Coagulation factor V (FV) plays an important role in maintaining the hemostatic balance in both the formation of thrombin in the procoagulant pathway as well as in the protein C anticoagulant pathway. FV deficiency is a rare bleeding disorder with variable phenotypic expression. Little is known about the molecular basis underlying this disease. This study identified 5 novel mutations associated with FV deficiency in 3 patients with severe FV deficiency but different clinical expression and 2 unaffected carriers. Four mutations led to a premature termination codon either by a nonsense mutation (single-letter amino acid codes): A1102T, K310Term. (FV Amersfoort) and C2491T, Q773Term. (FV Casablanca) or a frameshift: an 8–base pair deletion between nucleotides 1130 and 1139 (FV Seoul1) and a 1–base pair deletion between nucleotides 4291 and 4294 (FV Utrecht). One mutation was a novel missense mutation: T1927C, C585R (FV Nijkerk), resulting in the absence of mutant protein despite normal transcription to RNA. Most likely, an arginine at this position disrupts the hydrophobic interior of the FV A2 domain. The sixth detected mutation was a previously reported missense mutation: A5279G, Y1702C (FV Seoul2). In all cases, the presence of the mutation was associated with type I FV deficiency. Identifying the molecular basis of mutations underlying this rare coagulation disorder will help to obtain more insight into the mechanisms involved in the variable clinical phenotype of patients with FV deficiency.
Introduction
Human coagulation factor V (FV) is a single-chain glycoprotein that plays an important role in maintaining the hemostatic balance. It circulates in blood as an inactive procoagulant with a Mr of 330 kd and a structure consisting of 3 homologous A-type domains and 2 homologous C-type domains connected by a heavily glycosylated B domain in the order A1-A2-B-A3-C1-C2. Proteolytic cleavage by thrombin at R709, R1018, and R1545 (single-letter amino acid codes) results in removal of the B domain and converts the procofactor into the fully active cofactor FVa, which consists of a Mr 105-kd heavy chain (A1-A2) and a Mr 74- or 71-kd light chain (A3-C1-C2), associated via a single Ca++ion.1-3 The difference in molecular weight of the light chain reflects the presence of 2 isoforms of FVa (FVa1 and FVa2) due to alternative glycosylation of the C2 domain, which leads to different affinities for biologic membranes and subsequent overall procoagulant activity.4,5 In its active form, FVa forms an essential part of the prothrombinase complex that catalyzes the conversion of prothrombin to thrombin by factor Xa in the presence of calcium and a phospholipid membrane.1-3Activated protein C (APC) inactivates FVa through cleavage of the active cofactor at R306, R506, and R679 and requires FV as a cofactor in the APC-mediated inactivation of factor VIIIa (FVIIIa).6,7 Thus, FV plays an important role in the procoagulant pathway as well as in the protein C anticoagulant pathway. The structure of FV is similar to FVIII (both cofactors share approximately 40% homology in their heavy and light chains) and ceruloplasmin, the copper-binding protein in plasma.8,9 Recently, the crystal structure of the C2 domain of FV has been established10 and molecular models for the A and C domains of FV have been proposed.11 12
The gene for coagulation FV has been mapped to chromosome 1q2313 and spans more than 80 kilobases (kb). It consists of 25 exons and the messenger RNA (mRNA) encodes a leader peptide of 28 amino acids and a mature protein of 2196 amino acids. Roughly, the heavy chain is encoded by exons 1 to 12 and the light chain by exons 14 to 25. The entire B domain is encoded by exon 13, which contains 2 tandem repeats of 17 amino acids and 31 tandem repeats of 9 amino acids that are absent in the B domain of FVIII.14 15
Deficiency of FV, or parahemophilia, was first described in 1947 by Owren.16 It is a rare autosomal recessive bleeding disorder with an estimated frequency of one in one million. The phenotypic expression of FV deficiency is variable; heterozygotes are usually asymptomatic, whereas homozygous patients show mild, moderate, or severe bleeding symptoms. Identifying the molecular basis underlying this disease will help to obtain more insight into the mechanisms involved in this variable clinical expression. The recently published complete nucleotide sequence of the FV gene (GenBank accession number Z99572) has facilitated the molecular characterization underlying FV deficiency and reports have identified mutations in theFV gene that result in FV deficiency.17-30 To date, no compound heterozygous patients have been described. In the present study we used DNA sequence analysis to detect 5 novel mutations and one previously reported mutation in the FV gene of 3 patients, one of whom is the first characterized compound heterozygote, with severe FV deficiency and 2 asymptomatic carriers. All mutations resulted in type I FV deficiency.
Patients, materials, and methods
Patients
Patient 1 is a 19-year-old woman from South Korea. She was adopted by a Dutch family at the age of 3 months. At the age of 19 months she developed bleeding of the soft tissue of the mouth. Severe FV deficiency, reflected by an FV activity less than 1% (Table1), was diagnosed. At the age of 4 years she experienced a large subdural hematoma, which completely resolved after frequent transfusions of fresh frozen plasma. Her bleeding pattern mostly showed soft tissue bleeds of the mouth, epistaxis, and hematomas for which she received fresh frozen plasma once every 3 months. In the last years her bleeding pattern changed to spontaneous muscle bleedings.
Patient 2 is a 5-year-old boy of Turkish ancestry. He was diagnosed at the age of 1 year, because of soft tissue bleed in his mouth that did not stop after conservative treatment. His laboratory tests demonstrated an FV activity less than 1%. His consanguineous parents both had lowered FV activity and antigen levels, suggesting a FV deficiency carrier status (Table 1). No clinical bleeding problems in the parents were apparent. The following years he frequently had bleeds of his mouth, hematomas, and epistaxis. Severe bleeding episodes were treated with fresh frozen plasma. Until now he has not had muscle or joint bleeds.
Patient 3 is a 15-year-old girl from Morocco. The diagnosis of severe FV deficiency, FV activity less than 1% (Table 1), was made following family screening. She does not have a bleeding tendency, but so far she has not had any operative procedures or injuries. Her parents are first-degree cousins. Her 23-year-old brother also has a severe FV deficiency. Other than prolonged bleeding after injuries, he has had no bleeding problems. He has been described before.31
Patients 4 and 5 are both asymptomatic Dutch individuals who were discovered during routine laboratory testing. They presented with normal to slightly prolonged activated partial thromboplastin time (APTT), respectively, 31 and 34 seconds (reference values, 24-32 seconds), and slightly prolonged prothrombin time (PT), respectively, 13.6 and 13.8 seconds (reference values, 11.0-13.0 seconds). Liver failure and all other possible causes of aberrant APTT and PT results were excluded. FV activity and antigen levels were decreased (Table 1).
Informed consent was obtained from all patients and family members.
Control group
The control group consisted of 50 unrelated healthy laboratory employees of Caucasian origin. Their DNA was used to establish the allelic frequencies of exonic polymorphisms and the occurrence of the T1927C missense mutation by testing the appropriate base change either by restriction enzyme analysis or by DNA sequence analysis. Informed consent was obtained from all control individuals.
Blood collection for coagulation tests
Venous blood was collected into plastic tubes in 1/10 volume of 3.8% sodium citrate. Platelet-poor plasma was obtained by centrifugation at 1200g for 20 minutes at room temperature and aliquots were immediately stored at −80°C until testing.
FV coagulant activity and FV antigen
Activity of FV was measured on an STA coagulation analyzer (Roche, Mannheim, Germany) by performing a PT in a plasma sample diluted with FV-deficient plasma. FV antigen was determined by Professor Rogier M. Bertina by measuring FV light chain by an enzyme-linked immunosorbent assay (ELISA) as described.20
Isolation of DNA and RNA
DNA was isolated from peripheral white blood cells using the Puregene DNA isolation kit (Gentra Systems, Minneapolis, MN) according to the manufacturer's instructions. Lymphocytes were isolated from whole blood using Ficoll-Pague (Pharmacia, Uppsala, Sweden) and total RNA was extracted as described.32
Amplification of the FV gene
Nucleotides are numbered according to Jenny and colleagues.14 Nucleotides in the putative promoter region are numbered relative to the initiation codon. The primers used for polymerase chain reaction (PCR) amplification and DNA sequence analysis of the promoter and the individual exons are listed in Table2. Intronic primers were chosen at appropriate distance from the splice sites. The large exon 13 was divided into 5 fragments (a-e). The PCR reactions were carried out with 50 to 100 ng DNA in 100 μL volumes containing 10 mM Tris-HCL, pH 8.3, 50 mM KCL, 1.5 mM MgCl2, 0.01% (wt/vol) gelatin, 0.2 mM of each dNTP, 0.3 μM of each primer, and 2.5 U AmpliTaq DNA polymerase. All reagents were obtained from Perkin Elmer (Roche Molecular Systems, Branchburg, NJ). The samples were subjected to 30 or 35 cycles of amplification with denaturation at 94°C for 30 seconds (5 minutes at 95°C prior to the first cycle), annealing for 30 seconds at 50 to 64°C (Table 2) and extension at 72°C for 30 to 60 seconds followed by an elongated extension time of 10 minutes after the last cycle.
DNA sequence analysis
Automated DNA sequence analysis was performed with the ABI Prism dRhodamine Terminator Cycle Sequencing Ready Reaction Kit (PE Biosystems, Warrington, England), according to the manufacturer's instructions. Sequencing reactions were all carried out in forward and reverse direction, and samples were analyzed on an Applied Biosystems ABI 310 Genetic Analyzer (Applied Biosystems, Foster City, CA). The PCR products were purified prior to DNA sequence analysis with the QIAquick PCR purification kit (Qiagen, Valencia, CA) or excised from the agarose gel and purified using the Prep-A-Gene DNA Purification Kit (Biorad Laboratories, Hercules, CA) according to instructions.
Reverse transcription-PCR
Reverse transcription-PCR (RT-PCR) to detect the T1927C mutation was performed using the GeneAmp RNA PCR Kit from Perkin Elmer (Roche Molecular Systems, Branchburg, NJ) according to the instructions of the manufacturer. Briefly, 0.3 to 1.0 μg total RNA was reverse transcribed using random hexamers as primers. After addition of 30 pmol of primers HCFV-C11F 5′-ATGAGGTGAAACGTGATGACC-3′, exon 11 nts 1802 to 1822 and HCFV-C12R 5′-ACCGTCACAGATTCTCCACG-3′, exon 12 nts 2048 to 2029, the samples were subjected to 35 cycles of amplification with denaturation at 94°C for 30 seconds (5 minutes at 95°C prior to the first cycle), annealing at 58°C for 30 seconds, and extension at 72°C for 30 seconds, followed by an elongated extension time of 10 minutes after the last cycle. Total liver RNA was used as a positive control and controls without RNA as well as controls in which the reverse transcription step was omitted were included.
Restriction enzyme analysis
All mutations were confirmed by restriction enzyme analysis on a newly amplified PCR product (Table 3). The 8-bp deletion in exon 7 was confirmed by MboII digestion. The PCR product as amplified with primers HCFV-7F and HCFV-7R is 241 bp and contains one restriction site forMboII. Digestion of the wild-type allele results in fragments of 134 and 107 bp. The deletion abolishes this site, rendering a single fragment of 233 bp.
To confirm the T1927C mutation, exon 12 was amplified with primers HCFV-12F and HCFV-12R and subjected to BslI digestion. The 286-bp PCR product from the wild-type allele contains one restriction site for BslI, yielding fragments of 214 and 72 bp. The mutation creates a second restriction site resulting in additional fragments of 117 and 97 bp. The 247-bp PCR product containing exon 12, as obtained by RT-PCR with primers HCFV-C11F and HCFV-C12R, yields fragments of 228 and 19 bp for the wild-type allele and 131, 97, and 19 bp for the T1927C allele, following digestion with BslI.
The C2491T nonsense mutation in exon 13 was confirmed byHphI digestion of the 5′ end of exon 13 as amplified with primers HCFV-13aF and HCFV-13aR. The 605-bp PCR product normally contains one recognition site for HphI, producing fragments of 458 and 147 bp, which is abolished by this base change and thus results in an uncut PCR product from the mutant allele.
To detect the A5279G mutation, the PCR product (600 bp) amplified with primers HCFV-15F2 and HCFV-15R, containing exon 15, was subjected to digestion with Cac8I. The mutation adds an extra restriction site to the one normally present in this fragment; 338-, 55-, and 207-bp fragments are produced in contrast to the wild-type allele, 338 and 262 bp.
In general, 20 μL of the appropriate PCR product was incubated with 10 to 20 U of the respective enzymes. All enzymes were purchased from New England Biolabs (Beverly, MA).
Molecular modeling
Results
FV activity and antigen
Factor V activity and antigen results of the propositae and relevant family members are summarized in Table 1. In all subjects, excluding patient 3 from whom plasma was unavailable, the reduction of FV activity correlated with a similar reduction of FV antigen, indicating a type I FV deficiency.
Molecular analysis
During the course of the DNA sequence analysis of the completeFV gene of the patients, relevant relatives, and a control subject, a number of novel base changes as compared to the published sequence were identified. One was located in the promoter, 15 in the coding region, and 19 in the noncoding region. The intronic base changes were excluded from being the (primary) genetic defect underlying the FV deficiency, based on the fact that they were not located in or near consensus sequence motifs considered critical for RNA processing, neither did they create any such sequence.35 36 They were therefore considered to be polymorphisms. Likewise, the promoter base change at nt −426 was considered to be polymorphic given its high frequency in the control group. We report the allelic frequencies of all applicable exonic base changes, except for the 2 frameshift mutations and nonsense mutants, as determined in the control group. The results obtained from the molecular analysis are summarized in Table 3.
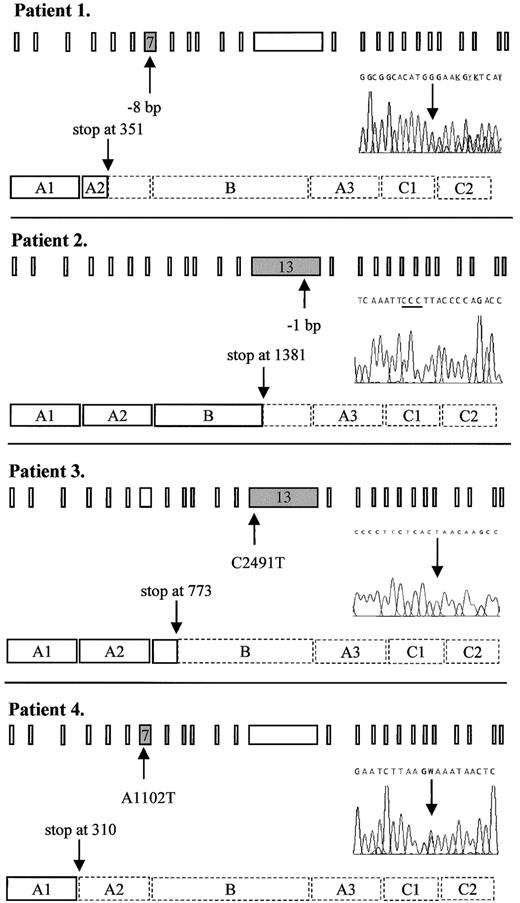
Patient 1
By DNA sequence analysis, this patient was found to be heterozygous for a novel deletion in exon 7, where 8 bp ((TG)AAGAGG(TG)) were deleted between nts 1130 and 1139 (Figure1), resulting in a frameshift and a premature stop codon at residue 351. This mutation was confirmed byMboII digestion. Because the patient had undetectable levels of FV activity and antigen (Table 1), we postulated her to be a probable compound heterozygote. Further DNA sequence analysis revealed, apart from 6 previously published polymorphisms (G1628A,37,38 (A2663G, A2684G, A2863G),14,15,39,40 C4279T41 and A5380G,42-44) one other base change in the heterozygous state: exon 15: A5279G, Y1702C. Because she was an orphan without any known relatives, it was not possible to determine which changes occurred in trans of the 8-bp deletion by linkage analysis. However, the A5279G mutation has recently been associated with FV deficiency25 and is therefore most likely the causative mutation on the other FV allele of this patient. This missense mutation was confirmed by Cac8I digestion. We named this compound heterozygous variant FV Seoul1,2.
Predicted truncated FV molecules as encoded by the FV frameshift and nonsense mutations detected in this study.
Schematic representation shows the 25 exons of the FV gene and the procoagulant protein that consists of a heavy chain (A1-A2), a connecting region or B domain, and a light chain (A3-C1-C2). The position of the mutation in the gene and in the DNA sequencing electropherogram as well as the predicted premature translation stop in the protein are indicated by arrows. The 3 cytosines resulting from the 1-bp deletion in patient 2 are underlined. Dashed lines in the protein indicate the part that is lacking, as compared to the wild-type protein. Patient 1: heterozygous 8-bp deletion between nts 1130 and 1139 in exon 7 leading to a premature stop at codon 351. Patient 2: homozygous 1-bp deletion between nts 4291 and 4294 in exon 13 leading to a premature stop at codon 1381. Patient 3: homozygous C→T nonsense mutation at nt 2491 in exon 13, changing the codon for glutamine at residue 773 into a stop codon. Patient 4: heterozygous A→T nonsense mutation at nt 1102 in exon 7, changing the codon for lysine at residue 310 into a stop codon. W: A or T
Predicted truncated FV molecules as encoded by the FV frameshift and nonsense mutations detected in this study.
Schematic representation shows the 25 exons of the FV gene and the procoagulant protein that consists of a heavy chain (A1-A2), a connecting region or B domain, and a light chain (A3-C1-C2). The position of the mutation in the gene and in the DNA sequencing electropherogram as well as the predicted premature translation stop in the protein are indicated by arrows. The 3 cytosines resulting from the 1-bp deletion in patient 2 are underlined. Dashed lines in the protein indicate the part that is lacking, as compared to the wild-type protein. Patient 1: heterozygous 8-bp deletion between nts 1130 and 1139 in exon 7 leading to a premature stop at codon 351. Patient 2: homozygous 1-bp deletion between nts 4291 and 4294 in exon 13 leading to a premature stop at codon 1381. Patient 3: homozygous C→T nonsense mutation at nt 2491 in exon 13, changing the codon for glutamine at residue 773 into a stop codon. Patient 4: heterozygous A→T nonsense mutation at nt 1102 in exon 7, changing the codon for lysine at residue 310 into a stop codon. W: A or T
Patient 2
DNA sequence analysis revealed a novel homozygous deletion in exon 13 where one cytosine was deleted from a series of 4 cytosines present between nts 4291 and 4294 (Figure 1). This resulted in a frameshift leading to a premature translation stop at codon 1381. Both parents, who were first cousins, were heterozygous for the same deletion thereby excluding the possibility of hemizygosity in the propositus due to a large deletion. We designated this variant FV Utrecht.
Patient 3
The FV gene of this patient showed a novel homozygous nonsense mutation in exon 13 where a C→T substitution at nt 2491 predicted a stop codon at residue 773 instead of the wild-type glutamine (Figure 1). This mutation was confirmed by HphI digestion and we named this variant FV Casablanca.
Patient 4
This patient was found to have a single heterozygous A→T base change at nt 1102 in exon 7 (Figure 1), thereby changing the codon for lysine at residue 310 into a termination codon. All available family members were tested for this mutation and the nonsense mutation cosegregated with reduced FV activity and antigen levels (Table 1). This mutant was designated FV Amersfoort.
Apart from this nonsense mutation and a previously described heterozygous polymorphism (G409C),45 we identified a novel homozygous base substitution in exon 24: T6533C, M2120T. Because this substitution concerns a conserved residue in the C2 domain of FV, its occurrence was investigated in the control group (Table 3). T6533C appeared to be a rare polymorphism of which all of the children in this family are obligate carriers, whereas the spouse of the patient is a wild-type homozygote (data not shown). The 2 children in this family who lack the A1102T mutation had normal FV activity levels (Table 1), so any (additional) effect of this amino acid substitution to the more pronounced reduction in FV activity of the propositus compared to his 2 sons (34% versus 62% and 60%) was not likely to be due to the fact that he was homozygous for this rare polymorphism.
Patient 5
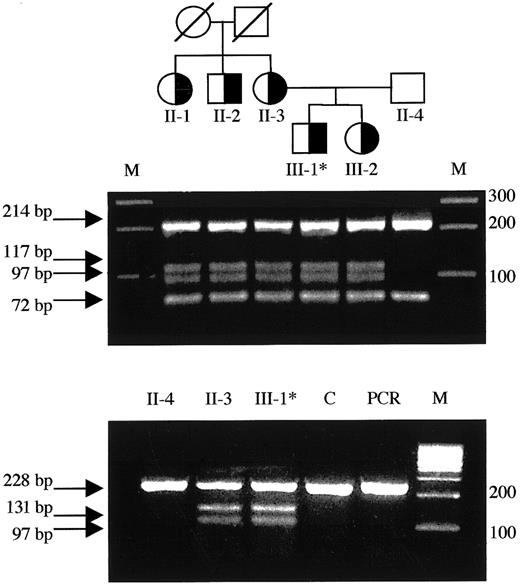
We identified 6 nucleotide substitutions linked to lowered activity and antigen levels in this family. Five were previously reported polymorphisms: (A2663G, A2684G, A2863G),14,15,39,40 C4300T,41 and A5380G,42-44 whereas one was a novel T→C substitution at nt 1927 in exon 12, coding for a C→R change at residue 585. We investigated the occurrence of T1927C in a normal population but found no control subjects carrying this allele (Table 3). We postulated the T1927C base change to cause FV deficiency (see “Discussion”) in this family (Table 1 and Figure 2) and named this variant FV Nijkerk. The mutant allele was transcribed to RNA, because the mutant RNA could be detected, by RT-PCR, in both the patient and his mother (Figure 2).
Detection of the T1927C missense mutation in the family pedigree and mutant RNA.
(Top) Family pedigree: individuals heterozygous for the T1927C (FV Nijkerk) missense mutation are indicated by a half-closed symbol. (Middle) Exon 12 was amplified from genomic DNA and subjected toBsl I digestion as described. The mutation creates an additional restriction site and the resulting fragments are indicated by arrows. Each lane represents the DNA from the individuals located directly above in the family pedigree in the top panel. (Bottom) Total RNA was reverse transcribed and amplified as described. SubsequentBsl I digestion revealed the presence of both the wild-type allele as well as the mutant allele in the RNA of patient 5 (III-1) and his mother (II-3). Asterisk indicates propositus; M, marker; C, liver tissue32 RNA control; PCR, uncut RT-PCR product (247 bp).
Detection of the T1927C missense mutation in the family pedigree and mutant RNA.
(Top) Family pedigree: individuals heterozygous for the T1927C (FV Nijkerk) missense mutation are indicated by a half-closed symbol. (Middle) Exon 12 was amplified from genomic DNA and subjected toBsl I digestion as described. The mutation creates an additional restriction site and the resulting fragments are indicated by arrows. Each lane represents the DNA from the individuals located directly above in the family pedigree in the top panel. (Bottom) Total RNA was reverse transcribed and amplified as described. SubsequentBsl I digestion revealed the presence of both the wild-type allele as well as the mutant allele in the RNA of patient 5 (III-1) and his mother (II-3). Asterisk indicates propositus; M, marker; C, liver tissue32 RNA control; PCR, uncut RT-PCR product (247 bp).
Discussion
Little is known about the molecular basis underlying FV deficiency due to its low frequency in the population combined with the complexity of the gene itself. In this study we investigated 3 patients with severe FV deficiency and 2 asymptomatic carriers from 5 different families. We identified 5 novel mutations and a recently published mutation associated with FV deficiency.
Patients 1, 2, and 3 all had severe FV deficiency and undetectable levels of FV. DNA sequence analysis of the FV gene revealed 2 novel frameshift mutations and a novel nonsense mutation associated with an FV null allele. Patients 2 and 3 were both homozygous for a mutation in exon 13, respectively, a 1-bp deletion between nts 4291 and 4294 and a C2491T nonsense mutation. Patient 1 was compound heterozygous for an 8-bp deletion in exon 7 and a missense mutation in exon 15: A5279G, Y1702C (FV Seoul2). The missense mutation was recently described in the heterozygous state as causative for FV deficiency.25 Our study provides further evidence for the postulation that the Y1702C change leads to FV deficiency, because neither FV activity nor FV antigen could be detected in our patient.
The 2 small deletions and the nonsense mutation in the FVgenes of patients 1, 2, and 3 would, if translated, result in truncated FV molecules. The 8-bp deletion between nts 1130 and 1139 in exon 7, −(TG)AAGAGG(TG) (FV Seoul1), in patient 1 results in a frameshift and would code for a peptide of only 350 amino acids long, lacking approximately half the heavy chain and the entire B domain and light chain (Figure 1). In patient 2, the deletion of a cytosine between nts 4291 and 4294 in exon 13 (FV Utrecht) results in a frameshift that would code for a premature translation stop at codon 1381. Consequently, this protein would lack approximately 20% of the B domain and the entire light chain (Figure 1). In patient 3, the C2491T nonsense mutation in exon 13 (FV Casablanca) predicts a premature stop instead of a glutamine at residue 773 and thereby encodes a protein that would consist of a FV heavy chain and only 63 amino acids of the B domain (Figure 1). Thus, this variant FV would lack approximately 90% of the B domain and the complete light chain.
In general, nonsense mRNAs resulting from frameshift and nonsense mutations are highly unstable, because they are subjected to nonsense-mediated mRNA decay (NMD),46-48 preventing the potentially deleterious effects of truncated proteins. Recent studies of FV RNA metabolism demonstrated the absence of RNA of the in trans non-FV Leiden allele in patients who are “pseudo homozygous” for FV Leiden due to either a nonsense mutation21 or a frameshift mutation19 in exon 13. Consequently, the frameshift and nonsense mutations in patients 1, 2, and 3 most likely are associated with an FV null allele. The patients presented with different phenotypes: patients 1 and 2 had moderate bleeding symptoms, whereas patient 3 was asymptomatic. Furthermore, patient 1 suffered from spontaneous muscle bleedings, whereas these were absent in patient 2. This variability in phenotypic expression has previously been observed in other FV-deficient patients. Moreover, as mentioned in earlier reports,49,50 there is a striking discrepancy between mice and man. FV null mice die either in utero or of fatal perinatal hemorrhage,49 whereas human patients with undetectable FV levels do survive. Table4 summarizes the clinical phenotype of all patients with severe FV deficiency described to date who have been characterized at the molecular level. There is a marked difference in age at diagnosis and clinical presentation. As shown by patients I, VI, XI, and XII in Table 4, similar residual amounts of FV are still associated with a different phenotypic expression. Also, individuals with the same mutation, between families (patients I and VI) and even within families (patients II and VII), show a different clinical bleeding pattern. Therefore, the clinical expression of FV deficiency most likely does not depend solely on the type of mutation but also on other, yet unknown, modulating factors.
Previously it has been proposed50,51 that in patients with severe FV deficiency a very low level of expression of FV may occur. Because trace amounts of FV are capable of generating a significant amount of thrombin,52 this would then result in a level of FV synthesis sufficient to mitigate the clinical phenotype. For instance, “ribosomal frameshifting” has previously been recognized as a mechanism able to partially rescue protein synthesis from genes harboring frameshift mutations,53-55and it has also been proposed to play a role in the phenotypic expression of FV deficiency caused by a frameshift mutation in exon 13.50 Mutations in exon 13 occur frequently in theFV gene (Table 5) and because the B domain is relatively unimportant for FV procoagulant function, “ribosomal frameshifting” may thereby reflect one of the modulating factors involved in the phenotypic differences observed in these patients. As shown in Table 4, however, mutations in exon 13 still can lead to a severe phenotypic expression of the disease.
In mammals, the basic mechanism by which nonsense transcripts are recognized and subjected to NMD is still poorly understood.48 Therefore, it cannot be excluded that any residual amount of stable nonsense FV mRNA could actually produce small amounts of (truncated) protein. These molecules could escape detection by the methods used, but still be of interest for their remaining function or potentially harmful effects in the procoagulant and anticoagulant pathways. In humans, the role of NMD as a modifier of the phenotypic consequences of nonsense mutations is becoming increasingly evident, for example, in β-thalassemia.56-58 Clearly, multiple factors are involved in modulating the phenotypic expression of FV deficiency. Therefore, studies using genotypic and phenotypic data are warranted to obtain insight in the mechanisms leading to the observed differences in phenotypic expression of severe FV deficiency.
