic T lymphocytes (CTLs) from the donor to the recipient after transplantation to restore CMV-specific cellular immunity. Proof of principle has been demonstrated by Walter and coworkers who showed that patients could be protected from CMV reactivation by CMV-specific T-cell clones expanded in culture from donor cells.15However, large-scale T-cell cloning using CMV-infected fibroblasts as stimulator cells is difficult to establish as a clinical routine. There is therefore a growing interest in developing widely applicable techniques using T cells induced by synthetic CMV peptides to prevent CMV infection after transplantation. In this study we describe a practical approach to generate CMV-specific T cells on a clinical scale. As a source of antigen we used the immunodominant HLA-A*0201–restricted CMV pp65 matrix protein peptide, pp65495-503. Pp65 is recognized by more than 70% of CMV-specific CTLs,12,20-24 and the HLA-A*0201 phenotype occurs at high frequency in all ethnic groups.15-19Because pp65 is processed and presented before endogenous viral replication, pp65-specific CTLs may preempt CMV spread.12As an alternative strategy to cloning by limiting dilution, we generated peptide-specific CTL from bulk cultures stimulated by peptide-pulsed dendritic cells (DCs) and selected them using HLA-A*0201/pp65 tetramers prior to nonspecific expansion to generate large numbers of CMV-specific CTLs for adoptive immunotherapy.
Patients, materials, and methods
Patients and samples
This study was approved by the South Carolina Cancer Center Institutional Review Board, and samples were obtained after informed consent. Peripheral blood (200 mL) was collected in acid-citrate-dextrose anticoagulant from normal, healthy volunteers or bone marrow donors. CMV status was determined by serologic techniques. HLA-A*0201 molecular types of samples and the fibroblast cell line MRC5 were confirmed by sequencing exons 2 and 3 of the HLA-A locus using an Applied Biosystems 377 DNA sequencer.25 Peripheral blood mononuclear cells (PBMNCs) were isolated by density centrifugation over Ficoll-Hypaque (Amersham Pharmacia-Biotech, Uppsala, Sweden). Autologous plasma (AP) was collected and heat inactivated by incubation at 56°C for 30 minutes, followed by centrifugation at 3300 rpm for 10 minutes to prevent coagulation.
Generation of donor-derived Epstein-Barr virus–transformed B-cell lines
The B95-8 EBV-producing cell line (a gift from Dr Cliona Rooney, Texas Children's Hospital, Houston, TX) was used for the culture of EBV. Supernatant from this cell line was harvested and filtered through a 0.45-μm filter and stored at −80°C. PBMNCs (2 × 106) were infected with 2 mL viral supernatant for 2 hours at 37°C. The PBMNCs were cultured in RPMI complete plus 10% fetal bovine serum (FBS) (Hyclone, Logan, Utah) and cyclosporin A (CsA) (Sandoz Pharmaceuticals, Cincinnati, OH) 1 μg/mL in a 24-well plate. The LCLs were fed weekly with half fresh media and CsA 1 μg/mL. The LCLs were expanded to 25-cm2 and 75-cm2 flasks over a period of 4 to 5 weeks.
Preparation of CD40-ligated B lymphocytes
The B lymphocytes were isolated from PBMNCs by positive selection using CD19+ paramagnetic beads (Miltenyi, Auburn, CA). B lymphocytes (2 × 106) were cocultured with a feeder layer of 0.5 × 106 NIH3T3 cells transfected with human CD40 ligand in 4 mL Iscoves modified Dulbecco medium (IMDM) supplemented with 10% AB serum, 2 ng/mL interleukin (IL)-4, 1 μg/mL CsA, and 2.5 μg/mL insulin. The NIH3T3-CD40L feeder layer (a gift from Dr Gordon Freeman at the Dana Farber Cancer Institute, Boston, MA) was prepared in 45% Dulbecco modified Eagle medium (DMEM), 45% F-12, and 10% FBS, irradiated to 7500 cGy, plated, and allowed to adhere 24 hours before addition of B cells. The B cells were harvested and plated over fresh NIH3T3-CD40L layers on days 3, 7, and 10, and expanded as necessary thereafter for 14 days. In some experiments soluble CD40 ligand (Immunex, Seattle, WA) was used instead of NIH3T3-CD40L cells. The CD40-ligated B cells were subsequently harvested and cryopreserved for use as stimulators. Up-regulation of HLA class I and II, costimulatory, and adhesion molecule expression following CD40 ligation was verified by flow cytometry (data not shown). Further, CD40-ligated B cells were demonstrated to be potent stimulators of immune responses compared to DCs and nonligated B lymphocytes in allogeneic mixed lymphocytic reactions (MLRs) with limiting numbers of stimulators (data not shown).
Infection of MRC5 fibroblasts with AD169 CMV
The CMV viral supernatant was prepared by infecting a monolayer of MRC5 cells for 2 hours with the CMV strain AD169. The MRC5 cells were subsequently cultured in DMEM with 2% FBS. Supernatant was harvested at day 7 to 9 when a clear cytopathic effect was present. The supernatant was cleared by centrifugation and stored at −80°C prior to use.
Culture of DCs
Donor-derived CD14+ cells were positively selected with CD14+ paramagnetic beads (Miltenyi). This method yielded 12 × 106 CD14+ cells for every 100 × 106 peripheral blood leukocytes (PBLs) with a mean purity of 75%. CD14+ cells were cultured in RPMI with 10% FBS or 10% AP, and cytokines IL-4 (1000 IU/mL) (R&D Systems, Minneapolis, MN) and granulocyte-macrophage colony-stimulating factor (GM-CSF, 100 ng/mL, R&D Systems) for 7 days. DCs were cultured for 2 additional days in culture media with 500 ng/mL CD40 ligand (Immunex) to induce maturation.
Mixed lymphocyte reactions
Responder T cells were negatively selected from PBMNCs using immunomagnetic beads (Dynal, Oslo, Norway) coated with CD14, CD19, CD33, and CD56 antibodies (Immunotech, Marseilles, France). In all instances there were less than 5% contaminating cells in the resulting T cells. Decreasing numbers of irradiated, third-party PBMNCs were incubated with 105 T cells in sextuplets in 96-well round-bottom plates for 5 days prior to the addition of 0.5 μCi3H-thymidine. T-cell proliferation was assessed by3H- thymidine after 16 hours.
Measurement of endocytic capacity
Fifty microliters of 1 × 105 DCs in PBS and 5% AB serum were added to 6 tubes containing either 25 μL fluorescein isothiocyanate (FITC)-dextran (Sigma, St Louis, MO), 25 μL lucifer yellow (Sigma), or 25 μL PBS. Each tube was prepared in duplicate and incubated for 2 hours, one set at 37°C and the other set on ice. DCs were washed and kept on ice prior to analysis by flow cytometry.
T2 peptide-binding assay
The T2 cells were incubated at 3 × 106/mL in acid-stripping buffer (0.131 M citric acid, 0.066 M Na2HPO4, pH 3.3) for 45 seconds. The T2 cells were washed twice with 50 mL RPMI supplemented with 1% HEPES and then resuspended at 3 × 106/mL. Then, the T2 cells (100 μL) were incubated with 3 μg/mL β2-microglobulin and 100 μg/mL peptide for 3.5 hours in a 20°C water bath. After incubation, 10 μL HLA-A2 antibody (One Lambda, Canoga Park, CA) and 7-AAD (Pharmingen, San Diego, CA) was added and the T2 cells were stained for HLA-A2 expression for 30 minutes at 4°C. The T2 cells were washed and fixed prior to flow cytometry.
Induction of CMV-specific CTLs
Mature AP-DCs (2 × 105) were pulsed with 100 μg/mL pp65495-503 and 3 μg/mL β2-microglobulin for 4 hours at room temperature (Figure1). DCs were irradiated to 2500 cGy prior to adding autologous 2 × 106 CD8+ purified cells (prepared by negative selection) or 2 × 106 PBMNCs depleted of CD14+ cells. Cultures were fed every 3 days with half fresh media (RPMI and 10% AP with IL-2 10 IU/mL) and expanded as necessary. Cultures were restimulated on days 7 and 14 with autologous irradiated peptide-pulsed CD40-ligated B cells or LCLs at a ratio of 1 stimulator to 4 CTLs. To some cultures IL-7 10 ng/mL was added at the onset of the culture, and on a weekly basis thereafter.
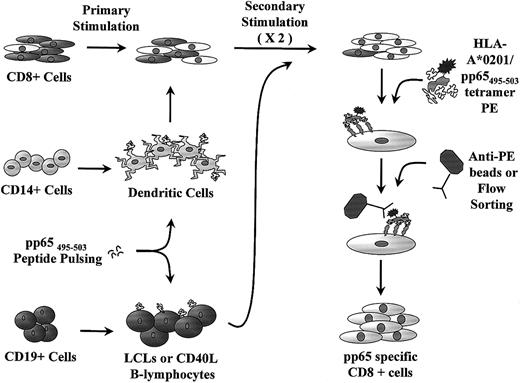
Schematic representation of generation of pp65495-503-specific CTL lines.
Mature DCs are cultured from CD14+ monocytes and used for primary stimulation of CD8+ cells. Peptide-pulsed LCLs or CD40-ligated B cells are used for restimulation. The CTL line is further purified with anti-PE–coated paramagnetic beads or flow sorting prior to rapid expansion.
Schematic representation of generation of pp65495-503-specific CTL lines.
Mature DCs are cultured from CD14+ monocytes and used for primary stimulation of CD8+ cells. Peptide-pulsed LCLs or CD40-ligated B cells are used for restimulation. The CTL line is further purified with anti-PE–coated paramagnetic beads or flow sorting prior to rapid expansion.
Cytotoxicity assays
Cytotoxicity was tested in triplicate in a 4-hour51CrO4 release assay, at E/T ratios shown, against autologous or recipient phytohemagglutinin-induced blasts (PHA-blasts) pulsed with pp65495-503 or a HLA-A*0201–binding control peptide, the natural killer (NK)–sensitive cell line K562 and HLA-A*0201–positive MRC5 fibroblasts. MRC5 fibroblasts were infected overnight with 2 mL CMV supernatant in the presence of 100 μCi 51Cr/106 target cells. Peptide-pulsed cells were pulsed with 100μg/mL peptide in the presence of 3 μg/mL β2-microglobulin for 4 hours, prior to labeling for 1 hour with 51Cr. Nonpeptide-pulsed targets were directly incubated with 51Cr. Specific lysis was calculated as follows: % lysis = [(maximum release − experimental release) / (maximum release − spontaneous release)] × 100.
Intracellular cytokine staining of CTLs
Th/Th2 (CD4+ helper cells) and Tc1/Tc2 (CD8+ cytotoxic cells) profiling was performed using the cytofix/cytoperm kit for intracellular staining (Pharmingen). The CTLs were incubated with monensin, phorbol 12-myristate 13-acetate (25 ng/mL, Sigma), and ionomycin (1 μg/mL, Sigma) for 4 hours at 37°C to stimulate cytokine build-up. Unstimulated CTLs and stimulated PBMNCs were included as controls. Then, 10 μL CD4 APC and CD8 PerCP–conjugated antibodies (Becton Dickinson, San Diego, CA), and 1 μL CMV pp65495-503 tetramer PE (NIAID Tetramer Facility, Atlanta, GA) were incubated with the CTLs for 30 minutes at 37°C. The cells were washed and permeabilized with 250 μL cytofix/cytoperm (Pharmingen). After washing with saponin-containing buffer, the CTLs were stained with FITC-labeled antibodies: IgG1 (Pharmingen), tumor necrosis factor-α (TNF-α), IL-4, or interferon γ (IFN-γ, R&D Systems) for 30 minutes at 4°C. The CTLs were washed, fixed, and analyzed on the flow cytometer. Quadrant assignments were based on isotype controls designed specifically for intracellular staining.
Immunophenotyping of CTLs
Cells were stained using standard methods with PE- and/or FITC-labeled antibodies for the surface molecule of interest and analyzed on the FACScan flow cytometer (Becton Dickinson). At least 5000 events in the gate were acquired for analysis. Optimal voltage settings and electronic subtraction for spectral fluorescence overlap correction were determined by including single stains for all FITC-labeled antibodies. Isotype-specific negative controls were included in all experiments. The following FITC-labeled antibodies were used: CD3, CD45RA, CD45RO, CD49d (VLA-4), CD49e (VLA-5), HLA-DR, cutaneous lymphocyte antigen (CLA), CD8 (all from Becton Dickinson) and PE antibodies: CD3, CD19, CD8, CD4, CD56, CD57, CD62L (L-selectin), CD95(FAS) (all from Becton Dickinson), and PE-conjugated HLA-A*0201/pp65495-503 tetramer. The number of antigen-specific cells in each culture was calculated by dividing the number of CD8bright/HLA-A*0201/pp65495-503tetramer–positive cells by CD8bright/ HLA-A*0201/pp65495-503 tetramer–positive cells plus CD8bright/HLA-A*0201/pp65495-503tetramer–negative cells.
Purification of CTLs with HLA-A*0201/pp65495-503tetramer
Cells were incubated with tetramer-PE for 25 minutes at 37°C (Figure 1). One microliter of tetramer-PE was used per 106cells in PBS plus 5% AP; the final dilution of tetramer-PE was 1:10. After washing, tetramer-positive cells were isolated using the MoFlo Cell Sorter (Cytomation, Fort Collins, CO) or with anti-PE antibody-coated paramagnetic beads (Miltenyi). Prior to positive selection of cells gated in the FL2 (PE) parameter on the MoFlo Cell Sorter, the cell suspension was passed through a microaggregate filter to remove clumps. Cells were collected in RPMI media containing 10% AP and 1 μg/mL Fungizone (Gibco BRL, Life Technologies, Grand Island, NY). Intensely staining, high-avidity tetramer-positive cells were selected for cell sorting. Bead selection was accomplished using manufacturer's instructions (Miltenyi). Cells were subsequently checked for purity by flow cytometry.
Rapid expansion of HLA-A*0201/pp65495-503tetramer–positive cells
After sorting, 1 to 2 × 106 tetramer-positive cells were resuspended in 50 mL RPMI containing IL-2 50 U/mL, OKT3 10 ng/mL, IL-7 10 ng/mL, 10 × 106 irradiated allogeneic feeder cells from 3 randomly chosen individuals, and 5 × 106 irradiated autologous LCLs pulsed with pp65495-503 tetramer peptide in the presence of 3 μg/mL β2-microglobulin for 4 hours at room temperature (Figure1). The rationale for using the 3 pool irradiated allogeneic feeder cells is that these may secrete additional cytokines that support the rapid expansion of CTLs. The cells were cultured in the smallest ledge of a 75-cm2 flask for optimal density (45° angle). On day 10, the flask was placed upright, the cells were harvested or restimulated, and given half fresh medium with replenishment of cytokines.
Vβ spectratyping
Between 2 and 5 × 106 CMV-specific CTLs were harvested for spectratype analysis by centrifugation and lysed by the addition of 800 μL Trizol reagent (Life Technologies, Gaithersburg, MD). Total RNA was extracted essentially according to the manufacturer's instructions with one significant modification—the inclusion of 200 μL Phaselock gel (Brinckman Instruments, Westbury, NY) prior to the addition of 0.2 volumes of chloroform and subsequent phase separation, to prevent contamination of RNA samples with genomic DNA. Aliquots of 2 μg of each RNA sample were then reverse transcribed in 40 μL reactions containing 1 × polymerase chain reaction (PCR) buffer II (10 mM Tris-HCl, pH 8.3, 50 mM KCl), 5 mM MgCl2, 4 mM dNTP, 2.5 mM oligo dT primer, 1 U recombinant RNase inhibitor, and 2.5 U reverse transcriptase. Reverse transcriptions were incubated at 37°C for 1 hour, and terminated by heat inactivation at 95°C for 5 minutes. Each 25-μL PCR reaction contained 1 × PCR buffer II, 2 mM MgCl2, 0.8 mM dNTP, 0.5 μL target complementary DNA (cDNA), 0.625 U AmpliTaq Gold DNA polymerase, one of 23 forward primers specific for the TCR Vβ 1, 2, 3, 4, 5.1, 5.2, 6, 7, 8, 9, 11, 12, 13, 14, 15, 16, 17, 18, 20, 21, 22, 23, and 24 genes (each at a concentration of 1 μM), and a common constant region-specific reverse primer labeled at its 5′ end with a 6-FAM moiety (also at a concentration of 1 μM). All primers were supplied by Life Technologies. Amplifications were performed using a PerkinElmer PCR2400 thermocycler using the following cycling parameters: 95°C, 10 minutes (activation of DNA polymerase), 35 cycles of 94°C/20 seconds, 55°C/20 seconds, 72°C/30 seconds, and a final extension at 72°C for 10 minutes. For high-resolution analysis, 3-μL aliquots of PCR reactions were paired as indicated in the relevant figure legends, mixed with 4 μL deionized formamide/blue dextran (5:1) and denatured at 95°C for 5 minutes. Fluorescent-labeled PCR products were resolved by electrophoresis through 4.75% polyacrylamide sequencing gels, and visualized using an FMBIO II laser-scanning fluorescent imager. The optical density (OD) of each individual band in each TCR Vβ family was calculated and plotted in histogram format to obtain a quantitative graphical representation of the data.
Results
DCs cultured in AP and FBS have comparable morphologic, immunophenotypic, and functional features
We first established that AP-DCs are as efficient in inducing immune responses as FBS-DCs. The median yields for FBS- and AP-DCs derived from 107 CD14+ cells were 2.0 × 106 (range, 1.6-2.8 × 106) and 2.4 × 106 (range, 0.7-4.5 × 106), respectively. The median purities on day 9 after 2 days of maturation with CD40 ligand, based on scatter plots and expression of CD1a or CD83, were 94% (range, 92%-95%) and 84% (range, 63%-97%). FBS- and AP-DCs were similar with respect to morphology, immunophenotype (data not shown), endocytosis (data not shown), induction of allogeneic MLR (Figure 2A), and antigen processing and presentation (Figure 2B). Both FBS- and AP-DCs lost expression of CD14. CD83, a marker for mature DCs, was up-regulated by day 9, whereas CD1a, a marker for immature DCs, was down-regulated. HLA-DR and the costimulatory molecules B7.1, B7.2, and CD40 were up-regulated on both FBS- and AP-DCs. The adhesion molecule ICAM-1 (CD54) and the αx subunit of integrin CR4 (CD11c), which enhances the interaction between DCs and T cells, were both highly expressed. Endocytosis of the tracer lucifer yellow internalized by macropinocytosis and FITC-labeled dextran taken up by the scavenging mannose receptor were down-regulated on maturation. The ability of decreasing numbers of irradiated DC mature FBS- and AP-DCs to stimulate an immune response from purified, allogeneic T cells was similar with routine detection of alloresponses at 10 to 50 cells/well (Figure 2A).
T-cell proliferation assays.
(A) Mature DCs grown in either FBS or AP are equally efficient at inducing allogeneic T-cell proliferation. (B) FBS-DCs induce autologous T-cell proliferation due to recognition of FBS-derived peptides. Primary responses were stimulated from autologous T cells by FBS-DCs with KLH added (●) and in the FBS-DC autologous MLR without antigen (▴) suggesting that xenogeneic FBS peptides are presented to autologous T cells. In contrast, the AP-DCs stimulated a response only when the foreign KLH antigen was present (▪), versus without KLH (♦).
T-cell proliferation assays.
(A) Mature DCs grown in either FBS or AP are equally efficient at inducing allogeneic T-cell proliferation. (B) FBS-DCs induce autologous T-cell proliferation due to recognition of FBS-derived peptides. Primary responses were stimulated from autologous T cells by FBS-DCs with KLH added (●) and in the FBS-DC autologous MLR without antigen (▴) suggesting that xenogeneic FBS peptides are presented to autologous T cells. In contrast, the AP-DCs stimulated a response only when the foreign KLH antigen was present (▪), versus without KLH (♦).
Autologous MLR stimulated by FBS-DC is due to recognition of xenogeneic, FBS-derived peptides
We compared the ability of FBS- and AP-DCs to stimulate a primary immune response. Decreasing numbers of mature DCs were coincubated with autologous T cells in the presence of keyhole limpet hemocyanin (KLH; 50 μg/mL). The strongest response was noted in the FBS–DC and T-cell coculture with KLH added; however, a response was also observed without antigen (Figure 2B). Presumably, this response is to peptides derived from FBS. In contrast, the AP-DCs stimulated a response only when KLH antigen was introduced. Thus, culture of DCs in AP avoids exposure to potential xenogeneic pathogens and recognition of FBS peptides.
pp65495-503 binds to HLA-A*0201
The affinity of pp65495-503 to HLA-A*0201 was confirmed by pp65495-503 binding to acid-stripped T2 cells in a dose-dependent fashion with a maximum at 100 μg/mL (Figure3). Control HLA-A*0201–binding peptides were derived from the Wilms tumor protein.
T2 peptide-binding assay.
Dose-dependent, increased expression of HLA-A*0201 is seen with addition of peptides pp65495-503 (▪), Wt242-249 (♦) and Wt225-233(●). WT indicates Wilms tumor protein.
T2 peptide-binding assay.
Dose-dependent, increased expression of HLA-A*0201 is seen with addition of peptides pp65495-503 (▪), Wt242-249 (♦) and Wt225-233(●). WT indicates Wilms tumor protein.
The pp65495-503-specific CTLs generated from seropositive donors kill peptide-pulsed cells and CMV-infected fibroblasts
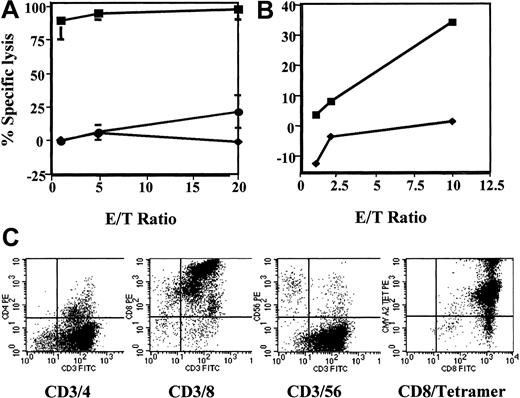
Cultures were commenced with donor-derived CD14-depleted cells or purified CD8+ cells as responders. Of 19 CTL cultures, 17 CMV-specific lines were generated from 3 HLA-A*0201, CMV-seropositive donors (Table 1). From 2 × 106 CD8+ cells at the onset of the culture, a median of 13 × 106 cells was obtained (range, 4-45.6 × 106) after 2 restimulations from 14 CTL lines. Five cultures were commenced with both CD4+and CD8+ responders (CD14-depleted PBLs), and a median of 17 × 106 cells was collected (range, 7.9-46.2 × 106). Cytotoxicity to pp65495-503peptide-pulsed autologous PHA-blasts was measured using a standard51Cr release assay 3 to 4 days after the second restimulation (Figure 4). In 2 cases a third restimulation was necessary to increase cell number before cytotoxicity could be determined. Appropriate controls, such as PHA-blasts pulsed with an irrelevant HLA-A2–restricted peptide, K562 targets to determine nonspecific NK cell cytotoxicity, and recipient PHA-blasts, were included in the assays. Generally, we observed higher nonspecific lysis in cultures started with a substantial CD56 population (> 15%). The most specific results were obtained from cultures commenced with CD8+ purified cells, not containing any CD56+ cells or CD4+ cells. The average specific lysis at a ratio of 1 target cell to 10 CTLs was 62% for CD8+ cultures with IL-7 added and 68% with no IL-7. Immunophenotyping showed that these CTLs were predominantly CD8+ (Figure 4). In contrast, CD14-depleted CTLs killed only 27% of pp65495-503-pulsed targets (Table 1). The pp65495-503-specific CTLs also killed CMV-infected MRC5 fibroblasts (see Figure 6). We observed 33% killing of CMV-infected fibroblasts and no lysis of uninfected cells above background.
Summary of cytotoxicity assays for CMV peptide–induced CTL lines
| Culture conditions . | No. lines . | No. of CMV-specific lines . | Lysis of CMV peptide–pulsed targets at 1:1 range (average) . | Nonspecific lysis at 1:1 of control targets . | Lysis of CMV peptide–pulsed targets at 1:5 range (average) . | Nonspecific lysis at 1:5 of control targets . | Lysis of CMV peptide–pulsed targets at 1:10 range (average) . | Nonspecific lysis at 1:10 of control targets . |
|---|---|---|---|---|---|---|---|---|
| CD8+ | 5 | 4 (80%) | 17-22% (20%) | < 3% | 59% | < 1% | 66-70% (68%) | 10% |
| CD8+, IL-7+ | 9 | 9 (100%) | 5-54% (22%) | < 1% | 18-96% (57%) | < 1% | 31-93% (62%) | < 3% |
| CD14− | 2 | 2 (100%) | 4-5% (5%) | < 1% | 16% | — | 27% (27%) | 9% |
| CD14−, IL-7+ | 3 | 2 (67%) | 4-7% (6%) | < 1% | — | — | 26-29% (28%) | < 6% |
| Total | 19 | 17 (89%) | 4-50% (11%) | < 1-3% | 16-96% (50%) | — | 26-93% (52%) | < 3-10% |
| Culture conditions . | No. lines . | No. of CMV-specific lines . | Lysis of CMV peptide–pulsed targets at 1:1 range (average) . | Nonspecific lysis at 1:1 of control targets . | Lysis of CMV peptide–pulsed targets at 1:5 range (average) . | Nonspecific lysis at 1:5 of control targets . | Lysis of CMV peptide–pulsed targets at 1:10 range (average) . | Nonspecific lysis at 1:10 of control targets . |
|---|---|---|---|---|---|---|---|---|
| CD8+ | 5 | 4 (80%) | 17-22% (20%) | < 3% | 59% | < 1% | 66-70% (68%) | 10% |
| CD8+, IL-7+ | 9 | 9 (100%) | 5-54% (22%) | < 1% | 18-96% (57%) | < 1% | 31-93% (62%) | < 3% |
| CD14− | 2 | 2 (100%) | 4-5% (5%) | < 1% | 16% | — | 27% (27%) | 9% |
| CD14−, IL-7+ | 3 | 2 (67%) | 4-7% (6%) | < 1% | — | — | 26-29% (28%) | < 6% |
| Total | 19 | 17 (89%) | 4-50% (11%) | < 1-3% | 16-96% (50%) | — | 26-93% (52%) | < 3-10% |
CTL lines capable of killing CMV peptide-pulsed targets were produced in 17 of 19 cultures derived from seropositive donors. Cultures were initiated with purified CD8+ T cells or PBMCs depleted of CD14+ cells. In general, CTL lines generated from purified CD8+ cells achieved higher specific lysis of targets than CD14− lines. This is probably due to the presence of expanded nonspecific CD4+ and CD56+cell populations in the CD14− cultures.
Immunophenotypic and cytolytic profile of pp65495-503-specific CTL line.
This line killed (A) autologous PHA-blasts pulsed with pp65495-503 (▪) and (B) the CMV infected HLA-*A0201+ MRC5 fibroblast cell line (▪). There is little or no killing of autologous PHA-blasts pulsed with the human papillomavirus-derived peptide E711-20 (♦, A), K562 cells (●), and noninfected MRC5 fibroblasts (♦, B). The pp65495-503-specific CTL line is predominantly CD8+ and stains intensely with HLA-*A0201/pp65495-503 tetramer (C).
Immunophenotypic and cytolytic profile of pp65495-503-specific CTL line.
This line killed (A) autologous PHA-blasts pulsed with pp65495-503 (▪) and (B) the CMV infected HLA-*A0201+ MRC5 fibroblast cell line (▪). There is little or no killing of autologous PHA-blasts pulsed with the human papillomavirus-derived peptide E711-20 (♦, A), K562 cells (●), and noninfected MRC5 fibroblasts (♦, B). The pp65495-503-specific CTL line is predominantly CD8+ and stains intensely with HLA-*A0201/pp65495-503 tetramer (C).
The pp65495-503-specific CTLs stain positively with HLA-A*0201/pp65495-503 tetramer
The CTL lines were stained with HLA-A*0201/pp65495-503tetramer to determine the percentage of antigen-specific cells present in the CTL lines (Figure 5). The median percentage of CD8bright and HLA-A*0201/pp65495-503 tetramer double-positive cells was 50.5% (range, 28%-97%). In all cases CD8+single stain controls were used to determine the optimal electronic subtraction for spectral fluorescence overlap correction.
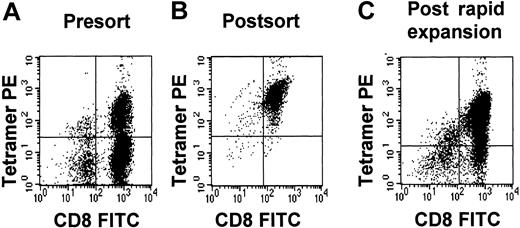
Tetramer purity.
Tetramer purity of a pp65495-503-specific CTL line before sorting (A), immediately after sorting (B), and after rapid expansion (C).
Tetramer purity.
Tetramer purity of a pp65495-503-specific CTL line before sorting (A), immediately after sorting (B), and after rapid expansion (C).
The pp65495-503-specific CTLs from CMV-seronegative donors
The method used to prepare CTL lines was identical to our induction protocol for seropositive donors with the exception that 2 additional restimulations were required before specific cytotoxicity was observed. Only 2 of 10 CTL lines, both initiated with donor CD14-depleted PBLs (containing both CD4+ and CD8+ cells), lysed peptide-pulsed targets in a cytotoxicity assay. For both CMV-specific lines, immunophenotyping revealed mostly CD8+ (74% and 86%) cells with few CD4+ (16% and 23%) and CD56+ (8% and 5%) cells. In contrast no specific lines were generated if only purified CD8+cells were used at the initiation of the culture indicating that CD4 help is essential for seronegative donors.
Purification and rapid expansion of pp65495-503/HLA-A*0201 tetramer–positive cells
The CTL preparations were intended for the reconstitution of CMV immunity in recipients of haploidentical allografts. We generated an additional 5 pp65495-503-specific CTL lines starting with larger numbers of purified CD8+ cells as responders (Table 2). These CTL lines were positively selected for pp65495-503-specific cells with HLA-tetramers using either a clinical grade flow sorter (MoFlo, Cytomation) or anti-PE paramagnetic beads. The number of antigen-specific cells isolated varied from 7 to 26.8 × 106 cells with a purity of 94% to 99.8% (Table2, Figure 5). Four purified fractions were rapidly expanded using peptide-pulsed LCLs, allogeneic feeders, IL-2, and a submitogenic dose of OKT3. The fold expansion varied from 4.3 to 8.4. Higher purities were obtained with the paramagnetic bead selection of CD8/tetramer-positive cells. The purities of CD8/tetramer-positive cells dropped to 75.4% to 86% after rapid expansion (Table 2). We demonstrated that the expanded cells were capable of killing CMV-infected fibroblasts (Figure 6).
Yield of tetramer-positive cells
| CTL no. . | CD8+ cells at onset . | Presorting . | Postsorting . | Method of sorting . | After rapid expansion . |
|---|---|---|---|---|---|
| 1 | 9 | 100 (49) | 7 (95) | Flow cytometry | 59 (81) |
| 2 | 13.8 | 95 (50) | 12.7 (94) | Flow cytometry | Failed |
| 3 | 13.8 | 95 (33) | 14.8 (99) | Paramagnetic beads | 106.9 (86) |
| 4 | 12 | 220 (28) | 22 (94.5) | Flow cytometry | 95 (75.4) |
| 5 | 10 | 150 (81) | 26.8 (99.8) | Paramagnetic beads | ND |
| CTL no. . | CD8+ cells at onset . | Presorting . | Postsorting . | Method of sorting . | After rapid expansion . |
|---|---|---|---|---|---|
| 1 | 9 | 100 (49) | 7 (95) | Flow cytometry | 59 (81) |
| 2 | 13.8 | 95 (50) | 12.7 (94) | Flow cytometry | Failed |
| 3 | 13.8 | 95 (33) | 14.8 (99) | Paramagnetic beads | 106.9 (86) |
| 4 | 12 | 220 (28) | 22 (94.5) | Flow cytometry | 95 (75.4) |
| 5 | 10 | 150 (81) | 26.8 (99.8) | Paramagnetic beads | ND |
Yield of tetramer-positive cells of 5 pp65495-503-specific CTLs that were purified by flow sorting or with anti-PE–coated paramagnetic beads. The number in the second column represents the number of CD8+ cells with which the culture was commenced. The numbers in the other columns indicate the total number of cells in culture (× 106); the percentage tetramer-positive cells is indicated in parentheses. One bead-purified pp65495-503-specific CTL lines (no. 5) was not expanded; the rapid expansion of line 2 failed probably due to technical problems during flow sorting, which increased the sorting time substantially and resulted in dead cells in the sorted population.
ND indicates not done.
Cytolytic assays of expanded CTLs.
A rapidly expanded pp65495-503-specific CTL line shows specific killing of (A) autologous PHA-blasts pulsed with pp65495-503 (▪) and (B) the CMV-infected HLA-*A0201+ MRC5 fibroblast cell line (♦). The specific lysis of the PHA-blasts is higher because they will present more exogenous peptide at the cell surface compared to the CMV-infected MRC5 fibroblast cell line, which presents endogenously processed, pp65-derived peptide. Negative controls were not killed: ♦, PHA blasts plus E711-20; ●, K562; and ▴, noninfected MRC-5 cells.
Cytolytic assays of expanded CTLs.
A rapidly expanded pp65495-503-specific CTL line shows specific killing of (A) autologous PHA-blasts pulsed with pp65495-503 (▪) and (B) the CMV-infected HLA-*A0201+ MRC5 fibroblast cell line (♦). The specific lysis of the PHA-blasts is higher because they will present more exogenous peptide at the cell surface compared to the CMV-infected MRC5 fibroblast cell line, which presents endogenously processed, pp65-derived peptide. Negative controls were not killed: ♦, PHA blasts plus E711-20; ●, K562; and ▴, noninfected MRC-5 cells.
Expanded pp65495-503-specific CTLs are of activated and memory phenotype and are able to cross endothelial barriers
The pp65495-503-specific CTLs cultured from purified CD8+ cells were predominately CD8+ (92%) with few CD4 (< 8%) and CD56 cells (< 7%). Gating on the CD8+ CTLs, we observed that CD45RO, a marker for memory cells was expressed, whereas CD45RA, a marker for naive cells, was not. There was very little expression of the terminal differentiation marker, CD57. The VLA-4 integrin (CD49d) was expressed on 95% of CTLs and VLA-5 (CD49e) was expressed on 4.2% of cells. These integrins are important in adhesive interactions between lymphocytes and APCs and are associated with the ability of T cells to cross endothelial barriers. CD62L (L-selectin), down-regulated after T-cell activation, was expressed on 30% of cells. The CTLs also expressed HLA class II-DR activation markers (90%) (Figure 7).
Immunophenotype of a rapidly expanded pp65495-503-specific CTL line shows virtually exclusively CD8+ cells with some cells triple staining for CD3, CD8, and CD56.
The CTLs are CD45RO+ and CD45RA− indicative of memory phenotype. The cell line is activated (HLA-DR+ and CD62L− or L-selectin negative) and expresses CD49d (VLA-4), which is associated with the ability of cells to transgress across epithelium.
Immunophenotype of a rapidly expanded pp65495-503-specific CTL line shows virtually exclusively CD8+ cells with some cells triple staining for CD3, CD8, and CD56.
The CTLs are CD45RO+ and CD45RA− indicative of memory phenotype. The cell line is activated (HLA-DR+ and CD62L− or L-selectin negative) and expresses CD49d (VLA-4), which is associated with the ability of cells to transgress across epithelium.
pp65495-503-specific CTLs have a highly restricted T-cell repertoire
The T-cell repertoire of the CTL line was highly restricted and oligoclonal as demonstrated by Vβ spectratyping (data not shown). However, examination of the expanded cells by Vβ spectratyping showed some additional laddering, which is in keeping with the drop-off in the purities of CD8/tetramer-positive cells after expansion, indicating the expansion of a small population of nonspecific cells despite the initial purification with tetramer. However, these additional bands resolved after 2 further restimulations with pp65495-503peptide-pulsed autologous LCLs.
Expanded pp65495-503-specific CTLs are of Tc1 phenotype
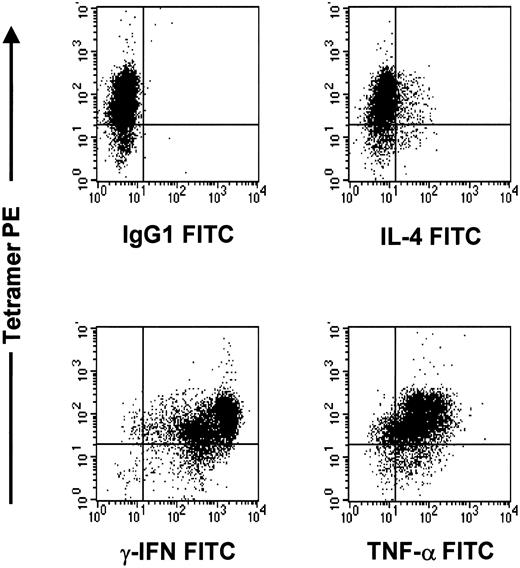
The CTL lines were of the Tc1 phenotype. There was strong intracellular staining for TNF-α (42%) and IFN-γ (77%), with little IL-4 expression (13%). The expanded tetramer-positive CMV-specific CTLs were of Tc1 phenotype, because intracellular staining was strongly positive for TNF-α and IFN-γ, with little IL-4 expression (Figure 8).
The pp65495-503-specific CTL line after rapid expansion is of Tc1 phenotype.
Cells were stained with tetramer PE, cytokine FITC, and CD4APC or CD8PERCP. The CD8 population is depicted in this graph.
The pp65495-503-specific CTL line after rapid expansion is of Tc1 phenotype.
Cells were stained with tetramer PE, cytokine FITC, and CD4APC or CD8PERCP. The CD8 population is depicted in this graph.
Discussion
The present study describes a convenient method for obtaining CMV-specific CTLs for clinical immunotherapy from a single blood draw using donor-derived DCs and HLA-tetramers. We chose to focus on HLA-A*0201+ individuals because the population frequency of HLA-A*0201 is nearly 50% in several ethnic groups allowing for the future treatment of a significant proportion of the patient population. All cultures were carried out using the donor's AP to support in vitro expansion of CMV-specific CTLs rather than xenogeneic FBS or allogeneic, human AB serum. We first established that donor DCs cultured in FBS and AP are equipotent for the induction of immune responses. Next we generated donor-derived CMV-specific CTLs using pp65495-503-pulsed autologous DCs. Finally, we were able to purify our CMV-specific CTLs with pp65495-503/ HLA-A*0201 tetramers. This last step was necessary because our main transplantation population consists of recipients of haploidentical grafts. Dose-escalation studies in our unit have shown that transfer of even a small number of alloreactive cells can cause severe graft-versus-host disease in the haplo setting.
The tetramer-purified CMV-specific CTLs were rapidly expanded in 10 days to achieve sufficient CTLs for adoptive immunotherapy. Tetramer staining and Vβ spectratyping revealed that there was some reduction in the number of CMV-specific CTLs after the expansion. This does raise the concern of expanding some alloreactive cells due to use of allogeneic feeders during the rapid expansion period. However, specificity testing showed killing of both peptide-pulsed targets and CMV-infected cells. Extrapolation of the data in Table 2 indicates that the rapid expansion step might not be necessary to isolate sufficient cells for clinical immunotherapy. It is feasible to routinely isolate 30 × 106 CD8+ cells from a 200-mL blood draw, which would yield approximately 1.3 to 3.2 × 107 CMV-specific cells/m2 prior to expansion. We were not able to test this hypothesis in large-scale experiments due to the limited availability of tetramer. HLA-tetramers have also been used for the rapid cloning for adoptive immunotherapy with MAGE-3–specific CTLs from the peripheral blood, tumor-infiltrated lymph nodes, and skin metastases in patients with melanoma.26 However, it is clear that the place of complex cellular therapies in the management of posttransplant CMV reactivation and disease requires careful further consideration, and that its efficacy needs to be compared to drug prophylaxis in future studies.
We were able to obtain functional information regarding the properties of tetramer-positive CMV-specific CTLs. Intracellular staining for the cytokines IFN-γ, TNF-α, and IL-4 demonstrated that the cytotoxic T cells were of Tc1 phenotype consistent with their cytolytic potential. Analysis of the T-cell receptor by spectratyping revealed highly restricted, oligoclonal T-cell populations of different Vβ types suggesting that isolation of a single Vβ type by monoclonal antibodies and beads will not capture all peptide-specific T cells. Analysis of the T-cell receptors in tetramer-positive melan-A–specific CD8+ T cells in melanoma patients also revealed that tetramer-positive cells have a restricted Vβ repertoire with both interpatient and intrapatient variability in Vβ usage.27 Further characterization of the pp65495-503-specific T cells by flow cytometry determined that virtually all cells expressed HLA-DR, indicative of T-cell activation, and were of memory phenotype (CD45RO+and CD45RA−). Most cells expressed CD49d (VLA-4) and to a lesser extent CD49e (VLA-5) associated with the ability to transgress endothelium and exit into tissues. However, we did not formally demonstrate that the expanded CTLs are capable of transgression into tissues. The terminal maturation marker CD57 was expressed only on a minority of cells, indicating that at least some of the expanded cells retained their ability to further expand in vivo. Of interest was that nearly all cells stained positive for FAS, suggesting that the cultured cells may be susceptible to apoptosis. However, it has recently been reported in a mouse model that antigen-specific memory cells have increased expression of BCL2, which may offer some protection against apoptosis.28 We have made similar observations with in vitro–generated human EBV-specific CTLs.29
Several suggestions have been made for the application of HLA class I tetramers including direct isolation of CMV-specific T cells from leukapheresis products.30 A disadvantage would be that the donor would need to undergo leukapheresis and that a substantial amount of tetramer is required. In addition, this method would not be suitable to isolate CMV-specific cells from CMV− donors. Similarly, it has been suggested that antigen-specific cells labeled with HLA molecule/peptide complexes could be captured with immunomagnetic beads coated directly with HLA molecule/peptide complexes.31 It has recently been suggested that a CMV-specific T-cell line could be further purified by stimulating with CMV antigen or peptide, and removing cells that secrete IFN-γ using an antibody to IFN-γ and an immunomagnetic bead labeled with a cell surface marker such as CD8. This method is elegant; however, it is unlikely to be approved for clinical application in the near future.32 Alternatives to the use of tetramer include the transfection of pp65 into LCLs or DCs and the generation of EBV- and CMV-bispecific T-cells lines.33-35 This approach is attractive because pp65 is naturally processed and there is no requirement for matching HLA allele with peptide. Furthermore, these polyclonal lines will contain T-helper cells, which will increase the longevity of the transfused cells. Our approach uses only a single pp65 epitope as a target for T cells, whereas use of the complete pp65 protein would also allow for the killing of CMV-infected cells that present other, subdominant epitopes, and limit the possibility of viral escape.
The present method of generating CMV-specific CTLs with the aid of tetramers does not allow for the transfer of CD4+cells. It has been shown that adoptively transferred CD8+ CMV-specific T-cell clones do not persist long-term without endogenous recovery of CD4+cells. Similar observations have been made in a mouse model of lymphocytic choriomeningitis virus.36,37 On the other hand, in patients with acquired immunodeficiency syndrome, the function of CMV-specific T cells is not affected by a low CD4 count and CMV-specific T cells can persist for prolonged periods in the absence of circulating peripheral CD4+ T cells.38 39 It is important to realize that our current haploidentical transplantation protocol only uses partial T-cell depletion and that endogenous recovery of helper cells may contribute to the survival of transfused pp65495-503-specific CD8+ cells. An alternative approach could be to give 2 transfusions of CMV-specific T cells (eg, on day 30 and day 75) to protect the patient from CMV disease during the most vulnerable, first 120 days after allografting. It may also prove feasible to select with HLA class II tetramers CMV-specific T-helper cells in the case of common haplotypes, or alternatively provide pan-helper epitopes to CD4+ cells.
The authors would like to thank Dr Cliona Rooney from the Texas Children's Hospital for donating the B95-8 cell line and the Immunex Corporation, Seattle, WA, for providing CD40 ligand. The CD40-ligated NIH3T3 cells were a gift from Dr Gordon Freeman at Dana Farber Cancer Institute. HLA-tetramer was in part provided by the NIAID Tetramer Facility, Atlanta, GA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Frits van Rhee, Prof of Medicine and Director of Immunotherapy, Myeloma and Transplantation Research Center, University of Arkansas for Medical Sciences, Little Rock, AR 72205; e-mail: vanrheefrits@uams.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal