Abstract
Abnormal isoforms of the prion protein (PrPSc) that cause prion diseases are propagated and spread within the body by “carrier” cell(s). Cells of the immune system have been strongly implicated in this process. In particular, PrPSc is known to accumulate on follicular dendritic cells (FDCs) in individuals affected by variant Creutzfeld-Jakob disease. However, FDCs do not migrate widely and the natural history of prion disorders suggests other cells may be required for the transport of PrPSc from the site of ingestion to lymphoid organs and the central nervous system. Substantial evidence suggests that the spread of PrPSc requires bone marrow-derived cells that express normal cellular prion protein (PrPC). This study examined the expression of PrPC on bone marrow–derived cells that interact with lymphoid follicles. High levels of PrPC are present on myeloid dendritic cells (DCs) that surround the splenic white pulp. These myeloid DCs are ontologically and functionally distinct from the FDCs. Consistent with these observations, expression of PrPC was strongly induced during the generation of mature myeloid DCs in vitro. In these cells PrPCcolocalized with major histocompatibility complex class II molecules at the level of light microscopy. Furthermore, given the close anatomic and functional connection of myeloid DCs with lymphoid follicles, these results raise the possibility that myeloid DCs may play a role in the propagation of PrPSc in humans.
Introduction
The recent emergence of variant Creutzfeld-Jakob Disease (vCJD) in humans has raised the specter of large-scale primary and secondary infections in human populations.1-4 In late stages of vCJD infection, abnormal prion protein (PrPSc) accumulates, particularly in brain, leading to a distinctive clinical and pathologic disorder.5However, preventive and therapeutic strategies will be most effective if targeted at the initial pathways through which ingested PrPSc is propagated within the body and transferred to nerve tissue.
It is now widely accepted that follicular dendritic cells (FDCs) have an important role in the pathogenesis of prion disease. Histopathologic studies of mice and other species exposed to PrPSc have highlighted the accumulation of PrPSc on the FDCs in lymphoid follicles.6-8 Furthermore, in a mouse model, it has now been shown that FDCs are required for the replication of PrPSc that precedes neuroinvasion.9,10 In support of this view, B-cell–deficient mice, which fail to form functional lymphoid follicles,11,12 are resistant to PrPSc infection. However, involvement of FDCs alone is not sufficient to explain how infective forms of the prion protein are transferred from the gastrointestinal tract to the lymphoid organs and the central nervous system (CNS). FDCs reside within B-cell areas of lymphoid organs where they bind and retain antigens on their cell surface over months or even years.13 The cells have a slow turnover and are not believed to recirculate. Moreover, cellular (PrPC) has not been found to be associated with the FDCs of enteric tissue.14
It is important therefore to understand how FDCs in central lymphoid organs become exposed to PrPSc and how PrPScmay be transferred from FDCs to other tissues. In this regard, evidence suggests that circulating cells may have a role. Results from animal models have emphasized that infective material can be isolated from the cellular fraction of the spleen soon after the ingestion of PrPSc,15 whereas in mice, marrow-derived cells have been shown to be required for the propagation and spread of PrPSc.16
Attention has therefore focused on the circulating cells that express normal PrPC and that may therefore act as carrier cells for the spread and circulation of its abnormal isoform.17,18In humans, peripheral B and T lymphocytes express PrPC at low levels, whereas higher levels of PrPC have been found on subsets of natural killer (NK) cells, platelets, and monocytes.19-21 However, the expression of PrPC on blood cells in the peripheral circulation does not provide a full description of the possible routes of propagation and spread of PrPSc. Recent evidence suggests that the expression of PrPC may vary with the developmental stage of cells and with their activation state21 22; thus the expression of PrPC on circulating cells will not necessarily reflect the expression of the molecule on the derived, differentiated, or activated cells within tissues.
Understanding the cellular expression of the normal form of the prion protein (PrPC) within lymphoid tissues may provide important clues to the pathogenesis of prion disorders. We have therefore studied the expression of PrPC on cells within human spleen, focusing particularly on those cells that interact closely with lymphoid follicles and with FDCs. We describe a distinctive cellular and intracellular distribution of PrPCthat may have important implications for its normal function and the spread of its abnormal isoforms(s).