Patients 4 and 5 were both asymptomatic and carriers of a novel FV null allele. However, because the presence and function of these FV variants could be studied only in individuals heterozygous for the mutation, we cannot exclude the presence of small amounts of mutant protein that would go by undetected because of the wild-type background. In patient 4, a novel nonsense mutation was identified: A1102T, K310Term (FV Amersfoort) that would code for a truncated protein lacking the A2 domain of the heavy chain, the complete B domain and the entire light chain (Figure 1). This mutation was found to cosegregate within the affected family with decreased FV activity and antigen levels. In patient 5, a novel base change was detected: T1927C, C585R (FV Nijkerk) that most likely represents the mutation associated with the decrease in FV activity and antigen levels. This missense mutation cosegregated with FV deficiency in the affected family and was not detected among 100 normal alleles. Although mutant RNA was detected, indicating transcription from the FV Nijkerk allele, FV antigen and activity levels were consistent with heterozygous type I FV deficiency. Apparently, substitution of C585 by arginine results in rapid degradation of FV, either before or immediately after secretion into the circulation. C585 is conserved among human, bovine, and murine FV, but not in FVIII and ceruloplasmin. Analysis of disulfide bridges and free cysteines in bovine FVa have not yielded conclusive data on the state of C589 (C585 in human FV).59 However, homology modeling of FV A domains based on the crystal structure of ceruloplasmin indicates that C585 is not involved in disulfide-bond formation.12 In the FV homology model C585 is completely buried inside the hydrophobic interior of the A2 domain. Substitution of a small and hydrophobic cysteine by a large and hydrophilic arginine would disrupt the tight packing interactions typical of protein interiors. As a consequence, the protein might be unstable and prone to intracellular proteolysis. If stable FV Nijkerk would be synthesized, functionality is doubtful because a potential thrombin cleavage site is created by the introduction of an arginine at residue 585, adjacent to S586. Cleavage by thrombin at this site would result in excision of a fragment from amino acids 585 to the next thrombin cleavage site at R709. This would disrupt the C-terminal end of the A2 domain, resulting in a loss of function or unstable protein.
Noteworthy with respect to the sister of the propositus in the family carrying the FV Nijkerk variant is the fact that her FV levels were consistently lower than in the other carriers of the T1927C mutation (Table 1). This may be explained by the fact that she inherited a FV HR2 allele from her father (data not shown). This variant FV, characterized by the H1299R substitution in the FV B domain, has been associated with reduced FV levels.60-62Phenotypically, she is therefore “pseudo homozygous” for FV HR2, a rare FV genotype that has been reported previously once by Castoldi and coworkers.25 They reported a family in which one member carried a FV missense mutation (Y1702C), associated with absence of the mutant protein in plasma, on the allele in trans of the FV HR2 allele. This family member also had lower FV levels as compared to carriers of only the missense mutation in the family.25
This study brings the total number of mutations associated with FV deficiency described to date to 17. An overview is presented in Table 5where the mutations are listed according to their proposed mode of action. The majority of these mutations have been found in unique families; only the C1690T,22,23 C3571T,28A5279G (this study and reference 25) and G6395A29 have been found in more than one (unrelated) family. FV Seoul1,2 in this study is the first compound heterozygous patient described to date who is fully characterized at the molecular level and in whom 2 rare FV deficiency–associated mutations are combined that completely abolish FV synthesis. Identifying the molecular basis underlying this rare coagulation disorder will help to obtain more insight into the mechanisms involved in the variable clinical phenotype of FV-deficient patients.
The authors wish to sincerely thank Richard Dirven and Rogier Bertina (Department of Hematology, Hemostasis and Thrombosis Research Center, University Medical Center, Leiden, The Netherlands) for providing the FV light chain data and Bruno Villoutreix (Department of Bioinformatics-Blood Coagulation, University of Paris, France) for kindly providing the PDB file containing the model structure of the 3 FV A domains.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Wouter W. van Solinge, Department of Clinical Chemistry, University Medical Center, Postbus 85500, 3508 GA, Utrecht, The Netherlands; e-mail: wsolinge@lab.azu.nl.