Abstract
Red blood cell (RBC) and platelet transfusion requirements in patients given nonmyeloablative versus conventional peripheral blood stem cell (PBSC) transplants from HLA-matched siblings were compared. Between December 1997 and March 2000, 40 patients, aged 21 to 67 years (median 51), with hematologic malignancies underwent nonmyeloablative allografts after either 2 Gy total body irradiation alone (n = 30) or 2 Gy total body irradiation preceded by fludarabine 30 mg/m2/d on days −4, −3, and −2 (n = 10). All received postgrafting mycophenolate mofetil and cyclosporine. Controls included 67 concurrent patients, aged 23 to 66 years (median, 46 years), given conventional PBSC transplants following high-dose conditioning and postgrafting methotrexate and cyclosporine. Among patients given nonmyeloablative transplants, 23% required platelet transfusions compared with 100% among patients given conventional grafts (P < .0001). Further, the number of platelet units given to nonmyeloablative recipients was reduced, with a median of 0 (range, 0 to 214) compared with a median of 24 (range, 4 to 358) after conventional transplantation (P < .0001). Sixty-three percent of nonmyeloablative recipients required RBC transfusions compared with 96% of those with conventional grafts (P = .0001). The number of RBC units transfused was also reduced, with a median of 2 (range, 0 to 50) compared with 6 (range, 0 to 34) after conventional transplantation (P = .0001). High transfusion requirements before transplantation and donor-recipient ABO incompatibility increased transfusion requirements in both patient groups, though neither significantly influenced the outcome of the analysis. Neither patient age, splenomegaly at transplantation, development of graft-versus-host disease, nor posttransplantation cytomegalovirus antigenemia or cytomegalovirus disease had statistically significant influences on posttransplantation transfusions.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) has been used successfully to treat patients with hematologic malignancies. Current standard protocols rely on maximally tolerated doses of systemic chemoradiation to both eradicate cancer and achieve host immunosuppression. The allografts then serve to rescue patients from treatment-induced pancytopenia and contribute a graft-versus-tumor effect of uncertain magnitude. During the pancytopenic period, virtually every patient undergoing transplantation requires intensive red blood cell (RBC) and platelet transfusion support. Based on extensive preclinical studies,1 we have developed a nonmyeloablative HSCT approach in which the burden of tumor eradication has been shifted from cytotoxic agents to the graft-versus-tumor effect.2-7 In our clinical trials, we have applied this approach to elderly or medically infirm patients who were ineligible for conventional transplantation.8 The initial regimen consisted of only 2 Gy of total body irradiation (TBI) delivered at a low dose rate followed by postgrafting immunosuppression with the antimetabolite mycophenolate mofetil (MMF) and the T-cell activation blocker cyclosporine (CSP). More recently, 3 doses of fludarabine have been added to the regimen. The regimen was found to be safe and minimally toxic. Sustained engraftment was established in most patients and, importantly, impressive antitumor responses have been seen in a wide variety of hematologic malignancies.8 9 Due to both the mild conditioning regimen and the use of granulocyte colony-stimulating factor–mobilized peripheral blood mononuclear cells (PBSCs) as the hematopoietic stem cell source, only modest and transient declines in blood counts were seen, with a median platelet nadir of 75 × 103/μL (75 000/μL) on day 11 and a median granulocyte nadir of 750/μL. Here, we examined the platelet and RBC transfusion requirements in 40 patients given nonmyeloablative HSCT and compared results to those in a control group of 67 slightly younger patients treated with standard myeloablative conditioning.
Patients, materials, and methods
Patients and disease characteristics
Patients and disease characteristics are described in Table1. All patients had hematologic malignancies, myelodysplasia, or myeloproliferative diseases. Results from all patients who survived longer than 60 days after HSCT were analyzed. Between December 1997 and March 2000, 40 patients were treated at the Fred Hutchinson Cancer Research Center with a nonmyeloablative regimen consisting of 2 Gy TBI (0.07 Gy/min [7 cGy/min]) on day 0 before HSCT and immunosuppression with MMF 30 mg/kg/d orally from day 0 to 27 and cyclosporine 12.5 mg/kg/d orally from day −1 to +35 with a subsequent taper. Ten of the 40 patients received fludarabine 30 mg/m2 at days −4 to −2 in addition to TBI. The 40 patients were ineligible for conventional HSCT because of age or medical contraindications.
For comparison, data from a group of 67 patients given conventional myeloablative transplants for equivalent diseases during the same time interval were analyzed. Their conditioning regimens included combinations of busulfan and cyclophosphamide, busulfan and TBI, or cyclophosphamide and TBI.10 Busulfan was administered orally every 6 hours for 4 days before transplantation, with levels targeted from 600 to more than 900 ng/mL. Cyclophosphamide was administered for 2 days at 60 mg/kg/d. TBI was given over 3 or 4 days with total doses of 12 to 14.4 Gy. One patient with multiple myeloma received busulfan and 9 Gy total marrow irradiation. Two patients conditioned with busulfan and cyclophosphamide received additional therapy with 131I-labeled anti-CD45 antibody.
Splenomegaly, as defined either by imaging studies or through clinical palpation, was documented in 17.5% of nonmyeloablative and 23.9% of conventional HSCT patients. The disease diagnoses were not well balanced among the 2 patient groups. Most patients (77.5%) with nonmyeloablative grafts had B-cell malignancies, including multiple myeloma, chronic lymphocytic leukemia, and non-Hodgkin and Hodgkin lymphoma. By comparison, most conventional patients (83.5%) had acute leukemia, myelodysplastic syndrome, chronic myelogenous leukemia in chronic phase, or agnogenic myeloid metaplasia.
Transplantation characteristics
All patients were given PBSC grafts from genotypically HLA-identical siblings, collected after granulocyte colony-stimulating factor mobilization. None of the grafts were processed (ie, RBC- or plasma-depleted) before transplantation. The RBC content was less than 15 mL and the plasma volume less than 300 mL. The numbers of CD34+ cells transplanted and the numbers of ABO-incompatible transplants were not significantly different (P = .36) between nonmyeloablative and myeloablative recipients (Table 1).
Transfusion policies
Platelet support before transplantation was given when platelet counts were less than 10 × 109/L in the outpatient setting and less than 20 × 109/L in the inpatient setting or when patients had signs of bleeding. Patients received random donor platelets unless they developed refractoriness. Random platelets were either whole-blood pooled random platelets or random single-donor apheresis platelets. Before transplantation, all patients received leukocyte-reduced platelets. After transplantation, the leukocyte reduction was no longer required. Packed RBCs were transfused when hematocrits were less than 26% (0.26) or when patients were symptomatic. The numbers of patients who required pretransplantation platelet or RBC transfusions are shown in Table 1. In addition to the total numbers of transfusions, the transfusion requirements were adjusted to the average numbers of units transfused per day during the pretransplantation period.
Posttransplantation platelet and plasma transfusions were of recipient type, and all RBCs transfused were type O in patients with minor RBC incompatibilities (presence of isohemagglutinins in donors against RBC antigens of recipients). For patients with major incompatibilities (presence of isohemagglutinins in recipients against RBC antigens of donors) and bidirectional incompatibilities (bidirectional presence of isohemagglutinins), the first-choice blood groups were AB for posttransplantation platelet transfusions and plasma infusions, and all RBCs were type O. In case of ABO-incompatible platelet transfusions, the platelets were volume-reduced to minimize the amount of isohemagglutinins contained in the plasma. All patients received blood products of donor-type rhesus factor unless recipients had high anti-D titers.
Statistical analysis
Unadjusted comparisons of platelet and RBC transfusion requirements for the first 60 days after transplantation were made between recipients of nonmyeloablative transplants and recipients of conventional transplants using the Wilcoxon rank sum test. To account for potential differences in variables related to transfusion requirements, multiple linear regression models were fit with the rank of the numbers of transfusion units serving as outcome variables. Potential explanatory variables included advanced underlying disease, risk for transfusions, pretransplantation transfusion requirements, ABO compatibility, spleen size, age at transplantation, and posttransplantation graft-versus-host disease and cytomegalovirus antigenemia. Proportions of patients requiring transfusion support were compared between groups using the χ2 test. Pvalues from regression models were obtained from the Wald test, and no adjustments were made for multiple comparisons.
Results
Posttransplantation platelet transfusion requirements
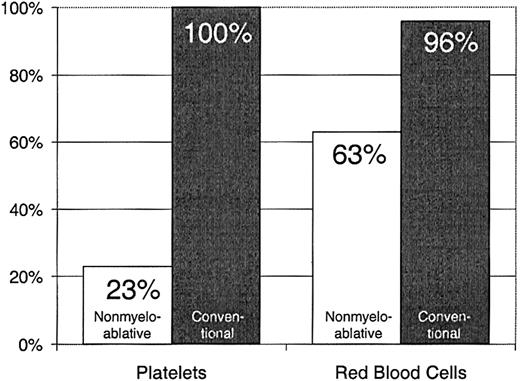
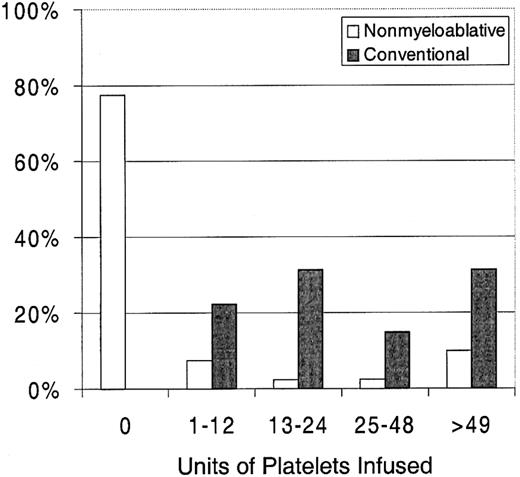
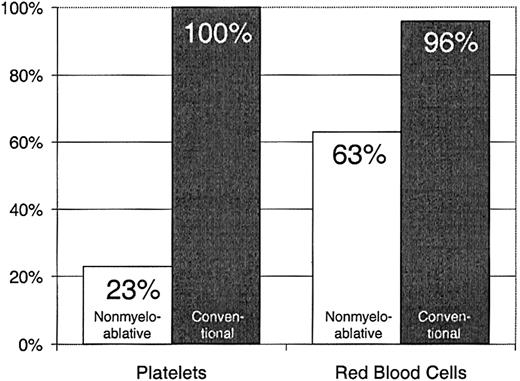
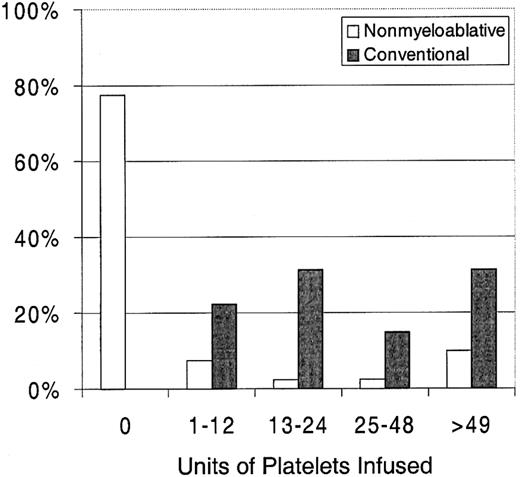
Twenty-three percent of patients with nonmyeloablative HSCT required platelet transfusions compared with 100% of patients given conventional HSCT (P < .0001) (Figure1). The overall numbers of platelet units transfused were also statistically significantly less after nonmyeloablative HSCT, with a median of 0 units (range, 0 to 214) compared with a median of 24 units (range, 4 to 358) after standard HSCT (unadjusted P < .0001). Figure2 illustrates this comparison in more detail. This difference in numbers of units was maintained even after adjusting for risk for platelet transfusions, pretransplantation transfusion requirements, and ABO compatibility (adjustedP < .0001). Both the risk for platelet transfusions and risk for pretransplantation transfusion requirements were statistically significantly associated with numbers of units transfused (P = .01 for each). ABO compatibility was suggestively associated with transfusion requirements but was not statistically significantly associated. The multivariable regression model is summarized in Table 2. One nonmyeloablative transplant recipient became refractory to platelet transfusions after transplantation compared with conventional transplant recipients. Twenty-seven platelet transfusion products in 3 nonmyeloablative transplantation patients were volume-reduced compared with 80 transfusion products in 20 conventional transplantation patients.
Platelet and RBC transfusion requirements in patients after nonmyeloablative versus conventional HSCT.
Platelet and RBC transfusion requirements in patients after nonmyeloablative versus conventional HSCT.
Percentages of patients requiring platelet transfusions after nonmyeloablative (n = 40) versus conventional (n = 67) HSCT.
Percentages of patients requiring platelet transfusions after nonmyeloablative (n = 40) versus conventional (n = 67) HSCT.
Posttransplantation RBC transfusion requirements
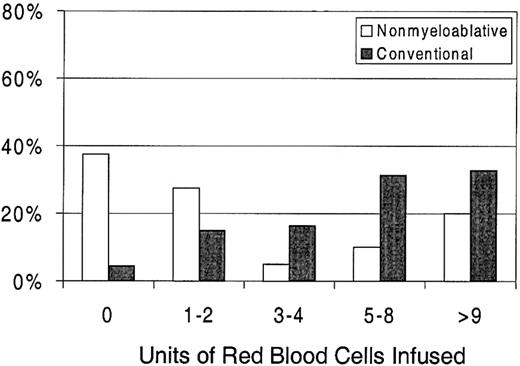
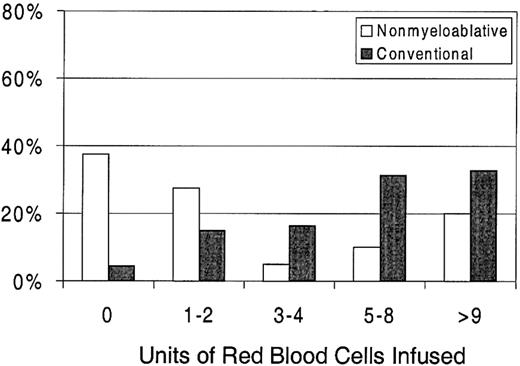
Sixty-three percent of patients with nonmyeloablative HSCT required RBC transfusions compared with 96% of patients undergoing standard transplantation (P < .0001) (Figure 1). The numbers of RBC units transfused were also statistically significantly less for patients after nonmyeloablative HSCT, with a median of 2 units (range, 0 to 50) compared with a median of 6 units (range, 0 to 34) after standard HSCT (unadjusted P = .0002). Figure3 illustrates this comparison in greater detail. As with platelet transfusions, the difference in RBC units remained statistically significant after adjusting for risk for RBC transfusions, pretransplantation RBC transfusion requirements, and ABO compatibility (adjusted P = .0004). Risk for RBC transfusions and risk for pretransplantation requirements were each statistically significantly associated with numbers of RBC units transfused (P = .0005 for each). Major ABO mismatches required more RBC units compared with compatible patients (P = .0001). The multivariable regression model is summarized in Table 3.
Percentages of patients requiring RBC transfusions after nonmyeloablative (n = 40) versus conventional (n = 67) HSCT.
Percentages of patients requiring RBC transfusions after nonmyeloablative (n = 40) versus conventional (n = 67) HSCT.
There was no suggestion of interactions between type of transplantation and any of the nontransplantation variables that were included in the regression models. In other words, the increased transfusion requirements seen with conventional PBSC grafts occurred at all levels of these other factors.
Neither development of acute graft-versus-host disease, seen in 55.0% of nonmyeloablative and 37.3% of conventional HSCT recipients; positive cytomegalovirus antigenemia or cytomegalovirus disease, seen in 15.0% of nonmyeloablative and 32.8% of conventional recipients before day 60; splenomegaly; nor increasing patient age were statistically significantly associated with increased posttransplantation transfusion requirements.
Transfusion requirements of the patient subgroup receiving fludarabine as part of the nonmyeloablative conditioning regimen
The 10 patients conditioned with fludarabine and TBI were transfused with a median of 0 platelet units (range, 0 to 214) and a median of 5 RBC units (range, 0 to 22). The 30 patients given only TBI received a median of 0 platelet units (range, 0 to 172) and a median of 2 RBC units (range, 0 to 50), and these differences were not statistically significant (unadjusted P = .43 for platelets, P = .27 for RBCs).
Discussion
The use of conventional high-dose cytotoxic conditioning regimens before allogeneic HSCT eliminates recipient marrow function. To reduce toxicities to nonhematopoietic organs, those regimens are generally administered over 5 to 7 days. At the time of allograft infusions, peripheral blood granulocyte counts are low or absent, and platelet counts have declined. Typically, conventional allogeneic PBSC recipients remain neutropenic (granulocytes < 500/μL) for a median of 16 days (range, 11-29 days) and thrombocytopenic (platelets < 20 × 103/μL [< 20 000/μL]) for a median of 13 days (range, 5-41 days).10 Recovery of RBC production and RBC transfusion independence are usually later, with medians of 60 to 70 days. Because of the profound and prolonged pancytopenia, virtually all conventional allograft recipients required both multiple platelet and RBC transfusions, and the current control group was no exception.
By comparison, only 23% of current nonmyeloablative recipients required platelet transfusions and 63% needed RBC transfusions. Furthermore, the numbers of platelet and RBC units transfused were significantly reduced. To account for the fact that clinical practice requires platelet transfusions for inpatients when counts decline below 20 × 103/μL (20 000/μL) and for outpatients when counts decline below 10 × 103/μL (10 000/μL), we made the theoretical assumption that all outpatients with platelet counts ranging from 10 × 103/μL to 20 × 103/μL received 6 units of platelets. Even with this assumption, the average number of platelet transfusions was still significantly lower among nonmyeloablative compared with myeloablative HSCT recipients (P = .0001). Also, the proportion of patients given platelet transfusions remained statistically significantly lower among nonmyeloablative compared with myeloablative HSCT (P < .0001).
There are at least 3 possible reasons for the decreased transfusion requirements. First, the single dose of 2 Gy TBI delivered at the low exposure rate of 0.07 Gy/min, while causing moderate pancytopenia, did not lead to irreversible marrow damage as conclusively documented in canine studies.11 The declines in peripheral blood counts in dogs given 2 Gy TBI and no subsequent marrow grafts were slow, and granulocyte and platelet nadirs were not reached until 14 to 20 days after TBI. Virtually all dogs showed prompt and complete hematopoietic recoveries within a few weeks of irradiation. In line with these experimental observations, the median granulocyte nadir in a group of 45 nonmyeloablative patients comparable to the current study was 750/μL and the platelet nadir 75 × 103/μL (75 000/μL).8
Second, the low-dose conditioning regimen was delivered just hours before HSCT. This led to a considerable reduction of the period of pancytopenia compared with the conventional 7 days of high-dose conditioning therapy with busulfan and cyclophosphamide. The addition of 3 doses of fludarabine to the 2 Gy TBI, while extending the regimen by several days, increased the transfusion requirement.
Third, other factors that might lead to increased transfusion needs in conventionally conditioned patients, such as gastrointestinal damage, veno-occlusive disease of the liver, hemorrhagic cystitis, and bacterial sepsis, were either completely absent or significantly reduced (bacterial sepsis) in current nonmyeloablative patients.
Not surprisingly, both transfusion needs before transplantation and major ABO incompatibility between donors and recipients resulted in increased platelet and/or RBC transfusions in all affected patients regardless of the HSCT regimen used. With the relatively small numbers of patients studied, it is as yet unclear whether ABO-incompatible nonmyeloablative recipients will require extended RBC transfusion support compared with conventional HSCT recipients, owing to the theoretical possibility that host and donor isohemagglutinins might be slower to disappear than after a myeloablative HSCT.
Finally, graft-versus-host disease,12splenomegaly,13,14 cytomegalovirus infection15 or its treatment with ganciclovir,16 or infection prophylaxis with trimethoprim-sulfamethoxazole17 have all been reported to affect the tempo of hematopoietic recovery and transfusion needs after conventional HSCT. Whether any of these factors will play a role in nonmyeloablative HSCT can be answered only with the study of larger numbers of transplanted patients.
A number of other nonmyeloablative HSCT regimens have been described, all based on combinations of chemotherapeutic drugs.7,18-22 Although much is known about transfusions following myeloablative transplants,23 24 careful studies of posttransplantation transfusion requirements have as yet not been reported for any of the nonmyeloablative chemotherapy regimens.
We thank the medical, nursing, clinical, and data processing staffs at the Fred Hutchinson Cancer Research Center for their important contributions to this study through their careful and dedicated care of patients. We are grateful to Bonnie Larson and Helen Crawford for their assistance in preparing the manuscript.
Supported in part by grants CA78902, CA18029, CA15704, and HL36444 from the National Institutes of Health, Department of Health and Human Services, Bethesda, MD. W.I.B. is also supported by the Jose Carreras International Leukemia Foundation, Barcelona, Spain.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Rainer Storb, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave North, D1-100, PO Box 19024, Seattle, WA 98109-1024; e-mail: rstorb@fhcrc.org.