Materials and methods
Immunohistochemistry and immunocytology
Human tissue samples were obtained from the Pathology Department at the John Radcliffe Hospital, Oxford. Formalin-fixed tissue sections were dewaxed and rehydrated. Sections were heat-treated with 2 mM HCl and PrPC was visualized using antihuman PrPC monoclonal antibody (clone 3F4). Signal was amplified and visualized using the ABComplex system (Dako, Cambridge, United Kingdom).
Cryostat tissue sections of spleen (8 μm) were fixed in acetone and examined using dual-color immunofluorescence. Cell surface antigens were detected with the antibodies shown in Table1.
Antibodies used to detect cell surface antigens
| Antigen . | Antibody/use* . | Source† . | Recognition . |
|---|---|---|---|
| PrP | 6H4 (fl, cyt, WBs) | 1 | |
| PrP | R29 (cryo) | 1 | |
| PrP | 3F4 (paraffin) | 2 | |
| CD3 | 3D4 (cryo) | 3 | T cells |
| CD11c | KB40 (cryo) | 3 | DC and macrophage |
| CD13 | WM47 (cryo) | 3 | Myeloid origin (DC and macrophage) |
| CD20 | L26 (cryo) | 3 | B cells |
| CD21 | CR4/23 (cryo) | 3 | FDCs (CD21 isoform) |
| CD68 | PGM1 (cryo) | 3 | Macrophages (high) |
| CD83 | HB15 (cryo) | 3 | DCs |
| HLA II | DK22 (cyt) | 2 |
| Antigen . | Antibody/use* . | Source† . | Recognition . |
|---|---|---|---|
| PrP | 6H4 (fl, cyt, WBs) | 1 | |
| PrP | R29 (cryo) | 1 | |
| PrP | 3F4 (paraffin) | 2 | |
| CD3 | 3D4 (cryo) | 3 | T cells |
| CD11c | KB40 (cryo) | 3 | DC and macrophage |
| CD13 | WM47 (cryo) | 3 | Myeloid origin (DC and macrophage) |
| CD20 | L26 (cryo) | 3 | B cells |
| CD21 | CR4/23 (cryo) | 3 | FDCs (CD21 isoform) |
| CD68 | PGM1 (cryo) | 3 | Macrophages (high) |
| CD83 | HB15 (cryo) | 3 | DCs |
| HLA II | DK22 (cyt) | 2 |
fl indicates indirect immunofluorescence and flow cytometry; cyro, indirect immunofluorescence on frozen sections; paraffin, indirect immunofluorescence on paraffin section; WBs, Western blots.
Antibodies used in the detection of PrPc in tissues or slide preparations, together with antibodies used for cellular recognition. A wider range of antibodies for the characterization of DCs by indirect immunofluorescence is given in “Materials and methods” under generation and assessment of DCs.
Sources of antibodies were 1, Prionics (Zurich, Switzerland); 2, Dako (Cambridge, United Kingdom); and 3, LRF Immunodiagnostics Unit (Oxford, United Kingdom).
Antibody-labeled cells were then detected using class-specific goat antisera to either mouse or rabbit immunoglobulin (fluorescein isothiocyanate [FITC] or Texas red conjugated as indicated) (Cambridge Bioscience, Cambridge, United Kingdom). Fluorescence-labeled sections were viewed and images captured using standard immunofluorescence microscopy and video capture equipment.
The immunocytology of monocyte-derived dendritic cells (DCs) was also studied. DCs were cultured (see below), washed 4 times with cold phosphate-buffered saline (PBS), and allowed to attach and spread on glass slides or coverslips for 60 minutes. Preparations were air dried and fixed in acetone/methanol. DCs were immunostained and visualized as described above for cryostat sections.
Western blot analysis
Tissue samples or centrifuged cell pellets were lysed directly in boiling sample buffer. Protein concentrations were compared using scanning densitometry of Coomassie-stained gels. Human serum was diluted 1:2 in PBS and mixed with double-strength sample buffer. Samples were run on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membrane. PrPC was detected using Mab 6H4 and goat antimouse horseradish peroxidase (HRP) conjugate (Dako) and visualized using enhanced chemoluminescence (Amersham Life Sciences, Amersham, United Kingdom) according to the manufacturer's instructions.
Generation and assessment of DCs
Immature myeloid DCs were derived from peripheral blood cells using standard procedures.23 24 Briefly, monocytes were cultivated in RPMI 1640 supplemented with 2 mM glutamine, 50 μg/mL kanamycin, 1% nonessential amino acids (Gibco BRL, Glasgow, United Kingdom), 10% human AB serum, and 50 ng/mL each of interleukin 4 (IL-4; specific activity > 2 × 106 U/mg; Peprotech, London, United Kingdom) and granulocyte-macrophage colony-stimulating factor (GM-CSF; specific activity > 1 × 107 U/mg; Schering-Plough, Welwyn Garden City, United Kingdom). After 6 days, DCs were matured with 100 ng/mL lipopolysaccharide (LPS; Salmonella typhimurium) for 48 hours. PrPC present in culture medium was assessed using Western blotting and densitometry and was found to be less than 10% of that expressed by the harvested DCs. Immature DCs expressed the surface molecules CD54 (clone 6.5B5), CD40 (clone LOB7/6), CD86 (clone BU63), and HLA DR (clone BF-1) as confirmed by staining with the respective monoclonal antibodies and fluorescence-activated cell sorter (FACScan; Becton Dickinson, Oxford, United Kingdom) analysis. Expression of CD14 was low. On maturation with LPS, 98% of DCs expressed CD83 (clone HB15b) (Serotec, Oxford, United Kingdom) and the levels of expression of CD54, CD40, CD86, and HLA-DR were increased. Early endocytotic vesicles in immature DCs were labeled by exposure of cells to FITC-dextran (1mg/mL; Molecular Probes, Leiden, Netherlands) for 7 minutes and for 15 minutes at 4°C and 37°C.
Amplification of the PrP gene
Total RNA was prepared from immature myeloid DCs generated from monocytes (7 days after culture with IL-4 and GM-SCF as described above), mature LPS-stimulated myeloid DCs, and on 2 independent preparations of monocytes freshly isolated from peripheral blood. The gene encoding PrP was amplified by reverse transcription-polymerase chain reaction (RT-PCR) using the Titan One Tube RT-PCR Kit (Roche), used according to manufacturer's instructions or by PCR using Taq polymerase (Roche). RNA (200 pg) or genomic DNA (50 ng) was used in the respective reactions. The primers 5′-GGCAGTGACTATGAGGACCGTTAC-3′ and 5′-GGCTTGACCAGCATCTCAGGTCTA-3′ were used to amplify the PrP gene to generate a 528-bp product. The primers 5′-CCATGTTCGTCATGGGTGTGAACCA-3′ and 5′-GCCAGTAGGCAGGGATGATGATGTT-3′ were used to amplify a 251-bp product by RT-PCR from the glyceraldehyde-3-phosphate dehydrogenase (G3PDH) gene in RNA prepared from all cell types as a positive control (data not shown).
Results
PrPC is strongly expressed by myeloid DCs in spleen
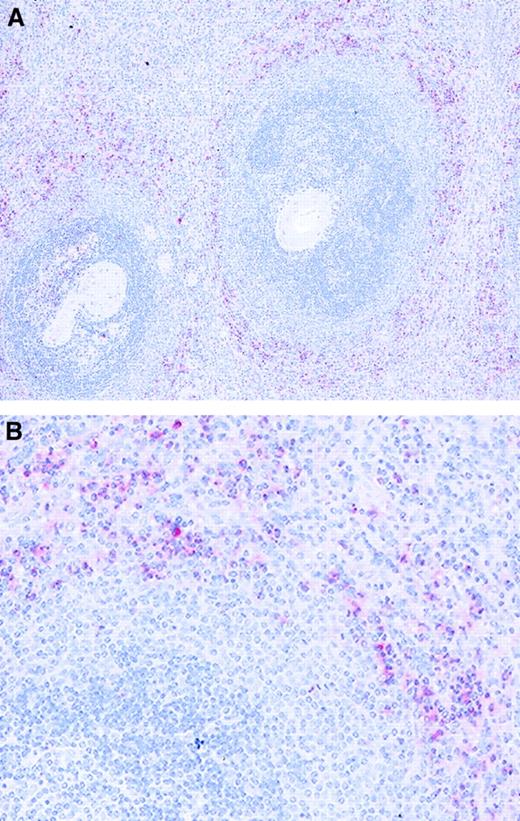
The cellular expression of the normal form of prion protein PrPC was assessed in normal human spleen using immunohistochemistry. The expression of PrPC was weak or absent on most cell types and on stromal elements, and in contrast to the reported accumulation of PrPSc within lymphoid follicles in infected tissue, strong expression of PrP was not detected within lymphoid follicles in these normal spleen sections. However, cells that strongly expressed PrPC were scattered throughout the splenic red pulp and particularly concentrated adjacent to the marginal zone of the white pulp (Figure1). To establish the identity of these cells we performed immunocytochemistry with lineage-specific antibodies. Double immunofluorescence staining revealed that these PrPC+ cells also expressed CD13, CD11c, and CD86, but did not express markers of FDCs (CR4/23), T cells (CD3), B cells (CD20 and CD79), or macrophages (CD68) (Figure 2and data not shown). We concluded that the distribution of the PrPC-expressing cells and their expression of lineage-defining antigens was consistent with the distribution and phenotype of myeloid DCs.
Localization of PrPC-expressing cells in tissue from human spleen.
The bulk of strongly PrPC-expressing cells (clone 3F4, red) are found in the red pulp (RP) immediately adjacent to the white pulp (WP). Tissue sections were counterstained with hematoxylin.
Localization of PrPC-expressing cells in tissue from human spleen.
The bulk of strongly PrPC-expressing cells (clone 3F4, red) are found in the red pulp (RP) immediately adjacent to the white pulp (WP). Tissue sections were counterstained with hematoxylin.
The human spleen cells that strongly express PrPC have an immunophenotype consistent with myeloid DCs.
(A-H) Identification of PrPC-expressing cells by dual-color immunolocalization. PrPC was identified using rabbit antiserum R26 and detected using Texas red-labeled antirabbit immunoglobulin (red) in all cases. Lineage-restricted mouse monoclonal antibodies (as indicated) to normal splenic cell types were detected using FITC-labeled antimouse immunoglobulin (green). Cell nuclei were identified by DAPI (blue). Sensitivity of the detection system was adjusted to identify only the strongly expressing cells. Strong PrPC-expressing cells are rarely found in the T-cell area (panel A, CD3) or B-cell area (panel B, CD20); the antigen is not strongly expressed on FDCs (panel C, CR4/23). Cells strongly expressing PrPC are clearly separate from surrounding monocytes/macrophages (panel D, CD68). Unstained cells (top right) represent an adjacent lymphoid area. Coexpression of PrPCwith CD13 (panel E and detail panel G) and CD11c (panel F and detail panel H) confirms myeloid origin and is indicated by an orange-yellow tone. Note that for CD11c (panel H) the cells coexpress PrPC and CD11c, but the intracellular localization is not identical. CD13+ or CD11c+, which do not express PrPC, are likely to represent monocyte-macrophage cells (panels E and F).
The human spleen cells that strongly express PrPC have an immunophenotype consistent with myeloid DCs.
(A-H) Identification of PrPC-expressing cells by dual-color immunolocalization. PrPC was identified using rabbit antiserum R26 and detected using Texas red-labeled antirabbit immunoglobulin (red) in all cases. Lineage-restricted mouse monoclonal antibodies (as indicated) to normal splenic cell types were detected using FITC-labeled antimouse immunoglobulin (green). Cell nuclei were identified by DAPI (blue). Sensitivity of the detection system was adjusted to identify only the strongly expressing cells. Strong PrPC-expressing cells are rarely found in the T-cell area (panel A, CD3) or B-cell area (panel B, CD20); the antigen is not strongly expressed on FDCs (panel C, CR4/23). Cells strongly expressing PrPC are clearly separate from surrounding monocytes/macrophages (panel D, CD68). Unstained cells (top right) represent an adjacent lymphoid area. Coexpression of PrPCwith CD13 (panel E and detail panel G) and CD11c (panel F and detail panel H) confirms myeloid origin and is indicated by an orange-yellow tone. Note that for CD11c (panel H) the cells coexpress PrPC and CD11c, but the intracellular localization is not identical. CD13+ or CD11c+, which do not express PrPC, are likely to represent monocyte-macrophage cells (panels E and F).
Prion protein expression is specifically up-regulated during the generation of myeloid DCs in vitro
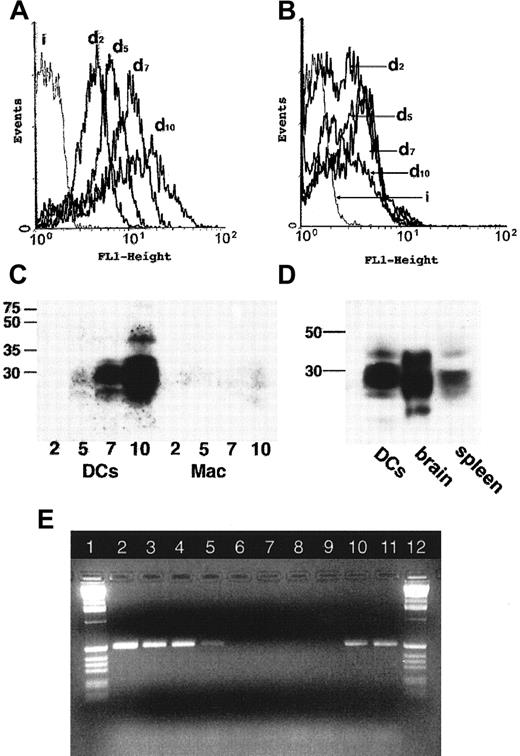
To confirm our findings that myeloid DCs strongly express prion protein, we generated DCs from monocytes. Such cells are widely used as a model of myeloid DCs23,24; these DCs generated in vitro cannot fully model cells in tissues that receive different stimuli and may vary in phenotype. However, DCs of splenic red pulp and marginal zone share many features with cells of monocyte lineage,25and thus monocyte-derived DCs were selected as a relevant system to confirm our in vivo findings. Freshly isolated peripheral blood monocytes express PrPC at low levels.20 We found that during the differentiation of monocytes to immature DCs in vitro the surface expression of PrPC increased 4-fold; this increased further when the DCs were matured with LPS to give an approximately 8-fold increase compared to monocytes (Figure3A,B). When the total cellular expression of PrPC was determined by SDS-PAGE and Western blotting a similar trend of increased expression was observed; however, increases in total expression were much greater than for surface expression, suggesting a significant intracellular pool of PrPC in myeloid DCs (Figure 3C). By contrast, macrophages generated from monocytes in vitro and subsequently exposed to LPS showed only a 3-fold increase in the surface expression of PrPC (Figure 3B) and a small increase in the total level of expression of PrPC(Figure 3C).
The expression of cellular PrPC is substantially up-regulated during differentiation of monocytes to myeloid DCs.
Flow cytometric analysis of PrPC expression during the maturation of DCs (A) or macrophages (B). Adherent peripheral blood mononuclear cells (PBMCs) were differentiated to DCs (A) or macrophages (B) in vitro. PrPC surface expression was monitored by staining PrPC (monoclonal antibody 6H3) and subsequent flow cytometry at days 2, 5, 7, and 10 of cell culture. PrPC expression is progressively up-regulated on successive days (d2-d10) for DCs (A), but there is no substantive change for macrophages (B). Isotype controls are indicated by i. (C) Western blots confirm the substantive up-regulation of total cellular PrPC during differentiation of adherent PBMCs to DCs (left lanes, days 2, 5, 7, 10), but not in macrophages (right lanes, days 2, 5, 7, 10). Note that the high-molecular-weight species visible at day 10 of DC maturation (left-hand lanes) represents a minor band only visible at very high levels of expression. (D) Molecular weight species of PrPC in lysates prepared from cultured human DCs compared with lysates of tissue from human brain and human spleen. The protein loading has been adjusted to allow comparison of tissues. DCs and spleen contain equal protein loading; brain loading is 10% of the total protein level of splenic tissue (determined by scanning densitometry of Coomassie-stained gels). Myeloid DCs generated in vitro and spleen express similar glycoform species. (E) Agarose gel electrophoresis of RT-PCR products of the PrP gene. A 528-bp fragment of the gene coding for PrP was amplified by RT-PCR from messenger RNA from myeloid DCs (lane 2), LPS-stimulated myeloid DCs (lane 3), and monocytes (lanes 4 and 5). No amplification of thePrP gene in the same samples by Taq polymerase (lanes 6-9) shows that no DNA was present in these samples. Amplification of the PrP gene from genomic DNA is shown for positive controls (lanes 10 and 11). One-Kb standard ladders (Gibco-BRL) are shown (lanes 1 and 12).
The expression of cellular PrPC is substantially up-regulated during differentiation of monocytes to myeloid DCs.
Flow cytometric analysis of PrPC expression during the maturation of DCs (A) or macrophages (B). Adherent peripheral blood mononuclear cells (PBMCs) were differentiated to DCs (A) or macrophages (B) in vitro. PrPC surface expression was monitored by staining PrPC (monoclonal antibody 6H3) and subsequent flow cytometry at days 2, 5, 7, and 10 of cell culture. PrPC expression is progressively up-regulated on successive days (d2-d10) for DCs (A), but there is no substantive change for macrophages (B). Isotype controls are indicated by i. (C) Western blots confirm the substantive up-regulation of total cellular PrPC during differentiation of adherent PBMCs to DCs (left lanes, days 2, 5, 7, 10), but not in macrophages (right lanes, days 2, 5, 7, 10). Note that the high-molecular-weight species visible at day 10 of DC maturation (left-hand lanes) represents a minor band only visible at very high levels of expression. (D) Molecular weight species of PrPC in lysates prepared from cultured human DCs compared with lysates of tissue from human brain and human spleen. The protein loading has been adjusted to allow comparison of tissues. DCs and spleen contain equal protein loading; brain loading is 10% of the total protein level of splenic tissue (determined by scanning densitometry of Coomassie-stained gels). Myeloid DCs generated in vitro and spleen express similar glycoform species. (E) Agarose gel electrophoresis of RT-PCR products of the PrP gene. A 528-bp fragment of the gene coding for PrP was amplified by RT-PCR from messenger RNA from myeloid DCs (lane 2), LPS-stimulated myeloid DCs (lane 3), and monocytes (lanes 4 and 5). No amplification of thePrP gene in the same samples by Taq polymerase (lanes 6-9) shows that no DNA was present in these samples. Amplification of the PrP gene from genomic DNA is shown for positive controls (lanes 10 and 11). One-Kb standard ladders (Gibco-BRL) are shown (lanes 1 and 12).
We were also interested in whether the glycoforms of PrPCexpressed by myeloid DCs derived in vitro resembled the in vivo glycoforms of PrPC found in spleen. PrPC is glycosylated in a tissue-specific manner, and these differences are reflected by corresponding changes in the apparent molecular weight of these species on SDS-PAGE.26 When molecular weights of forms of PrPC found in lysates from monocyte-derived DCss were compared with those from brain or spleen, the distributions of apparent molecular weights most closely resembled those of spleen (Figure 3D). Finally, nonquantitative RT-PCR of total RNA from myeloid DCs showed that the PrP gene was transcribed in these cells (Figure 3E). We concluded that the expression of PrPC was strongly up-regulated during the differentiation and maturation of myeloid DCs in vitro.
