Abstract
Factor VIII (FVIII) inhibitor antibodies are classified into 2 groups according to the kinetic pattern of FVIII inactivation. Type 2 antibodies are more commonly observed in patients with acquired hemophilia A and do not completely inhibit FVIII activity; in most cases, substantial levels of circulating FVIII are detected. Three type 2 autoantibodies from patients who had normal levels of FVIII antigen despite having low levels of FVIII activity were studied. The antibodies reacted exclusively with the light chain of FVIII but not with the C2 domain, and their epitopes were therefore ascribed to the regions in the A3-C1 domains. Heavy and light chains of FVIII were detected in plasma-derived immune complexes extracted by using protein G Sepharose. Direct binding assays using anhydro-activated protein C (anhydro-APC), a catalytically inactive derivative of activated protein C (APC) in which the active-site serine is converted to dehydroalanine, were used to examine the relation between immune complexes and APC. The intact FVIII, 80-kd light chain, and 72-kd light chain bound in a dose-dependent manner to anhydro-APC, with Kdvalues of 580, 540, and 310 nM, respectively, whereas no appreciable binding was detected for the heavy chain. The 3 autoantibodies blocked FVIII binding to anhydro-APC by approximately 80% and consequently inhibited APC-induced FVIII proteolytic inactivation. These antibodies also bound to a synthetic peptide, His2009-Val2018, which contains the APC binding site. The findings suggest that binding of type 2 autoantibodies, recognizing residues His2009 to Val2018, protects FVIII from APC-mediated proteolysis and might contribute to the presence of FVIII immune complexes in the circulation.
Introduction
Factor VIII (FVIII) inhibitors develop as alloantibodies in patients with hemophilia A who have received multiple transfusions or as autoantibodies in previously hemostatically normal individuals, particularly elderly people, patients with autoimmune diseases, pregnant women, and women in the postpartum period.1 Appearance of the autoantibodies causes a reduction in FVIII activity (FVIII:C) and usually results in a severe bleeding tendency known as acquired hemophilia A.
FVIII is a glycoprotein cofactor that accelerates the blood coagulation tenase complex.2 Mature FVIII is synthesized as a single-chain polypeptide consisting of 2332 amino acid residues,3,4 and it consists of 3 domains arranged in the following order: A1-A2-B-A3-C1-C2.5 As a result of proteolytic processing, FVIII circulates as a divalent, cation-linked heterodimer formed by a heavy chain (HCh) composed of the A1, A2, and B domains and a light chain (LCh) composed of the A3, C1, and C2 domains.4,5 FVIII is transformed into its active form, an A1/A2/A3-C1-C2 heterotrimer, by limited proteolysis at Arg372, Arg740, and Arg1689 by thrombin and factor Xa.6 Additional proteolytic cleavage at Arg336 by activated protein C (APC) inactivates FVIII.6 The binding site for APC was found within the residues His2009 to Val2018 in the LCh.7 In plasma, FVIII is noncovalently bound to von Willebrand factor (vWF), and this complex formation protects FVIII from inactivation by APC.8
Common epitopes of autoantibodies, as well as hemophilic alloantibodies, have been found in either the A2 domain, the C2 domain, or both.9 Most autoantibodies, however, appear to be directed against a single domain rather than both domains, with anti-C2 antibodies being more common than anti-A2 antibodies.9 In contrast, most hemophilic alloantibodies appear to recognize both domains.10 Most inhibitor antibodies with a C2 epitope inhibit binding of FVIII to both vWF and phospholipid (PL).11,12 However, a new dominant epitope in the A3-C1 domains has been identified, and some antibodies that recognize this epitope also prevent the association between FVIII and factor IXa (FIXa).13
Inhibitor antibodies are classified into 2 types on the basis of the pattern of FVIII inactivation.14 Type 1 inhibitors completely inhibit FVIII:C, following second-order kinetics,15 whereas type 2 inhibitors follow a more complex kinetics and do not completely inhibit FVIII:C.16Hemophilic alloantibodies characteristically are type 1 inhibitors, whereas autoantibodies tend to be type 2 inhibitors. These different patterns of FVIII inactivation suggest that inhibitors may react with different antigenic determinants.14 The reason for partial inhibition of FVIII by type 2 antibodies remains unclear, however, and the possible importance of circulating immune complexes in these instances has not been fully explored.
In this study, we identified immune complexes in the plasma of 3 patients with type 2 acquired hemophilia A. We found that the binding of the autoantibodies protected FVIII from APC-mediated proteolysis and might have contributed to the presence of the circulating immune complexes.
Patients, materials, and methods
Proteins
FVIII was affinity purified from plasma by using the monoclonal antibody (mAb) NMC-VIII/10, which recognizes the FVIII A3 domain, as described previously.17 The specific activity of purified FVIII was 2700 U/mg. vWF antigen (vWF:Ag) was not detected in the purified FVIII fraction. The 80-kd LCh, the thrombin-cleaved 72-kd LCh, the HCh, and A2 fragments of FVIII were prepared from plasma FVIII as described previously.18-20 The recombinant C2 domain was produced and purified as described previously.21 vWF was purified from a commercially available FVIII/vWF concentrate (Confact F; Chemo-Sero-Therapeutic Research Institute, Kumamoto, Japan) by using gel filtration on a Sepharose CL-4B column (Amersham Pharmacia Biotech, Uppsala, Sweden).22 FVIII antigen (FVIII:Ag) was not detected in the vWF fraction. Purified FIXa and APC were obtained from American Diagnostica Inc (Greenwich, CT) and Chemo-Sero-Therapeutic Research Institute, respectively.
Antibodies
Three patients with acquired hemophilia A were studied; patient 1 (aged 61 years) and patient 3 (aged 51 years) were women; patient 2 (aged 78 years) was a man. No pathologic cause for the inhibitor was evident in any of the patients. Plasma from a fourth patient with acquired FVIII deficiency, with typical features of a type 1 inhibitor, was used as a control. IgG was prepared from each plasma sample after acidification by the addition of 0.06 M acetate buffer (pH 4.0) and precipitation of nonimmunoglobulin proteins with n-caprylic acid.23 Each supernatant was subjected to protein A Sepharose chromatography (Amersham Pharmacia Biotech) for further purification, and autoantibody IgG was obtained. F(ab′)2fragment from the purified IgG was prepared as described previously.24 Specific F(ab′)2(immune-depleted F(ab′)2) was affinity purified by removal of anti-C2 antibody with use of immobilized recombinant C2 domain on cyanogen bromide–activated Sepharose 4B (Amersham Pharmacia Biotech) in a modification of a previously described method.25 An alloantibody with a C2 epitope was obtained from a patient with severe hemophilia A and was used for FVIII:Ag assays.26 We also used the following mAbs: NMC-VIII/5, which recognizes residues 2170 to 2327 in the C2 domain26; NMC-VIII/10, with the epitope 1675 to 1684 in the acidic region of the A3 domain22; and C5, which recognizes residues 351 to 365 in the acidic region of the A1 domain and was provided by Dr C. A. Fulcher (Scripps Clinic Research Institute, La Jolla, CA).27 JR8, a mAb directed against the A2 domain, was obtained from JR Scientific Inc (Woodland, CA); mAb 3A6, directed against FIXa, was obtained as described previously.28 Peroxidase-conjugated antibodies were prepared as described previously.24 IgG subclasses of antibodies were determined with enzyme-linked immunosorbent assay (ELISA).11 Peroxidase-conjugated goat-antihuman IgG4 F(ab′)2 was purchased from ICN Pharmaceuticals Inc (Aurora, OH). The antibody had no cross-reactivity with IgG1 or IgG2 on ELISA.
Protein iodination
Intact FVIII was radiolabeled with 18.5 MBq (0.5 mCi) sodium iodide 125 (Na 125I; Amersham Pharmacia Biotech) by immobilized Iodo-Gen (Pierce, Rockford, IL) as described previously.29 Residual-free Na 125I was removed by chromatography on a PD-10 column (Amersham Pharmacia Biotech). Specific FVIII radioactivity was 0.3 MBq (8 μCi/μg) protein. Radiolabeled FVIII was divided into aliquots and stored at −80°C for up to 1 month.
Preparation of anhydro-APC
Anhydro-APC, a catalytically inactive derivative of APC in which the active-site serine is replaced by dehydroalanine, was prepared as described previously for preparation of anhydro-thrombin.30 Briefly, APC was chemically modified with phenylmethylsulfonyl fluoride (PMSF; Wako Pure Chemical Industries, Osaka, Japan). To convert PMSF residues of modified APC to dehydroalanine residues, the product was diluted with 0.05 M sodium hydroxide and incubated for 12 minutes at 0°C; the pH was then adjusted to 7.5. After dialysis against 50 mM Tris-hydrochloric acid (HCl; pH 7.5) containing 1 M sodium chloride (NaCl), anhydro-APC was purified by benzamidine-Sepharose 4B column chromatography (Amersham Pharmacia Biotech). Its molecular weight was estimated to be 62 kd on assessment with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), similar to that of the native APC. The purified anhydro-APC did not inactivate FVIII:C.
Synthetic peptides
Synthetic peptides 1804KNFVKPNETKTYFWKV1819 and 2009HAGMSTLFIV2018, corresponding to the known FVIII binding sites of FIXa and APC,7,31 respectively, were prepared by the method of simultaneous multiple peptide synthesis described previously.32 The peptides were analyzed and purified with reversed-phase high-pressure liquid chromatography (purity > 95%).
Coagulation assays
FVIII:C was assayed in a one-stage clotting assay. Anti-FVIII:C was quantified with a Bethesda assay.33 Inhibitor neutralization assays were performed as described previously.9 The inhibitor titer for each autoantibody was adjusted to 2 Bethesda U/mL, which corresponded to the concentrations of 150, 190, and 130 μg/mL for the IgGs of patient 1, patient 2, and patient 3, respectively. All dilutions for the neutralization assay were prepared in 45 mM sodium acetate, 7 mM sodium barbital, 0.1 M NaCl (pH 7.4), and 2% human serum albumin (HSA).
FVIII:Ag and vWF:Ag assays
Inhibition of FVIII fragments by autoantibody binding
Each FVIII fragment was incubated with 0.5 μM autoantibody F(ab′)2 for 2 hours at 37°C and added to immobilized intact FVIII (4 nM). F(ab′)2 binding was detected with peroxidase-conjugated goat-antihuman IgG4F(ab′)2, and absorbance at 492 nm (A492) was measured by using o-phenylenediamine. The percentage of inhibition was calculated as follows: 100 − (bound − nonspecific A492)/(maximum − nonspecific A492) × 100 (%). The absorbance in the absence of F(ab′)2 was considered nonspecific; that in the absence of FVIII fragment was considered maximal reactivity.
ELISA for measuring the inhibitory effects of autoantibodies on FVIII binding
FVIII LCh binding to FIXa.
Binding of FIXa to immobilized FVIII LCh was assessed as described previously.31 FIXa (40 nM) was added to immobilized 80-kd LCh (60 nM) and incubated overnight at 4°C. Bound FIXa was detected by peroxidase-conjugated mAb 3A6. To investigate the inhibitory effects of autoantibodies, serial dilutions of autoantibody F(ab′)2were mixed with FIXa before they were added to LCh-coated wells.
FVIII binding to vWF or phosphatidylserine.
Binding of FVIII to vWF or phosphatidylserine (PS) was assessed as described previously.26 35 Briefly, 1 nM FVIII was added to immobilized vWF (40 nM) or PS (5 μM; Sigma Chemical, St Louis, MO). Bound FVIII was detected by using peroxidase-conjugated mAb JR8. To determine the inhibitory effects of antibodies, serial dilutions of F(ab′)2 or immune-depleted F(ab′)2 were incubated with FVIII for 2 hours at 37°C before immobilized vWF or PS was added.
ELISA for binding of autoantibodies to a synthetic peptide, Lys1804-Val1819 or His2009-Val2018
Microtiter plates were coated with the synthetic peptide (100 μM) in 0.1 M sodium bicarbonate buffer (pH 9.6). Autoantibody F(ab′)2 was serially diluted in triethanolamine-buffered saline (TBS) with 4% HSA and added to each well. Binding was detected with peroxidase-conjugated goat-antihuman IgG4F(ab′)2. Peptide specificity was confirmed in competitive inhibition experiments in which each F(ab′)2 fragment (1 μM) was preincubated with excess amounts of the soluble peptide (2 mM). The competitive effects of the synthetic peptide (Lys1804-Val1819 or His2009-Val2018) on autoantibody binding to LCh were determined by incubation of serial dilutions of each peptide and 1 μM F(ab′)2 for 2 hours at 37°C followed by addition of the mixture to immobilized 80-kd LCh (60 nM). F(ab′)2 binding was detected with peroxidase-conjugated goat-antihuman IgG4F(ab′)2. The absorbance in the absence of F(ab′)2 was considered nonspecific; that in the absence of peptide was considered maximum.
Gel-filtration analysis for evaluation of the association between FVIII and vWF
Plasma (500 μL) was applied to a Sepharose CL-4B column equilibrated with 20 mM Tris-HCl and 150 mM NaCl (TBS; pH 7.4). Elution was done by using TBS (pH 7.4) at a flow rate of 12 mL/hour, and 500-μL fractions were collected. FVIII:Ag and vWF:Ag levels in each fraction were measured with ELISA.
Direct detection of FVIII and autoantibody immune complex in plasma
Protein G Sepharose (Amersham Pharmacia Biotech) was suspended in an equal volume of TBS (pH 7.4). Plasma (3 mL) and protein G Sepharose suspension (50 μL) were mixed and incubated overnight at 4°C. After centrifugation, the supernatant was removed and the beads were washed at least 5 times with TBS (pH 7.4) containing 0.05% Tween 20. TBS (50 μL; pH 6.8) containing 2% sodium dodecyl sulfate (SDS), 10% glycerol, and 0.005% bromophenol blue was added to the washed beads. FVIII was eluted after boiling for 5 minutes. For thrombin treatment, 100 nM human α thrombin (specific activity, 3000 National Institutes of Health U/mg; Sigma) was added to the suspension mixture before the SDS elution step. Samples obtained before and after thrombin treatment were analyzed with 7.5% SDS-PAGE. After electrophoresis, the protein was electrophoretically transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA). After blocking with phosphate-buffered saline (pH 7.4) containing 5% skim milk, the membrane was incubated with mAb NMC-VIII/10 or JR8 and peroxidase-conjugated goat anti-mouse IgG (ICN Pharmaceuticals) was added. Reactive bands were detected with a Western blot chemiluminescence reagent (NEN Life Science Products, Boston, MA).
Effects of autoantibodies on FVIII cleavage at Arg336 by APC independent of PL
The mAb NMC-VIII/10 (0.2 μM), which recognizes the acidic region of the A3 domain, was immobilized on each well of a microtiter plate. FVIII (10 nM) was added to each well, followed by 50 nM APC and 2.5 mM calcium chloride (CaCl2) at 37°C in the absence of PL. To quench the APC reaction at timed intervals, the supernatant was removed from each well with the captured FVIII and 2 mM PMSF was added to each well. FVIII cleavage at Arg336 was detected by the reduction in the binding activity of peroxidase-conjugated mAb C5, which recognizes residues 351 to 365 in the acidic region of the A1 domain. The mAbs NMC-VIII/10 and C5 alone did not inhibit FVIII cleavage by APC (data not shown). To assess the inhibitory effects of antibodies on FVIII cleavage by APC, 0.5 μM autoantibody F(ab′)2 was added to FVIII and left for 2 hours at 37°C before APC was added. The rate of APC cleavage was calculated as follows: 100 − (bound − nonspecific A492)/(bound at time zero − nonspecific A492) × 100 (%). The absorbance in the absence of FVIII was considered nonspecific.
ELISA for binding to immobilized anhydro-APC
For this assessment, 150 μM anhydro-APC in TBS (pH 7.4) was immobilized on each well of a microtiter plate. After blocking with 4% HSA, serial dilutions of FVIII fragments in coating buffer containing 2.5 mM CaCl2 were added and incubated for 2 hours at 37°C. Bound FVIII fragments were detected by using peroxidase-conjugated mAb NMC-VIII/5 or JR8, which did not affect FVIII and APC interaction. The dissociation constant (Kd) of the interaction between FVIII fragment and anhydro-APC was measured by using an indirect ELISA as described previously.36 Competitive experiments were also performed by preincubating soluble anhydro-APC with FVIII fragment (0.3 μM) in the fluid phase. In addition, the competitive effects of unlabeled FVIII fragments on 125I-FVIII binding to anhydro-APC were determined by mixing 0.3 μM 125I-FVIII with serially diluted FVIII fragments before adding the mixture to immobilized anhydro-APC. The bound 125I-FVIII was measured in a γ counter. The percentage of inhibition was calculated as follows: 100 − (bound count − nonspecific count)/(maximum − nonspecific) × 100 (%). The count in the absence of FVIII fragments was considered maximum; that in the absence of125I-FVIII was considered nonspecific.
Inhibitory effects of autoantibodies on the binding of FVIII fragments to anhydro-APC
Serially diluted autoantibody F(ab′)2 or immune-depleted F(ab′)2 was incubated with 0.2 μM FVIII fragment for 2 hours at 37°C, and the mixtures were added to immobilized anhydro-APC. Bound FVIII was detected by using peroxidase-conjugated mAb NMC-VIII/5. The absorbance in the absence of FVIII fragment was considered nonspecific; that in the absence of F(ab′)2 was considered maximum.
Results
FVIII levels and characterization of anti-FVIII autoantibodies in patients with acquired hemophilia A
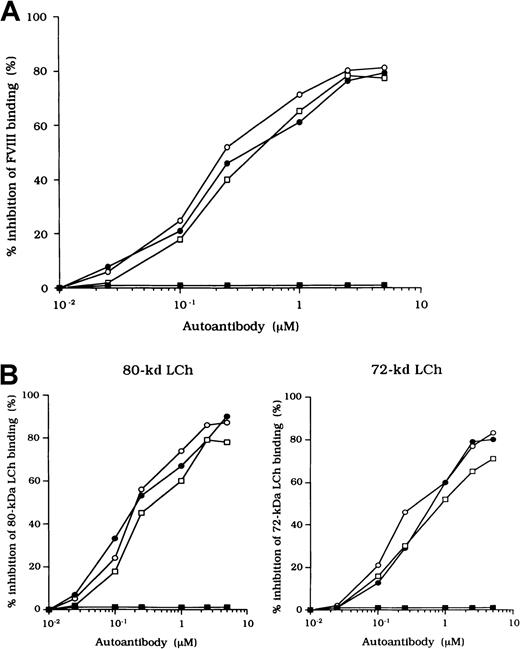
FVIII levels and the basic characteristics of the test autoantibodies are shown in Table 1. Treatment of each patient's plasma sample with acidification andn-caprylic acid resulted in removal of FVIII from IgG, and no FVIII:Ag or FVIII:C was detected in the supernatant autoantibody IgG fraction. Therefore, FVIII was not detected in the purified IgG fraction after protein A Sepharose chromatography. FVIII:Ag levels in the patients' plasma were within the normal range and were disproportionately higher than FVIII:C levels in the presence of anti-FVIII:C. Similarly disproportionate FVIII:Ag levels were observed in mixtures of purified normal FVIII and autoantibody IgG incubated in vitro for 2 hours at 37°C (data not shown). All autoantibodies were classified as type 2 inhibitors. In all cases, the IgG subclass of antibodies was restricted to IgG4. To localize the epitope reactivity of the autoantibodies, we initially performed the neutralization assay and the competitive inhibition assay using FVIII fragments. In all cases, the anti-FVIII:C was neutralized completely (> 95%) by the 80-kd or 72-kd LCh fragment. The inhibitor activity was not neutralized (< 5%), however, by the HCh, A2 domain, or C2 domain. The competitive binding assay yielded similar results (Table1). Because the inhibitory effects of the 80-kd LCh and the 72-kd LCh (free of the acidic region) were similar, we concluded that the epitopes of the 3 autoantibodies may be in the A3-C1 domain but that they are not associated with the acidic region.
Effects of autoantibodies on FVIII and FIXa or PL association
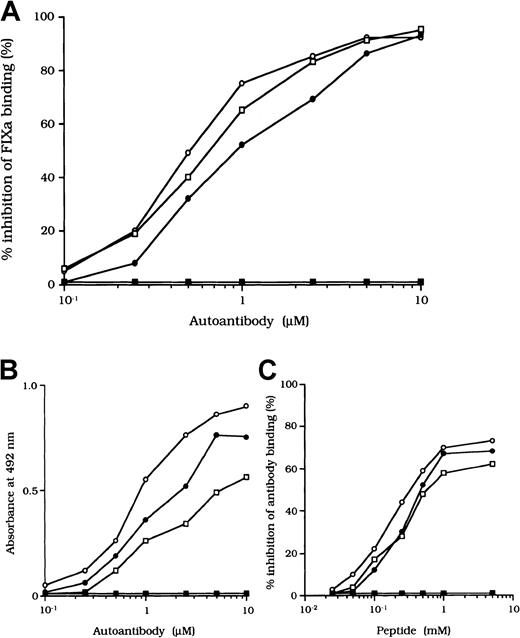
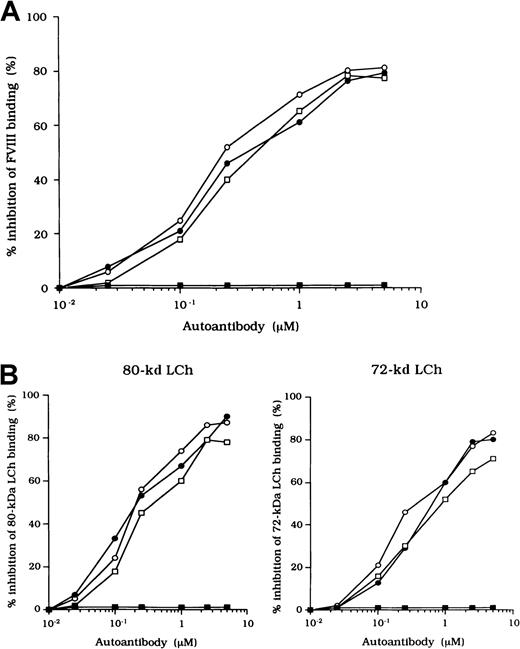
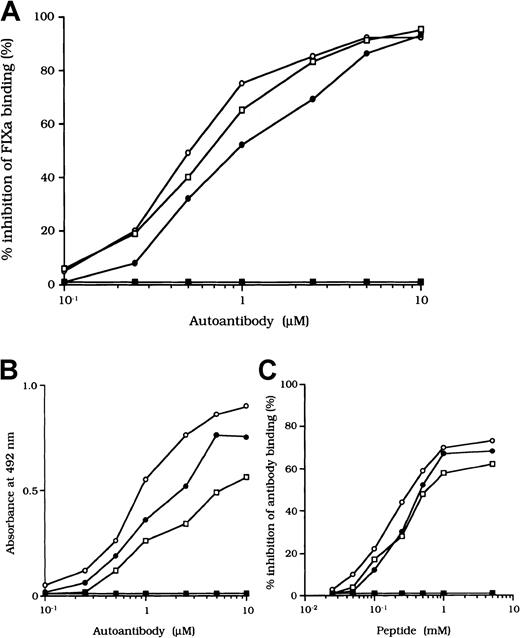
Previous studies showed that most inhibitor antibodies with A3-C1 epitopes inhibit FVIII binding to FIXa.13 We therefore assessed the inhibitory capacity of the studied autoantibodies on FVIII and FIXa interaction by using a specific ELISA (Figure1A). F(ab′)2 fragments of the 3 autoantibodies inhibited FIXa binding to FVIII LCh by 92%, 93%, and 95%, respectively. The values for the concentration that inhibits 50% (IC50) were 0.47, 0.85, and 0.62 μM, respectively. The FIXa binding region for LCh was previously identified in residues Lys1804 to Val1819 in the A3 domain.31 To determine whether the epitopes of the studied antibodies contain a critical FIXa binding region in the A3 domain, we examined direct binding to the synthetic peptide, Lys1804-Val1819. Dose-dependent binding patterns were observed for all 3 antibodies (Figure 1B). The type 1 antibody, which did not inhibit FVIII binding to FIXa, did not bind to the peptide. The specificity of the binding was confirmed by the demonstration that binding was completely blocked by preincubation with excess amounts of soluble peptide. In addition, a random version of the peptide Lys1804-Val1819 (VKWFYTKTENPKVFNK) did not bind to any of the antibodies (data not shown).
Effects of autoantibodies on the association between FVIII and FIXa.
(A) Inhibitory effects of autoantibodies on FIXa binding to LCh. Serial dilutions of autoantibodies F(ab′)2 were incubated with FIXa (40 nM) for 2 hours at 37°C before being added to immobilized 80-kd LCh (60 nM). Bound FIXa was detected by using peroxidase-conjugated mAb 3A6. Case 1, ○; case 2, ●; case 3, ■; type 1 autoantibody F(ab′)2, ▪. (B) Direct binding of autoantibodies to peptide Lys1804-Val1819. Serial dilutions of autoantibody F(ab′)2 were added to immobilized peptide (100 μM). Bound F(ab′)2 was detected with peroxidase-conjugated goat-antihuman IgG4F(ab′)2. Case 1, ○; case 2, ●; case 3 ■; type 1 autoantibody F(ab′)2, ▪. (C) Inhibitory effects of synthetic peptide Lys1804-Val1819 on autoantibody binding to LCh. Serial dilutions of peptide were incubated with F(ab′)2 (1 μM) for 2 hours at 37°C before being added to immobilized 80-kd LCh. Bound F(ab′)2 was detected by using peroxidase-conjugated antihuman F(ab′)2. Case 1, ○; case 2, ●; case 3, ■; type 1 autoantibody F(ab′)2, ▪.
Effects of autoantibodies on the association between FVIII and FIXa.
(A) Inhibitory effects of autoantibodies on FIXa binding to LCh. Serial dilutions of autoantibodies F(ab′)2 were incubated with FIXa (40 nM) for 2 hours at 37°C before being added to immobilized 80-kd LCh (60 nM). Bound FIXa was detected by using peroxidase-conjugated mAb 3A6. Case 1, ○; case 2, ●; case 3, ■; type 1 autoantibody F(ab′)2, ▪. (B) Direct binding of autoantibodies to peptide Lys1804-Val1819. Serial dilutions of autoantibody F(ab′)2 were added to immobilized peptide (100 μM). Bound F(ab′)2 was detected with peroxidase-conjugated goat-antihuman IgG4F(ab′)2. Case 1, ○; case 2, ●; case 3 ■; type 1 autoantibody F(ab′)2, ▪. (C) Inhibitory effects of synthetic peptide Lys1804-Val1819 on autoantibody binding to LCh. Serial dilutions of peptide were incubated with F(ab′)2 (1 μM) for 2 hours at 37°C before being added to immobilized 80-kd LCh. Bound F(ab′)2 was detected by using peroxidase-conjugated antihuman F(ab′)2. Case 1, ○; case 2, ●; case 3, ■; type 1 autoantibody F(ab′)2, ▪.
We also tested the effects of the peptide on autoantibody binding to LCh in the competitive assays and showed that binding was inhibited by 73%, 68%, and 61%, respectively, with the 3 autoantibodies (Figure1C). These results indicate that the studied autoantibodies recognize the region encompassing residues Lys1804 to Val1819 of the A3 domain or its near region and that they block interaction between FVIII and FIXa. In contrast, the antibodies did not appreciably inhibit binding of FVIII to PS (< 5%; data not shown). The C2 domain is involved in FVIII binding to PS,12 and our results are consistent with the absence of an anti-C2 inhibitor.
Association between FVIII and vWF
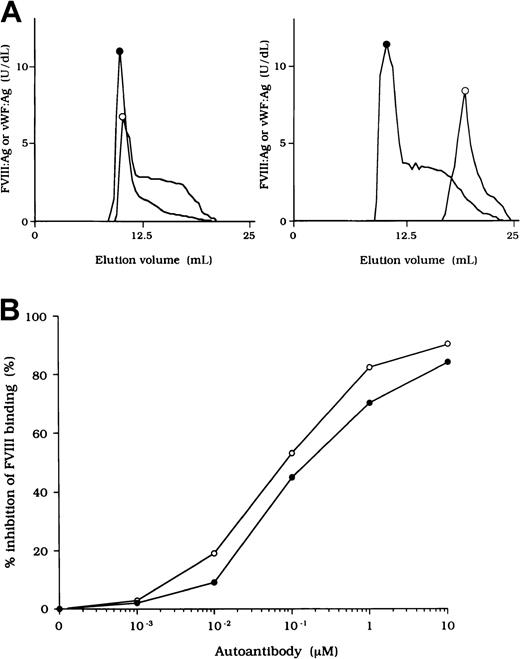
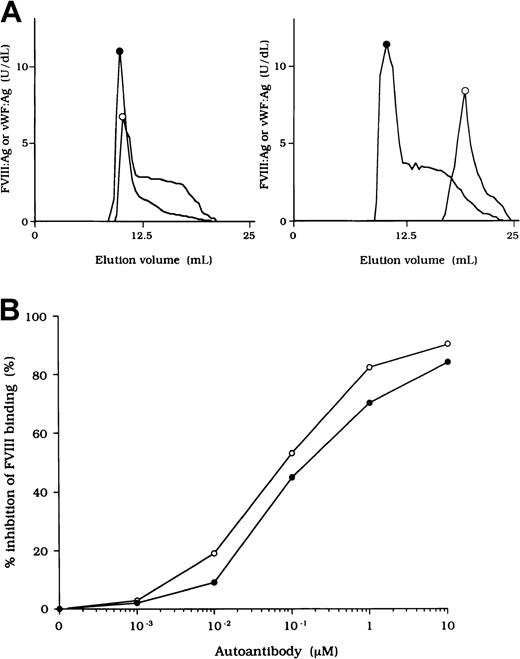
To investigate the association of FVIII and vWF in plasma, we measured FVIII:Ag and vWF:Ag in fractions after gel filtration on Sepharose CL-4B. In normal plasma, the elution peak of both FVIII and vWF appeared at or near the void volume (Figure2A, left). In contrast, in each sample of plasma from the patients, elution of FVIII was delayed and FVIII:Ag was not detected in any of the early fractions containing vWF (Figure 2A, right). These results indicate that FVIII was dissociated from vWF in the patients' plasma. Figure 2A shows a representative gel-filtration pattern from an experiment using plasma from patient 2.
FVIII and vWF interaction in plasma from a patient with hemophilia A.
(A) Sepharose CL-4B gel-filtration pattern of plasma from patient 2. Normal plasma (500 μL, left panel) or plasma from patient 2 (right panel) was applied to a Sepharose CL-4B column and eluted with TBS. FVIII:Ag (○) and vWF:Ag (●) were measured in each fraction. (B) Inhibitory effect of autoantibody from patient 2 on FVIII binding to vWF. Serial dilutions of autoantibody F(ab′)2 or immune-depleted F(ab′)2 were incubated with FVIII (1 nM) for 2 hours at 37°C before being added to immobilized vWF (40 nM). Bound FVIII was detected by using peroxidase-conjugated mAb JR8. F(ab′)2, ○; immune-depleted F(ab′)2, ●.
FVIII and vWF interaction in plasma from a patient with hemophilia A.
(A) Sepharose CL-4B gel-filtration pattern of plasma from patient 2. Normal plasma (500 μL, left panel) or plasma from patient 2 (right panel) was applied to a Sepharose CL-4B column and eluted with TBS. FVIII:Ag (○) and vWF:Ag (●) were measured in each fraction. (B) Inhibitory effect of autoantibody from patient 2 on FVIII binding to vWF. Serial dilutions of autoantibody F(ab′)2 or immune-depleted F(ab′)2 were incubated with FVIII (1 nM) for 2 hours at 37°C before being added to immobilized vWF (40 nM). Bound FVIII was detected by using peroxidase-conjugated mAb JR8. F(ab′)2, ○; immune-depleted F(ab′)2, ●.
Inhibition of association of FVIII and vWF was also observed in an ELISA using autoantibody F(ab′)2 together with normal FVIII and vWF. In all cases, F(ab′)2 preparations inhibited FVIII binding to vWF by more than 90% (Figure 2B). Because the vWF binding site is in the C2 domain,37 we examined the inhibitory effect of immune-depleted F(ab′)2 after C2-column chromatography to exclude completely the presence of anti-C2 reactivity in the studied autoantibodies. In all cases, the immune-depleted F(ab′)2 blocked the binding of normal FVIII and vWF by greater than 80% and in a dose-dependent manner. Figure 2B shows a representative inhibitory pattern from an experiment using antibody from patient 2.
Direct detection of FVIII antibody complexes in plasma
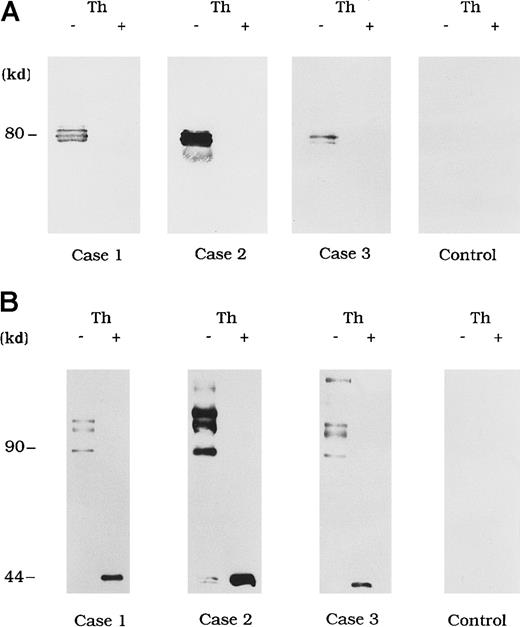
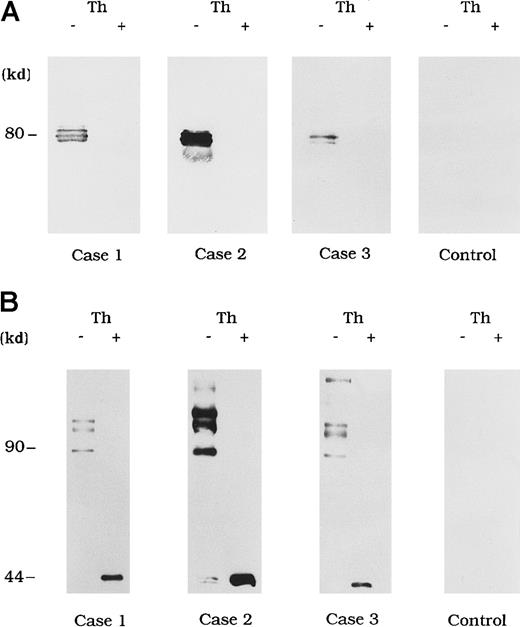
Circulating immune complexes were recovered from plasma by using protein G Sepharose and identified with immunoblotting using anti-A3 mAb NMC-VIII/10 and anti-A2 mAb JR8 to detect bound FVIII. No FVIII band was detected in control type 1 autoantibody plasma (Figure3A and 3B, control). In contrast, in the 3 plasma samples from patients with acquired hemophilia A, 80-kd LCh bands were detected by using mAb NMC-VIII/10 (Figure 3A). These bands disappeared after thrombin treatment of the plasma, reflecting the known LCh reactivity of NMC/VIII-10.22 Similarly, HCh bands ranging from 90 to 210 kd were detected with mAb JR8 and, in all cases, these bands were abolished and converted into 44-kd bands after thrombin treatment of plasma (Figure 3B).
Direct detection of FVIII and autoantibody immune complexes in plasma.
Each FVIII band was detected by using mAb NMC-VIII/10 (A), which recognizes the 80-kd LCh but not the 72-kd LCh, or mAb JR8 (B), which recognizes the 44-kd HCh. Th (−) represents untreated material and Th (+) represents the material treated with thrombin. Type 1 autoantibody plasma was used as a control.
Direct detection of FVIII and autoantibody immune complexes in plasma.
Each FVIII band was detected by using mAb NMC-VIII/10 (A), which recognizes the 80-kd LCh but not the 72-kd LCh, or mAb JR8 (B), which recognizes the 44-kd HCh. Th (−) represents untreated material and Th (+) represents the material treated with thrombin. Type 1 autoantibody plasma was used as a control.
Inhibitory effects of autoantibodies on FVIII proteolytic cleavage at Arg336 by APC
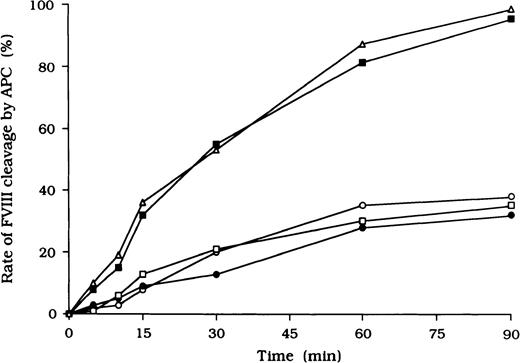
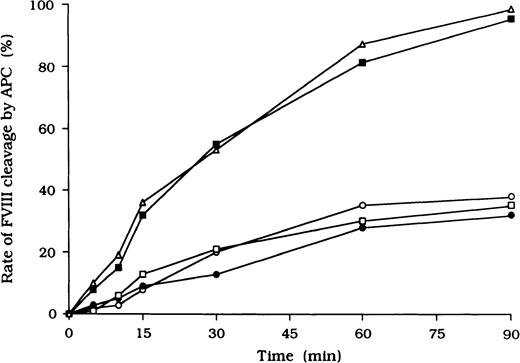
Because it is known that free FVIII is rapidly degraded by APC, we focused on the action of APC as a potential mechanism for the persistence of immune complexes in the 3 patients. To evaluate the quantitative effects of autoantibodies on proteolytic cleavage of FVIII by APC, we developed an ELISA to measure FVIII inactivation by APC independent of PL. In this assay, mAb C5, which recognizes the acidic region of the A1 domain (epitope in the residues 351-365), was used for detection.27 This mAb loses its reactivity after cleavage of FVIII by APC at Arg336 in the A1 domain, and hence a reduction in binding to C5 reflects APC-dependent proteolysis of FVIII. In the presence of test autoantibody F(ab′)2, the reactivity of C5 in the 3 samples was reduced to 38%, 30%, and 35%, respectively, by 90 minutes after the addition of APC (Figure4). In contrast, reactivity with the mAb remained higher than 95% in the presence of F(ab′)2 from normal IgG or control type 1 autoantibody. Furthermore, assays of FVIII activity showed that FVIII was inactivated by APC, with time courses similar to those observed in the assays of FVIII cleavage by APC (data not shown).
Inhibitory effects of autoantibodies on FVIII proteolytic cleavage at Arg336 by APC in the absence of PL.
Autoantibody F(ab′)2 (0.5 μM) was added to 10 nM FVIII, which was bound to immobilized NMC-VIII/10 (0.2 μM). Then, 50 nM APC and 2.5 mM CaCl2 were added. At timed intervals, supernatants were removed and APC activity was stopped by adding 2 mM PMSF to the microtiter wells. FVIII cleavage at Arg336 was detected with peroxidase-conjugated mAb C5. Case 1, ○; case 2, ●; case 3, ■; type 1 autoantibody F(ab′)2, ▪; normal F(ab′)2, ▵.
Inhibitory effects of autoantibodies on FVIII proteolytic cleavage at Arg336 by APC in the absence of PL.
Autoantibody F(ab′)2 (0.5 μM) was added to 10 nM FVIII, which was bound to immobilized NMC-VIII/10 (0.2 μM). Then, 50 nM APC and 2.5 mM CaCl2 were added. At timed intervals, supernatants were removed and APC activity was stopped by adding 2 mM PMSF to the microtiter wells. FVIII cleavage at Arg336 was detected with peroxidase-conjugated mAb C5. Case 1, ○; case 2, ●; case 3, ■; type 1 autoantibody F(ab′)2, ▪; normal F(ab′)2, ▵.
Direct binding of FVIII to immobilized anhydro-APC
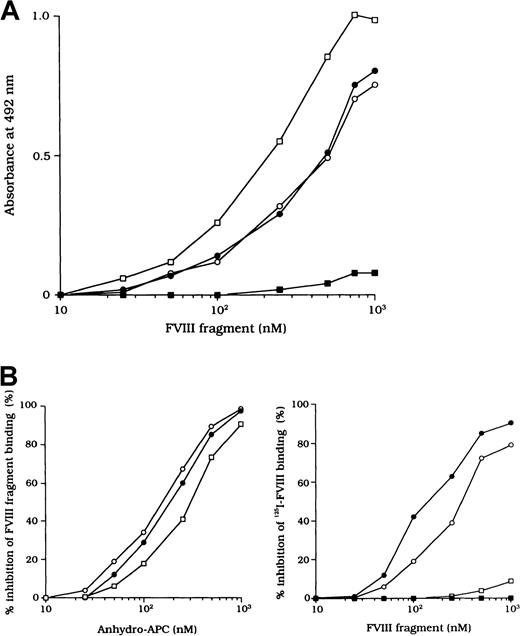
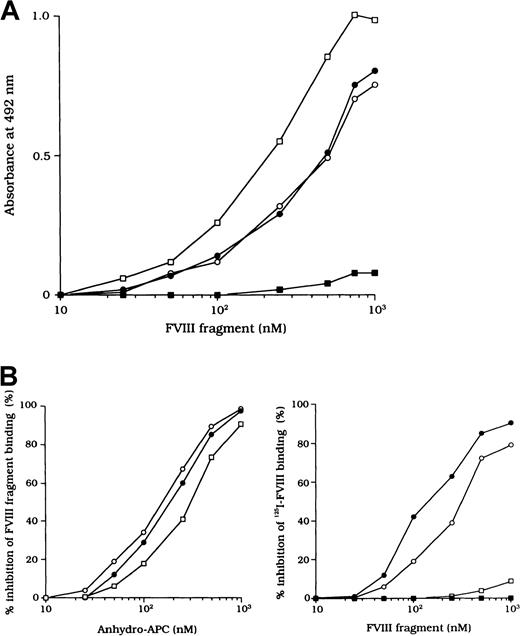
Because the 3 type 2 autoantibodies prevented APC-induced proteolytic inactivation of FVIII, we considered that they interfered with FVIII and APC interaction directly. To confirm this idea, we initially examined direct binding in an ELISA by using active-site modified APC (anhydro-APC), which lacks procoagulant activity. The binding of FVIII was detected by using mAb NMC-VIII/5 or JR8, which did not affect the APC reactivity. Intact FVIII bound in a dose-dependent fashion to the immobilized anhydro-APC (Figure5A) and remained detectable by the ELISA for at least 12 hours. The 80-kd and 72-kd LCh fragments also bound to anhydro-APC in a dose-dependent manner. The affinity (Kd) value for intact FVIII was 580 nM; values for the 80-kd LCh and the 72-kd LCh were 540 nM and 310 nM, respectively. The HCh fragment did not bind appreciably to anhydro-APC.
ELISA for FVIII binding to anhydro-APC.
(A) Dose-dependent patterns on FVIII fragments to immobilized anhydro-APC. Serial dilutions of FVIII fragments were added to immobilized anhydro-APC (150 nM). Bound FVIII was detected by using peroxidase-conjugated mAb NMC-VIII/5 or JR8. Intact FVIII, ○; 80-kd LCh, ●; 72-kd LCh, ■; HCh, ▪. (B) Competitive inhibition on FVIII binding to anhydro-APC. Competitive experiments were performed by preincubating serial dilutions of soluble anhydro-APC with FVIII fragments (0.3 μM) in the fluid phase (left panel). Intact FVIII, ○; 80-kd LCh, ●; 72-kd LCh, ■. In addition, 0.3 μM125I-FVIII was preincubated with serial dilutions of FVIII fragments before the assay of anhydro-APC binding (right panel). Bound125I-FVIII was measured in a γ counter. 80-kd LCh, ○; 72-kd LCh, ●; HCh, ■; C2 domain, ▪.
ELISA for FVIII binding to anhydro-APC.
(A) Dose-dependent patterns on FVIII fragments to immobilized anhydro-APC. Serial dilutions of FVIII fragments were added to immobilized anhydro-APC (150 nM). Bound FVIII was detected by using peroxidase-conjugated mAb NMC-VIII/5 or JR8. Intact FVIII, ○; 80-kd LCh, ●; 72-kd LCh, ■; HCh, ▪. (B) Competitive inhibition on FVIII binding to anhydro-APC. Competitive experiments were performed by preincubating serial dilutions of soluble anhydro-APC with FVIII fragments (0.3 μM) in the fluid phase (left panel). Intact FVIII, ○; 80-kd LCh, ●; 72-kd LCh, ■. In addition, 0.3 μM125I-FVIII was preincubated with serial dilutions of FVIII fragments before the assay of anhydro-APC binding (right panel). Bound125I-FVIII was measured in a γ counter. 80-kd LCh, ○; 72-kd LCh, ●; HCh, ■; C2 domain, ▪.
To assess the specificity of binding, soluble anhydro-APC was preincubated with FVIII fragments in the fluid phase before the ELISA. The binding of all FVIII fragments to the immobilized anhydro-APC was inhibited by greater than 95% by soluble anhydro-APC (1 μM), thereby confirming the specificity of the direct binding assay (Figure 5B, left). Furthermore, direct binding of FVIII to anhydro-APC was confirmed in a competitive assay using 125I-FVIII preincubated with FVIII fragments. The 80-kd and 72-kd LCh fragments inhibited binding of 125I-FVIII to anhydro-APC by 80% to 90%. In contrast, the HCh and C2 domains showed little or no inhibition, even at a concentration of 1 μM (Figure 5B, right).
Inhibitory effects of autoantibodies on FVIII binding to anhydro-APC
The F(ab′)2 preparations of the 3 type 2 autoantibodies inhibited FVIII binding to anhydro-APC by 82%, 79%, and 77%, respectively (Figure 6A). The IC50 values were 0.20, 0.31, and 0.40 μM, respectively. In contrast, F(ab′)2 from control type 1 autoantibody did not inhibit FVIII binding to anhydro-APC. Furthermore, all autoantibodies inhibited 80-kd or 72-kd LCh binding to anhydro-APC by 70% to 90% (Figure 6B). In addition, the immune-depleted F(ab′)2 prepared by using C2-affinity columns inhibited binding to a similar extent (data not shown). These results suggest that the inhibition of FVIII binding to anhydro-APC by the studied antibodies led to restricted APC-induced proteolytic inactivation of FVIII.
Inhibitory effects of autoantibodies on binding of FVIII fragments to anhydro-APC.
Serial dilutions of autoantibody F(ab′)2 were incubated with 0.2 μM intact FVIII (A), 80-kd LCh, or 72-kd LCh (B) for 2 hours at 37°C before being added to immobilized anhydro-APC. Case 1, ○; case 2, ●; case 3, ■; type 1 autoantibody F(ab′)2, ▪.
Inhibitory effects of autoantibodies on binding of FVIII fragments to anhydro-APC.
Serial dilutions of autoantibody F(ab′)2 were incubated with 0.2 μM intact FVIII (A), 80-kd LCh, or 72-kd LCh (B) for 2 hours at 37°C before being added to immobilized anhydro-APC. Case 1, ○; case 2, ●; case 3, ■; type 1 autoantibody F(ab′)2, ▪.
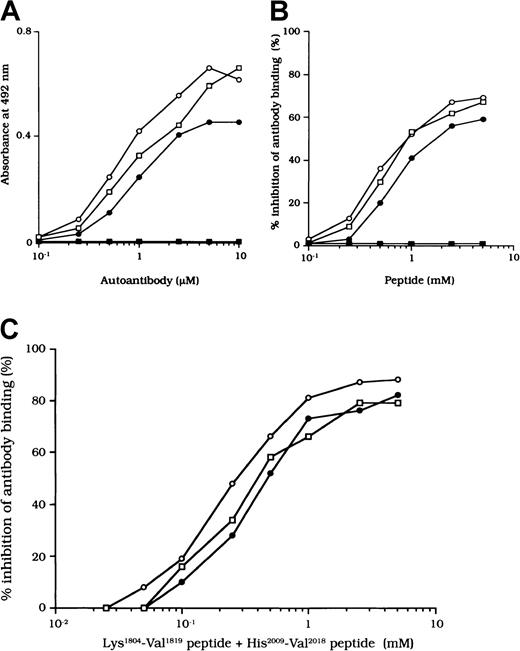
Binding of autoantibodies to a synthetic peptide composed of residues His2009 to Val 2018
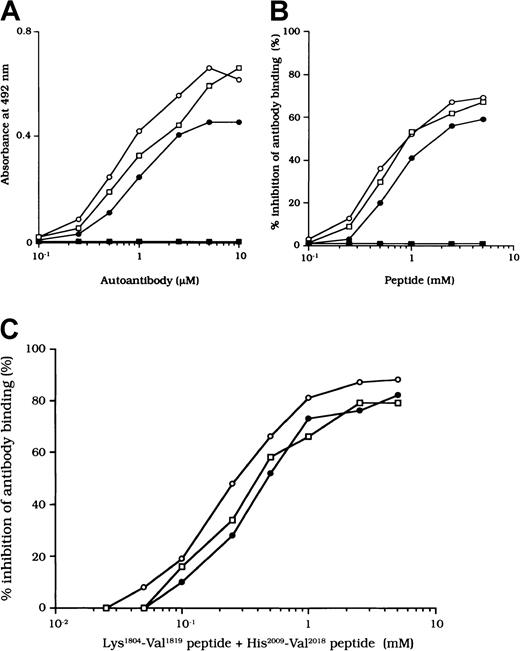
The binding site for APC is in residues His2009 to Val2018 in the LCh.7 To confirm that the studied autoantibodies recognized this critical APC binding region, we examined the direct binding to a synthetic peptide composed of these amino acids. Dose-dependent binding patterns were observed for all 3 antibodies (Figure 7A). In contrast, the type 1 autoantibody, which did not affect FVIII and APC interaction, did not bind to the peptide. Furthermore, the random version of the peptide His2009-Val2018 (VIFLTSMGAH) did not bind to any of the autoantibodies (data not shown). The specificity for direct binding was confirmed by demonstrating complete inhibition after preincubation with excess amounts of soluble peptide.
Binding of autoantibodies to a synthetic peptide with residues His2009 to Val2018.
(A) Direct binding of autoantibodies to peptide His2009-Val2018. Serial dilutions of autoantibody F(ab′)2 were added to immobilized peptide (100 μM). Bound F(ab′)2 was detected with using peroxidase-conjugated goat-antihuman IgG4F(ab′)2. Case 1, ○; case 2, ●; case 3, ■; type 1 autoantibody F(ab′)2, ▪. (B) Inhibitory effects of peptide His2009-Val2018 on autoantibody binding to 80-kd LCh. Serial dilutions of synthetic peptide were incubated with 1 μM autoantibody F(ab′)2 for 2 hours at 37°C before being added to immobilized 80-kd LCh (60 nM). Case 1, ○; case 2, ●; case 3, ■; type 1 autoantibody F(ab′)2, ▪. (C) Inhibitory effects of both peptide Lys1804-Val1819 and peptide His2009-Val2018 on autoantibody binding to 80-kd LCh. The mixtures of serial dilutions of both peptides were incubated with 1 μM autoantibody F(ab′)2 for 2 hours at 37°C before being added to immobilized 80-kd LCh (60 nM). Case 1, ○; case 2, ●; case 3, ■.
Binding of autoantibodies to a synthetic peptide with residues His2009 to Val2018.
(A) Direct binding of autoantibodies to peptide His2009-Val2018. Serial dilutions of autoantibody F(ab′)2 were added to immobilized peptide (100 μM). Bound F(ab′)2 was detected with using peroxidase-conjugated goat-antihuman IgG4F(ab′)2. Case 1, ○; case 2, ●; case 3, ■; type 1 autoantibody F(ab′)2, ▪. (B) Inhibitory effects of peptide His2009-Val2018 on autoantibody binding to 80-kd LCh. Serial dilutions of synthetic peptide were incubated with 1 μM autoantibody F(ab′)2 for 2 hours at 37°C before being added to immobilized 80-kd LCh (60 nM). Case 1, ○; case 2, ●; case 3, ■; type 1 autoantibody F(ab′)2, ▪. (C) Inhibitory effects of both peptide Lys1804-Val1819 and peptide His2009-Val2018 on autoantibody binding to 80-kd LCh. The mixtures of serial dilutions of both peptides were incubated with 1 μM autoantibody F(ab′)2 for 2 hours at 37°C before being added to immobilized 80-kd LCh (60 nM). Case 1, ○; case 2, ●; case 3, ■.
We also examined the inhibitory effect of the peptide on autoantibody binding to 80-kd LCh. The peptide inhibited binding of all type 2 F(ab′)2 preparations to 80-kd LCh in a similar dose-dependent manner, by 67%, 58%, and 66%, respectively, for the 3 autoantibodies (Figure 7B). The random peptide (VIFLTSMGAH) did not inhibit any of the binding reactions. As described above, the peptide Lys1804-Val1819, corresponding to the FIXa binding region, inhibited antibody binding to LCh. We also tested the inhibitory effects of mixtures of both peptides (Lys1804-Val1819 and His2009-Val2018) on antibody binding to LCh. Inhibition was enhanced to 87%, 80%, and 79%, respectively (Figure 7C), consistent with the presence of at least 2 populations of antibodies with epitopes including residues 2009 to 2018 and 1804 to 1819.
Discussion
We found normal circulating levels of FVIII:Ag in 3 patients with acquired hemophilia A, despite the presence of inhibitors and very low levels of FVIII:C. The presence of FVIII:Ag in these patients appeared to be similar to that observed in patients with cross-reacting material-positive (CRM+) hemophilia A. In CRM+hemophilia A, FVIII functioning is thought to be impaired as a result of point mutations at crucial residues in the FVIII molecule associated with FVIII cofactor activity, such as thrombin cleavage sites.38 39 Such mutations do not usually cause serious conformational changes, however, and the circulating FVIII:Ag bound to vWF is protected from proteolytic degradation. It seemed possible that the endogenous FVIII in the patients we studied also may have escaped degradation by binding to the antibodies. More rapid clearance from the circulation by the formation of immune complexes could be expected, however, and the reasons for the persistence of FVIII:Ag in these patients with CRM+ acquired hemophilia A prompted further investigations.
The antibodies we studied showed complex kinetic inactivation patterns typical of type 2 FVIII inhibitors. We used anti-C2 antibodies to measure FVIII:Ag, and the normal plasma levels of FVIII:Ag we found suggested that the antibody epitopes were likely to be different from those of more common FVIII inhibitors. These results were in agreement with the suggestion of Gawryl and Hoyer,14 who proposed that differences between the epitopes in type 1 and type 2 antibodies could explain the different inactivation kinetics. It is generally accepted that both FVIII autoantibodies and FVIII alloantibodies mainly recognize C2 sequences,9 and although all the autoantibodies we studied exclusively recognized the FVIII LCh, they did not react with the C2 domain in the neutralization assay or the competitive binding assay. FVIII inhibitors with C2 epitopes are known to inhibit FVIII binding to PL,12 and Zhong et al13 reported that some FVIII inhibitors with A3-C1 epitopes inhibit binding to FIXa. The antibodies we studied did not significantly inhibit FVIII binding to PL, but they did inhibit binding to FIXa, findings that are consistent with the lack of reaction with the C2 domain.
We also prepared highly specific antibodies by means of immune depletion on C2-affinity columns to exclude the contribution of anti-C2 antibodies completely. The results obtained with the immune-depleted antibodies were similar to those with nondepleted antibodies. Furthermore, we found that the antibodies interacted with 2 synthetic peptides, Lys1804-Val1819 and His2009-Val2018, in the direct binding and competitive experiments. Altogether, these results provide persuasive evidence that the studied antibodies had the epitopes in the A3-C1 domain. The greater inhibitory effect of the mixture of the 2 synthetic peptides compared with the effect of each of them alone is consistent with the presence of 2 different populations of antibodies recognizing the epitopes Lys1804 to Val1819 and His2009 to Val2018. It is also possible that one population of clonally related antibodies targets both peptides, since the inhibitory effects of the mixtures were not complete and the 2 LCh segments are in close proximity in the 3-dimensional arrangement of the LCh.
The normal levels of FVIII:Ag found in plasma samples from all 3 patients with the type 2 inhibitors suggested the possibility that FVIII was circulating as immune complexes with the autoantibodies. Moreover, when antibodies F(ab′)2 were added to FVIII in vitro, similar patterns of residual FVIII:Ag were observed, strongly suggesting that the CRM+ patterns were caused by antibody binding and supporting the concept that FVIII existed in immune complexes. FVIII was not detected in IgG fractions prepared by precipitation with n-caprylic acid followed by protein A Sepharose chromatography. It seems possible that treatment withn-caprylic acid, with reduction of the pH to 4.0, dissociated FVIII:Ag from the immune complexes. Alternatively, this procedure might have induced conformational changes that altered the antigenic reactivity of the FVIII protein irreversibly. In contrast, the presence of immune complexes containing FVIII:Ag was confirmed by immunoblotting experiments using plasma extracts with protein G Sepharose beads. Both FVIII LCh and HCh were detected in all 3 plasma samples, indicating that intact, heterodimeric FVIII circulated as the immune complexes. Furthermore, immune complexes can be expected to be cleared rapidly from the circulation by the reticuloendothelial system, and our results suggest that FVIII is protected from this clearance mechanism.
In normal plasma, FVIII is protected and stabilized by vWF.40 We therefore initially considered that FVIII and vWF might be more tightly associated by the formation of immune complexes. It is pertinent to this idea that Saenko et al41reported that anti-FVIII mAb with the epitope comprising the C2-domain residues 2248 to 2285 and a C2-inhibitor alloantibody prevented dissociation of FVIII from vWF. The opposite effects observed with the 3 autoantibodies we studied, which inhibited the binding of FVIII to vWF, were quite unexpected. Both the amino-terminal acidic region of the A3 domain and the C2 domain have been proposed as binding regions for vWF,36,42 although the precise sites have not been identified. The inhibitory effect of antibodies on FVIII binding to vWF observed in the current study was consistent with the gel-filtration patterns of plasma, which revealed the existence of FVIII independent of vWF. In addition, the anti-FVIII:C effects of autoantibodies were neutralized completely by 72-kd LCh but not by the C2 domain. The results we obtained did not reveal the presence of antibodies against the vWF binding sites in the amino-terminal A3 and C2 domains. Furthermore, the inhibitory effects on FVIII binding to vWF remained in F(ab′)2 fractions after immune depletion on C2-affinity columns. Jacquemin et al43 reported that anti-C1 antibodies inhibited FVIII binding to vWF, indicating a possible role for the A3 and C1 sequences in the association between FVIII and vWF, although steric hindrance could not be excluded.
The binding site for APC was previously localized to the A3-C1 domain in FVIII LCh, and a synthetic peptide (His2009-Val2018) in the A3 domain was shown to inhibit APC-catalyzed FVIII inactivation.7 Although the precise binding sites and the binding mechanism have not been fully defined, the A3 peptide region seems likely to be directly or indirectly involved in APC binding. A close relation between APC and vWF binding is suggested by the finding that vWF protects FVIII from proteolytic inactivation by APC,8 and the FVIII, which was dissociated from vWF in our cases, could be expected to have been rapidly degraded by APC. The finding of normal levels of FVIII:Ag therefore indicates that the antibodies can replace the protective effect of vWF effectively. Consequently, we attempted to determine whether the formation of immune complexes restricted APC-induced FVIII proteolysis.
We found that, indeed, the 3 autoantibodies diminished APC-induced FVIII proteolytic inactivation. Inhibition of inactivation was partial, however, and repression of other proteolytic processes could not be discounted. For instance, FIXa is also known to inactivate FVIII by cleavage at Arg33644 and all 3 studied autoantibodies inhibited FIXa binding to FVIII. It is therefore possible that the inhibition of binding of FVIII to FIXa contributed to the observed protective mechanisms. It has been reported that removal of FVIII from the circulation involves a low-density lipoprotein receptor–related protein (LRP) on cells. Although binding regions for LRP have been found in both the A2 and C2 domain,45 46 we cannot completely rule out the possibility that the autoantibodies we studied also protect patients' circulating FVIII from the cell-mediated uptake system by blocking a yet unknown region in the A3-C1 domain for LRP participating in interaction with LRP. Nevertheless, our study clearly demonstrated that the type 2 autoantibodies blocked FVIII binding to anhydro-APC and bound to a synthetic peptide (His2009-Val2018) containing the APC binding site. The findings suggest that inhibition of proteolytic cleavage by APC at least partly accounts for the persistence of FVIII:Ag in the circulation.
Our study demonstrated directly for the first time the presence of circulating FVIII immune complexes in patients with acquired hemophilia A. The autoantibodies manifested characteristic phenotypic features of type 2 inhibitors, although the epitopes appeared to be located in the A3-C1 FVIII domains—an unusual finding. We also found that the protection of FVIII from APC-mediated proteolysis by FVIII-bound type 2 autoantibodies might contribute to the presence of these complexes. Because acquired hemophilia is viewed as an autoimmune disease caused by simple antigen-antibody reactions, the demonstration of circulating immune complexes in these circumstances provides further insights into the pathophysiologic aspects of autoimmune disorders.
Acknowledgments
We thank Dr A. C. Beddall for referring patient 2, Dr O. Yonekawa for referring patient 3, and Dr D. Scandella, who kindly provided the recombinant C2 domain for our study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Midori Shima, Department of Pediatrics, Nara Medical University, 840 Shijo-cho. Kashihara City, Nara 634-8522, Japan.