Abstract
CD38 is a transmembrane glycoprotein expressed on the surface of leukemic cells in a significant percentage of patients with B-cell chronic lymphocytic leukemia (B-CLL). A recent study suggested that CD38 expression has prognostic value in CLL. Peripheral blood samples from 218 patients with B-CLL were analyzed by flow cytometry for CD38 expression on CD5/19+ leukemic cells. Various patient characteristics were studied including age, sex, Rai and Binet stages, splenomegaly, hepatomegaly, hemoglobin (Hgb) level, β-2 microglobulin (β2M) level in the serum, number of nodal sites involved with disease, and length of survival. The Kaplan-Meier method was used to construct survival curves, and the log-rank statistic was used to compare these curves. CD38 was expressed in 20% or more of leukemic cells in 43% of the patients. Patients with high CD38 expression (20% or more) had significantly shorter survival times (P =.00005). Multivariate analyses showed that CD38 expression is an important prognostic factor associated with high incidence of lymph node involvement (P = .004), lower hemoglobin level (P = .001), hepatomegaly (P = .05), and high β2M level (P = .00005). CD38 expression identified a group of patients with aggressive disease that was considered by Rai staging to be early-stage disease (Rai stages 0-II). Patients with CD38+ samples have significantly aggressive disease regardless of their clinical stage. Measurement of CD38 expression by flow cytometry should become a routine test in the evaluation of patients with CLL.
Introduction
CD38 (also referred to as T10 antigen) was initially characterized in 1980 as a T-cell differentiation antigen.1 In the following years, several studies showed that CD38 expression is not limited to T cells but is widely expressed on different hematopoietic and nonhematopoietic tissues.2-5 The strength of expression of CD38 on hematopoietic cells varies, depending on their stage of maturation and activation. It is expressed on CD34+ progenitor cells, unstimulated pregerminal center B cells, germinal center B cells, activated mature lymphocytes, plasma cells, and myeloid precursors.6,7 The function of CD38 in B cells is not clearly defined, and its expression level varies with the stage of maturation, the type of activation, and the milieu in which activation takes place.8-14 CD38 expression on different types of leukemic cells and its value as a prognostic indicator has been the subject of intense investigation.15-19
Although CLL is the most common leukemia in western countries, it is still a poorly understood disease. CLL is responsible for more than 5000 deaths yearly in the United States.20 Some patients with CLL die within 2 years of diagnosis, yet others have a normal lifespan. Several staging systems have been developed to guide the management of these patients, but the decision to treat or not to treat patients, particularly in earlier stages of the disease, is still difficult to make. The expression of CD5, CD19, and CD23 and the absence of FMC7 and CD22 surface markers are routinely used as diagnostic criteria in patients with B-CLL.21,22 However, these markers do not help in determining the aggressiveness of the disease or in predicting the outcome. Fais et al23 were the first to reported that the presence or absence of immunoglobulin gene mutation could classify patients with B-CLL into 2 groups. Hamblin et al24 and Damle et al18 independently confirmed this observation and demonstrated that patients without the immunoglobulin gene mutation had more aggressive disease with shorter survival times, whereas patients with the immunoglobulin gene mutation had less aggressive disease and prolonged survival times. Damle et al18 studied CD38 expression in 37 samples from patients with B-CLL and reported that cells from patients with unmutated immunoglobulin genes were positive for CD38 expression (30% or more of cells), and this was associated with aggressive disease. In contrast, cells from patients with mutated immunoglobulin genes had low CD38 expression and indolent disease with significantly longer survival times.
In this study, we measured the levels of CD38 expression in 218 patients with B-CLL and correlated these values with various clinical characteristics. Univariate and multivariate statistical analyses showed that CD38 expression in B-CLL cells can identify a subgroup of patients with significantly more aggressive disease.
Patients, materials, and methods
Patient population
Two hundred eighteen patients with B-CLL seen at the M. D. Anderson Cancer Center from 1990 to 1999 were included in this study. Patients were excluded or included in the study strictly based on the availability of samples. The demonstration that known prognostic factors (such as Rai staging, β2M, hemoglobin) are also prognostically relevant in this group of patients (see “Results”) suggests that this group of patients is representative of patients with CLL and is not biased. Choosing 218 patients was also random, without any particular selection. Diagnosis was confirmed using peripheral blood and bone marrow samples examined in morphologic, cytochemical, and immunologic studies (CD3, CD5, CD10, CD11c, CD19, CD23, FMC7, Igκ, and Igλ), cytogenetics, and molecular studies (immunoglobulin heavy- and light-chain gene rearrangement and T-cell receptor rearrangement). Patients were selected for the study based on the availability of peripheral blood samples at the time of presentation, and complete clinical data were recorded from time of diagnosis (time of collecting samples) to the date of the study. Samples were collected at the time of presentation, before therapy was initiated. Of the 218 patients, 142 patients were previously untreated and the rest were previously treated at other institutions. All patients were selected randomly, and patients were not selected based on their history of therapy. All samples were collected under an approved protocol with consent forms signed by the patients. Therapy was mainly based on fludarabine.
Quantification of β-2 microglobulin
Serum levels of β2M were determined using microparticle enzyme immunoassay with IMX autoanalyzer (Abbott, Chicago, IL) as recommended by the manufacturer.
Measurement of CD38 expression by flow cytometry
Cryopreserved peripheral blood samples were obtained at the time of presentation and diagnosis and were used for flow cytometry analysis. Samples were prepared using a 3-color staining method. Isotype-matched negative control antibodies were used to separate positive from negative cells. Directly labeled monoclonal antibodies (mAbs) against the lymphoid antigens CD5-phycoerythrin (PE), CD19-PerCP (peridinin chlorophyll protein), and CD38-allophycocyanin (APC; Becton Dickinson Immunocytochemistry Systems, San Jose, CA) were used. Immunophenotyping expression was measured by FACScalibur (Becton Dickinson). B-CLL cells (CD5/19+) were gated. The degree of CD38 expression in this gated population was expressed as percentage positivity. Samples from 10 patients were analyzed when fresh and after freezing to test what effect freezing had on the surface expression of CD38. Spearman correlation showed complete correlation between the 2 measurements (P < .0001) and no significant difference between them. Mean and variance were 21.7 and 642.8 for the fresh samples and 21.5 and 642.88 for the frozen samples, confirming the reliability of the assay and that freezing had no effect on CD38 expression.
Statistical analyses
The univariate Cox proportional hazard model was used to evaluate the possible associations between overall survival and each risk factor singly. Variables identified as statistically significant (P < .05) in univariate analyses were subsequently included in the stepwise multivariate Cox proportional hazards model. Survival time was measured from the test date to either the last follow-up date (censored) or to the time of death by any cause. Variables examined were age, sex, Rai and Binet stages, splenomegaly, hepatomegaly, hemoglobin (Hgb) level, β-2 microglobulin (β2M) level in the serum, white blood cell (WBC) count, platelet count, lymphocyte count in the peripheral blood, lymphocyte percentage in the bone marrow, cellularity of the bone marrow, number of nodal sites involved with disease, history of therapy, and survival time. All data were collected from reviewing the patient's record and were entered into the leukemia database. The Kaplan-Meier method was used to construct survival curves, and results were compared using the log-rank test. Spearman and Kruskal-Wallis tests were used for comparing correlations and for performing nonparametric hypothesis tests between more than 2 subgroups.
Results
Patient characteristics
Patient characteristics are summarized in Table1. Overall, our patient population was representative for this disease. The male to female ratio was 1.6:1, and the median age was 62 years. Seventy-four percent of patients had Rai stages 0 to II disease, and 66% were untreated. CD38 expression varied in patients with CLL (Figures 1,2). Forty-four percent of the patients had CD38 expression in 20% or more of the cells, and 56% of the patients had CD38 expression in less than 20% of the cells. Sixteen percent of the patients had CD38 expression in more than 80% of the leukemic cells. Only 7 (3%) patients had CD38 expression in 20% to 30% of the cells. Using 20% as a cut-off point in our analysis was arbitrary, and most of the analysis was repeated using CD38 as a continuous variable. Of the 218 patients we studied, 142 (65%) were previously untreated. Our institution is a referral center, and it is possible that a significant number of our patients who had undergone previous therapy were heavily treated and by various therapies.
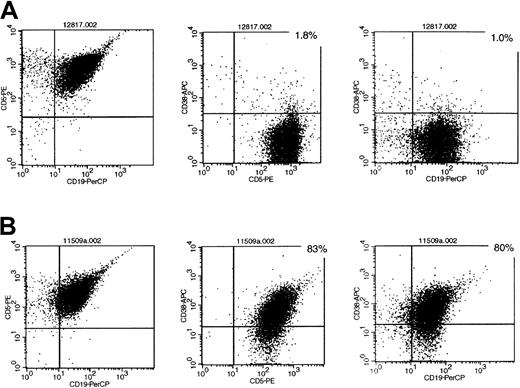
Representative flow cytometry profiles of CD38 expression in patients with B-CLL.
(A) Sample of patients negative for CD38 expression (less than 20%). (B) Sample of patients positive for CD38 expression (20% or more). Directly labeled antibody for CD5-PE, CD19-PerCP, and CD38-APC.
Representative flow cytometry profiles of CD38 expression in patients with B-CLL.
(A) Sample of patients negative for CD38 expression (less than 20%). (B) Sample of patients positive for CD38 expression (20% or more). Directly labeled antibody for CD5-PE, CD19-PerCP, and CD38-APC.
CD38+ B cells.
Percentage of CD38+ B cells as calculated by flow cytometry in patients with B-CLL.
CD38+ B cells.
Percentage of CD38+ B cells as calculated by flow cytometry in patients with B-CLL.
Comparison of CD38+ and CD38− patient subgroups
When the percentages of CD38+ cells were plotted for the entire patient population (data not shown), the patients could be segregated into 2 groups around the 20% level. Patients with 20% or more B cells expressing CD38 were considered positive, and those with less than 20% were considered negative. As shown in Table2, there was no significant difference between the 2 groups in terms of Rai or Binet staging, splenomegaly, age, or sex. In addition, there was no significant difference between the 2 groups in WBC count, number of circulating lymphocytes, or level of bone marrow involvement. The Kruskal-Wallis test indicated that there was a trend toward more palpable hepatomegaly in the CD38+ patients (P = .05). Most striking was the association between the number of nodal sites involved with disease and CD38 expression (P = .004). CD38+ patients also had significantly lower hemoglobin levels (P = 0.001) and higher levels of β2M (P = .00001).
CD38 expression is associated with poor outcome
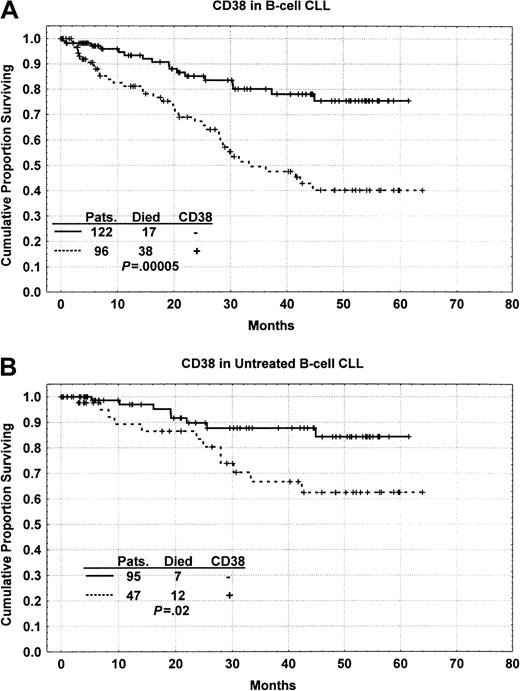
As shown in Figure 3A, patients whose samples were positive for CD38 had significantly shorter survival times (median, 30 months) than patients whose samples were negative for CD38 (P = .00005). Of the 122 patients who did not express CD38, only 17 (14%) died whereas 38 (40%) of the CD38+patients died. This remained true when comparisons were made for previously untreated patients with CLL (Figure 3B). Furthermore, CD38 positivity identified a subgroup of CLL patients with aggressive disease staged by Rai as indolent and intermediate (Rai stage 0-II) (Figure 4A). CD38 expression also distinguished a subgroup of patients with less aggressive disease whose Rai stage indicated aggressive disease (Rai III-IV) (Figure 4B). We also analyzed patients after separating them into 3 groups according to the National Cancer Institute-modified staging system for Rai classification. CD38+ patients in the Rai intermediate stage (Rai I-II) had poor outcome (P = .04). However, CD38+ patients in the low-risk group (Rai 0) had outcome not significantly different from those negative for CD38 (P = .16). Similarly, CD38 expression distinguished a group of patients with favorable prognoses among those with high (greater than 2.5 mg/L) β2M levels, which are considered indicative of unfavorable prognosis (Figure 4C). Analysis of the role of CD38 in predicting overall survival in various subgroups was also performed using the Cox proportional hazard model and CD38 as a continuous variable rather than using the 20% cut-off. This analysis also showed similar results in all subgroups.
Kaplan-Meier survival curves.
(A) Kaplan-Meier survival curve comparing patients with B-CLL whose samples were positive (20% or more) or negative (less than 20%) for CD38 expression; the difference is significant atP = .00005. (B) Kaplan-Meier survival curve showing the survival difference between CD38+ and CD38−patients in the group of patients with CLL who were previously untreated.
Kaplan-Meier survival curves.
(A) Kaplan-Meier survival curve comparing patients with B-CLL whose samples were positive (20% or more) or negative (less than 20%) for CD38 expression; the difference is significant atP = .00005. (B) Kaplan-Meier survival curve showing the survival difference between CD38+ and CD38−patients in the group of patients with CLL who were previously untreated.
Length of survival.
(A) Kaplan-Meier curve based on CD38 expression comparing the length of survival among patients with B-CLL stratified as Rai 0-II; the difference is significant at P = .001. (B) Kaplan-Meier curve based on CD38 expression comparing the length survival among patients with B-CLL stratified as Rai III-IV; the difference is significant at P = .001. (C) Kaplan-Meier curve based on CD38 expression comparing length of survival among patients with B-CLL stratified as having a high β2M (greater than 2.5 mg/L) levels; the difference is significant at P = .0005.
Length of survival.
(A) Kaplan-Meier curve based on CD38 expression comparing the length of survival among patients with B-CLL stratified as Rai 0-II; the difference is significant at P = .001. (B) Kaplan-Meier curve based on CD38 expression comparing the length survival among patients with B-CLL stratified as Rai III-IV; the difference is significant at P = .001. (C) Kaplan-Meier curve based on CD38 expression comparing length of survival among patients with B-CLL stratified as having a high β2M (greater than 2.5 mg/L) levels; the difference is significant at P = .0005.
To investigate the effects of therapy on CD38 expression, we used the Kruskal-Wallis test and compared CD38 expression between previously untreated patients and those previously treated. We found significantly higher levels of CD38 expression in previously treated patients (P = .0001). This raises the possibility that patients with high CD38 levels more frequently require therapy or that patients acquire CD38 expression after therapy. To investigate, we correlated CD38 expression with the length of time between appearance of disease and the time of testing. We found no correlation between CD38 expression and length of time from onset of disease and testing. This suggests that CD38 expression is more likely to be a feature of the disease at the time of presentation, and patients with CD38 expression are more likely to need therapy. Furthermore, history of therapy was associated with shorter survival in patients with low CD38 expression (P < .001) and in patients with high CD38 expression (P < 0.001).
Statistical analyses for CD38 expression
We used the univariate Cox proportional hazard model to assess associations between survival time and various risk factors in this group of patients with CLL (Table 3). As expected, Rai and Binet stages, age, β2M, hemoglobin, WBC count, peripheral blood lymphocytosis, history of therapy, splenomegaly, number of sites with lymph node involvement, and CD38 positivity were all significant factors in determining overall survival. Hepatomegaly, bone marrow cellularity, and percentage of lymphocytic infiltration were factors not significantly associated with survival time. When some prognostic factors, including Rai staging, hemoglobin, β2M, and CD38 were considered in a multivariate analysis that included 150 patients with all variables (Table 4), only hemoglobin and CD38 emerged as significant prognostic factors associated with overall survival time. In this group of patients, the prognostic value of CD38 masked that of β2M.
Analyses of sequential samples
Sequential blood samples from 7 patients obtained at different time points during the course of the disease (from 4 to 56 months) were analyzed (Table 5). CD38 expression did not significantly change in 6 of the 7 patients. However, in one patient the percentage of CD38+ cells changed from 9% to 89% in a 36-month period, during which the patient received no treatment. This increase in CD38 expression was associated with significant worsening in the patient's clinical condition, manifested as liver and spleen enlargement with a decrease in platelet count and an increase in WBC count. Change in disease staging was also manifested as Rai 0 to 2 and Binet A to B.
Discussion
In this study, we found that patients with B-CLL could be divided into 2 prognostically different groups—those with worse prognoses had high CD38 expression on malignant cells (20% or more), and those with a good prognoses had low CD38 expression (less than 20%). Multivariate analyses confirmed that CD38 expression is an important prognostic factor. We also correlated this observation to most of the known prognostic parameters for B-CLL to bolster our findings that CD38 is an important prognostic factor. Whether CD38 expression reflects the mutation status of the VgH gene will be further evaluated. Regardless of this association (or lack of it), CD38 expression is a powerful prognostic tool that should be considered a standard clinical test for all patients with B-CLL.
CD38 is a 45 000-d transmembrane type II glycoprotein with a short amino-terminal cytoplasmic tail, a single membrane-spanning region, and a long extracellular carboxy-terminal domain.14 It has ecto-enzymatic activity that serves in the conversion of nicotinamide adenine dinucleotide to cyclic adenosine diphosphate ribose, or cADP, an important regulator of intracellular Ca++release.9,25 CD38 has no lineage restriction and mediates variable functions.13 In T cells it functions as an adherence molecule to the CD31 ligand expressed on vascular endothelium, and its ligation mediates the production of qualitatively different cytokines compared with ligation of the CD3 complex.26,27 In myeloid cells, CD38 is expressed on immature precursors and can be up-regulated using all-transretinoic acid. Its presence has been associated with better prognosis in acute myeloid leukemia (AML), with the exception of AML-M3.19
CD38 has been reported to play a complex role in lymphocyte proliferation.13 Ligation of CD38 using an agonistic monoclonal antibody produced diverse responses manifested as growth or apoptosis.28 CD38 ligation on mature B cells protected against apoptosis and up-regulated the expression of the Bcl-2 proto-oncogene.10,29 In contrast, ligation of the CD38 molecule suppressed the growth of immature B cells in the bone marrow micro-environment.11
Clinically and morphologically, it is known that B-CLL is a heterogeneous group of diseases under the same name. These diseases have different progression patterns and require variable therapeutic modalities. Zupo et al30 reported that patients with B-CLL could be divided into groups based on their lymphocyte CD38 expression.
Recent studies divided patients with B-CLL into 2 prognostic groups based on their IgH gene mutation status, one with mutation and good outcome and the other without mutation but with poor outcome.18,23,24 Damle et al18 found that patients with B-CLL with high CD38 expression did not haveIgH mutations and that this correlated with poor prognosis, whereas patients with low CD38 expression had IgH mutations that correlated with good prognosis. Based on this observation, they proposed that the B-CLL subset with high CD38 expression arises from pregerminal center B cells (naive) and that the subset with low CD38 expression arises from postgerminal center (memory) cells.
In contrast to immunoglobulin gene mutations, which are not reversible, CD38 expression could be up-regulated or down-regulated based on the stage of maturation or the antigenic stimulation. Although CD38 expression was constant (negative or positive) over a variable period of time in 6 of 7 patients examined, it increased significantly in 1 patient (9% to 89%). This increase in CD38 expression was associated with marked worsening of the patient's clinical parameters. This was an unexpected finding and contradicted the concept that CD38 expression reflects the origin of B cells from pregerminal or postgerminal centers. Although we cannot rule out the possibility of an evolving new clone of pregerminal center origin, the fact that the patient was untreated suggests an evolution of the same disease. Omede et al15 also reported that patients with multiple myeloma in relapse showed an increase in CD38 expression levels. This observation and ours—that the higher the percentage of CD38+ cells, the more aggressive the disease—imply that CD38 expression is a parameter for disease progression in this B-cell neoplasm.
CD38 expression stands as an excellent prognostic indicator because it is readily available from a peripheral blood sample and is easily measured; this adds a new dimension to the care available to patients with CLL. In addition, it adds information to other prognostic factors such as Rai and Binet stages or β2M serum levels. Furthermore, measurement of CD38 expression allows the identification of a subset of patients with better prognoses within a presumably poor prognostic group (Rai stages III-IV or high β2M levels) and the identification of patients with poorer prognoses among what is considered good prognostic group (Rai stages 0-II). This is particularly important in managing patients in the early stages of the disease (Rai 0-II), which includes a subgroup whose disease will progress quickly. CD38 expression or the IgV mutation status may help in identifying the subgroup of patients whose disease will have an aggressive course. It remains to be seen whether CD38 expression correlates with disease response or resistance to a particular therapy. In conclusion, CD38 expression is a strong predictor of survival time in patients with B-CLL, and it should be measured and used for the stratification of patients for optimum care.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Maher Albitar, Dept of Hematopathology, The University of Texas M. D. Anderson Cancer Center, Box 72, 1515 Holcombe Blvd, Houston, TX 77030-4095; e-mail: malbitar@mdacc.tmc.edu.