Abstract
The activation of discrete T-cell responses depends on the triggering of individualized threshold numbers of T-cell receptors (TCRs). The results of this study indicate that the lipocalin placental protein 14 (PP14), a T-cell inhibitor produced by cells of the reproductive and hematopoietic systems, mediates its anti-inflammatory activity by elevating the T-cell activation threshold, thereby rendering T cells less sensitive to stimulation. Significantly, the data demonstrate hierarchical sensitivity of selected cytokine responses to PP14-mediated inhibition, with the hierarchy reflecting their respective activation thresholds. These findings suggest a novel paradigm for immunoinhibition wherein negative regulators can finely tune, rather than inactivate, T-cell responses, and thereby skew the cytokine output of immunologic responses.
Introduction
The T-cell activation threshold model proposes that (1) T-cell activation ensues once a threshold number of receptors have been triggered, as measured by down-modulation of T-cell receptors (TCRs)1; (2) distinct cytokine and other functional responses within individual T cells are pegged to different levels of TCR occupancy, and hence, there is a hierarchy of individualized thresholds at the single cell level2,3; and (3) the threshold number is flexible, in that costimulation can significantly lower the number of receptors required for individual responses.1 3
Whereas the effect of costimulation on T-cell activation thresholds has been evaluated, the possibility that T-cell inhibitors might also function through the tuning of such thresholds has not been explored. Hence, although T-cell inhibitors are generally presumed to act by blocking activation pathways in an all-or-none fashion, the possibility emerges that at least some of these inhibitors might function in a “rheostatic” manner to desensitize TCR signaling and elevate the number of TCRs required for activation of specific T-cell responses.
Placental protein 14 (PP14; progesterone-associated endometrial protein; glycodelin) is a 28-kd glycoprotein of the lipocalin structural superfamily with documented immunoinhibitory properties.4-7 This glycoprotein is produced by cells of the female and male reproductive tracts and is present at especially high levels in amniotic fluid (AF) and maternal serum.8,9Interestingly, we have demonstrated that PP14 is also present in platelets7 and is released from activated platelets (N. Xiong and M.L.T., unpublished observations, 1996). Significantly, we have recently demonstrated that PP14 directly inhibits human T cells and accounts for the T-cell inhibitory activity of AF.10 Thus, PP14 joins transforming growth factor β (TGF-β) as a second major T-cell immunomodulatory protein produced by platelets. Of note, the set of immunomodulatory proteins known to directly inhibit T cells is quite limited.
Interestingly, in contrast to TGF-β, PP14 targets a proximal event in TCR signaling. This was first suggested by the observation that protein kinase C activators, in combination with a calcium ionophore, can override PP14-mediated T-cell inhibition.10 Our more recent data have established that PP14 reduces the half-life of TCR-triggered phosphotyrosines, and this molecular effect within the proximal side of the TCR activation pathway is, at least in part, a consequence of CD45-dependent dephosphorylation (J.R. et al, submitted for publication). The PP14-induced decrease in the stability of TCR-triggered phosphotyrosines is in marked contrast to the documented opposite effect of the B7/CD28 costimulatory axis.11Because costimulation through CD28 is known to lower the TCR activation threshold,1 3 the opposing effects of PP14 inhibition and CD28 costimulation on phosphotyrosine stability prompted us to ask whether AF, and its active factor PP14, might act by functionally elevating the T-cell activation threshold. The data presented in the present study establish that this novel T-cell immunoinhibitory mechanism is indeed invoked by PP14.
Materials and methods
Cell culture
Peripheral blood mononuclear cells (PBMCs) were purified from the venous blood of healthy donors by density gradient centrifugation as described.7 T cells were isolated from the PBMC pool by first depleting monocytes by adherence to tissue culture flasks and then further purifying the nonadherent T cells with Lympho-Kwik isolation reagent (One Lambda, Canoga Park, CA). The purity of each T-cell preparation was verified by its failure to proliferate in response to phytohemagglutinin (PHA). The cells were maintained in RPMI medium (Whittaker Bioproducts, Walkersville, MD) supplemented with 10% heat-inactivated fetal calf serum (Sigma Chemical, St Louis, MO), 2 mM glutamine, and penicillin/streptomycin.
As a source of accessory cells (ACs), monocytes were isolated from mononuclear cell populations by adherence to plastic. PBMCs (2 × 106 cells/mL) were incubated in serum-free medium for 1 hour at 37°C. The adherent cells were washed extensively with medium to remove any residual nonadherent cells, and fresh complete medium was added. Following a 24-hour incubation period, adherent monocytes were removed by gentle scraping with a plastic cell scraper. Cell viability was more than 80% as determined by trypan blue exclusion. Cells were washed with phosphate-buffered saline (PBS) and fixed for 5 minutes with 0.4% paraformaldehyde (Sigma) in PBS at room temperature. The reaction was quenched by adding an equal volume of 0.2 M l-lysine solution (in PBS). The fixed cells were then washed 3 times with medium and incubated in culture medium at 37°C for 1 hour before adding them to cultures. The Jurkat cell line was obtained from the American Type Culture Collection (ATCC; Manassas, VA).
Cytokine production
Cultures containing 106 peripheral blood T cells, and 106 paraformaldehyde-fixed preactivated monocytes, were plated in 1 mL RPMI containing 10% fetal bovine serum (FBS) in individual wells of a 24-well culture plate. Cells were stimulated with varying PHA concentrations with the indicated amounts of AF for 48 hours. Interleukin-2 (IL-2) and interferon-γ (IFN-γ) levels in the conditioned media were assayed by enzyme-linked immunosorbent assay (ELISA; Genzyme, Cambridge, MA).
Jurkat cells (5 × 105) were cultured in a 1-mL volume of RPMI containing 10% FBS in individual wells of 24-well culture plates, and stimulated with different PHA concentrations, with or without AF, or cyclosporine A (CSA; Sigma) for 20 hours. Chinese hamster ovary (CHO) cells transfected with the glutamine synthetase amplification/expression construct pEE14/hB7-1 were fixed with 0.4% paraformaldehyde for 5 minutes at room temperature. Jurkat cells were stimulated as described above in the presence or absence of 5 × 105/well of these human B7-1–expressing CHO cell transfectants.
Flow cytometry
Down-modulation of TCR was measured by direct immunofluorescence using fluorescein isothiocyanate (FITC)–anti-CD3 monoclonal antibody (mAb; UCTH1). Similar results were obtained with indirect immunofluorescence (unpublished data, February 1998). The data were calculated using the mean fluorescence values of the cell. For intracellular cytokine staining, monensin (2 mM) was added in the last 5 hours of a 48-hour culture. Cells were fixed and coimmunostained with phycoerythrin (PE)–anti–IL-2 and FITC–anti–IFN-γ antibodies (Pharmingen, San Diego, CA) as described,3 and then analyzed, (1 × 104 cells/sample) on a FACScan flow cytometer (Becton Dickinson, San Jose, CA) using Lysis II software.
Cell sorting
Jurkat cells were stained with FITC-conjugated UCTH1 mAb and then sorted into 2 fractions of low and high expressors. Cells were incubated for 4 days, and the level of CD3 expression was confirmed by flow cytometric analysis. Cells were cultured as described above and were stimulated with a combination of protein A beads bearing immobilized anti-CD3 mAb (OKT3) and soluble anti-CD28 mAb (9.3; 1 μg/mL).
AF samples and PP14 immunoabsorption
Discarded human AF samples were obtained from the Center for Human Genetics Laboratory at University Hospitals of Cleveland and stored at −80°C. Samples obtained from several patients (collected at 14-16 weeks of gestation) were pooled and filter-sterilized before use. Anti-PP14 polyclonal antibodies7 were coupled to protein A-Sepharose beads (Sigma) to generate an immunoabsorbent. Control beads consisted of protein A-Sepharose beads coupled to preimmune rabbit serum. Immunoabsorption was carried out by adding AF to antibody-coupled beads and incubating the mixture overnight at 4°C with gentle rotation. The beads were pelleted by centrifugation, and the supernatant was filtered and used in assays as described. The presence of PP14 was verified by Western blotting.
Production of PP14 · Fcγ1
The PP14 complementary DNA (cDNA),7 including the signal sequence, was fused to the 5′ end of the cDNA for the hinge, CH1 and CH2 domains of human IgG1 in the vector pIgG/REP7β.12 pPP14 · Fcγ1/REP7β was transfected into 293 cells (ATCC). Stable transfectants secreting PP14 · Fcγ1 were grown in Ultraculture (Whittaker Bioproducts) supplemented with Hygromycin (200 μg/mL; Calbiochem, La Jolla, CA) at 37°C with 5% CO2. To purify the protein, conditioned media was mixed with an equal volume of binding buffer (3.0 M sodium chloride; 1.5 M glycine, pH 8.6). Protein A-Sepharose (Sigma) was added and the suspension was mixed overnight at 4°C. The matrix was collected and washed with 10 column volumes of 100 mM citrate pH 5.0. PP14 · Fcγ1 was eluted with 100 mM citrate, pH 3.0, into 0.4 volumes of 0.2 M sodium phosphate (dibasic). The purified protein was concentrated and buffer exchanged into PBS using Centricon 10 (Millipore, Bedford, MA).
Results
To test whether PP14 elevates T-cell activation thresholds, we evaluated the effects of AF on IL-2 secretion by Jurkat cells stimulated with increasing concentrations of PHA. In the absence of AF, PHA induced dose-dependent IL-2 secretion (reaching a plateau at approximately 1.25 μg/mL PHA) (Figure1A). AF increased the concentration of PHA required for a minimal measurable IL-2 response (Figure 1A). Interestingly, and as expected for a threshold-centered mechanism, the AF-mediated inhibitory effect was inversely correlated with activator concentration, with the highest concentrations of PHA totally attenuating the inhibitory effect of AF (Figure 1A). Higher AF concentrations (50%) resulted in a requirement for higher PHA concentration (5 μg/mL) to override the inhibition (data not shown). Similar results were obtained with cells stimulated with solid-phase OKT3 and CD28 mAbs (data not shown). Moreover, immunodepletion of PP14 from the AF abrogated its immunoinhibitory activity, verifying the PP14 dependence of the observed AF inhibitory effect (Figure 1A).
The inhibitory activity of AF, but not CSA, is inversely correlated to stimulus and is overridden by increasing receptor engagement.
Jurkat cells were stimulated with various concentrations of PHA in the absence (▪) or presence of either AF (25% ■) or PP14-depleted AF (▴) (A); or in the presence of CSA (100 pg/mL [■] or 2 ng/mL [▵]) (B). IL-2 secretion was determined in the conditioned medium. The data represent the mean of triplicate samples. Comparable results were obtained in 4 separate experiments. (C) Change in absolute IL-2 levels as a function of PHA concentration in the presence of either AF (25% ▪) or CSA (100 pg/mL [■] or 2 ng/mL [▵]), as calculated from the data in panels A and B.
The inhibitory activity of AF, but not CSA, is inversely correlated to stimulus and is overridden by increasing receptor engagement.
Jurkat cells were stimulated with various concentrations of PHA in the absence (▪) or presence of either AF (25% ■) or PP14-depleted AF (▴) (A); or in the presence of CSA (100 pg/mL [■] or 2 ng/mL [▵]) (B). IL-2 secretion was determined in the conditioned medium. The data represent the mean of triplicate samples. Comparable results were obtained in 4 separate experiments. (C) Change in absolute IL-2 levels as a function of PHA concentration in the presence of either AF (25% ▪) or CSA (100 pg/mL [■] or 2 ng/mL [▵]), as calculated from the data in panels A and B.
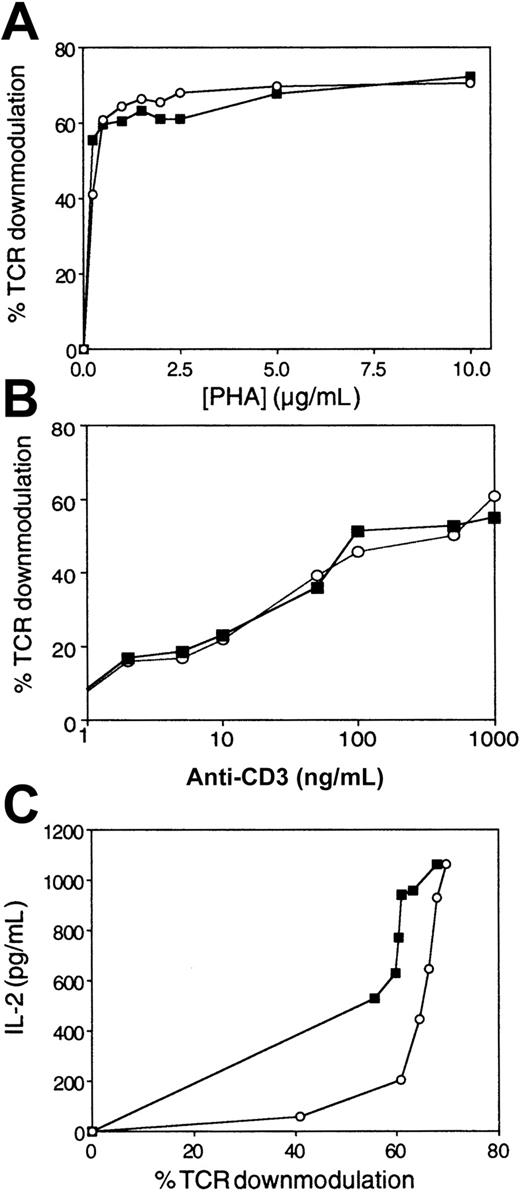
We next determined whether the pattern of inhibition in Figure 1A, characterized by override of inhibition with increased TCR triggering, is unique to PP14, or instead, is associated with any T-cell inhibitory agent used at nonsaturating concentrations. To test this possibility, we used CSA, an intensively studied inhibitor of the Ca++-dependent phosphatase, calcineurin, which blocks IL-2 induction.13 14 When used at saturating concentrations, CSA totally blocked IL-2 secretion at all PHA doses tested (data not shown). IL-2 secretion in the presence of nonsaturating CSA concentrations, which attenuate without fully blocking signal transmission, reached a plateau at a similar PHA concentration to untreated cells, but as expected, at lower absolute IL-2 levels (Figure1B). In marked contrast to AF, the CSA inhibitory effect was not inversely correlated to PHA concentration, and most significantly, could not be overcome by increasing TCR occupancy. The difference between PP14 and CSA is highlighted in Figure 1, panel C, in which changes in IL-2 levels, as a function of PHA concentration, have been recalculated from the data in Figure 1, panels A and B. The plot demonstrates the marked difference between the 2 T-cell inhibitors. Whereas CSA results in greater reduction in IL-2 production with increasing PHA concentrations, AF/PP14-mediated inhibition is characterized by less of a change in the IL-2 response as the PHA stimulus increases.
In light of these results (Figure 1A), which showed a shift in the dose-response curve typical of an increase in activation threshold, the effect of AF/PP14 on the number of triggered TCRs was next tested. We simultaneously evaluated the effects of AF on TCR triggering and IL-2 secretion by Jurkat cells stimulated with increasing concentrations of PHA. In the absence of AF, PHA induced dose-dependent TCR down-modulation (as measured by loss of surface CD3) (Figure2A), consistent with previous observations using other TCR ligands.1 15 AF, though inhibiting IL-2 secretion (Figure 1A), did not significantly change the number of engaged TCRs (Figure 2A). Similar results were obtained when peripheral T cells were stimulated with various concentrations of either PHA (data not shown) or solid-phase OKT3 (Figure 2B). Together, these observations establish that the number of triggered TCRs required for inducing a response is elevated in the presence of AF (Figure 2C).
AF elevates the threshold number of TCRs required for IL-2 secretion.
Either Jurkat cells or T cells were stimulated with various concentrations of PHA or solid-phase OKT3 in the absence (▪) or presence of AF (○). (A) Percentage of TCR down-modulation in Jurkat cells as a function of PHA concentration, as measured by CD3 immunofluorescence and flow cytometry. (B) Percentage of TCR down-modulation in T cells as a function of OKT3 concentrations. (C) IL-2 secretion by Jurkat cells as a function of the percentage of TCR down-modulation, as calculated from the data in Figure 1, panel A, and panel A herein. The data represent the mean of triplicate samples. Comparable results were obtained in 3 separate experiments.
AF elevates the threshold number of TCRs required for IL-2 secretion.
Either Jurkat cells or T cells were stimulated with various concentrations of PHA or solid-phase OKT3 in the absence (▪) or presence of AF (○). (A) Percentage of TCR down-modulation in Jurkat cells as a function of PHA concentration, as measured by CD3 immunofluorescence and flow cytometry. (B) Percentage of TCR down-modulation in T cells as a function of OKT3 concentrations. (C) IL-2 secretion by Jurkat cells as a function of the percentage of TCR down-modulation, as calculated from the data in Figure 1, panel A, and panel A herein. The data represent the mean of triplicate samples. Comparable results were obtained in 3 separate experiments.
These results clearly demonstrate that AF increases the relative TCR occupancy required for elicitation of a specific cytokine (IL-2) response. Therefore, by increasing the absolute number of triggered TCRs (via increasing stimulus concentrations), one can override the AF-mediated inhibitory effect. This AF effect, dependent on the presence of PP14, contrasts with the previously described effects of costimulators, which function in an opposite fashion to lower the number of triggered TCRs required for T-cell activation.1,3 Furthermore, the AF/PP14 effect differs from that of partial agonists, with the lower response of the latter attributable to fewer triggered TCRs,15 and from that of CSA, the inhibitory activity of which does not depend on the extent of TCR occupancy.
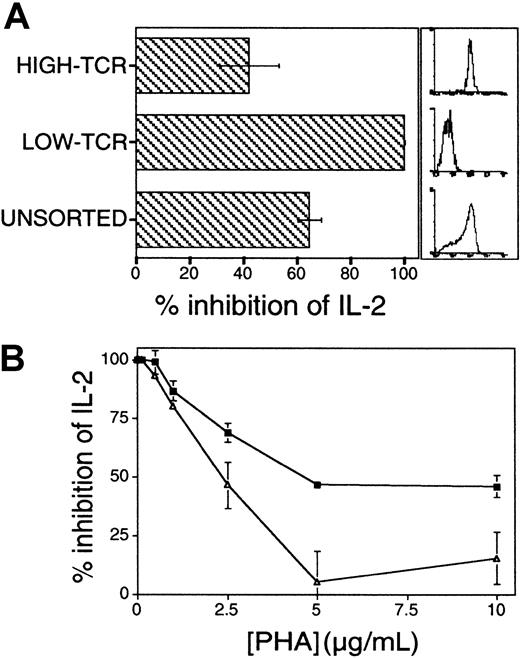
Additional experiments were performed to support the proposed functional link between AF/PP14 and T-cell activation thresholds. According to the threshold model, the capacity of a given T cell to respond to a defined concentration of stimulus is positively correlated with the absolute number of TCRs on that cell.1 16 Jurkat cells were preparatively sorted into high and low TCR expressors, and the cells were then stimulated with solid-phase OKT3 and CD28 mAbs. The respective susceptibilities of these sorted Jurkat cell subpopulations to inhibition by AF/PP14 were compared. As expected, low TCR expressors secreted significantly lower levels of IL-2 at each OKT3 concentration, as compared to Jurkat cells expressing high levels of TCR; at low concentrations of OKT3, the low TCR expressors did not respond at all (data not shown). Significantly, Jurkat cells expressing low levels of TCR were more sensitive to AF-mediated inhibition of IL-2 secretion at all OKT3 concentrations (data not shown), and this was most prominent at OKT3 concentrations in the lower range, where inhibition by AF reached 100% in the low-TCR cells (Figure 3A). Thus, sensitivity to inhibition is inversely proportional to TCR numbers at the cell surface. The sensitivity to inhibition by AF was inversely correlated with agonist concentration in the earlier experiments (Figure 1A), and with the number of available TCRs in the latter experiments (Figure3A). In both instances, the magnitude of activation and the sensitivity to inhibition is eventually determined by the number of TCRs engaged.
Influence of absolute numbers of surface TCRs and costimulation on sensitivity to inhibition by AF.
(A) Absolute numbers of TCRs determine sensitivity to inhibition by AF. Jurkat cells expressing low or high levels of TCRs were preparatively sorted and stimulated with solid-phase OKT3 and anti-CD28 mAbs in the presence or absence of AF (25%). Histograms on the right show the relative CD3 immunostaining of the sorted cell populations. Comparable results were obtained in 2 repeat experiments in which cell populations were sorted independently. (B) Costimulation lowers the threshold number of TCRs required for IL-2 production and renders cells less sensitive to inhibition by AF. Jurkat cells were stimulated with varying concentrations of PHA, with (▵) or without (▪) addition of B7-1–transfected CHO cells. Results in panels A and B are presented as a percentage of inhibition of IL-2 secretion.
Influence of absolute numbers of surface TCRs and costimulation on sensitivity to inhibition by AF.
(A) Absolute numbers of TCRs determine sensitivity to inhibition by AF. Jurkat cells expressing low or high levels of TCRs were preparatively sorted and stimulated with solid-phase OKT3 and anti-CD28 mAbs in the presence or absence of AF (25%). Histograms on the right show the relative CD3 immunostaining of the sorted cell populations. Comparable results were obtained in 2 repeat experiments in which cell populations were sorted independently. (B) Costimulation lowers the threshold number of TCRs required for IL-2 production and renders cells less sensitive to inhibition by AF. Jurkat cells were stimulated with varying concentrations of PHA, with (▵) or without (▪) addition of B7-1–transfected CHO cells. Results in panels A and B are presented as a percentage of inhibition of IL-2 secretion.
Next, the interplay between AF/PP14-mediated inhibition and costimulation was examined. Because costimulatory signals delivered to CD28 on T cells are known to lower the activation threshold,1 3 we predicted that such threshold-lowering costimulatory signals would counteract any increase in activation threshold mediated by AF/PP14. To this end, PHA-stimulated Jurkat cells were inhibited with AF in the presence or absence of human B7-1–expressing CHO cell transfectants. Addition of B7-1 transfectants markedly augmented IL-2 secretion at each level of TCR stimulus (data not shown). Importantly, the presence of B7-1 costimulator markedly reduced AF-mediated inhibition of IL-2 secretion (Figure 3B). Similar results were obtained when anti-CD28 mAbs (9.3) were used in similar assays, instead of B7-expressing CHO cells (data not shown), and hence, the observed effects are not attributable to the CHO cells per se. Taken together, the findings that AF-mediated inhibition can be reduced by either increasing TCR numbers on the cell surface or by lowering activation thresholds through costimulation, are consistent with the notion that this inhibition operates via rheostatic modulation of activation thresholds.
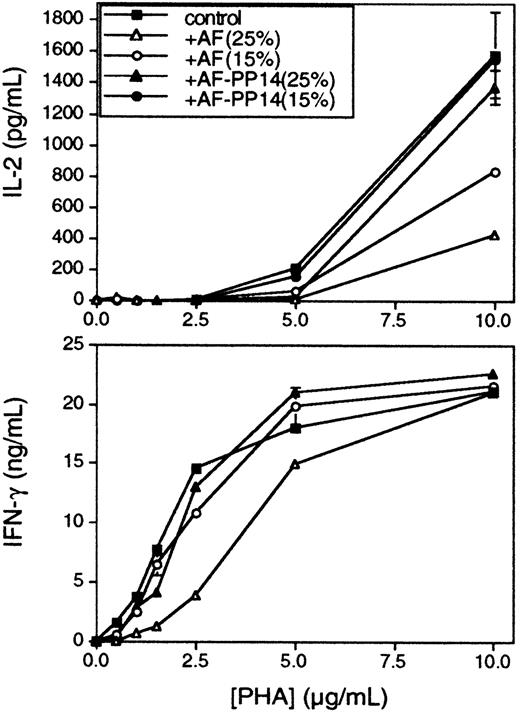
Subsequent experiments used peripheral blood T cells in place of Jurkat cells as responders. As with Jurkats, the number of TCRs down-modulated in anti-CD3–induced T cells was not significantly changed in the presence of AF (Figure 2B). In the case of Jurkat cells, there is no significant difference in the activation thresholds for the cytokines IL-2 and IFN-γ, and consequently, AF does not differentially affect the secretion of these cytokines (data not shown). On the other hand, in the case of peripheral T cells, a lower PHA concentration was required to elicit IFN-γ production (Figure4, bottom panel), as compared with IL-2 (Figure 4, top panel), confirming earlier data suggesting distinct TCR signaling thresholds for each of these cytokine responses.3 Significantly, whereas the inhibition of both IFN-γ and IL-2 responses by AF was inversely correlated to PHA concentration, the 2 responses exhibited differential sensitivity to AF-mediated inhibition, in accordance with their known TCR signaling thresholds. Hence, IFN-γ was inhibited only at relatively high concentrations of AF, whereas IL-2 was more susceptible to AF-mediated inhibition, even at lower AF concentrations. Furthermore, the effect on IFN-γ was reversed at PHA concentrations of 10 μg/mL, whereas the effect on IL-2 was not reversed at this concentration (Figure 4), nor even at the highest concentration of PHA tested (20 μg/mL; data not shown). Again, no significant inhibition was observed when the AF was immunodepleted of its PP14 (Figure 4). Similar results were obtained using flow cytometric analysis of intracellular IL-2 and IFN-γ expression in individual cells (data not shown). The data demonstrate a hierarchical sensitivity to AF-mediated inhibition for different cytokines, and the observed hierarchy is consistent with their respective, previously established activation thresholds. Therefore, it seems that the higher the activation threshold is for a given T-cell response, the more sensitive this response to inhibition by PP14. Similar analyses of several other responses will be required to further substantiate the correlation between the response activation threshold and its respective sensitivity to inhibition by PP14.
Hierarchical inhibition by AF of IFN-γ and IL-2 production in peripheral blood T cells.
Peripheral blood T cells were stimulated in the presence of fixed activated monocytes, with increasing PHA concentrations, with or without AF or PP14-depleted AF at the indicated concentrations. Conditioned media were collected 48 hours later, and IL-2 (upper panel) and IFN-γ (lower panel) secretions were determined by ELISA. Comparable results were obtained in 4 separate experiments.
Hierarchical inhibition by AF of IFN-γ and IL-2 production in peripheral blood T cells.
Peripheral blood T cells were stimulated in the presence of fixed activated monocytes, with increasing PHA concentrations, with or without AF or PP14-depleted AF at the indicated concentrations. Conditioned media were collected 48 hours later, and IL-2 (upper panel) and IFN-γ (lower panel) secretions were determined by ELISA. Comparable results were obtained in 4 separate experiments.
To verify that the observed pattern of inhibition (ie, inversely related to stimulus concentration) is due to PP14 per se, and not a consequence of the AF milieu, a recombinant PP14 · Fcγ1 fusion protein that we produced (G.J.R., manuscript in preparation) was used in an analogous experiment. PP14 · Fcγ1 was used because Fc fusion proteins generally feature extended half-lives and are readily produced in high quantity. PP14 · Fcγ1 inhibited IL-2 secretion from either PHA-induced or immobilized OKT3-induced Jurkat cells (data not shown), as well as OKT3-induced T-cell proliferation (Figure5). The pattern and extent of inhibition by PP14 · Fcγ1 (48 μg/mL; the molar equivalent to 16 μg/mL native PP14), characterized by decreased inhibition with increased stimulus dose, is the same as that seen with a similar amount of PP14 in AF (12.5%; which corresponds to an estimated PP14 concentration of approximately 12.5 μg/mL).
PP14.Fcγ1 increases OKT3 dose required for T-cell activation.
Purified T cells were stimulated with increasing amounts of solid-phase OKT3 mAbs either alone (♦), in conjunction with anti-CD28 mAb (▪), in conjunction with anti-CD28 mAb plus AF (12.5% vol/vol; ■), or in conjunction with anti-CD28 mAb plus purified PP14 · Fcγ1 (48 μg/mL; ▵). [3H]-thymidine incorporation was determined 72 hours later as described in the legend for Figure 1. Comparable results were obtained in 3 experiments.
PP14.Fcγ1 increases OKT3 dose required for T-cell activation.
Purified T cells were stimulated with increasing amounts of solid-phase OKT3 mAbs either alone (♦), in conjunction with anti-CD28 mAb (▪), in conjunction with anti-CD28 mAb plus AF (12.5% vol/vol; ■), or in conjunction with anti-CD28 mAb plus purified PP14 · Fcγ1 (48 μg/mL; ▵). [3H]-thymidine incorporation was determined 72 hours later as described in the legend for Figure 1. Comparable results were obtained in 3 experiments.
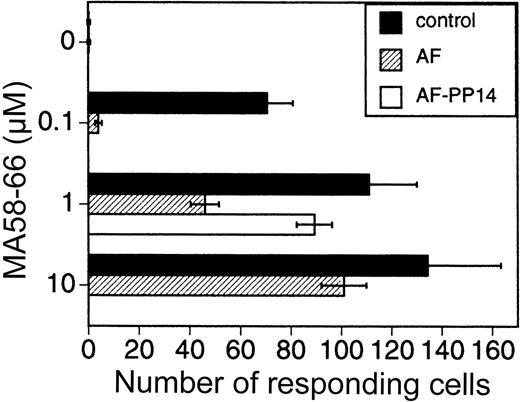
The previous set of experiments demonstrated the effect of AF/PP14 on activation thresholds of PHA- and CD3-induced cells. We next determined the effect of AF/PP14 on antigen-induced cells. For this purpose PBMCs were stimulated with the influenza virus matrix peptide antigen (MA 58-66),17 and the number of IFN-γ–producing cells was determined by ELISAspot.18 19 The number of cells responding to the peptide antigen stimulus was lowered in the presence of AF. This AF-mediated inhibitory effect was attenuated by increasing antigen concentrations, as well as by depleting PP14 from the AF (Figure 6). Thus, activation threshold modulation by AF/PP14 is not limited to polyclonal T-cell activators, but is also observed with a more physiologic antigenic stimulus.
AF increases the dose of peptide antigen required for T-cell activation.
PBMCs were stimulated for 24 hours with various MA58-66 peptide antigen concentrations, with or without either AF (10%) or AF immunodepleted of its PP14. The number of cells producing IFN-γ was determined by ELISAspot.18 19
Discussion
This study clearly demonstrates that AF, and its active immunoregulatory factor PP14, function to increase the number of triggered TCRs required for T-cell activation. As predicted by a model wherein PP14 increases T-cell activation thresholds, the present findings establish that inhibition is sensitive to: (1) the extent of TCR triggering (and thus can be overridden by increasing the dose of stimulus); (2) the number of TCRs expressed on the cell surface; (3) the combined presence of costimulators that reciprocally lower activation thresholds; and (4) the hierarchical threshold of the particular response being measured. In contrast to AF/PP14, the inhibitory activity of CSA, even at nonsaturating concentrations, was not attenuated with increasing PHA doses. Hence, activation threshold modulation is not a common feature of all T-cell inhibitors, although this functional property may ultimately prove to extend beyond PP14. CSA functions by blocking the activity of the phosphatase calcineurin, resulting in a failure to activate IL-2.14 By contrast, it would seem that the mode of action of PP14 is to attenuate rather than to eliminate or block downstream signaling events, resulting in functionally increased activation thresholds.
In the present study we demonstrated a PP14-mediated shift in the dose-response curve to a TCR stimulus. This shift does not stem from a lower number of engaged TCR molecules, because the same number of TCR molecules were down-regulated in the absence or presence of PP14. Likewise, costimulation through CD28, which also functions by modulating the TCR activation threshold (in this case, lowering it1), was shown to do so without affecting the number of TCR triggering events.11 That said, it should be noted that TCR down-regulation does not necessarily give a complete picture of TCR engagement, because only those engagement events that result in full ζ-chain and ZAP-70 phosphorylation lead to receptor down-regulation.20 Thus, the extent of TCR engagement is substantially underestimated when down-regulation is the readout, with interactions leading to partial signaling events not translated into internalization events. The failure of PP14 to inhibit TCR engagement or down-regulation, while inhibiting other T-cell activation events, most probably puts the signaling defect events downstream of ζ-chain and ZAP-70.
Our mechanistic insight into PP14's threshold modulation activity at the cellular level provides a useful framework for further exploring the intracellular pathways influenced by this molecular factor. In fact, in a parallel series of experiments, we have recently shed some light on the molecular underpinnings of the threshold effect and the consequent desensitization of receptor signaling that we have formally documented here. Specifically, our other data demonstrate that PP14 decreases the half-lives of TCR-triggered phosphotyrosines by promoting their dephosphorylation in a CD45-dependent fashion (J.R. et al, manuscript submitted for publication). In this way, PP14's effect on T cells contrasts with that of the B7/CD28 costimulation axis that stabilizes tyrosine phosphates linked to TCR activation.11This is significant from our standpoint because, as we have shown here, PP14 and costimulation also have opposing effects on TCR activation thresholds (Figure 3).1 3 Modulation of the stability of TCR-triggered phosphoproteins provides a compelling explanation at the molecular level for TCR activation threshold modulation by PP14, whereby the balance between tyrosine kinase and phosphatase activity sets the T-cell activation threshold.
In aggregate, the data point to a novel immunoregulatory mechanism for T-cell inhibition based on the modulation of T-cell activation thresholds. By this mechanism, immunoregulatory factors (in this case, PP14) may dictate not only quantitative, but also qualitative, aspects of immune responses. This is highlighted by our demonstration that PP14 differentially influences cytokines according to their activation thresholds (such as IFN-γ versus IL-2). It is especially interesting to note, in this regard, that pregnancy is associated with a markedly altered immunologic status, encompassing among other things the improvement of clinical manifestations in patients with autoimmune diseases and a skewing of the cytokine profile (from a Th1 to Th2 phenotype).21 Interestingly in this regard, IL-4, a Th2-like cytokine, was reported to have a lower activation threshold than IFN-γ, the paradigmatic Th1-like cytokine,22,23 and thus is expected to be less sensitive to inhibition by PP14. Because PP14 is highly expressed in maternal serum, endometrial decidua, and AF during early to mid gestation, it could well explain, at least in part, these alterations in the gravid immune status (without generalized immunosuppression). Of note, the concentrations of PP14 reported for maternal serum8 are in the order of that used in our in vitro experiments. Interestingly, previous reports have demonstrated that a factor(s) present in serum from pregnant women is capable of inhibiting proliferation and the production of IL-2 during lymphocyte activation.24 25 Furthermore, it will be of interest to determine whether platelet-derived PP14, by virtue of its unique T-cell immunoregulatory properties, accounts for at least some of the immunologic perturbations associated with disseminated intravascular coagulation and other coagulopathies.
The authors thank Drs M. Tary-Lehmann, P. V. Lehmann, and R. Trezza for assistance with ELISAspot assays. We appreciate Dr R. Germain's (National Institutes of Health) and Dr M. I. Greene's (University of Pennsylvania) review of the manuscript. We thank Dr M. Lamm for his long-term support, and Drs A. Hochberg and N. de Groot for helpful discussions. We also thank Ms Susi Brill for her expert secretarial assistance.
Supported by grant RO1 AI 38960 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mark L. Tykocinski, Department of Pathology and Laboratory Medicine, University of Pennsylvania, 6 Gates Bldg, 3400 Spruce St, Philadelphia, PA, 19104-4283; e-mail:mlt4@mail.med.upenn.edu.

![Fig. 1. The inhibitory activity of AF, but not CSA, is inversely correlated to stimulus and is overridden by increasing receptor engagement. / Jurkat cells were stimulated with various concentrations of PHA in the absence (▪) or presence of either AF (25% ■) or PP14-depleted AF (▴) (A); or in the presence of CSA (100 pg/mL [■] or 2 ng/mL [▵]) (B). IL-2 secretion was determined in the conditioned medium. The data represent the mean of triplicate samples. Comparable results were obtained in 4 separate experiments. (C) Change in absolute IL-2 levels as a function of PHA concentration in the presence of either AF (25% ▪) or CSA (100 pg/mL [■] or 2 ng/mL [▵]), as calculated from the data in panels A and B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/13/10.1182_blood.v98.13.3727/6/m_h82411873001.jpeg?Expires=1769277453&Signature=0aYHcrPMY~SlgL6llklcrmsae8hl2dciQUx6WEzx87bh708nUGMoro3H~CWxYYc~~C~krqN906Su9G7RK6LtW7aTQP177TVVe5Jwr543c53vnd3qpzTpvaDc5e4L8v2IwqnjYKt-gYezhGi7I879TaJ-puODTIhgzZM-6brdvQebpMFCOZEXFrNiO5NwCJ5KeTs8oIE9QBCuPt8lCoc6YuktaEnBjnw3zgve9UBw3m5utT3qj9iS8SzVIdJZyD7oh7-Inf1rK25PkIWPq33Kpaco3J5nigmkyPeNg38H6UiXIDH-WIvI3cPz5U-3zK8UUukEAzsMqxIx14v44MWlRQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. PP14.Fcγ1 increases OKT3 dose required for T-cell activation. / Purified T cells were stimulated with increasing amounts of solid-phase OKT3 mAbs either alone (♦), in conjunction with anti-CD28 mAb (▪), in conjunction with anti-CD28 mAb plus AF (12.5% vol/vol; ■), or in conjunction with anti-CD28 mAb plus purified PP14 · Fcγ1 (48 μg/mL; ▵). [3H]-thymidine incorporation was determined 72 hours later as described in the legend for Figure 1. Comparable results were obtained in 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/98/13/10.1182_blood.v98.13.3727/6/m_h82411873005.jpeg?Expires=1769277453&Signature=EKQEGlAu2Ew-8A2X1Szqv8~nLEW5Ag~Yu0Z~6pc2AGnbj~Iz6U2Sq~noa7hme5w4wMFu435I6X-DDqqN-cZ6HMYzblrHs1BAgCj9zR3~G8jGB3LX9MO9cyKi7do9AonkST-S4PD9JR2F-iPnoMgBQxHv~YloyCx7PCh7fxRgtFN8-~8-P6Q5UJJq8ulKBLqgm2uL4JaO~msubsNS4DNQJ99HCwzFVTiLCO~EyziJyu5wvJ3tKRCbp5kM9nrdZuMjOJSMGh4BmkMxd7x5-TDzyl6~9paz~Ralv5MGFq-Y0Ruo~jrXZE0W3Set~FOtOZ2wLLvqhW-3fUKAm5JAMbReqw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)