Abstract
The hypothesis was tested that amino acid substitutions in specific positions within human leukocyte antigen class I heavy chain would have different impacts on transplant-related mortality (TRM) in patients receiving transplanted bone marrow from unrelated donors. One hundred patients and their unrelated donors were typed by sequence-based typing for the human leukocyte antigen (HLA)–A, -B, and -C loci. All pairs were matched for DRB1, DRB3, DRB4, DRB5, DQA1, and DQB1 loci. Forty pairs were also matched at class I, and 60 pairs had one or more mismatches at class I loci. It was found that substitutions at positions 116 and 114 of class I heavy chain significantly increased the risk for TRM in univariate and bivariate Cox analyses. Conversely, no association between number of multiple mismatches or number of amino acid substitutions and TRM was seen when positions 116 and 114 were adjusted for. Variables predictive of TRM in multivariate Cox analysis were number of cells infused, diagnosis (chronic myeloid leukemia [CML] or non-CML), and amino acid substitution at position 116 or 152. The only variable predictive of severe acute graft-versus-host disease (GVHD) in multivariate Cox analysis was substitution at position 116. Actuarial risk for acute GVHD grade III-IV, TRM, and relapse in pairs with substitutions at position 116 (n = 37) compared to other pairs (n = 63) was, respectively, 36% versus 14% (P = .01), 59% versus 28% (P = .001), and 25% versus 31% (P = .4). In conclusion these data suggest that substitutions at position 116 of class I heavy chain increase the risk for acute GVHD and TRM in patients who receive transplanted bone marrow from unrelated donors.
Introduction
Graft-versus-host disease (GVHD), graft rejection, and delayed immune recovery are more severe and frequent after unrelated donor bone marrow transplantation (UD-BMT) than after human leukocyte antigen (HLA)–identical sibling transplantation.1 One interpretation is that siblings matched by serology are likely to be genotypic matches, whereas unrelated persons with the same serotype may have different alleles. This has suggested the need for molecular matching of most HLA loci in UD-BMT. The outcome is improved when donor and recipient are matched for DRB1 and DQA-DQB,2 whereas the role of DP is less well defined.3
As to class I antigens, the general agreement is that multiple mismatches have a negative impact on survival but that a single mismatch does not modify the outcome significantly.3,4However, we do not know whether different class I mismatches have a different influence on transplant-related mortality (TRM).2,3 In organ transplantation there are so-called taboo combinations,5 associated with a high risk for graft rejection, and so-called permissive mismatches,6-8that will not reduce graft survival. This phenomenon probably depends on the functional relevance of the amino acid differences between the mismatched alleles. We reasoned that permissive and nonpermissive combinations could be relevant in bone marrow transplantation, especially because the immune reaction is directed in 2 directions, the graft-versus-host (GVH) and the host-versus-graft (HVG) vectors. We have, therefore, studied the role of class I loci mismatches in patients who have undergone UD-BMT to identify specific amino acid substitutions increasing the risk for TRM.
Patients and methods
Patients
Patients underwent transplantation at 4 different transplantation units between January 1994 and December 1999: Ospedale San Martino Genova, Ospedale Gaslini Genova, Cattedra Ematologia Bologna, and Clinica Pediatrica Torino. Donor-recipient pairs at the time of transplantation were all DRB1- and DRB3-matched by sequence-specific primers or by sequence-specific oligonucleotide probes (SSOP). Typing for HLA-A and -B loci at the time of transplantation was performed by complement-dependent cytotoxicity test.9
Transplantation procedures
All patients were cared for in single rooms with positive pressure and absolute filtered air. The conditioning regimen consisted of 60 mg/kg cyclophosphamide (CY) per day for 2 consecutive days, followed by total body irradiation (TBI) (9.9 Gy or 12 Gy), or CY 120 mg/kg and 16 mg/kg busulfan, or 15 mg/kg thiotepa. All patients received unmanipulated bone marrow cells provided by the National Donor Registries and facilitated through the Italian Bone Marrow Donor Registry. GVHD prophylaxis consisted of 2 mg/kg per day cyclosporin A and 10 mg/m2 short-course methotrexate on days 1, 3, and 6. Acute GVHD was scored as 0 to IV according to established criteria.10
HLA high-resolution typing
Donor-recipient pairs were retrospectively typed by polymerase chain reaction–sequence-based typing (PCR-SBT) for the HLA-A, -B, -C, -DRB1, -DRB3, -DRB4, -DRB5, -DQA1, and -DQB1 loci, starting from genomic DNA or frozen samples of peripheral blood. When peripheral blood was used as the DNA source, genomic DNA was isolated with the salting-out technique. Amplification and sequencing protocols for typing by PCR-SBT of class I loci were described in previous reports.11-13 Briefly, for HLA-B and -C loci, only one locus-specific amplification of exons 2 and 3 was performed using primers located in introns 1 and 3, near the polymorphic exons. For HLA-A locus, the amplification spanned exon 1 to exon 4. Four sequencing reactions covering exons 2 and 3 allowed complete HLA-A typing in most samples. Additional sequencing of exons 1 and 4 led to complete resolution of all HLA-A polymorphisms.11 The ambiguous heterozygous patterns were solved through allele-specific amplification followed by direct sequencing of the product. An analogous approach was used for the DR and DQ loci typed according to previously described techniques.14 For HLA-DRB1, 7 group-specific amplifications of exon 2 were performed. All group-specific primers were designed with a fluorescently labeled 5′ end tail of a -21M13 sequence (-21M13 Big-dye Primer cycle-sequencing reaction kit (Perkin Elmer, Foster City, CA). For HLA-DRB3, -DRB4, and -DRB5 loci, 2 specific primers at the 5′ and 3′ regions of exon 2 were designed to selectively amplify exon 2 of each locus, intron 1 and exon 3 of the DRB4 locus, and exon 3 for the DRB5 locus. Positive PCR mixes were sequenced in both directions using the same amplification primers and the standard AmpliTaq FS Big Dye Terminator cycle-sequencing protocol. HLA-DQA1 exon 2 was amplified using 5 group-specific PCRs that allowed the separation of deleted from nondeleted DQA1 alleles. To increase typing resolution, DQA1 exons 1, 3, and 4 and DQB1 exon 3 also were eventually analyzed by PCR sequence-specific primers.15 HLA-DQB1 exon 2 was amplified using 2 group-specific amplifications. PCR products were purified and then used for cycle-sequencing reactions (Big Dye Terminator cycle-sequencing kit; Perkin Elmer) followed by automated analysis (ABI Prism 377 DNA Automatic Sequencer; Perkin Elmer). Sequence analysis and allele assignment were performed using MatchTools and MTNavigator software (Perkin Elmer).14
Evaluation of amino acid substitution
Amino acid sequences of HLA class I molecules were obtained from the Anthony Nolan site on the World Wide Web (www.anthonynolan.org.uk/HIG/seq/pep/text/). Visual analysis was carried out by using program O16 on an SGI workstation.
Statistical analysis
The effect of the number and position of amino acid substitutions on TRM and on acute GVHD was evaluated in univariate and multivariate analyses (including other clinical variables) using a Cox model with a backward selection procedure based on the likelihood ratio test. Kaplan-Meier actuarial survival estimates were calculated for acute GVHD, TRM, relapses, disease-free survival (DFS), and overall survival (OS) and were compared among different patient categories using the log-rank test. In tests of significance, no correction for multiple comparisons was made because of the exploratory nature of these analyses. As a consequence, all P values should be considered with caution. Cumulative incidence rates, based on the competing risks method, were also calculated for TRM and relapses.17
Results
Effect of position of amino acid substitution on transplant-related mortality
All 100 donor–recipient pairs were matched for DRB1, DRB3, DRB4, DRB5, DQA1, and DQB1 loci. Forty pairs were also matched for A, B, and C loci, whereas 60 had one or more mismatches at class I. In detail, 16 pairs had 2 mismatches, always at different loci, and 44 had only one.
Step 1.
The occurrence of a single mismatch slightly increased the risk for TRM (hazard ratio [HR] = 1.54; P = .27), whereas the occurrence of multiple mismatches significantly increased the risk for TRM (HR = 3.38; P = .004). A single mismatch occurred 4 times at the HLA-A allele, 8 times at the B allele, and 32 times at the C allele.
Step 2.
We then evaluated whether there was any difference in the risk for TRM in mismatches at different loci (A, B, or C). No statistically significant heterogeneity was seen (χ = 2.20, P = .4).
Step 3.
We then analyzed the impact on TRM of number and position of amino acid substitutions. The frequency of substitutions at each amino acid position of the class I heavy chain is detailed in Table1: some positions were rarely involved, such as position 10 with 1 of 60 (2%) patients, and some were frequently involved, such as position 156 with 38 of 60 (63%) patients. Table 2 reports the results of univariate analysis on the association between TRM and total number and the position of amino acid substitutions. The latter association was evaluated only for the most frequently involved positions, that is, substitutions present in 20 or more pairs. Total number of amino acid substitutions (HR = 1.06; P = .02) and substitutions at position 116 (HR = 2.75; P = .002) and 114 (HR = 2.30;P = .01) were significantly associated with the risk for TRM, whereas substitutions at position 9, 156, 24, and 152 showed a weak and nonsignificant association (HR = 1.5-2;P = .05-.10). Because the total number of substitutions and the presence of a substitution at any position are highly correlated variables, bivariate Cox analysis was performed for each amino acid position correcting also for the number of other substitutions (Table 2). When the number of other substitutions was included in the model, using a stepwise procedure, substitutions at positions 116 and 114 were the only ones that excluded the number of other substitutions from the model. Conversely, no association between number of amino acid substitutions and TRM was seen when positions 116 and 114 were adjusted for.
Step 4.
Multivariate Cox analysis was then carried out that included all the positions examined at univariate analysis and several clinical variables affecting risk for TRM—diagnosis (chronic myeloid leukemia [CML] vs non-CML), time interval from diagnosis to transplantation, recipient age, donor age, conditioning regimen (TBI vs no TBI), number of cells infused, donor–recipient gender combinations (female donor–male recipient vs others), and disease phase (first remission for acute leukemia or chronic phase for CML vs advanced phase). Variables retained in the final model (Table3) were number of cells infused (HR = 0.71; P = .003), diagnosis (HR = 0.33;P = .003), amino acid substitution at position 116 (HR = 2.36; P = .01), and amino acid substitution at position 152 (HR = 2.33; P = .03). Parameters estimated using multivariate Cox analysis including interaction terms suggested no interaction between substitution at positions 116 and 152, indicating that these substitutions act independently on the risk for TRM.
Effect of position of amino acid substitution on graft-versus-host disease
The same analysis carried out on TRM was performed on GVHD (Table 3). Total number of amino acid substitutions (HR = 1.07;P = .02) and substitutions at positions 116 (HR = 2.79;P = .01) and 114 (HR = 2.88; P = .01) were significantly associated with the risk for GVHD, whereas substitutions at positions 77, 80, 156, and 152 showed weak and nonsignificant association (HR = 1.5-2; P = .05-.10). In addition, after adjusting for the number of other substitutions, bivariate analysis for GVHD indicated that substitutions at positions 116 and 114 excluded from the model the number of other substitutions. No association between number of other amino acid substitutions and GVHD resulted when positions 116 and 114 were adjusted for. Multivariate Cox analysis was carried out that included all the positions examined at univariate analysis and all the clinical variables in the model for TRM. Only substitution at position 116 was included in the final model (HR = 2.79; P = .01).
Table 4 shows the clinical data of patients, grouped by absence or presence of amino acid substitution at position 116. There appeared to be no difference in the way each parameter was distributed in the 2 groups.
Effect of amino acid substitutions at position 116 on outcome
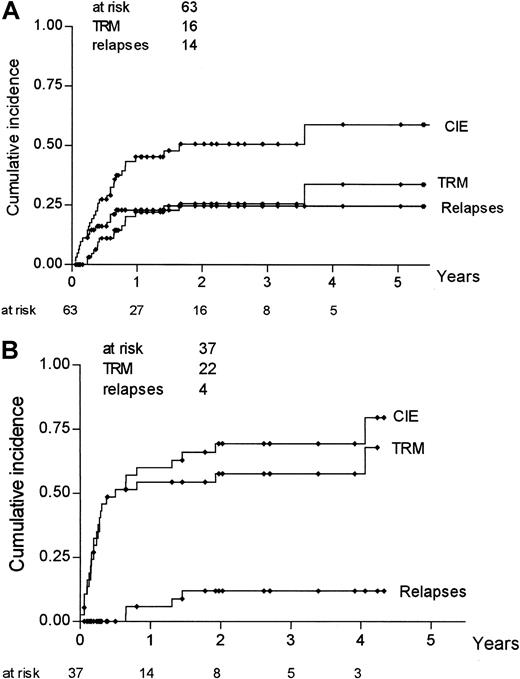
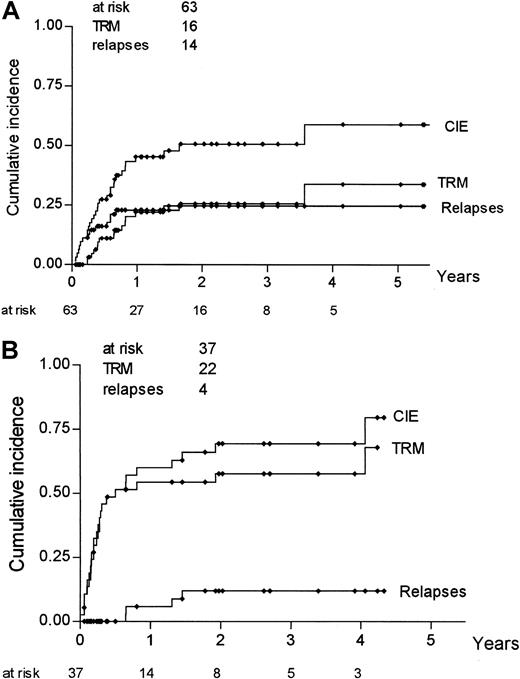
Actuarial risk estimates of severe (grade III-IV) acute GVHD, TRM, relapse, DFS, and OS were then calculated, comparing mismatched pairs with substitutions at position 116 with all the other pairs. As shown in Table 5, the risk for acute GVHD at 100 days was higher in donor–recipient pairs with an amino acid substitution at position 116 than in pairs with no substitution at the same position (36% vs 14%; P = .01); risk for relapse was not statistically different between the 2 groups (25% vs 31%;P = .41), whereas both DFS and OS were higher in donor–recipient pairs with no substitution at position 116. Kaplan-Meier estimates of TRM and severe acute GVHD for pairs with a substitution at position 116 and pairs without such a substitution are shown in Figure 1. Because relapse and TRM are mutually exclusive events, we calculated the cumulative incidence rates based on the competing risks method17; it is known, in fact, that Kaplan-Meier estimates tend to overestimate the proportion of patients in whom each event taken separately develops. Figure2 outlines the cumulative incidence curves, which sum up the cumulative incidence of events in the 2 patient groups. Risks for relapse and TRM at 3 years were estimated as 25% and 26%, respectively, for donor–recipient pairs with no substitution at position 116 and as 12% and 57% for pairs with a substitution at position 116 (Table 5).
Kaplan-Meier estimates of TRM and severe acute GVHD according to involvement of position 116.
TRM (A) and severe (grade III-IV) acute GVHD (B) incidence for pairs with a substitution at position 116 (p116+) compared to pairs without such a substitution (p116−).
Kaplan-Meier estimates of TRM and severe acute GVHD according to involvement of position 116.
TRM (A) and severe (grade III-IV) acute GVHD (B) incidence for pairs with a substitution at position 116 (p116+) compared to pairs without such a substitution (p116−).
Cumulative incidence rates for TRM, relapse, and CIE according to involvement of position 116.
Analysis was based on competing risk methods for pairs without a substitution at position 116 (A) and for pairs with a substitution at position 116 (B).
Cumulative incidence rates for TRM, relapse, and CIE according to involvement of position 116.
Analysis was based on competing risk methods for pairs without a substitution at position 116 (A) and for pairs with a substitution at position 116 (B).
Discussion
These results suggest that a molecular mismatch involving position 116 of the HLA class I heavy chain can influence the outcome of unrelated BMT in donor–recipient pairs, otherwise matched for DRB and DQ loci. Patients with substitution at position 116 are at increased risk for TRM, primarily because of the concomitant increased risk for severe acute GVHD. In these patients, a nonsignificant reduction of relapse was also observed. The resultant net effect for patients with a mismatch involving position 116 was a decrease in DFS and OS. These findings are not in contrast with the correlation between postgraft events and total number of amino acid substitutions or frequency of multiple mismatches; in fact, the higher the number of substitutions or mismatches, the higher the chance position 116 will be involved. Our results may also explain the controversy about the relevance of locus-specific class I mismatches in transplants from unrelated donors18 19; it is possible that the effect of mismatches involving position 116 is diluted by the null effect of mismatches not involving this position.
Based on the available class I crystal structures, some considerations on the peculiar behavior of position 116 may be discussed. The overall structure of different class I molecules is similar, diverging only at the polymorphic residues, which are relatively few and located mostly in the peptide-binding groove.20,21 Bound peptides interact with class I molecules, in human and in mouse, through specific pockets in the groove.22,23 Of particular relevance are the B and F pockets, which harbor, respectively, the N- and C-terminal common motifs of the peptides bound by class I molecules. A slight structural variation affecting one of these pockets can dramatically change the requirements for binding peptides and may result in a different set of bound peptides. Amino acid 116 forms the floor of the F pocket, selecting the size of the peptide C-terminal residue.22,24 Analysis of the different crystal structures indicates that amino acid 116 specifically interacts with residue P9 of the bound peptide.22,24,25 It also shows that at the level of the F pocket, the cleft for the peptide is tight, restricting the requirements for P9 residue accommodation.20 In this regard it has been reported that the different specificities of the same pocket in the different alleles depend primarily on steric, rather than chemical, changes within the pocket.26 Analysis of the 6 Protein Data Bank27 entries representing 5 distinct HLA class I molecules displaying different residues at position 116 (Ser, Tyr, Asp) shows that residue replacement at position 116 is likely to affect steric conformation of the F pocket, changing the requirements for the common motif located at the peptide C-terminus and modifying the set of bound peptides. This is confirmed by the analysis of peptide binding and by the study of T-cell reactivity.28-30 The substitution of Tyr for Ser at position 116 in HLA-B3501 reduces the affinity of peptides carrying Tyr at P9 while it enhances that of peptides with Ile or Leu at P9.28 Furthermore, changes in the sole C-terminal residue of a peptide are sufficient to produce heterogeneity in the responses of different CTL clones with the same peptide specificity.29 In contrast, mutations in the F pocket involving residues other than 116 have no effect on CTL recognition.30
The possibility that differences at position 116 could select for binding of peptide residues other than P9 should also be taken into account. Differences in the residues at positions 114 and 116 modify the conformation outside the F pocket of a peptide that binds with similar efficiency to different B35 alleles and contribute to allele-specific recognition by different CTL clonotypes.31In addition, the occurrence of indirect allorecognition, with peptides including the substituted residue 116 presented here, cannot be ruled out.
If amino acid substitution at position 116 affects the outcome of BMT, it would be difficult to prove the effect of the different amino acid substitutions at this position. The replacement of Tyr with Phe induces less dramatic changes in bulkiness or charge of the side chain than the replacement of Tyr with Ser or the replacement of His with Asp, respectively. Therefore, the type of amino acid substitution occurring at position 116 may have an additional impact on transplantation outcome. A much larger cohort is required to verify this hypothesis.
Conflicting results of substitutions at positions 114 and 152, statistically significant in univariate or multivariate analysis, respectively, suggest that an association caused by pure chance cannot entirely be ruled out. Indeed, the small sample size of the current study is a limiting factor for the power of the analysis. In terms of TRM, with 38 events we have a power of 80% (at a significance level of 5%) to detect a hazard ratio equal to 2.65. A lower HR could be not significant because of insufficient statistical power. The large number of statistical comparisons and the high number of variables included in the multivariate models should be also considered. Exploratory analyses on the robustness of the statistical models suggest caution in interpreting these results and warrant further studies in larger, independent data sets.
We thank our nursing staff for their great work. We thank the Italian Bone Marrow Donor Registry for giving us the pretransplantation typing of patients and donors and for supplying us with blood samples for the SBT typing. We also thank the Centro Ricerche Immunoematologiche AVIS di Bergamo for supplying us with blood samples for the set-up of molecular typing.
Supported by Associazione Italiana Ricerca contro il Cancro, Associazione Ricerca Trapianto Midollo Osseo, CNR Target Project on Biotechnology 1999, Ministero della Sanità R.F. 1998, MURST National Research Programs 1998, and Regione Liguria 1999 and 2000. S.P. is a recipient of an FIRC grant 1999-2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Giovanni B. Ferrara, Servizio di Immunogenetica, IST, c/o CBA torre A2, Largo Rosanna Benzi 10, 16132 Genova, Italy, e-mail: ferrara@cba.unige.it.