Severe T-cell immunodeficiency after solid organ or bone marrow transplantation may result in the uncontrolled outgrowth of latently Epstein-Barr virus–infected B cells, leading to B-lymphoproliferative disorder (BLPD). Given the potentially important pathogenic role of IL-6 in BLPD, it was tested whether the in vivo neutralization of IL-6 by a monoclonal anti–IL-6 antibody could contribute to the control of BLPD. Safety and efficacy were assessed in 12 recipients of transplanted organs who had BLPD refractory to the reduction of immunosuppression over 8 days. Five patients received 0.4 mg/kg per day. The next 7 patients received 0.8 mg/kg per day. Treatment was scheduled to last 15 days. It was completed in 10 patients, and in the other 2 patients was discontinued early (days 10 and 13, respectively) because of disease progression. Treatment tolerance was good, and no major side effects were observed. High C-reactive protein levels were found in 9 patients before treatment but were normalized under treatment in all patients, demonstrating efficient IL-6 neutralization. Complete remission (CR) was observed in 5 patients and partial remission (PR) in 3 patients. Relapse was observed in 1 of these 8 patients in whom remission was observed. This relapse was unresponsive to treatment. Disease was stable in 1 patient, but it progressed in 3 patients. Seven patients are alive and well. Two patients died because of disease progression, and 3 patients died while in CR (chronic rejection in 2 patients and BLPD sequelae in 1 patient). These data suggest that the anti–IL-6 antibody is safe and should be further explored in the treatment of BLPD.
Introduction
B-lymphoproliferative disorder (BLPD) is a severe complication of organ and bone marrow transplantation (BMT) caused by the Epstein-Barr virus (EBV).1-7 EBV latently infects B cells, which become immortalized by expressing part of the viral genome that persists in an episomal form.8 The growth of EBV-infected B cells is controlled by cytotoxic T cells.9In vivo severe T-cell immunodeficiency, such as occurs in patients with inherited cellular immunodeficiency10 or acquired immunodeficiency syndrome11 and in recipients of solid organ or bone marrow transplants,12-14 may result in the uncontrolled outgrowth of EBV-infected B cells, leading to BLPD. BLPD occurs in 1% to 15% of organ recipients and in 0.5% to 24% of bone marrow recipients, depending on the type of transplantation, the ages of donor and recipient, the intensity of immunosuppression, and the method of T-cell depletion.15-18 The overall prognosis of BLPD is poor, and the disease is fatal in 40% to 60% of affected recipients of transplanted organs15,19-22 and in 90% of affected BMT recipients.15,23-25 Treatment strategy is still controversial. Antiviral therapy (acyclovir, ganciclovir) is ineffective at preventing the persistence of episomal EBV associated with the latent phase. Although such treatment has not been definitively shown to be effective against BLPD, remission has occasionally been reported.15,26 Chemotherapy and radiotherapy are of limited value, at least in early-onset EBV-associated BLPD.15,27 Surgery may save the patient's life in cases of localized BLPD.15 Preliminary data have suggested improved survival with the use of interferon-α and the intravenous infusion of high-dose immunoglobulins.28 For BLPD occurring after BMT, the infusion of donor T lymphocytes or EBV-specific donor cytotoxic T lymphocytes can be effective in bringing B-cell proliferation under control.12,29 The use of anti–B-cell monoclonal antibodies (mAbs) (anti-CD24 and anti-CD21) appeared to be a safe and relatively efficient therapy for post-transplant early-onset BLPD.30-32 However, these anti–B-cell mAbs are no longer available. The use of a humanized anti-CD20 mAb may be an attractive alternative, as recently reported in a small number of patients.33-35
We investigated the effect of a monoclonal anti–interleukin-6 (IL-6) antibody on B-cell growth in patients with BLPD. IL-6 is a multifunctional cytokine produced by monocytes, fibroblasts, endothelial cells, and other cell types. It plays an important role in the proliferation and maturation of B cells.36-39Overproduction of this cytokine is thought to be involved in the pathogenesis of lymphoid malignancies, high-grade B-cell lymphomas,40,41 and myelomas42-44 in particular. It has also been shown that IL-6 promotes the growth of EBV-infected B cells,45,46 that patients with BLPD produce abnormally high levels of IL-6,45,47 and that B-cell lines derived from BLPD express the p80 chain of the IL-6 receptor.48 In addition, the transfection of EBV-transformed B cells with human IL-6 cDNA greatly increases the proliferation of these cells both in vivo and in vitro.49,50 Durandy et al48 showed that anti–IL-6 mAb could inhibit the growth of several BLPD-derived B-cell lines in severe combined immunodeficiency disease (SCID) mice in vivo. This treatment led to complete remission in most mice and to tumor-free, long-term survival in 40% of mice.
Given the probable importance of IL-6 in BLPD pathogenesis, we tested, in a phase 1-2 clinical trial, the toxicity and efficacy of anti–IL-6 mAb treatment against BLPD occurring after organ transplantation. We report here the results of a trial in 12 patients.
Materials and methods
Monoclonal anti–IL-6 antibody
The mouse monoclonal anti–IL-6 antibody (B-E8)51was produced and supplied by Diaclone (Besançon, France; specific activity, 1 μg B-E8 neutralizes 6000 U IL-6).
Protocol design
This study consisted of a multicenter phase 1-2 trial of monoclonal anti–IL-6 antibody administration to patients with post-transplant BLPD. Detailed informed consent was obtained from all patients or from parents of children younger than 10 years of age, in accordance with French legislation and as approved by the ethics committee of Necker Hospital (Paris, France). We evaluated anti–IL-6 antibody-related toxicity and effects on BLPD.
Patient enrollment
BLPD was diagnosed based on the presence of a lymphoproliferative syndrome with detectable tumors consisting of EBV-positive B lymphocytes. EBV was detected immunohistologically, with anti-LMP antibodies, by polymerase chain reaction (PCR) detection of the EBV genome, or by Southern blot analysis or by in situ hybridization (see below).
For inclusion in the study, patients could be of any age but had to have acquired BLPD after organ transplantation and to have satisfied at least one of the following criteria: (1) be unresponsive to the tapering of immunosuppression for a minimum of 8 days; (2) have histologically invasive disease with nodal capsule disruption; (3) have rapidly progressive multiple lymphoproliferative lesions, excluding those from surgery. BLPD unresponsive to the tapering of immunosuppression treatment for a minimum of 8 days was defined as no change in C-reactive protein (CRP) level, fever, or other general manifestations, and no decrease in tumor size detected by clinical assessment and appropriate imaging tests.
No other BLPD treatments were given in association with the anti–IL-6 antibody. If disease progression was observed during the administration of anti–IL-6 antibody, treatment could be stopped and another treatment could be proposed. Twelve patients were included in the study between September 1995 and August 1998. The final data were collected on June 30, 1999.
Anti–IL-6 antibody dose and administration
The monoclonal anti–IL-6 antibody BE-8 was diluted in 2 mL/kg 5% glucose serum and administered as a 30-minute intravenous infusion once a day for 15 days. To evaluate toxicity and the dose dependency of the effects of the anti–IL-6 antibody, a dose escalation trial was performed. Patients 1 to 3 received a dose of 0.4 mg/kg per day. Patients 4 and 5 received a 1 mg/kg bolus on day 1, which was followed by injections of 0.4 mg/kg per day. These doses were well tolerated (see “Results”); hence, the remaining 7 patients received a third regimen consisting of a 1 mg/kg bolus followed by injections of 0.8 mg/kg per day. CRP was determined on day 3. In all patients, if CRP levels did not normalize by day 3, the dose given was doubled for the remaining 12 days of treatment, whatever the initial dose.
Study criteria
The end point of this dose escalation study was the determination of the toxicity of anti–IL-6 antibody administration and its efficacy against BLPD, as assessed 4 months after treatment. We evaluated the toxicity of BE-8 in patients using the World Heath Organization Toxicity Criteria. Blood pressure, temperature, and heart rate were monitored every 10 minutes during BE-8 infusion, then every hour for 3 hours and every 3 hours until the next injection. Hematologic, renal, and liver function tests were conducted every day during treatment and on days 22, 30, 60, and 120.
Clinical symptoms of BLPD were monitored (examination of all affected sites, fever) every day during treatment, then once per week for 2 weeks, and then once per month for 6 months. Tumor size was determined by radiologic imaging (computed tomography and/or magnetic resonance imaging and/or ultrasound imaging) on days 15, 30, and 120. Biologic variables were also studied. CRP was determined every 3 days during treatment, then once per week for 2 weeks, and then on days 60 and 120. PCR tests for the detection of EBV-DNA in blood and determinations of serum immunoglobulin levels, monoclonal immunoglobulin components, and specific antibodies against EBV were carried out every 2 weeks for 1 month and then on days 60 and 120.
Complete remission (CR) was defined as the complete clinical and radiologic disappearance of tumors at all sites, the disappearance of associated biologic signs (high levels of CRP, detection of circulating B cells expressing the EBV genome), and the absence of newly involved sites. Partial remission (PR) was defined as at least a 50% decrease in measurable tumor localization, with the disappearance of fever and no newly involved sites. Stable disease (SD) was defined as no significant change in tumor measurements and no newly involved sites. Progressive disease (PD) was defined as an increase in the size of tumor lesions or the appearance of new lesions.
Immunologic investigations
The following B- and T-cell–specific mAbs were used, as previously described,31 to characterize T and B lymphocytes in blood and in organ tissue samples when available: anti-immunoglobulin heavy-chain and light-chain isotypes30; anti-CD19, CD20, CD24, CD21, and CD23 antibodies (Immunotech, Marseilles, France); and anti-CD3, CD4, and CD8 antibodies (BD, San Diego, CA). Analyses were performed by indirect immunofluorescence cytofluorometry. Fresh cells were used for membrane immunofluorescence analysis and fixed cells for intracytoplasmic staining. Immunoperoxidase staining of biopsy sections was performed as previously described.52 Serum immunoglobulin levels were determined by nephelometry, and monoclonal immunoglobulin components were determined by immunofixation.53 Serum IL-6 levels were determined using a specific anti–IL-6 enzyme-linked immunosorbent assay (ELISA) assay (CLB, Amsterdam, The Netherlands), as previously described.48
Virologic investigations
EBV-DNA was detected in frozen material by Southern blot analysis using a randomly primed 32P-labeled probe specific for the BamHI W internal repeats of the virus, in situ hybridization with EBV-specific probes,54 PCR analysis,55 or any combination thereof. Specific antibodies (IgG and IgM isotypes) against EBV (viral capsid antigen, early antigen, EBV nuclear antigen) in organ transplant recipients were detected by an ELISA assay. Immunoperoxidase staining of biopsy sections was also performed for LMP1.52
Clonality studies
Immunoglobulin gene rearrangement studies of proliferative B cells were performed by Southern blotting, using a probe for the sequence encoding the heavy-chain joining region (JH). BLPD was considered to be monoclonal if a single immunoglobulin rearrangement was obtained for the abnormal specimen analyzed, regardless of whether a single monoclonal serum component had been detected by immunofixation. BLPD was considered to be oligoclonal if no unique immunoglobulin heavy-chain rearrangement was observed, several serum monoclonal immunoglobulin components were detected through immunofixation, or both.
Results
Patients and BLPD characteristics
Median age at the onset of BLPD was 35 years (range, 1 year to 62 years). Organs transplanted were lung (n = 5), kidney (n = 3), liver (n = 3), and heart (n = 1). The characteristics of the BLPD are shown in Table 1. In all patients, biopsy of BLPD lesions was performed to confirm the B-lymphocyte phenotype of infiltrating cells and the presence of EBV (Table1).
All patients were on immunosuppressive treatment at the time of BLPD onset (Table 2). Eight patients had received highly aggressive immunosuppressive therapy because of graft rejection; in 4 patients, this occurred less than 3 months before the onset of BLPD. In all patients, immunosuppression was reduced when BLPD was diagnosed. In none of these patients did immunosuppression tapering for at least 8 days affect the BLPD lesions (ie, tumors were not reduced, as shown by appropriate imaging and clinical examination). Tapering of immunosuppression also had no effect on fever or serum CRP levels. All patients were, therefore, considered unresponsive to the tapering of immunosuppression over an 8-day period.
One patient (patient 12) had been injected with an anti-CD20 antibody that had no effect on BLPD, 2 months before anti–IL-6 antibody treatment. In all other patients, no other treatment was performed before the initiation of anti–IL-6 mAb antibody therapy.
Treatment characteristics and tolerance
Treatment was scheduled to last 15 days. It was completed in 10 patients, and in the other 2 patients it was stopped early (on day 10 for patient 4 and on day 13 for patient 7) because of disease progression. One patient (patient 2) received a second course of anti–IL-6 antibody treatment after a relapse of BLPD. No major side effect was observed during any of the 13 courses of anti–IL-6 antibody treatment. One patient (patient 4) had moderate allergic manifestations—erythema on both hands after each anti–IL-6 antibody injection—that responded to antihistamine treatment. One patient (patient 3) had paresthesia on day 2, which then disappeared spontaneously. Two patients (patients 2, 9) had moderately high levels of arterial blood pressure, which resolved after sublingual nifedipine treatment. On day 2, patient 3 was examined for hyperkalemia associated with hyperphosphoremia and hypocalcemia, all of which resolved within 1 day. These biochemical manifestations were interpreted as a lytic syndrome.
Pharmacologic data
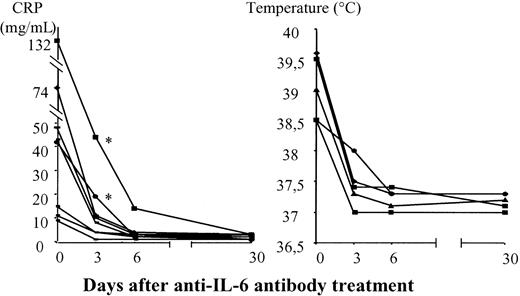
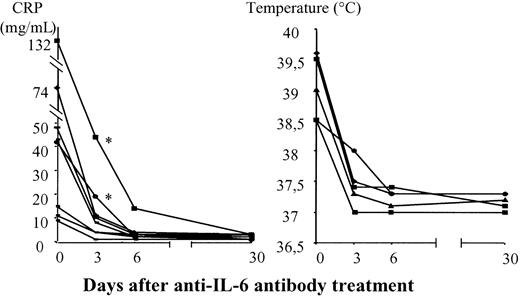
As high CRP concentration is known to be an in vivo hallmark of high levels of IL-6 synthesis.56-58 We assessed the effects of the treatment on CRP and IL-6 levels to determine the pharmacologic effects of the anti–IL-6 antibody. High serum CRP concentrations were found in 9 of 12 patients, and 5 of 9 patients had fever (Table 1). In all patients, CRP concentration decreased and fever disappeared after treatment, suggesting that systemic IL-6 was neutralized (Figure 1). CRP normalized within 3 days of the initiation of treatment in 7 of these 9 patients, whereas it was necessary to double the anti–IL-6 antibody dose on day 3 for 2 patients (patients 6, 11) even though the initial dose given to these patients was 0.8 mg/kg.
Evolution of CRP levels and fevers after anti–IL-6 antibody treatment.
Asterisks indicate patients in whom the absence of CRP level normalization on day 3 led to a 2-fold increase in the anti–IL-6 treatment dose.
Evolution of CRP levels and fevers after anti–IL-6 antibody treatment.
Asterisks indicate patients in whom the absence of CRP level normalization on day 3 led to a 2-fold increase in the anti–IL-6 treatment dose.
As shown in Table 3, serum IL-6 concentration was evaluated in 8 patients before treatment (after tapering immunosuppression) and was found to be elevated in 5 patients. In the 4 patients who survived for more than 2 months, IL-6 normalized or decreased significantly. Serum IL-6 concentration could not be analyzed during or immediately after treatment because of the formation of immune complexes between IL-6 and anti–IL-6 antibody that were also detected in the ELISA assay.
Evaluation of anti–IL-6 antibody treatment on disease outcome
Twelve patients were treated with 13 courses of anti–IL-6 antibody injection. Median follow-up time after treatment was 20 months (range, 9-33 months). CR was achieved in 5 patients (patients 1, 3, 5, 6, 8), 4 months after the initiation of treatment (Table 3). In patient 1, tumor size had decreased by 50% on day 12 of treatment, and CR was achieved on day 21. Examination of an endobronchial lesion on day 21 showed that no infiltrating tumor cells were present. Neither necrosis nor T-cell infiltration was detected. Patient 3 presented on day 2 with hyperkalemia associated with hyperphosphoremia and hypocalcemia, suggestive of a lytic syndrome. By day 3, the tumor size had decreased, and CR was achieved 5 weeks after the initiation of treatment. It was not possible to perform an examination of the lesion after CR was achieved. In patient 5, tumor size began to decrease 30 days after treatment, and CR was achieved 4 months after treatment. Examination of the lungs confirmed that infiltrating tumor cells were no longer present and that there was no necrosis, nor were there infiltrating T cells. In patient 6, tumor reduction was observed 30 days after treatment, and CR was achieved 2 months after treatment. Analysis showed that the infiltrating cells had been replaced by severe necrosis. In patient 8, pleuritis had disappeared by day 3, and CR was achieved on day 15. No pathologic examination was performed.
None of these patients had a relapse of BLPD. All can be considered cured of BLPD. However, 3 of these 5 patients died 10, 13, and 36 months after treatment because of chronic rejection in 2 patients and severe liver hilum necrosis in the other, which may be considered a sequela of BLPD. Two patients are alive and well 21 and 33 months, respectively, after anti–IL-6 antibody treatment.
Partial remission was observed in 3 patients (patients 2, 9, 12). In patient 2, pleurisy decreased by the third day after treatment, mediastinal nodes were half their size 1 month after treatment, and BLPD decreased by approximately 90% only 2 months after treatment. However, BLPD relapsed 5 months after treatment. This patient received a second course of anti–IL-6 antibody treatment, which was ineffective. This patient eventually died of progressive disease. In patient 9, a decrease in tumor size became detectable 15 days after the initiation of treatment. This PR made it possible to perform surgery and retransplantation that had not previously been feasible. Pathologic examination of the liver showed necrosis of 80% of the tumor that was infiltrated by T cells and histiocytic cells. This patient was alive and well, in complete remission, at the 21-month follow-up. In patient 12, a slight decrease in tumor size was observed by day 30. Three months later, the tumor decreased by 90%. Nine months after treatment, this patient was still in PR and had a small persistent lesion in the lung but no associated manifestations. Before the anti–IL-6 antibody treatment, this patient received a course of anti-CD20 antibody that had no effect on BLPD.
Anti–IL-6 antibody treatment was effective in 8 patients (5 in CR, 3 in PR). The reintroduction of highly aggressive immunosuppression in 3 of these patients because of episodes of rejection did not lead to BLPD relapse.
Stable disease was observed in one patient (patient 11), who was then treated with anti-CD20 antibody, which also failed to control BLPD. This patient was then treated by surgery and retransplantation and is alive and well, in complete remission, 19 months after treatment.
In 3 patients, treatment did not prevent disease progression (patients 4, 7, 10). Patient 10 died within 1 month because of disease progression, despite the use of chemotherapy. In patients 7 and 10, anti–IL-6 antibody treatment was stopped on days 10 and day 13, respectively, because of disease progression, but chemotherapy led to complete remission. These 2 patients are still alive and well and in CR 22 and 26 months, respectively, after treatment.
Anti–IL-6 antibody treatment was effective at controlling associated signs of BLPD such as high CRP concentration and fever. This treatment was also shown to be effective by PCR detection of the EBV genome in the blood and the determination of serum immunoglobulin concentration by immunofixation. For 7 patients, the EBV genome was detected in the blood before treatment. We were able to evaluate 6 of these patients after treatment. The EBV genome was no longer detectable in the blood 2 to 4 months after treatment in 5 of these 6 patients. Six of 10 patients with detectable serum monoclonal immunoglobulin components on immunofixation were evaluated after treatment. In 5 of them, the monoclonal immunoglobulin components had disappeared. Most of the survivors developed full antibody responses to EBV, including anti-EBNA antibodies (Table 2).
Analysis of factors that might have influenced disease outcome
BLPD characteristics such as clonality, EBV genome detection in blood, monoclonal immunoglobulin component, number or localization of affected sites, and time to BLPD onset and patient characteristics such as age, type of transplantation, and type of initial disease were tested as possible factors influencing outcome after anti–IL-6 antibody treatment.
The dose of anti–IL-6 antibody given (0.4 vs 0.8 mg/kg) did not affect its efficacy (Table 3). Although no firm conclusions can be drawn because of the small number of patients, time to BLPD onset seemed to be the only factor affecting treatment efficacy. Indeed, for the 7 patients in whom BLPD occurred within 6 months of transplantation, CR was achieved in 4 patients and PR was achieved in 2 patients (Table4). Conversely, for the 5 patients in whom BLPD occurred more than 5 years after BLPD, CR was achieved in only one patient and PR in another, who eventually had a relapse, whereas the disease progressed in 3.
Discussion
In this phase 1-2 clinical trial, 12 patients with BLPD after organ transplantation were treated intravenously by daily injections of 0.4 to 0.8 mg/kg per day of a mouse monoclonal anti–IL-6 antibody (B-E8)51 for 15 days in 10 patients and for 10 and 13 days, respectively, in 2 other patients. This antibody has previously been used to treat patients with myeloma,56 B lymphoma related to EBV infection in the patients with HIV,59Castleman disease,60 and severe rheumatoid arthritis.61 In all these studies, treatment was well tolerated. The treatment was also well tolerated in this study, and no major side effects were reported for any of the doses of anti–IL-6 mAb used. These data suggest that this mAb is safe for use in therapeutic trials at doses up to 0.8 mg/kg per day for 15 days.
To determine the pharmacologic kinetics of anti–IL-6 mAb antibody, we analyzed CRP and IL-6 levels. CRP is produced by human hepatocytes in response to IL-6 stimulation58 and is known to be indicative of the presence of high levels of IL-6.56-58Nine patients had high CRP levels. The normalization of serum CRP concentration by treatment in all 9 patients suggests that IL-6 was systematically neutralized. Two of these 9 patients, both of whom received an initial anti–IL-6 antibody dose of 0.8 mg/kg, had persistently high CRP levels after 3 days of treatment. The dose of anti–IL-6 mAb was therefore increased, resulting in the normalization of CRP concentration. This suggests that CRP determination during treatment may be useful for assessing whether a pharmacologic effect has been achieved. However, CRP concentration was not predictive of treatment efficacy.
Treatment was clinically effective in 8 patients (5 in CR, 3 in PR). BLPD relapsed in only one of these 8 patients and then became insensitive to treatment. These data indicate that the anti–IL-6 mAb may be useful in the treatment of severe BLPD.
In all cases, prior immunosuppression tapering for an 8-day period had no effect on BLPD. However, one could argue that this delay is too short and that the observed remissions could be related to a delayed effect of immunosuppression reduction. This is unlikely because the observed remission rate under immunosuppression tapering is generally lower than the one observed in our series (8 of 12 patients). Moreover, this 8-day period of immunosuppression reduction has been previously proposed as an appropriate delay to allow treatment by anti–B-cell monoclonal antibodies.32 The efficacy of anti–IL-6 antibody may be similar to that of anti-B-cell mAb, as complete remission was achieved in 61% of the 58 patients with BLPD treated with anti-B mAbs.32 Anti–IL-6 mAb treatment was not effective in 4 patients (1 in SD, 3 in PD). However, for 3 of these 4 patients, improvements were achieved with another treatment (surgery in 1 patient and chemotherapy in 2 patients). Thus, the lack of efficacy of the anti–IL-6 mAb did not preclude the use of alternative therapies such as surgery and chemotherapy.
These encouraging data require confirmation in a larger group of patients, allowing statistical analysis and including patients with post-BMT BLPD, which is known to induce a poorer outcome.32 It may also be of value to perform a phase 3 clinical trial in which anti–IL-6 antibody treatment could be compared to or associated with other forms of treatment (eg, anti-CD20 [anti-B cell]) mAbs or cytotoxic T lymphocytes in patients who have undergone BMT12 29).
Clonality, type of transplantation, and sites affected did not seem to influence treatment efficacy. The only factor that seemed to have a strong effect on efficacy was time to BLPD onset. These findings require confirmation in a larger series of patients. However, similar results were reported by Benkerrou et al,32 who showed in a multivariate analysis that the late onset of BLPD was a major risk factor for the failure of anti-B-cell mAb treatment. Late-onset BLPD may be caused by a different physiopathogenic mechanism, with secondary oncogenic events (such as bcl-2 rearrangements, c-myc, n-ras, and p53 mutations) and LMP1 deletions62-65 responsible for the formation of true lymphomas. In such cases, B-cell proliferation could be insensitive to anti–B-cell or anti–IL-6 antibody treatment but responsive to chemotherapy.
Our data, which are consistent with those obtained for SCID mice injected with B-cell lines derived from patients with BLPD,48 suggest that IL-6 may, in some cases, have a major role in BLPD growth. Pathologic examination after treatment was possible in 4 patients in whom treatment was effective. In 2 patients, extensive tumor necrosis was observed. In one, infiltrating T cells and histiocytic cells replaced infiltrating B cells. In 2 other cases, complete resolution of BLPD was observed. No firm conclusions can be drawn from these data concerning the mechanism of BLPD resolution after anti–IL-6 antibody treatment. There are several possibilities. First, IL-6 may act as an autocrine–paracrine growth factor and may promote the growth of EBV-infected B cells. Indeed, IL-6 has been shown to promote the growth of EBV-infected B cells45,46 and patients with BLPD produce abnormally high levels of IL-6.45,47 A tumorigenic role for IL-6 in BLPD was also suggested by the results of experiments in which EBV-transformed B cells were transfected with human IL-6 cDNA: transfection significantly increased the proliferation of these cells in vivo and in vitro.49,50 These observations suggest that original tumor cells (EBV-infected B cells), stromal cells, or both—as suggested for myeloma,66 immunoblastic lymphoma,40 and BLPD47—synthesize IL-6 that may act directly on the target EBV-infected B cells, promoting their growth. Alternatively, IL-6 has been shown to inhibit immune effector functions, such as natural killer (NK) cell activity, and cytotoxic functions of splenocytes in athymic mice, thereby permitting tumor development.67 Therefore, NK cells, the function of which might be restored by anti–IL-6 antibody, may be involved in disease regression. However, it has been shown that beige–SCID mice, which show little NK activity, display a similar response to the anti–IL-6 antibody treatment of implanted EBV-B cell tumors (A.D. et al, unpublished data, 1997). These 2 mechanisms are not mutually exclusive and may both be involved in the potential efficacy of the anti–IL-6 monoclonal antibody.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Elie Haddad, Service de Nephrologie Pediatrique, 48 Blvd Serrurier, 75019 Paris, France; e-mail:elie.haddad@rdb.ap-hop-paris.fr.