The intracellular localization of prion protein
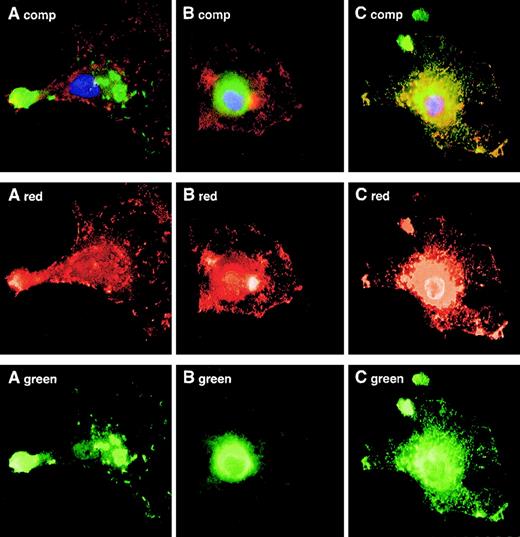
In mice, previous studies have suggested a functional role for PrPC in T-cell activation. We were, therefore, interested in determining the intracellular localization of PrPC within different functional compartments of the myeloid DCs, in particular those associated with adhesion or antigen processing. We allowed cultured monocyte-derived DCs to adhere and spread on glass slides, and used dual-fluorescence immunostaining to determine the colocalization of PrPC with cytoskeleton, major histocompatibility complex (MHC) class II, or endosomes within those cells. These experiments showed that PrPCdistribution was separate from that of adhesion plaque components mediating contact with the substratum or adjacent cells. PrPC was also absent from endocytotic vesicles visualized by the uptake of FITC-dextran (Figure4A,B). In contrast, when the distribution of PrPC was compared with that of MHC class II molecules in immature and mature DCs, a striking colocalization was revealed at the level of light microscopy (Figure 4C).
PrPC colocalizes with the MHC class II compartment in monocyte-derived DCs.
Adherent immature monocyte-derived DCs were allowed to ingest FITC-dextran for 15 minutes to reveal endocytic vesicles (A), stained for talin to reveal adhesion plaques (B), or stained for MHC class II molecules to reveal the MHC class II compartment (C). PrPCis shown in red in each instance. Colocalization of green-red signal gives yellow fluorescence.
PrPC colocalizes with the MHC class II compartment in monocyte-derived DCs.
Adherent immature monocyte-derived DCs were allowed to ingest FITC-dextran for 15 minutes to reveal endocytic vesicles (A), stained for talin to reveal adhesion plaques (B), or stained for MHC class II molecules to reveal the MHC class II compartment (C). PrPCis shown in red in each instance. Colocalization of green-red signal gives yellow fluorescence.
Discussion
In tissue from animals infected by transmissible spongiform encephalopathy, and in humans suffering vCJD, PrPSc is particularly concentrated on FDCs.6-8 In the present study we show that in the absence of prion disease, high levels of expression of the normal form of prion protein in human spleen occur principally on myeloid DCs immediately adjacent to the white pulp, whereas FDCs do not strongly express PrPC. In prion diseases FDCs heavily express PrPSc. PrPC is also found on FDCs in most, but not all, studies of mice.27,28 We are not aware of previous studies of myeloid DCs, but recent reports in sheep have identified PrPSc on myeloid cells with a “dendritic morphology”29; also PrPC expression has been identified on related microglial cells within the CNS.30
The distinction between FDCs and myeloid DCs is important because the 2 cell types are ontologically and functionally distinct.26Myeloid DCs are derived from bone marrow precursor cells or from monocytes and their precursors and are readily identified within circulating cell populations. In spleen, myeloid DCs are found in the red pulp and immediately adjacent to the white pulp. The cells migrate into the lymphoid areas after receiving a maturation stimulus where they are powerful mediators of T-cell activation. The origin of FDCs remains controversial; although evidence is accumulating that they too may be derived from bone marrow cells,31 their turnover and repopulation is slow, and they are not believed to recirculate.32 In spleen, FDCs are confined to lymphoid follicles and present surface-bound antigen to maturing B cells. The high levels of expression of PrPC on myeloid DCs, but not on surrounding macrophage cells, suggests that PrPC is selectively up-regulated during the ontogeny of myeloid DCs in vivo.
Our data showing a distinct cellular and subcellular expression of PrPC in antigen- presenting cells may help shed light on the, as yet, undefined functional role of PrPC. PrPC is a glycosylphosphatidylinositol (GPI)–linked molecule that may be expressed at the cell surface or be associated with intracellular vesicles.33,34It has recently been shown that GPI-linked molecules localize within lipid rafts where they may play an important role in costimulatory functions, including activation of T cells (reviewed by Ilangumaran and coworkers35). The protein has a high-affinity copper-binding site and has structural homology with signal peptidases,36 although no enzyme function has been demonstrated for PrPC. The protein has a widespread tissue distribution, but is particularly expressed in neurologic tissue and on cells of the immune system. Recent reports have shown an increase in PrPC expression in monocytes stimulated by γ-interferon.21 Taken together with our data these findings suggest that, at least in the immune system, PrPC expression is controlled during cellular development and activation.
Consistent with such a role, we have shown that the prion protein was colocalized with MHC class II molecules in immature and mature myeloid DCs. The colocalization of PrPC with MHC class II molecules reported in the present study does not define the function of PrPC; indeed, the MHC class II compartment is large, and our study does not precisely identify the role of PrPCwithin that compartment. Nevertheless, the coexpression of PrPC with MHC class II in monocyte-derived DCs is consistent with a potential role for PrPC in the stimulation of a variety of immunologic cells. Indeed, in mice, PrPC has been indirectly linked to a role in T-cell activation by observations that peptides from PrPC may directly induce T-cell activation.37,38 Although there have been no detailed reports of immune function in PrPC−/− knockout mice, in the absence of PrPCconcanavalin-A–induced activation of T cells is reduced.39 DCs are believed to be central to the ability of concanavalin-A to activate T cells.40 These data, taken together with our observations that PrPC is colocalized with MHC class II molecules in myeloid DCs, are consistent with an indirect or direct role for PrPC in T-cell activation by antigen-presenting cells. Recent data has suggested a role for PrPC in the processes of morphogenesis and adhesion41,42 and that the protein distributes to dendritic extensions on neurites.43 We did not, however, detect a link with adhesion structures or a distribution to dendritic extensions in our studies. The molecule is also known to be endocytosed in relation to various stimuli44 45; we did not however detect the presence of PrPC in early endocytotic vesicles.
Our findings may be relevant to the spread of abnormal forms of prion protein. The spread of PrPSc in an infected host must require the conversion of PrPC to PrPSc. In mice high titers of infectivity occur in the spleen soon after intraperitoneal inoculation with PrPSc, and subsequent spread to the CNS can be retarded, though not prevented, by splenectomy.15 PrPSc has been detected on FDCs, B cells, and T cells in the lymphoid follicles of mice, and recently, FDCs have been shown to be required for transmission of PrPSc in mice.9 However, splenic infectivity can be restored in PrPC knockout mice using transplanted normal bone marrow, suggesting an importance for a rapidly repopulated bone marrow-derived cell population,16 and in some mouse models spread of PrPSc from peripheral tissues to the CNS occurs in the absence of functional FDCs or T lymphocytes.46 These findings strongly suggest that PrPSc transmission and propagation is a complex process requiring the interaction of several cell types. In the present study we have established that the PrPC expressed by myeloid DCs is the major reservoir of normal prion protein in human spleen. The relationship between levels of PrPC expression, and the susceptibility of a cell to PrPSc infection, is not simple; overexpression of PrPC within an organism confers an increased susceptibility to prion diseases,47 48 but this is not true for all tissues or cell types. This suggests the characteristics of the cell(s) expressing PrPC are also important.
Dendritic cells are derived from precursors within the bone marrow and migrate widely.49-52 They make early contact with ingested or inoculated antigen and can transport it to secondary lymphoid organs, where there is extensive interaction with other immune cell types including B cells and other cells within the germinal centers.53 Given our novel findings we suggest these myeloid-derived DCs may be important in the spread of prion disease and that monocyte-derived DCs may provide a model for the study of PrPC function in human cells.
We would like to thank Professor K. Gatter for his help and encouragement in this work, Dr John Kurtz for helpful comments on the manuscript, Robin Roberts-Gant and Kingsley Micklem for their skilled and dedicated help in the preparation of the figures and other members of the Cellular Science Research Laboratory and Medical Informatics Unit at the John Radcliffe Hospital in Oxford for their help and advice.
Supported by the University of Oxford, the National Blood Service, and the Wellcome Trust. B.C.U. was supported by the Sir E. P. Abraham Trust (Oxford), and D.J.R. was a Wellcome Trust Senior Fellow in Clinical Science and a Howard Hughes International Research Scholar.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
David J. Roberts, National Blood Service, Oxford Centre, John Radcliffe Hospital, Headington, Oxford OX3 9DU, United Kingdom; e-mail: droberts@imm.ox.ac.uk.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal