Abstract
It has been unclear whether regimens containing topotecan + ara-C (TA) or fludarabine + ara-C (FA) ± idarubicin are superior to regimens containing idarubicin + ara-C (IA) without either fludarabine or topotecan for treatment of newly diagnosed acute myeloid leukemia (AML), refractory anemia with excess blasts in transformation (RAEB-t), or RAEB. Of 1279 patients treated here for these diagnoses between 1991 and 1999, 322 received IA regimens, 600 FA regimens, and 357 TA regimens. All regimens used ara-C doses of 1 to 2 gm/m2/d, given by continuous infusion in IA, and over 2 to 4 hours in FA and TA. Complete remission (CR) rates were lower with FA (55%) and TA (59%) than with IA (77%). Both event-free survival (EFS) in CR and survival were shorter: median EFS in CR (95% confidence interval) was 63 weeks (range, 55-76 weeks) for IA, 40 (range, 31-46 weeks) for FA, and 36 (range, 27-44 weeks) for TA; median survival was 77 weeks (range, 57-88 weeks) for IA, 30 (range, 27-35 weeks) for FA, and 41 (range, 35-50 weeks) for TA. These trials were not randomized, and patients with worse prognoses were disproportionately given the FA and TA regimens. Nonetheless, after accounting for prognosis the FA and TA regimens remained highly significantly associated with lower CR rates, shorter EFS in CR, and shorter survival. Accounting for possible effects of individual trials within each of the IA, FA, and TA groups did not alter these findings. It is unlikely that, as given here, either FA or TA is, in general, superior to IA, highlighting the need for new treatments.
Introduction
Over the past decade our group has emphasized the development of regimens containing fludarabine or topotecan combined with ara-C for treatment of newly diagnosed acute myeloid leukemia (AML), or those myelodysplastic syndromes (refractory anemia with excess blasts in transformation [RAEB-t], and in certain cases, RAEB), which are prognostically similar to AML.1Fludarabine + ara-C (FA) was first investigated in 1991. In 1992, granulocyte colony-stimulating factor (G-CSF) was added to the FA combination to form FLAG, and subsequently idarubicin was combined with FA and given with or without G-CSF and/or all-trans retinoic acid (ATRA). After its original use in 1997, topotecan + ara-C (TA) was given together with cyclophosphamide + G-CSF (CAT). At least partly as a result of reports of these trials,2-4FA, FLAG, FLAG + idarubicin, and TA have found widespread use.
Thus, it appeared appropriate to compare the FA- and TA-containing regimens with regimens containing idarubicin + ara-C (IA), but not fludarabine or topotecan. During the years we were investigating fludarabine and topotecan, we also gave such IA regimens to patients with newly diagnosed AML, RAEB-t, or RAEB, with IA being given ± G-CSF, lisofylline, or ATRA. The IA regimens were not “3 + 7”-type regimens but rather used daily ara-C doses of 1.5 gm/m2, as described below. Although the trials involving IA, FA, and TA were not randomized studies, over the last decade, each trial included adults of all ages, cytogenetic groups, and so forth, making it feasible to undertake comparisons. Here we report the results of these comparisons.
We will consider all the fludarabine-containing regimens as “FA,” all the topotecan-containing regimens as “TA,” and all the IA regimens, without fludarabine or topotecan, as “IA.” Regression analyses will use the IA treatment group as the baseline, so that FA and TA effects are defined relative to IA. Extended Cox model regression analyses of the FA and TA effects on survival and event-free survival (EFS), and logistic regression analyses of the FA and TA effects on the probability of complete remission (CR), each will account for patient prognostic covariates, including cytogenetic abnormalities, age, and performance status. Subsequently, these regression models will be expanded to include “trial effects,” that is, variations in outcome among each of the regimens comprising FA (FA, FLAG, FLAG + idarubicin, etc), TA (TA, CAT), or IA (IA, IA + G-CSF, etc), in order to account for these trial effects while evaluating the FA and TA effects. Similarly, because these trials were conducted over a 9-year period, we also adjust for calendar year in which treatment began in order to assess whether any apparent treatment effects merely reflected changes over time in supportive care or in patient characteristics that could not be captured by descriptions of age, cytogenetics, etc. Part of this work was presented at the 2000 American Society of Hematology meeting.5
Patients, materials, and methods
Patients
Between January 1, 1991, and January 1, 2000, 1279 of the 1347 patients (95%) treated for newly diagnosed AML (acute promyelocytic leukemia excepted), RAEB-t, or RAEB at M. D. Anderson Cancer Center received a regimen containing IA, FA, or TA. Of the 1279 patients, 322 (25%) received an IA regimen, 600 (47%) an FA regimen, and 357 (28%) a TA regimen. Table 1lists the various treatments comprising each of the IA, FA, and TA groups. A total of 119 of the patients in the IA group (37%) received idarubicin + high-dose ara-C per se in 1 of 2 sequential trials. In the first trial (1991), 84 patients were treated,6whereas in the second trial (1995-1996) 35 patients were randomly assigned to receive idarubicin + ara-C and 35 to receive idarubicin + ara-C + lisofylline.7 Noting that patients were considered in the FA group provided they received fludarabine + ara-C with or without idarubicin, 384 patients (64%) in the FA group received idarubicin in addition to FA (FAI). Of these 384 patients, 215 (55%) participated in a randomized trial comparing FAI ± G-CSF and/or ATRA,8 with an additional 136 patients receiving FAI + G-CSF and an additional 31 patients receiving FAI +G-CSF + ATRA prior to or subsequent to this randomized trial. Seventy-three percent of the TA group received cyclophosphamide + G-CSF in addition to TA.
Regimens administered to the 1279 patients
| Regimen . | No. of patients . |
|---|---|
| IA group (322 patients, 1991-1997) | |
| Idarubicin + ara-C | 119* |
| Idarubicin + ara-C + G-CSF | 145 |
| Idarubicin + ara-C + lisofylline | 35 |
| Idarubicin + ara-C + G-CSF + ATRA | 23 |
| FA group (600 patients, 1991-1998) | |
| Fludarabine + ara-C | 79 |
| Fludarabine + ara-C + G-CSF | 137 |
| Fludarabine + ara-C + idarubicin | 53 |
| Fludarabine + ara-C + idarubicin + G-CSF | 191 |
| Fludarabine + ara-C + idarubicin + ATRA | 55 |
| Fludarabine + ara-C + idarubicin + G-CSF + ATRA | 85 |
| TA group (357 patients, 1997-1999) | |
| Topotecan + ara-C | 98 |
| Topotecan + ara-C + cyclophosphamide + G-CSF | 259 |
| Regimen . | No. of patients . |
|---|---|
| IA group (322 patients, 1991-1997) | |
| Idarubicin + ara-C | 119* |
| Idarubicin + ara-C + G-CSF | 145 |
| Idarubicin + ara-C + lisofylline | 35 |
| Idarubicin + ara-C + G-CSF + ATRA | 23 |
| FA group (600 patients, 1991-1998) | |
| Fludarabine + ara-C | 79 |
| Fludarabine + ara-C + G-CSF | 137 |
| Fludarabine + ara-C + idarubicin | 53 |
| Fludarabine + ara-C + idarubicin + G-CSF | 191 |
| Fludarabine + ara-C + idarubicin + ATRA | 55 |
| Fludarabine + ara-C + idarubicin + G-CSF + ATRA | 85 |
| TA group (357 patients, 1997-1999) | |
| Topotecan + ara-C | 98 |
| Topotecan + ara-C + cyclophosphamide + G-CSF | 259 |
IA, idarubicin + ara-C; G-CSF, granulocyte colony-stimulating factor; ATRA, all-trans retinoic acid; FA, fludarabine + ara-C; TA, topotecan + ara-C.
35 of the 119 patients received IA in the IA − lisofylline arm of the IA ± lisofylline trial.
Assignment to treatment
Throughout the entire period, January 1, 1991, to January 1, 2000, our policy was to treat all patients with RAEB-t and those patients with RAEB and International Prognostic Scoring System9 scores of Int-2 or higher as if they had AML. Ninety-two percent of the patients seen with RAEB-t and 52% of those with RAEB were thus treated. The proportion of patients with RAEB treated did not change significantly during the 1991-1999 period. IA was our principal regimen during most of 1991. Later that year FA was introduced, with patients initially assigned to this regimen based on the presence of cytogenetic abnormalities other than inv(16) or t(8;21). After FA appeared safe in such patients, its use was generalized as was, beginning in mid-1992, that of its successor FLAG. In late 1993, the IA + G-CSF (IAG) regimen was begun. After FLAG + idarubicin was initiated in 1994, patients were assigned to it or to IAG according to their cytogenetics, as described for IA and FA above. If treatment was required, generally on the basis of a white blood cell (WBC) count more than 50 000, before the 3 days needed to receive cytogenetic results had elapsed, patients received the IA regimens. A trial randomizing patients between IA ± lisofylline was begun in 1995.7 Patients were eligible if they were aged younger than 65 years. Older patients were randomized among FAI ± G-CSF or ATRA (4-arm trial) mentioned above.8After completion of the lisofylline trial, the 4-arm trial was generalized. Beginning in 1997, TA was introduced and used primarily in RAEB or RAEB-t, although 24 patients with AML (> 30% blasts) were also treated. The CAT regimen combining cyclophosphamide and G-CSF with TA was our principal regimen for all patients beginning in mid-1998 and continuing through the end of the period in question.
Given that the IA, FA, and TA trials were open to patients regardless of age, regardless of whether they had myelodysplasia (MDS) or AML, and regardless of a documented history of abnormal blood counts for 1 month or more before M. D. Anderson presentation (antecedent hematologic disorder [AHD]), less than 10% of the 1279 patients included in this report had inv(16) or t(8;21), whereas 54% were considered to have worse prognosis cytogenetics [all abnormalities except inv(16) or t(8;21)] for purposes of assignment to treatment (Table2). Because, in some periods covered by this report, patients were preferentially assigned to the IA regimens if their cytogenetics were prognostically “better” (preceding paragraph), the IA group more often had a normal karyotype, or an inv(16) or t(8;21) and was younger than the FA or TA groups. The IA group also had a lower incidence of MDS or AHD. Similarly, because patients with MDS were preferentially assigned to TA in the 1997-1998 period, proportionately more patients with RAEB or RAEB-t received regimens containing TA (TA and CAT).
Patient characteristics by regimen
| . | All regimens . | IA regimens . | FA regimens . | TA regimens . |
|---|---|---|---|---|
| Patients | 1279 | 322 | 600 | 357 |
| Inv(16) or t(8;21) | 82 (6%) | 47 (15%) | 14 (2%) | 21 (6%) |
| − 5/− 7 | 340 (27%) | 29 (9%) | 219 (37%) | 92 (26%) |
| Other abnormality | 344 (27%) | 85 (26%) | 178 (30%) | 81 (23%) |
| Normal karyotype | 513 (40%) | 161 (50%) | 189 (32%) | 163 (46%) |
| MDS | 379 (30%) | 57 (18%) | 186 (31%) | 136 (38%) |
| AHD | 575 (45%) | 48 (15%) | 348 (58%) | 179 (50%) |
| Performance status Zubrod 3 or 4 | 149 (12%) | 44 (14%) | 79 (13%) | 26 (7%) |
| Protective environment | 744 (58%) | 146 (41%) | 367 (81%) | 231 (72%) |
| Median age, y (range) | 60 (16-88) | 53 (16-83) | 63 (16-88) | 61 (17-84) |
| Median marrow blast, % (range) | 37 (0-98) | 48.5 (0-97) | 36 (0-96) | 31 (0-98) |
| Median platelet count, × 109/L (range) | 44 (1-2292) | 50 (1-2292) | 40 (1-1355) | 44 (3-708) |
| Median hemoglobin, g/L (range) | 8.0 (2.1-15.1) | 8.15 (2.1-14.4) | 8.0 (2.8-15.1) | 7.6 (2.9-15.0) |
| Median WBC, × 109/L (range) | 8 (0.2-394) | 16.1 (0.4-367.2) | 7 (0.2-245) | 4.1 (0.5-394) |
| Median bilirubin, mg/dL (range) | 0.7 (0-12.1) | 0.6 (0-4.7) | 0.7 (0.1-9.2) | 0.7 (0.2-12.1) |
| . | All regimens . | IA regimens . | FA regimens . | TA regimens . |
|---|---|---|---|---|
| Patients | 1279 | 322 | 600 | 357 |
| Inv(16) or t(8;21) | 82 (6%) | 47 (15%) | 14 (2%) | 21 (6%) |
| − 5/− 7 | 340 (27%) | 29 (9%) | 219 (37%) | 92 (26%) |
| Other abnormality | 344 (27%) | 85 (26%) | 178 (30%) | 81 (23%) |
| Normal karyotype | 513 (40%) | 161 (50%) | 189 (32%) | 163 (46%) |
| MDS | 379 (30%) | 57 (18%) | 186 (31%) | 136 (38%) |
| AHD | 575 (45%) | 48 (15%) | 348 (58%) | 179 (50%) |
| Performance status Zubrod 3 or 4 | 149 (12%) | 44 (14%) | 79 (13%) | 26 (7%) |
| Protective environment | 744 (58%) | 146 (41%) | 367 (81%) | 231 (72%) |
| Median age, y (range) | 60 (16-88) | 53 (16-83) | 63 (16-88) | 61 (17-84) |
| Median marrow blast, % (range) | 37 (0-98) | 48.5 (0-97) | 36 (0-96) | 31 (0-98) |
| Median platelet count, × 109/L (range) | 44 (1-2292) | 50 (1-2292) | 40 (1-1355) | 44 (3-708) |
| Median hemoglobin, g/L (range) | 8.0 (2.1-15.1) | 8.15 (2.1-14.4) | 8.0 (2.8-15.1) | 7.6 (2.9-15.0) |
| Median WBC, × 109/L (range) | 8 (0.2-394) | 16.1 (0.4-367.2) | 7 (0.2-245) | 4.1 (0.5-394) |
| Median bilirubin, mg/dL (range) | 0.7 (0-12.1) | 0.6 (0-4.7) | 0.7 (0.1-9.2) | 0.7 (0.2-12.1) |
IA, idarubicin + ara-C; FA, fludarabine + ara-C; TA, topotecan + ara-c; MDS, myelodysplasia; AHD, antecedent hematologic disorder; WBC, white blood cell.
Descriptions of the regimens
The IA, FA, and TA regimens (Table3) delivered approximately the same amount of ara-C: 1000 to 2000 mg/m2/d × 4 to 5 days, with the 4-day duration used when ara-C was combined with idarubicin. A possibly significant difference between the regimens was that ara-C was given by continuous infusion in the IA studies, but over 2 to 4 hours daily in the FA and TA trials. Idarubicin in the IA regimen, and when used together with FA, was given at 12 mg/m2 daily on days 1 to 3 (IA) and 2 to 4 (FA). Fludarabine was given at 30 mg/m2/d, with each dose given 4 hours prior to a dose of ara-C. The topotecan dose was 1.5 mg/m2/d × 5 days by continuous infusion. When combined with TA, cyclophosphamide was given at 300 mg/m2 every 12 hours on days 1 to 3. When given with the IA, FA, or TA regimens, G-CSF at 200 to 400 μg/m2 was begun 1 day prior to chemotherapy and continued until the neutrophil count exceeded 1000/μL; if the presenting WBC count was more than 10 000/μL, G-CSF began 1 to 2 days after chemotherapy began. In both IA + G-CSF + ATRA and FAI + G-CSF + ATRA, the ATRA dose was 45 mg/m2/d, beginning 2 days prior to chemotherapy and continuing until response was known; if the presenting WBC count exceeded 10 000/μL, ATRA began on day 1 of chemotherapy. Lisofylline, a purported cytokine antagonist, had been reported to decrease complications of induction therapy10; it was given at 3 mg/kg every 6 hours for 28 days or until CR was observed, whichever came first.
Regimen doses and schedules
| Regimen . | Induction doses (mg/m2/d × d) . | Postremission therapy . | |||
|---|---|---|---|---|---|
| Ara-C . | Idarubicin . | Fludarabine . | Topotecan3-150 . | ||
| IA | 1500 × 4, CI | 12 × 3 | 0 | 0 | Ara-C, IA × 9-12 mo |
| FA | 1000-2000 × 4-5, bolus | 0 or 12 × 3 | 30 × 4-5 | 0 | Ara-C, FA ± I × 6-12 mo |
| TA | 1000-2000 × 5, bolus | 0 | 0 | 1.5 × 5 CI | TA, or IA, FA, TA × 6-9 m |
| Regimen . | Induction doses (mg/m2/d × d) . | Postremission therapy . | |||
|---|---|---|---|---|---|
| Ara-C . | Idarubicin . | Fludarabine . | Topotecan3-150 . | ||
| IA | 1500 × 4, CI | 12 × 3 | 0 | 0 | Ara-C, IA × 9-12 mo |
| FA | 1000-2000 × 4-5, bolus | 0 or 12 × 3 | 30 × 4-5 | 0 | Ara-C, FA ± I × 6-12 mo |
| TA | 1000-2000 × 5, bolus | 0 | 0 | 1.5 × 5 CI | TA, or IA, FA, TA × 6-9 m |
IA, idarubicin + ara-C; CI, continuous infusion; FA, fludarabine + ara-C; I, idarubicin; TA, topotecan + ara-C.
Cyclophosphamide dose when added to TA was 300 mg/m2 every 12 hours on days 1, 2, and 3.
Only 2% of the 789 patients entering CR after use of the regimens described in Table 3 received transplants in first CR (4% of the CRs obtained after IA, 1% of the CRs after FA, and 3% of the CRs after TA). Rather, post-CR therapy consisted of reduced doses of chemotherapy. With IA and FA, ara-C at 100 mg/m2 daily × 5 continuous infusion, alternated approximately every 5 weeks with reduced doses of the original regimen (Table 3). For IA, reduction entailed an idarubicin dose of 8 mg/m2 daily on days 1 to 2 and an ara-C dose of 1.5 gm/m2 daily × 2 days by continuous infusion. For FA, fludarabine was given at 30 mg/m2 and ara-C at 1 gm/m2 each day on days 1 to 3; when idarubicin was included (FAI-containing regimens), FA was administered on days 1 and 2, with idarubicin 8 mg/m2administered on day 3. After CR with TA, patients received continuing cycles of TA at a topotecan dose of 1.25 mg/m2 daily by continuous infusion on days 1 to 3 and an ara-C dose of 1 gm/m2 daily on the same 3 days. After CR with CAT, patients alternated one course of IA, one course of FA, each as described above, and one course of CAT with a cyclophosphamide dose of 300 mg/m2 every 12 hours, days 1 to 3, an ara-C dose of 1 gm/m2 daily on days 1 and 2, and a topotecan dose of 1.25 mg/m2 daily by continuous infusion on days 1 to 3. The daily dose of each postremission regimen was decreased approximately 25% if duration of myelosuppression exceeded 5 weeks or if toxicity had ensued on the previous course. Therapy continued somewhat longer with the IA regimens (9-12 months of postremission therapy), than with the FA (6-12 months) or TA (6-9 months) regimens.
Other practices
Throughout the 9-year period covered by this report, patients aged 50 years or older received induction therapy in laminar air flow rooms (LAFRs) if these rooms were available; 72% of the 907 patients aged 50 years or older were treated in an LAFR. Patients remained in the LAFR until the neutrophil count was more than 500 to 1000/μL or until more than 42 days had elapsed from the start of treatment. From 1991 to 1995, infection prophylaxis consisted of trimethoprim/sulfamethoxazole and oral fluconazole; from 1995 on quinolones replaced trimethoprim/sulfamethoxazole, and oral itraconazole was added to fluconazole. Between 1991 and 1998, patients not in CR after a first course of induction therapy generally received a second course of the same therapy, changing to alternate therapy if CR, as conventionally defined, was not evident after course 2. Thus, 76% of patients not in CR after course 1 of an IA regimen and 72% of those not in CR after course 1 of an FA regimen received such a second course. By 1998, patients not in CR after course 1 were usually changed to alternative therapies. Thus, only 42% of patients not in CR after course 1 of TA or CAT received a second identical course. Criteria for starting a second course were persistent disease (> 20% blasts in a marrow that was at least 20% cellular in AML or RAEB-t, > 5% blasts in a similarly cellular marrow in RAEB) 14 and 21 days after the start of chemotherapy without improvement between these dates, or the same picture in 2 consecutive marrows obtained after a marrow had shown decreased blasts or less than 20% cellularity as noted above. Relapse was defined by a marrow with more than 5% blasts unrelated to recovery from the previous course of chemotherapy. Marrow morphology was reviewed by a group of hematopathologists at M. D. Anderson.
Statistical methods
Unadjusted probabilities of survival and of EFS in CR were estimated, using the method of Kaplan and Meier.11Unadjusted between-group comparisons of survival and of EFS were made using the log-rank test.12 The extended Cox proportional hazards regression model13,14 was used to assess the ability of patient characteristics or treatments to predict survival and EFS, with goodness-of-fit assessed by the Grambsch-Therneau test,15 Schoenfeld residual plots, Martingale residual plots, and likelihood ratio statistics. The extended Cox model allows the possibility that one or more treatment or covariate effects on the risk of the event (death in survival analysis; death or relapse in analysis of EFS) may vary over time, rather than taking on a single numerical value. Logistic regression16 was used to assess the ability of patient characteristics or treatments to predict the probability of CR, or of achieving CR in the first course of therapy, with goodness-of-fit assessed by residual and partial residual plots. All scatter plots were smoothed by using the lowness method of Cleveland,17 with predictive variables transformed as appropriate based on these plots. Multivariate logistic regression and extended Cox models were obtained by first performing a backward elimination with cutoff of P = .05, then allowing any variable previously deleted to enter the final model if itsP value was < .05. Terms characterizing interactions between treatment and covariates were then added to the model and retained only if their P value was < .05. All computations were carried out on a DEC Alpha 2100 5/250 system computer (Digital Electronics Corporation, Nashua, NH) in Splus18 using standard Splus functions and the Splus survival analysis package of Therneau.19
Results
CR rates
Not surprisingly in view of their more favorable prognoses (Table2), overall CR rates were higher in patients given one of the IA regimens (248 of 322, 77%) than in patients given either an FA regimen (330 of 600, 55%) or a TA regimen (211 of 357, 59%). However, the IA regimens were still superior after accounting for these prognostic variables via logistic regression. This finding is illustrated in Table4, which lists the covariates that were significant predictors of the probability of CR in all 1279 patients. After accounting for the effects of patient prognostic variables, the null hypothesis that the FA regimens are equivalent to the IA regimens is rejected at P = .007; the corresponding Pvalue for the TA regimens is < .001. As previously, after accounting for the covariates listed in Table 4, diagnosis (RAEB-t or RAEB rather than AML) was not predictive. Furthermore, after accounting for these covariates, there was no suggestion that there was an “interaction” between diagnosis and treatment such that the effect of FA or the effect of TA differed in patients with AML as opposed to MDS. Specifically, testing for an interaction between diagnosis and FA gave a P value of .67, whereas the analogous P value for TA was .59.
Logistic regression model for the effects of covariates on the probability of CR in all 1279 patients
| Covariate . | Coefficient estimate . | Effect . | P . |
|---|---|---|---|
| Intercept | 0.854 (0.694) | — | — |
| Received TA | − 0.750 (0.201) | Negative | < .001 |
| Received FA | − 0.511 (0.190) | Negative | .007 |
| Performance status Zubrod 3 or 4 | − 1.190 (0.229) | Negative | < .001 |
| Age of the patient | − 0.022 (0.005) | Older age worse | < .001 |
| Inv(16) or t(8;21) | 1.255 (0.407) | Positive | .002 |
| Chromosome 5 and/or 7 abnormal | − 0.866 (0.166) | Negative | < .001 |
| Other cytogenetic abnormalities | − 0.374 (0.162) | Negative | .021 |
| Treated in laminar air flow room | 0.781 (0.156) | Positive | < .001 |
| Log duration of AHD | − 0.349 (0.057) | Longer duration worse | < .001 |
| Log platelet count | 0.950 (0.339) | ||
| (Log platelet count)2 | − 0.108 (0.045) | CR rate increases as platelet count increases from 1 to 80 000, then decreases at higher platelet counts | .005 |
| Log WBC count | − 0.171 (0.047) | Higher WBC worse | < .001 |
| Log serum bilirubin | − 0.323 (0.148) | Higher bilirubin worse | .030 |
| Covariate . | Coefficient estimate . | Effect . | P . |
|---|---|---|---|
| Intercept | 0.854 (0.694) | — | — |
| Received TA | − 0.750 (0.201) | Negative | < .001 |
| Received FA | − 0.511 (0.190) | Negative | .007 |
| Performance status Zubrod 3 or 4 | − 1.190 (0.229) | Negative | < .001 |
| Age of the patient | − 0.022 (0.005) | Older age worse | < .001 |
| Inv(16) or t(8;21) | 1.255 (0.407) | Positive | .002 |
| Chromosome 5 and/or 7 abnormal | − 0.866 (0.166) | Negative | < .001 |
| Other cytogenetic abnormalities | − 0.374 (0.162) | Negative | .021 |
| Treated in laminar air flow room | 0.781 (0.156) | Positive | < .001 |
| Log duration of AHD | − 0.349 (0.057) | Longer duration worse | < .001 |
| Log platelet count | 0.950 (0.339) | ||
| (Log platelet count)2 | − 0.108 (0.045) | CR rate increases as platelet count increases from 1 to 80 000, then decreases at higher platelet counts | .005 |
| Log WBC count | − 0.171 (0.047) | Higher WBC worse | < .001 |
| Log serum bilirubin | − 0.323 (0.148) | Higher bilirubin worse | .030 |
TA, topotecan + ara C; FA, fludarabine + ara-C; AHD, antecedent hematologic disorder; CR, complete remission; WBC, white blood cell.
Other variables considered for inclusion in the final model with the p-values obtained after adding each variable one-at-a-time to the final model: acute myeloid leukemia (rather than myelodysplasia)P = .95, percentage of marrow blasts P= .45, hemoglobin P = .33.
We observed that 92%, 91%, and 94% of the CRs with IA, FA, and TA, respectively, occurred on the first course of therapy. Among patients given a second course of their initial regimen, the CR rates were IA 20 of 39 (51%), FA 28 of 106 (26%), and TA 12 of 49 (24%), so that the superiority of IA in a second course was similar to that seen in the overall relative CR rates. However, as noted above, patients not in CR after an initial course of therapy were more likely to get a second course of their initial regimen if given an IA or FA regimen rather than a TA regimen. To account for the resultant possibility that there might be more CRs with TA if patients were equally likely to receive a second course of TA as of IA or FA, we repeated the logistic regression analysis with patients counted as CR only if CR was observed after course 1. The results were substantively the same; both FA (P = .06) and TA (P < .001) still had negative effects on the probability of achieving CR in one course after accounting for the prognostic covariates listed in Table 4.
EFS in CR
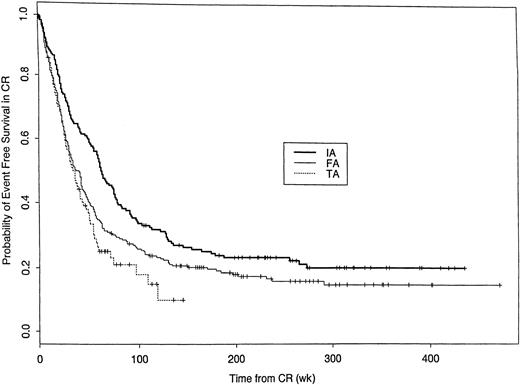
Among the 789 (62%) patients achieving CR, EFS (with an “event” defined as relapse or death in CR) was greatest in patients given the IA regimens (Figure 1). Covariates predictive of EFS (Table5) were similar to those associated with CR. As for CR, the superior EFS with the IA regimens was not merely due to the more favorable constellation of covariates in IA-treated patients. In particular, after adjusting for significant prognostic variables, the relative risk (RR) of relapse or death with the TA regimens was 1.53 times that with the IA regimens (Table 5, e.426 = 1.53). The covariate-adjusted effect of FA was more complex in that it varied with time from CR (Table 5). Compared with IA, the RR of an event with FA was higher during the first year after CR, with a beneficial effect thereafter. After accounting for the covariates in Table 5, diagnosis (AML versus MDS) was not predictive of EFS.
Probability of event-free survival among patients achieving CR following treatment with IA, FA, or TA regimens.
Kaplan-Meier estimates of the proportion of patients achieving CR who have neither relapsed nor died in CR dated from CR date. Log-rankP value < .001. Events have occurred in 191 of the 248 IA CRs, 279 of the 330 FA CRs, and 144 of the 211 TA CRs. Median censoring times (in weeks) are 238 (IA), 206 (FA), and 56 (TA).
Probability of event-free survival among patients achieving CR following treatment with IA, FA, or TA regimens.
Kaplan-Meier estimates of the proportion of patients achieving CR who have neither relapsed nor died in CR dated from CR date. Log-rankP value < .001. Events have occurred in 191 of the 248 IA CRs, 279 of the 330 FA CRs, and 144 of the 211 TA CRs. Median censoring times (in weeks) are 238 (IA), 206 (FA), and 56 (TA).
Extended Cox model for event-free survival in CR
| Covariate . | Coefficient estimate . | Effect . | P . |
|---|---|---|---|
| TA | 0.426 (0.144) | Negative | .003 |
| FAt | 0.558 (0.257) | Initially negative, then positive after about 1 y | .011 |
| FAt2 | − 0.347 (0.118) | ||
| Age | 0.011 (0.004) | Older age worse | .002 |
| Inv(16) or t(8;21) | − 0.641 (0.198) | Positive | .001 |
| Chromosome 5 and/or 7 abnormal | 0.803 (0.120) | Negative | < .001 |
| Treated in laminar air flow room | − 0.367 (0.116) | Positive | .002 |
| AHD present | 0.373 (0.113) | Negative | < .001 |
| Log platelet count | − 0.152 (0.049) | Higher better | .002 |
| Log WBC count | 0.106 (0.037) | Higher worse | .004 |
| Covariate . | Coefficient estimate . | Effect . | P . |
|---|---|---|---|
| TA | 0.426 (0.144) | Negative | .003 |
| FAt | 0.558 (0.257) | Initially negative, then positive after about 1 y | .011 |
| FAt2 | − 0.347 (0.118) | ||
| Age | 0.011 (0.004) | Older age worse | .002 |
| Inv(16) or t(8;21) | − 0.641 (0.198) | Positive | .001 |
| Chromosome 5 and/or 7 abnormal | 0.803 (0.120) | Negative | < .001 |
| Treated in laminar air flow room | − 0.367 (0.116) | Positive | .002 |
| AHD present | 0.373 (0.113) | Negative | < .001 |
| Log platelet count | − 0.152 (0.049) | Higher better | .002 |
| Log WBC count | 0.106 (0.037) | Higher worse | .004 |
CR, complete remission; TA, received topotecan + ara-C regimen; FA, received fludarabine + ara-C regimen; t, (weeks from CR)/20; AHD, antecedent hematologic disorder; WBC, white blood cell.
Other variables considered for inclusion in the final model with theP values obtained after adding each variable one at a time to the final model: performance status 3 or 4 (rather than 0-2)P = .09, percentage of marrow blasts P= .52, hemoglobin P = .10, log(bilirubin)P = .89, without either fludarabine or topotecan for treatment of newly diagnosed acute myeloid leukemia (rather than myelodysplasia) P = .69.
Survival
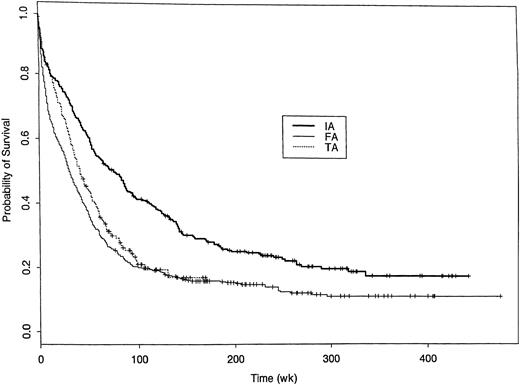
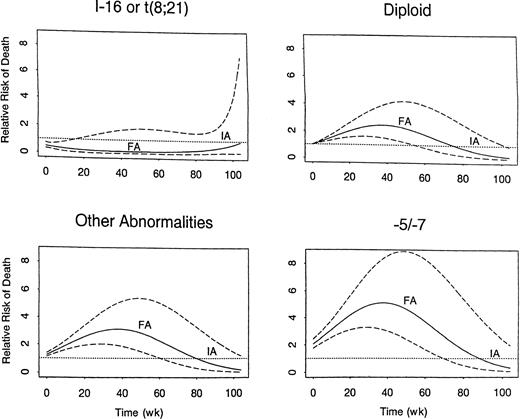
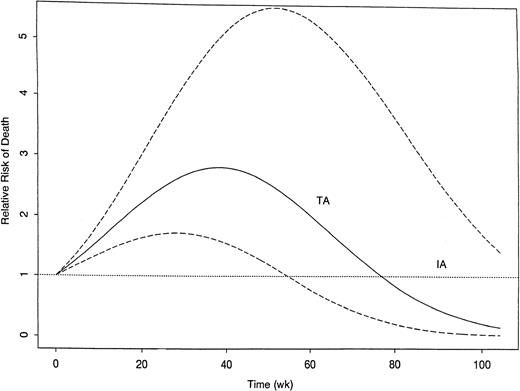
As might be expected given the effects of treatment on CR rate and EFS in CR, survival from start of treatment was shorter in both the FA and TA groups compared with the IA group (Figure2). Table6 summarizes the treatment and covariate effects in the fitted extended Cox model for survival. The unfavorable effects of both FA and TA relative to IA each varied over time from treatment, with both effects disappearing after 2 years. Additionally, the unfavorable effect of FA relative to IA was not seen in patients with inv(16) or t(8;21). Figure3 illustrates the time-varying effect of FA compared with IA within each of 4 cytogenetic subgroups. Note the unfavorable effect in all groups but inv(16) or t(8;21). Figure4 provides the analogous illustration of the TA effects, and this figure is simpler than Figure 3 because the TA effect does not change across the cytogenetic subgroups; rather TA is unfavorable relative to IA in all cytogenetic subgroups. The curvilinear patterns illustrated in the figures lead to the characterization of the effects of FA and TA as “quadratic” (Table6). For each plot in Figures 3 and 4, the RR of death corresponding to IA is 1.0 (dotted line) because this is the baseline group, and each estimated RR (solid line) is accompanied by a 95% confidence band (dashed line). As shown by the 95% confidence bands, the RR of death was significantly higher with FA regimens than with IA regimens for at least 1 year in all patients except those with inv(16) or t(8;21) in which there was a lower RR with FA for approximately the first 20 weeks after start of treatment. The RR of death with FA compared with IA was greatest in patients with −5/−7. The RR pattern was similar for patients given the TA regimens, as it increased over time to a maximum of 2.8 at approximately 38 weeks from start of therapy, subsequently decreasing; by 78 weeks and thereafter it was similar to IA. After adjustment for the prognostic covariates in Table 6, diagnosis (AML versus MDS) had no effect on survival, nor was there any suggestion that the effects of FA or TA on survival differed in AML as opposed to MDS (P = .16 for FA and P = .99 for TA).
Probability of survival following treatment with IA, FA, or TA regimens.
Kaplan-Meier estimates of the proportion of patients remaining alive dated from start of treatment. Log-rank P value < .001. Of the 322 IA patients 244 have died, 508 of the 600 FA patients, and 217 of the 357 TA patients. Median censoring times (in weeks) are 243 (IA), 206 (FA), and 58 (TA).
Probability of survival following treatment with IA, FA, or TA regimens.
Kaplan-Meier estimates of the proportion of patients remaining alive dated from start of treatment. Log-rank P value < .001. Of the 322 IA patients 244 have died, 508 of the 600 FA patients, and 217 of the 357 TA patients. Median censoring times (in weeks) are 243 (IA), 206 (FA), and 58 (TA).
Extended Cox model for survival
| Covariate . | Coefficient estimate . | Effect . | P . |
|---|---|---|---|
| FAt [t < 104] [not inv(16) or t(8;21)] | 0.048 (0.007) | Quadratic | < .001 |
| Fat2 [t < 104] [not inv(16) or t(8;21)] | − 0.0006 (0.00009) | — | — |
| TAt [t < 104] | 0.055 (0.009) | Quadratic | < .001 |
| TAt2 [t < 104] | − 0.0007 (0.0001) | — | — |
| PS34 [t < 26] | 1.242 (0.120) | Negative | < .001 |
| PS34 [26 ≤ t < 52] | 0.682 (0.272) | Negative | .012 |
| PE [t < 26] | − 0.595 (0.104) | Positive | < .001 |
| Log WBC [t < 26] | 0.104 (0.033) | Higher worse | .002 |
| Log WBC [26 ≤ t < 78] | 0.255 (0.039) | Higher worse | < .001 |
| Age | 0.021 (0.002) | Older worse | < .001 |
| Inv(16) or t(8;21) | − 0.720 (0.193) | Positive | < .001 |
| Chromosome 5 and/or 7 abnormal | 0.731 (0.083) | Negative | < .001 |
| Other cytogenetic abnormalities | 0.235 (0.081) | Negative | .004 |
| Log duration of AHD | 0.095 (0.027) | Longer worse | < .001 |
| Log platelet count | − 0.165 (0.035) | Higher better | < .001 |
| Log serum bilirubin | 0.164 (0.070) | Higher worse | .018 |
| Covariate . | Coefficient estimate . | Effect . | P . |
|---|---|---|---|
| FAt [t < 104] [not inv(16) or t(8;21)] | 0.048 (0.007) | Quadratic | < .001 |
| Fat2 [t < 104] [not inv(16) or t(8;21)] | − 0.0006 (0.00009) | — | — |
| TAt [t < 104] | 0.055 (0.009) | Quadratic | < .001 |
| TAt2 [t < 104] | − 0.0007 (0.0001) | — | — |
| PS34 [t < 26] | 1.242 (0.120) | Negative | < .001 |
| PS34 [26 ≤ t < 52] | 0.682 (0.272) | Negative | .012 |
| PE [t < 26] | − 0.595 (0.104) | Positive | < .001 |
| Log WBC [t < 26] | 0.104 (0.033) | Higher worse | .002 |
| Log WBC [26 ≤ t < 78] | 0.255 (0.039) | Higher worse | < .001 |
| Age | 0.021 (0.002) | Older worse | < .001 |
| Inv(16) or t(8;21) | − 0.720 (0.193) | Positive | < .001 |
| Chromosome 5 and/or 7 abnormal | 0.731 (0.083) | Negative | < .001 |
| Other cytogenetic abnormalities | 0.235 (0.081) | Negative | .004 |
| Log duration of AHD | 0.095 (0.027) | Longer worse | < .001 |
| Log platelet count | − 0.165 (0.035) | Higher better | < .001 |
| Log serum bilirubin | 0.164 (0.070) | Higher worse | .018 |
FA = received fludarabine + ara-C regimen; t = weeks from start of treatment; [t < 104] = 1 if t < 104, 0 if t ≥ 104, etc; TA, received topotecan + ara-C regimen; PS, performance status; PE, protected environment; WBC, white blood cell; AHD, antecedent hematologic disorder.
Other variables considered for inclusion in the final model with TheP values obtained after adding each variable one at a time to the final model: acute myeloid leukemia (rather than myelodysplasia)P = .94, percentage of marrow blasts P= .10, hemoglobin P = .054.
Time-varying covariate-adjusted relative risk of death following treatment with FA regimens relative to risk with IA regimens.
In each cytogenetic group, the risk with IA is 1.0 (dotted line), because IA is the baseline group. The risk with FA is given by the solid line, accompanied by the 95% confidence bands (dashed lines).
Time-varying covariate-adjusted relative risk of death following treatment with FA regimens relative to risk with IA regimens.
In each cytogenetic group, the risk with IA is 1.0 (dotted line), because IA is the baseline group. The risk with FA is given by the solid line, accompanied by the 95% confidence bands (dashed lines).
Time-varying covariate-adjusted relative risk of death following treatment with TA regimens relative to risk with IA regimens.
The risk with IA, the baseline group, is 1.0 (dotted line). The risk with TA is given by the solid line, accompanied by the 95% confidence bands (dashed lines). Unlike the FA effect, the TA effect does not vary across cytogenetic groups.
Time-varying covariate-adjusted relative risk of death following treatment with TA regimens relative to risk with IA regimens.
The risk with IA, the baseline group, is 1.0 (dotted line). The risk with TA is given by the solid line, accompanied by the 95% confidence bands (dashed lines). Unlike the FA effect, the TA effect does not vary across cytogenetic groups.
Possible confounding factors
Effects of individual trials.
We next asked whether variations in outcomes between the trials collectively comprising the IA, FA, or TA treatment groups (Table 1) were sufficiently large to account for the unfavorable effects heretofore attributed to FA and TA. To assess whether the apparent effects of FA or TA were due to variation between trials, we distinguished the following trial groups: (1) the original IA trial; (2) the IA + G-CSF trial; (3) the IA + G-CSF + ATRA trial; (4) the 2-arm randomized trial of IA ± lisofylline, having previously published7 that these, the 2 arms of a randomized trial, produced similar CR, disease-free survival in CR, and survival; (5) a randomized 4-arm trial of FAI, FAI + G-CSF, FAI + ATRA, FAI + ATRA + G-CSF, with a previous publication8 indicating that each of these 4 regimens produced similar outcomes; (6) an FAI + G-CSF trial given outside the context of the randomized trial; (7) a trial of FA itself and a trial of FLAG, with a previous publication20 indicating these were similar; (8) the original TA trial; and (9) the trial of TA + CAT. We used the IA trial (trial 1 above) as the “baseline” trial in defining between-trial effects. After fitting this more detailed extended Cox model and then dropping nonsignificant individual trial effects via a backward elimination, the only individual trial effect that remained was that of the IA ± lisofylline trial (trial 4, negative trial effectP = .023), whereas the negative effects of the FA and TA regimens persisted. In particular, the inclusion in the model, described in Table 6, of the additional term for the lisofylline trial effect had a trivial effect on the numerical values of the parameter estimates, and the substantive conclusions regarding the FA and TA effects were unchanged.
Treatment year.
An alternative to adjusting for trial effects is to account for possible latent effects over time by including factors for the year in which each patient's treatment began. The regimens comprising the IA group were more often used in the early 1990s, whereas the FA and TA regimens were more often administered in the mid- and late-1990s, respectively. However, the IA, FA, and TA regimens were given in overlapping periods (Table 1; IA 1991-1997, FA 1991-1998, and TA 1997-1999). This fact made it possible to ascertain whether the treatment effects described above merely reflected an effect of the year in which treatment began. When separate terms for each of the calendar years 1991-1999 were added to the model summarized in Table 6, none of these terms provided significant additional information; theP values of the 9 tests for calendar year effects ranged from .13 to .96, suggesting that the effects of latent variables that may have changed over time were negligible.
Therapies given after failure of IA, FA, or TA.
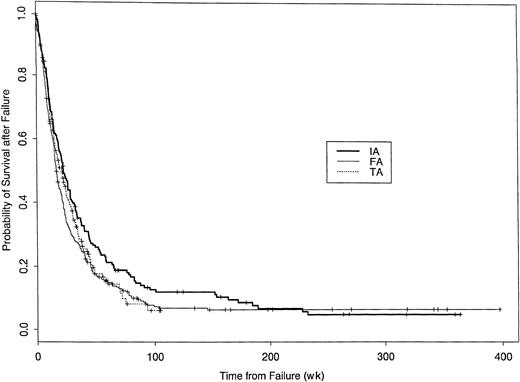
Might the longer survival in patients given an IA, rather than an FA or a TA, regimen reflect the superiority of treatments given afterfailure of IA, FA, or TA? Similar proportions of patients who failed (relapse, or alive but no initial CR) IA, FA, or TA regimens received an allogeneic transplant at failure: 7% of the 184 IA failures, 6% of the 324 FA failures, and 5% of the 230 TA failures; only one patient received an autologous transplant at failure. However, a higher proportion of IA failures received an investigational high-dose ara-C (≥ 1gm/m2/dose)–containing regimen (47% of IA failures, 24% of FA failures, and 28% of TA failures), with these regimens being essentially equivalent therapeutically.21 In contrast, FA or TA failures were more likely to receive phase I or phase II therapies not containing ara-C (32% IA, 40% FA, and 38% TA) or standard regimens not containing ara-C (eg, mitoxantrone + etoposide) (7% IA, 17% FA, and 21% TA). The proportion of failures receiving no further therapy was 7% IA, 13% FA, and 7% TA. High-dose ara-C may have been used more frequently at failure in the IA group because patients in this group were generally younger than patients in the FA or TA groups. Survival after failure was slightly, but not significantly, longer in the IA cohort (Figure 5). Moreover, this effect was insufficient to account for the overall superior survival, dated from start of IA, FA, or TA, seen with IA (compare Figures 2 and5). Furthermore, IA was superior to FA or TA (P < .0001, data not shown) considering time to failure from initial treatment (IA, FA, or TA), with failure defined as whichever came first among failure to enter CR (despite surviving remission induction), relapse, or death.
Probability of survival after failure of IA, FA, or TA regimens.
Kaplan-Meier estimates of the proportion of patients remaining alive dated from time of failure. Failure is defined as failure to achieve CR, despite surviving induction, or relapse. Log-rank Pvalue is .10.
Probability of survival after failure of IA, FA, or TA regimens.
Kaplan-Meier estimates of the proportion of patients remaining alive dated from time of failure. Failure is defined as failure to achieve CR, despite surviving induction, or relapse. Log-rank Pvalue is .10.
Non–model-based comparisons
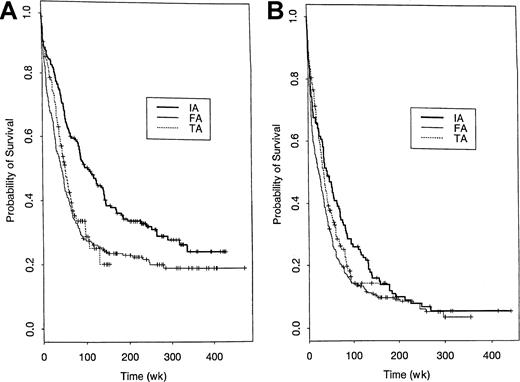
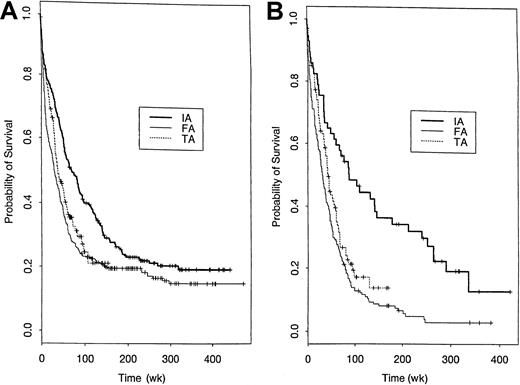
An important question is whether the conclusions from the model-based analyses described above are borne out by much simpler non–model-based comparisons within important patient subgroups. Figure6 shows that IA appears superior in either younger or older patients. Very similar comparative patterns were found in subgroup analyses based on performance status (< 3 versus > 2), presence of an AHD, or diagnosis (AML versus MDS) (Figure 7). Figure8 provides a similar comparison for the 4 cytogenetic groups considered here and illustrates longer survival with IA in patients with a normal karyotype and cytogenetic abnormalities other than −5/−7 or inv(16)/t(8;21). The longer survival with FA in the inv(16)/t(8;21) group is based on a relatively small number of patients (n = 47 IA, 14 FA). The slight inferiority of IA in the −5/−7 group must be interpreted in light of the very poor survival with all 3 regimens in this subgroup and the fact that, after accounting for factors other than cytogenetics that affect prognosis, IA was superior to FA and TA in this subset (Table 6, Figures 3,4). Table 7 provides median survival times for each of the 9 trials enumerated above, both overall and within the subgroups for those aged younger than 60 years and 60 years or older. A similar survival breakdown by cytogenetic subgroup is given in Table 8. These tables show that, although there was substantial trial-to-trial variability in survival when considering all 9 trials without regard to treatment, most of this variability was due to differences between the IA, TA, and FA regimens. In particular, each of the 4 IA trials was associated with a longer median survival than any of the 3 FA or 2 TA trials. Tables 7and 8 also show that in both younger and older patients and in the principal cytogenetic groups (normal and other abnormal) each IA regimen outperformed each FA regimen and, in general, each TA regimen.
Probability of survival in younger and older patients following treatment with IA, FA, or TA regimens.
Kaplan-Meier estimates of the proportion of patients aged younger than 60 years (A) and 60 years or older (B) who remain alive following treatment with the 3 regimens.
Probability of survival in younger and older patients following treatment with IA, FA, or TA regimens.
Kaplan-Meier estimates of the proportion of patients aged younger than 60 years (A) and 60 years or older (B) who remain alive following treatment with the 3 regimens.
Probability of survival in patients with AML and patients with MDS following treatment with IA, FA, or TA regimens.
Kaplan-Meier estimates of the proportion of patients with AML (A) and MDS (B) who remain alive following treatment with IA, FA, or TA regimens.
Probability of survival in patients with AML and patients with MDS following treatment with IA, FA, or TA regimens.
Kaplan-Meier estimates of the proportion of patients with AML (A) and MDS (B) who remain alive following treatment with IA, FA, or TA regimens.
Probability of survival according to cytogenetic subgroup following treatment with IA, FA, or TA regimens.
Kaplan-Meier estimates of the proportion of patients with inv(16) or t(8;21) (A), normal karyotype (B), −5/−7 (C), and other abnormal karyotypes (D) who remain alive following treatment with IA, FA, or TA regimens.
Probability of survival according to cytogenetic subgroup following treatment with IA, FA, or TA regimens.
Kaplan-Meier estimates of the proportion of patients with inv(16) or t(8;21) (A), normal karyotype (B), −5/−7 (C), and other abnormal karyotypes (D) who remain alive following treatment with IA, FA, or TA regimens.
Trial-to-trial variability in survival by age group
| Trial . | Group . | Median survival, wk (95% CI) . | ||
|---|---|---|---|---|
| Overall . | Age < 60 y . | Age ≥ 60 y . | ||
| IA | IA | 77 (56-132) | 169 (96 to —) | 47 (32-69) |
| n = 84 | n = 54 | n = 30 | ||
| IA + G-CSF (G) | IA | 86 (58-123) | 123 (86-182) | 36 (17-94) |
| n = 144 | n = 83 | n = 61 | ||
| IA + G + ATRA | IA | 83 (41 to —) | 81 (34 to —) | 83 (52 to —) |
| n = 25 | n = 20 | n = 5 | ||
| IA ± lisofylline | IA | 53 (44-88) | 61 (49-127) | 34 (10-81) |
| (randomized) | n = 69 | n = 47 | n = 22 | |
| FAI ± G and/or ATRA | FA | 30 (23-40) | 44 (30-63) | 23 (15-35) |
| (randomized) | n = 248 | n = 89 | n = 159 | |
| FAI + G or ATRA | FA | 28 (18-39) | 36 (16-66) | 25 (15-35) |
| (not randomized) | n = 136 | n = 60 | n = 76 | |
| FA or FLAG | FA | 32 (27-46) | 51 (41-88) | 20 (10-32) |
| n = 216 | n = 97 | n = 119 | ||
| TA | TA | 38 (30-48) | 45 (25-67) | 38 (27-52) |
| n = 98 | n = 23 | n = 75 | ||
| CAT | TA | 45 (35-58) | 58 (46-97) | 30 (23-41) |
| n = 259 | n = 136 | n = 123 | ||
| Trial . | Group . | Median survival, wk (95% CI) . | ||
|---|---|---|---|---|
| Overall . | Age < 60 y . | Age ≥ 60 y . | ||
| IA | IA | 77 (56-132) | 169 (96 to —) | 47 (32-69) |
| n = 84 | n = 54 | n = 30 | ||
| IA + G-CSF (G) | IA | 86 (58-123) | 123 (86-182) | 36 (17-94) |
| n = 144 | n = 83 | n = 61 | ||
| IA + G + ATRA | IA | 83 (41 to —) | 81 (34 to —) | 83 (52 to —) |
| n = 25 | n = 20 | n = 5 | ||
| IA ± lisofylline | IA | 53 (44-88) | 61 (49-127) | 34 (10-81) |
| (randomized) | n = 69 | n = 47 | n = 22 | |
| FAI ± G and/or ATRA | FA | 30 (23-40) | 44 (30-63) | 23 (15-35) |
| (randomized) | n = 248 | n = 89 | n = 159 | |
| FAI + G or ATRA | FA | 28 (18-39) | 36 (16-66) | 25 (15-35) |
| (not randomized) | n = 136 | n = 60 | n = 76 | |
| FA or FLAG | FA | 32 (27-46) | 51 (41-88) | 20 (10-32) |
| n = 216 | n = 97 | n = 119 | ||
| TA | TA | 38 (30-48) | 45 (25-67) | 38 (27-52) |
| n = 98 | n = 23 | n = 75 | ||
| CAT | TA | 45 (35-58) | 58 (46-97) | 30 (23-41) |
| n = 259 | n = 136 | n = 123 | ||
CI, confidence interval; IA, idarubicin + ara-C; G-CSF, granulocyte colony-stimulating factor; ATRA, all-trans retinoic acid; FAI, fludarabine + ara-C + idarubicin; FA, fludarabine + ara-C; FLAG, FA + G-CSF; TA, topotecan + ara-C; CAT, cyclophosphamide + G-CSF.
Trial-to-trial variability in survival by cytogenetic group
| Trial . | Group . | Median survival, wk (95% CI) . | ||||
|---|---|---|---|---|---|---|
| Overall . | Inv(16) or t(8;21) . | − 5/− 7 . | Other abnormal . | Normal . | ||
| IA | IA | 77 (56-132) | — (69 to —) | 1 (0.4, to —) | 75 (52-196) | 85 (29-264) |
| n = 84 | n = 15 | n = 3 | n = 27 | n = 39 | ||
| IA + G-CSF (G) | IA | 86 (58-123) | 269 (83 to —) | 34 (7 to —) | 64 (40-136) | 86 (51-140) |
| n = 144 | n = 18 | n = 9 | n = 36 | n = 81 | ||
| IA + G + ATRA | IA | 83 (41 to —) | 65.5 (0.4 to —) | 5 (— to —) | 68 (34 to —) | 83 (41 to —) |
| n = 25 | n = 4 | n = 1 | n = 4 | n = 16 | ||
| IA ± lisofylline | IA | 53 (44-88) | 115 (32 to —) | 22 (5-36) | 88 (51-178) | 63 (49-145) |
| (randomized) | n = 69 | n = 10 | n = 16 | n = 18 | n = 25 | |
| FAI ± G and/or ATRA | FA | 30 (23-40) | 78 (2 to —) | 15 (7-26) | 37 (17-59) | 47 (32-72) |
| (randomized) | n = 248 | n = 5 | n = 76 | n = 74 | n = 93 | |
| FAI + G or ATRA | FA | 28 (18-39) | — (— to —) | 18 (13-33) | 28 (12-61) | 53 (33-81) |
| (not randomized) | n = 136 | n = 1 | n = 70 | n = 42 | n = 23 | |
| FA or FLAG | FA | 32 (27-46) | — (— to —) | 26 (17-32) | 26 (12-51) | 49 (35-99) |
| n = 216 | n = 8 | n = 73 | n = 62 | n = 73 | ||
| TA | TA | 38 (30-48) | 64 (17 to —) | 27 (22-42) | 43 (28-80) | 53 (38-86) |
| n = 98 | n = 3 | n = 35 | n = 28 | n = 32 | ||
| CAT | TA | 45 (35-58) | — (— to —) | 31 (17-48) | 41 (23-71) | 52 (32 to —) |
| n = 259 | n = 18 | n = 57 | n = 53 | n = 131 | ||
| Trial . | Group . | Median survival, wk (95% CI) . | ||||
|---|---|---|---|---|---|---|
| Overall . | Inv(16) or t(8;21) . | − 5/− 7 . | Other abnormal . | Normal . | ||
| IA | IA | 77 (56-132) | — (69 to —) | 1 (0.4, to —) | 75 (52-196) | 85 (29-264) |
| n = 84 | n = 15 | n = 3 | n = 27 | n = 39 | ||
| IA + G-CSF (G) | IA | 86 (58-123) | 269 (83 to —) | 34 (7 to —) | 64 (40-136) | 86 (51-140) |
| n = 144 | n = 18 | n = 9 | n = 36 | n = 81 | ||
| IA + G + ATRA | IA | 83 (41 to —) | 65.5 (0.4 to —) | 5 (— to —) | 68 (34 to —) | 83 (41 to —) |
| n = 25 | n = 4 | n = 1 | n = 4 | n = 16 | ||
| IA ± lisofylline | IA | 53 (44-88) | 115 (32 to —) | 22 (5-36) | 88 (51-178) | 63 (49-145) |
| (randomized) | n = 69 | n = 10 | n = 16 | n = 18 | n = 25 | |
| FAI ± G and/or ATRA | FA | 30 (23-40) | 78 (2 to —) | 15 (7-26) | 37 (17-59) | 47 (32-72) |
| (randomized) | n = 248 | n = 5 | n = 76 | n = 74 | n = 93 | |
| FAI + G or ATRA | FA | 28 (18-39) | — (— to —) | 18 (13-33) | 28 (12-61) | 53 (33-81) |
| (not randomized) | n = 136 | n = 1 | n = 70 | n = 42 | n = 23 | |
| FA or FLAG | FA | 32 (27-46) | — (— to —) | 26 (17-32) | 26 (12-51) | 49 (35-99) |
| n = 216 | n = 8 | n = 73 | n = 62 | n = 73 | ||
| TA | TA | 38 (30-48) | 64 (17 to —) | 27 (22-42) | 43 (28-80) | 53 (38-86) |
| n = 98 | n = 3 | n = 35 | n = 28 | n = 32 | ||
| CAT | TA | 45 (35-58) | — (— to —) | 31 (17-48) | 41 (23-71) | 52 (32 to —) |
| n = 259 | n = 18 | n = 57 | n = 53 | n = 131 | ||
CI, confidence interval; IA, idarubicin + ara-C; G-CSF, granulocyte colony-stimulating factor; ATRA, all-trans retinoic acid; IA, idarubicin + ara-C; FAI, fludarabine + ara-C + idarubicin; FA, fludarabine + ara-C; FLAG, FA + G-CSF; TA, topotecan + ara-C; CAT, cyclophosphamide + G-CSF.
Discussion
The above data indicate that the various FA and TA regimens we investigated may be inferior to our IA regimens, recalling again that IA regimens are not equivalent to 3 + 7 regimens (Table 3). A possible exception is that the FA regimens appear at least equivalent to IA in patients with the best prognosis cytogenetics, ie, inv(16) or t(8;21) (Figures 3,8), although this finding is based on a relatively small number of patients. The limitations of our analysis must be acknowledged. First, the IA, FA, and TA regimens were not studied within the context of a randomized trial. Hence, it is possible that outcomes were influenced by latent covariates that, although unknown, were both unevenly distributed among the IA, FA, and TA groups (or among the various IA, FA, and TA subgroups) and prognostically important. The observation that patients in the IA ± lisofylline trial had worse outcomes than patients in the other IA trials even after accounting for known covariates such as age, cytogenetics, and so forth, supports the existence of this possibility. In particular, our previously published results indicate that, after accounting for observed prognostic covariates, the IA − lisofylline and IA + lisofylline regimens produced very similar outcomes in a small (70 patient) randomized trial.7 Although the IA − lisofylline regimen was identical in all respects to the IA regimen, patients in the IA ± lisofylline trial did worse than patients in other IA trials and, specifically, did worse than patients given the IA regimen itself. Thus, although there was no lisofylline effect whatsoever, there was a clear “lisofylline trial” effect. Furthermore, the unfavorable effect associated with the lisofylline trial was quantitatively similar to those associated with receiving the regimens comprising FA or the regimens comprising TA. However, it must be borne in mind that only 22% of the patients in the composite IA group were entered into the IA ± lisofylline trial. Thus, although a comparison of the FA or TA patients only with the patients in the IA ± lisofylline trial would presumably find these 3 groups were equivalent (given the quantitatively similar effects described above), such a comparison would lead us to ignore more than 75% of the total data pertaining to the IA group.
Our statistical methodology has limitations in addition to those inherent in the inability to account for latent covariates. In particular, multivariate regression analyses may not take appropriate account of substantial differences in known covariates. The method “regresses” in the sense that there is an overall shrinkage of outlying results to some overall mean function. In addition, when treatment and prognosis are partially confounded, as here, treatment and prognosis share the differential load, thereby exaggerating treatment differences. To address the issue of exaggeration, we established prognostic categories of patients (eg, younger and older, inv(16) or t(8;21), −5/−7 etc, AML and MDS) across the treatments and compared the treatments within these categories. The consistency of treatment effects favoring IA across prognostic categories (Figures6-8) and trials (Tables 7,8) seen with these relatively simple, non–model-based methods supported the findings reached, using the more sophisticated methods described in statistical methods and presented in earlier tables and figures.
Another possible confounding factor is the somewhat longer duration of post-CR treatment in the IA group (Table 3). However, we are unaware of data indicating that differences, in the range of those noted in Table3, in the duration of postremission therapy affect either EFS in CR or survival. Furthermore, the unfavorable effects of FA and TA on EFS in CR and survival were evident, while patients in each of the IA, FA, and TA groups were still receiving postremission therapy (Figures 3,4). A final confounder is that ara-C was administered solely by bolus (over 2-4 hours) in the FA and TA groups but solely by continuous infusion in the IA group (Table 2). Hence, the negative effects attributed to FA and TA may reflect a possible negative effect of receiving ara-C by bolus rather than by continuous infusion. There is little empirical evidence supporting this possibility. In a small randomized trial, Radomski et al22 reported a 24% CR rate in 24 children 15 days after beginning 2-CDA (9 mg/m2 daily × 5) + 500 mg/m2 ara-C over 2 hours daily × 5 versus a 64% rate in 25 children given 2-CDA + continuous infusion ara-C (500 mg/m2 daily × 5). In contrast, in similarly sized, sequential trials in adults with relapsed/refractory AML, we observed CR rates in 21 patients of 59 (36%) with fludarabine (30 mg/m2 daily × 5) + ara-C (1-3 g/m2) given as a short daily infusion (over 2-6 hours daily × 5) and in 5 patients of 21 (24%) with fludarabine (50 mg/m2 daily × 4) + continuous infusion ara-C (1.5 gm/m2 daily × 4)4 (E.H.E., unpublished observations, 2001). More specifically, in patients whose first CR duration exceeded 1 year, the bolus FA regimen gave a CR rate in 14 patients of 20 (70%) and the continuous FA regimen a CR rate in 3 patients of 6 (50%). In patients with shorter first CR durations, CR rates were 7 of 39 (18%) with bolus FA and 2 of 15 (13%) with continuous infusion FA.
The TA regimens used here might be inferior to our IA regimens because neither TA nor CAT contained an anthracycline. In this context, it is interesting that, within the FA group, regimens combining idarubicin with FA were similar to the basic FA regimen.
Although we lack convincing causal explanations for our findings, which cannot be viewed as definitive given the issues raised above, our results are of considerable clinical significance. As noted in the Introduction, TA- and FA-containing regimens, largely identical to those described here, are not uncommonly used outside the setting of a clinical trial, both in the United States and Europe. We hope that our data will lead to caution in the routine use of these regimens. Although it might fairly be debated whether FA and TA are trulyinferior to IA, we believe that our data make it unlikely that these regimens, as used here, are in fact superior to IA, as used here. Nonetheless, the data also indicate that IA regimens themselves are not “good” in any absolute sense for the majority of patients. Hence, emphasis must be placed on investigating new regimens, optimally within the context of randomized clinical trials. These trials should as much as possible contain a “standard” treatment arm. Although inclusion of such a standard has been criticized as allowing patients to receive what may be a very poor therapy, our results highlight the lack of any a priori assurance that the new therapies (eg, FA or TA) will be superior to the standard. Furthermore, the future is likely to see increasing application of “adaptive randomization” designs23 in which, based on the accruing data, patients are more likely to be randomized to a therapy that appears to be performing well. These designs often have desirable statistical properties (eg, probabilities of correctly or incorrectly declaring therapies as useful) and thus serve both the scientific goal of identifying a promising therapy and the medical goal of maximizing the number of patients receiving that therapy.
We thank Angela Beasley for her expert secretarial assistance and Dr Donald Berry of the Department of Biostatistics at M. D. Anderson for helpful suggestions regarding data presentation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elihu H. Estey, Dept of Leukemia, Box 428, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030; e-mail: eestey@mdanderson.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal