Abstract
This study examined changes in the biomechanical properties of cultured pulmonary microvascular endothelial cells (ECs) and neutrophils induced by adhesion of neutrophils to these ECs. The biomechanical properties of cells were evaluated using magnetic twisting cytometry, which measures the angular rotation of ferromagnetic beads bound to cells through antibody ligation on application of a specified magnetic torque. Adhesion of neutrophils to 24-hour tumor necrosis factor-α (TNF-α)–treated ECs, but not to untreated ECs, induced an increase in EC stiffness within 2 minutes, which was accompanied by an increase and a reorganization of F-actin in ECs. A cell-permeant, phosphoinositide-binding peptide attenuated the EC stiffening response, suggesting that intracellular phosphoinositides are required. The stiffening response was not inhibited by ML-7, a myosin light-chain kinase inhibitor, or BAPTA, an intracellular Ca2+ chelator. Moreover, the phosphorylation pattern of the regulatory myosin light chains was unaltered within 15 minutes of neutrophil adherence. These data suggested that the EC stiffening response appeared not to be mediated by myosin light-chain–dependent mechanisms. Concomitantly, neutrophil adhesion to 24-hour TNF-α–treated ECs also induced changes in the biomechanical properties of neutrophils compared to neutrophils bound to untreated ECs. Taken together, these results demonstrated that neutrophil adhesion to TNF-α–treated ECs induces changes in the biomechanical properties of both cell types through actin cytoskeletal remodeling. These changes may modulate neutrophil transmigration across the endothelium during inflammation.
Introduction
Neutrophil transmigration across the vascular endothelium is a highly regulated process that requires the up-regulation of neutrophil and endothelial cell (EC) adhesion molecules.1 During neutrophil adhesion to ECs, binding of the neutrophil or EC adhesion molecules to their ligands may induce intracellular signaling pathways and downstream events, which may in turn modulate neutrophil transmigration. Indeed, the ligation of various adhesion molecules can initiate signal transduction pathways and induce subsequent cellular changes. Cross-linking of neutrophil CD11/CD18 induces intracellular Ca2+ increases, CD11/CD18 up-regulation, and F-actin polymerization.2 Ligation of EC E-selectin, P-selectin, ICAM-1, and VCAM-1 by antibodies induces transient increases in cytosolic Ca2+.3-5Similarly, neutrophil adherence and emigration also induce increases in intracellular Ca2+ levels in ECs.3,6 One of the downstream targets of these signaling events in ECs after neutrophil adherence is the actin cytoskeleton. Neutrophil adherence induces stress fiber formation in ECs,3,7 and inhibition of the F-actin changes and actin polymerization in ECs reduces neutrophil transmigration as observed both in vivo and in vitro.7-9 However, the mechanisms involved in mediating the EC actin cytoskeletal changes on neutrophil adhesion are not understood.
In the systemic circulation, neutrophil emigration occurs within postcapillary venules and is initiated by selectin-mediated neutrophil tethering and rolling, followed by neutrophil activation and β2-integrin/ICAM-1–mediated firm adhesion to ECs.10 In contrast, within the pulmonary circulation, most of the neutrophil emigration occurs through capillaries, and neutrophil rolling does not occur because of anatomic restrictions.11,12 Whereas adhesion molecules are not required during the initial process of neutrophil sequestration, they are important for subsequent events during neutrophil response in pulmonary inflammation.13 14
This study examined neutrophil adhesion-induced changes in the biomechanical properties of both neutrophils and cytokine-activated human pulmonary microvascular ECs using magnetic twisting cytometry.15 16 This technique measures the angular rotation (strain) of ferromagnetic beads bound to cells through specific ligands on application of a magnetic torque (stress), and the apparent stiffness of the cells is defined as the ratio of stress to strain. This study also evaluated the mechanisms through which adhesion of neutrophils to ECs induced changes in the biomechanical properties of ECs, particularly the roles of intracellular signaling pathways in mediating the changes in the biomechanical properties of ECs. Finally, whether this increase in EC stiffening required remodeling of the actin cytoskeleton, the actin-myosin contraction, or both was also determined. The results show that cytoskeleton-dependent stiffening of neutrophils and ECs occurred during neutrophil-EC adhesion. The EC stiffening response involved a phosphoinositide-dependent and intracellular Ca2+-independent mechanism and required the remodeling of the actin cytoskeleton, but it appeared not to involve myosin light-chain–mediated EC contraction. These changes in the biomechanical properties of neutrophils and pulmonary microvascular ECs during adhesion may modulate neutrophil emigration across the endothelium during inflammatory responses.
Materials and methods
Materials
Cytochalasin D, forskolin, lysophosphatidylcholine, rhodamine-conjugated phalloidin, fMLP, thrombin, and IBMX were obtained from Sigma (St Louis, MO); jasplakinolide was obtained from Molecular Probes (Eugene, OR); BAPTA/am and ML-7 were obtained from Calbiochem (San Diego, CA); recombinant human tumor necrosis factor-α (TNF-α) was obtained from R&D Systems (Minneapolis, MN); mouse antihuman CD45 antibody (clone HI30) was obtained from Pharmingen (San Diego, CA); mouse antihuman β1-integrin antibody (clone P5D2) was obtained from Chemicon (Temecula, CA), and chromium-51 (51Cr) was obtained from NEN (Boston, MA).
Methods
Human neutrophil isolation.
Blood was obtained from healthy human subjects by venipuncture after informed consent was obtained. Human neutrophils were isolated using histopaque density gradients (Sigma) according to manufacturer's protocols. The purity of isolated neutrophils was greater than 95%.
Cultivation of human pulmonary microvascular endothelial cells.
Human pulmonary microvascular ECs were obtained from Clonetics (Walkersville, MD) and plated onto fibronectin-coated culture dishes according to manufacturer's protocols. They were used between passages 6 and 10. These cells can be induced to up-regulate ICAM-1 expression on TNF-α stimulation.17
Biomechanical properties of neutrophils and endothelial cells evaluated using magnetic twisting cytometry.
The biomechanical properties of neutrophils and ECs were measured using magnetic twisting cytometry. This technique measures the angular rotation of ferromagnetic beads bound to cells on application of a magnetic torque (stress). Ferromagnetic beads (4.5 μm in diameter) coated with goat antimouse immunoglobulin G Fc were obtained from Spherotech (Libertyville, IL). These beads were incubated in phosphate-buffered saline (PBS) with mouse antibodies against either human CD45 for studies of neutrophils or human β1integrin for studies of ECs, at a concentration of 1 μg/106 beads for 30 minutes at 4°C, followed by 3 washes in PBS. CD45 and β1 integrin were selected because they are transmembrane proteins linked to the actin cytoskeleton.
To measure the stiffness of neutrophils, ECs treated with 20 ng/mL TNF-α or buffer for 24 hours were washed twice with Dulbecco minimum essential medium (DMEM) containing 5% fetal bovine serum (FBS), and isolated neutrophils (2 × 105/well) and anti-CD45 antibody-coated beads (3.5 × 105/well) were combined in EC-plated wells. The well was gently centrifuged at 150g for 4 minutes. Unbound beads and neutrophils were washed off, and each well was placed in the magnetic twisting cytometer. Biomechanical properties of the cells were measured as previously described.15,16Briefly, the bound beads were exposed to a brief (10 μsec) but strong (1000 G) magnetic field, which magnetizes the beads in the horizontal direction. Twenty seconds later, the beads were twisted by a much weaker (30 G) but continuous (1 minute) vertical magnetic field. This twisting field was not strong enough to remagnetize the beads, but it caused the beads to rotate out of the plane of the EC membrane. The magnitude of magnetic vector in the horizontal direction (remnant magnetic field) was measured by an in-line magnetometer. From this value, the average bead rotation (angular strain) was calculated.15 16 The rotational stress was calculated by rotating the beads in a viscous standard. For these beads, a twisting field of 10 G at the start of twist produced a torque that corresponded to an initial stress of 7 dyne/cm2. The specific torque (stress) on the beads at the end of the 1-minute twist (stress1min) was calculated using the initial stress times the ratio of remnant field at the end of 1-minute twist and the remnant field at zero time. The apparent stiffness was measured at 1 minute of twist and was defined as the ratio of stress1min to the angular strain at this time point. The stiffness of neutrophils was measured after adhesion to ECs for 6, 15, and 30 minutes. Studies also evaluated the effect of fMLP (1 μM), which stimulates neutrophils but not ECs, on the stiffness of neutrophils adherent to ECs for 15 minutes.
To measure the stiffness of ECs, they were treated with 20 ng/mL TNF-α or buffer in culture medium for 24 hours at 37°C. After TNF-α was washed off, ECs were incubated with anti–β1-integrin antibody-coated beads at 37°C for 30 minutes. Unbound beads were gently washed off, and the well was placed in the magnetic twisting cytometer. Biomechanical properties of ECs were evaluated as described above. The apparent stiffness of ECs was measured before and after 2 to 15 minutes of neutrophil adherence. To determine the mechanisms by which ECs stiffened in response to adherent neutrophils, they were pretreated with the agents, as described in “Results.”
Role of phosphoinositides in mediating endothelial cell stiffening response.
A cell-permeant phosphoinositide-binding peptide corresponding to part of the phosphoinositide binding domain of gelsolin was provided by Rolands Vegners (Latvian Organic Synthesis Institute). This peptide of sequence QRLFQVKGRR is conjugated at the N-terminal residue with rhodamine B, which renders it permeable to the plasma membrane of many cell types.18 The nonconjugated peptide of the same sequence was used as the control.
F-actin visualization and quantification.
F-actin distribution in neutrophils or ECs was visualized using rhodamine-phalloidin stain. ECs were grown to confluence on glass coverslips and were treated with 20 ng/mL TNF-α or control vehicle for 24 hours. After TNF-α was washed off, neutrophils or buffer were then added to ECs and incubated for 2 to 15 minutes. The cells were fixed with 3.7% paraformaldehyde for 10 minutes, and this was followed by incubation in PBS containing 200 μg/mL L-lysophosphatidyl choline and 3.3 × 10−7 M rhodamine-phalloidin for 1 hour. Coverslips were mounted on slides and examined under a Leica TCS-NT laser scanning confocal microscope (Leica Microsystems, Mannheim, Germany). The F-actin staining in a slice of ECs that was under the luminal membrane but that did not contain any neutrophils was quantified by measuring the mean pixel intensity of the F-actin fluorescence in 10 randomly selected fields and the percentage of the observed area (1000×) occupied by F-actin staining.
Neutrophil adhesion assay.
Isolated neutrophils were labeled with 51Cr as previously described.19 Confluent ECs plated onto 96-well plates were treated with 20 ng/mL TNF-α for 24 hours. After 2 washes,51Cr-labeled neutrophils (neutrophil to EC ratio, 1:1) were added to the wells and allowed to adhere for 2 to 15 minutes. Nonadherent neutrophils were then washed off, and the fraction of adherent neutrophils was calculated.
Localization of neutrophils on endothelial cell monolayer.
To examine the position of neutrophils adherent to 24-hour TNF-α–activated ECs with respect to the EC borders, the cells were stained using silver nitrate as previously described.20 21Briefly, ECs were treated with 20 ng/mL TNF-α for 24 hours, followed by 2 washes. Neutrophils were then added to the well and allowed to adhere for 1 to 15 minutes. After 2 washes, the cells were fixed with 0.5% glutaraldehyde for 10 minutes at room temperature, washed twice with PBS, and stained with 0.25% AgNO3 for 30 seconds. After 2 washes in PBS, the stain was developed under UV light for 5 minutes. The cells were examined under light microscope, and the percentage of total adherent neutrophils present at EC borders was then determined at each time point.
Quantification of paracellular gap formation on neutrophil adherence.
ECs were grown to confluence on glass coverslips and treated with 20 ng/mL TNF-α for 24 hours. Neutrophils were added to ECs and incubated for 2 to 15 minutes. Cells were fixed with 10% buffered formalin, stained with Coomassie blue, and examined under a microscope with a drawing tube. Gaps formed between ECs were outlined on a digitizing pad interfaced with a computer and quantified using SigmaScan software (Jandel, San Rafael, CA). Five random fields were examined for each slide (magnification, 200 ×).
Phosphorylation of myosin light chain examined by 2-dimensional gel electrophoresis.
To characterize the phosphorylation pattern of the EC regulatory myosin light chain on neutrophil adherence, 2-D gel electrophoresis of the whole-cell lysates was performed using the 2-D system from Genomic Solutions (Ann Arbor, MI) according to manufacturer's protocols. In brief, ECs treated with TNF-α or buffer for 24 hours were washed twice with DMEM containing 5% FBS, and neutrophils (neutrophil to EC ratio, 1:1) were added. After 2, 6, and 15 minutes, the wells were washed twice with ice-chilled PBS, and the cells were lysed with boiled sample buffer I containing 0.3% sodium dodecyl sulfate, 200 mM dithiothreitol, 28 mM Tris HCl, and 22 mM Tris base. For positive controls, samples were also prepared from untreated ECs and ECs treated with thrombin (1 U/mL) for 15 and 30 minutes. The plate was immediately scraped, and the collected samples were acetone precipitated and dissolved in sample buffer mix containing 20% sample buffer I and 80% sample buffer II (9.9 M urea, 4% Triton X-100, 2.2% ampholytes, 100 mM dithiothreitol). For first-dimensional separation, isoelectric focusing gels with propidium iodide (PI) values ranging from 3 to 10 were prepared using the manufacturer's protocols (Genomic Solutions). Second-dimensional separation was performed using 15% sodium dodecyl sulfate gels as previously described.22 The gels were stained with silver stain according to the manufacturer's protocols (Genomic Solutions) and analyzed by scanning laser densitometry using the Hewlett-Packard Deskscan Software II (Palo Alto, CA). The myosin light-chain isoforms (the regulatory light chains that can become monophosphorylated or diphosphorylated and the essential light chains that do not become phosphorylated) were identified according to the molecular weights and the PI values of the human myosin light chains and by comparing the protein migratory pattern with the published 2-D gels separating myosin light chain isoforms in cultured human ECs.23,24 For each sample, the density of unphosphorylated (U), monophosphorylated (M), and diphosphorylated (D) forms of the regulatory myosin light chain were measured, and myosin light-chain phosphorylation was evaluated by determining the mol PO4/mol regulatory myosin light chain as previously described.23 24 The mol PO4/mol myosin light chain was calculated using (M + D × 2)/(U + M + D).
Statistical analysis.
Data were analyzed using the Student t test.P < .05 using a 2-tailed test was considered significant. Data were expressed as the mean value ± SEM.
Results
Changes in the biomechanical properties of neutrophils and their cytoskeleton on adherence to TNF-α–activated endothelial cells
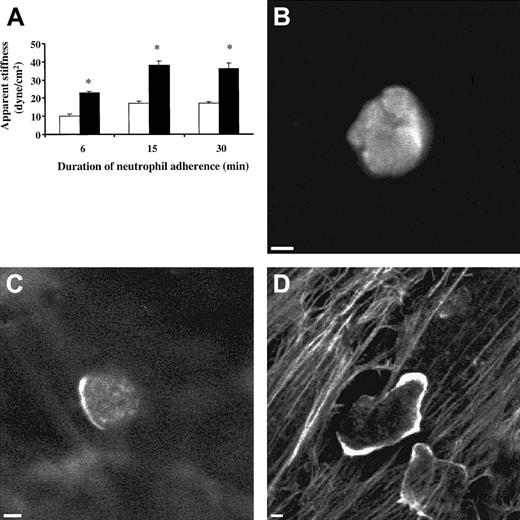
Adherence to ECs treated with 20 ng/mL TNF-α for 24 hours resulted in an increase in the apparent stiffness of neutrophils compared with neutrophils adherent to untreated ECs (Figure1A). When neutrophils were added to untreated ECs for 6, 15, and 30 minutes, the stiffness of neutrophils measured 10.0 ± 1.4 dyne/cm2, 16.9 ± 1.2 dyne/cm2, and 17.1 ± 0.6 dyne/cm2, respectively. Pretreatment of ECs with TNF-α for 24 hours increased the stiffness of neutrophils to 22.8 ± 0.9 dyne/cm2, 37.9 ± 2.5 dyne/cm2, and 36.2 ± 3.0 dyne/cm2. The effect of TNF-α was time-dependent; it became apparent by 2 hours and reached a plateau by 8 hours (data not shown).
Apparent stiffness of neutrophils and distribution of neutrophil F-actin when neutrophils were adherent to 24-hour TNF-α–treated ECs or untreated ECs.
After the TNF-α was washed off, the apparent stiffness of the neutrophils was measured using magnetic twisting cytometry, and F-actin was visualized using rhodamine-phalloidin stain as described in “Materials and methods.” (A) Apparent stiffness of neutrophils when they were adherent to TNF-α–treated ECs (▪) or untreated ECs (■) for the indicated times. (B) F-actin distribution in nonadherent neutrophils. (C) F-actin distribution in neutrophils bound to untreated ECs for 15 minutes. (D) F-actin distribution in neutrophils adherent to 24-hour TNF-α–treated ECs for 15 minutes. The stiffness measurement is presented as mean ± SEM of at least 5 separate experiments. *P < .05 when compared with neutrophils bound to untreated ECs. Scale bars, 2 μm.
Apparent stiffness of neutrophils and distribution of neutrophil F-actin when neutrophils were adherent to 24-hour TNF-α–treated ECs or untreated ECs.
After the TNF-α was washed off, the apparent stiffness of the neutrophils was measured using magnetic twisting cytometry, and F-actin was visualized using rhodamine-phalloidin stain as described in “Materials and methods.” (A) Apparent stiffness of neutrophils when they were adherent to TNF-α–treated ECs (▪) or untreated ECs (■) for the indicated times. (B) F-actin distribution in nonadherent neutrophils. (C) F-actin distribution in neutrophils bound to untreated ECs for 15 minutes. (D) F-actin distribution in neutrophils adherent to 24-hour TNF-α–treated ECs for 15 minutes. The stiffness measurement is presented as mean ± SEM of at least 5 separate experiments. *P < .05 when compared with neutrophils bound to untreated ECs. Scale bars, 2 μm.
This increase in neutrophil stiffness was accompanied with F-actin redistribution (Figure 1B-D). In nonadherent neutrophils (Figure 1B) and neutrophils adherent to untreated ECs (Figure 1C), F-actin was present mainly in the central region of the neutrophils. Occasional patches of F-actin were also observed along the cell periphery. In neutrophils adherent to TNF-α–treated ECs, most neutrophils were spread and elongated, and F-actin was often concentrated along the cell periphery (Figure 1D).
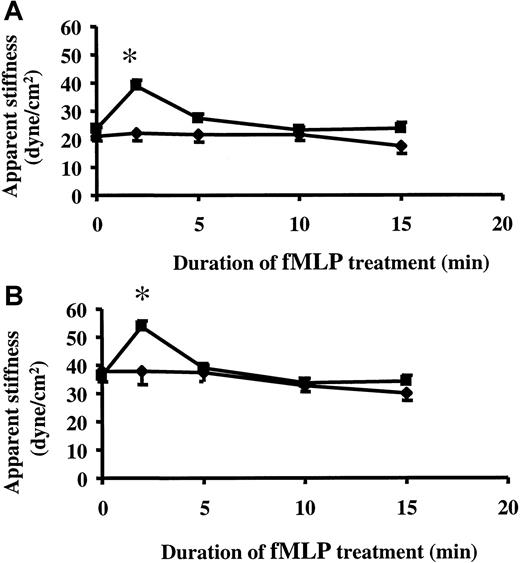
Although neutrophils adherent to 24-hour TNF-α–treated ECs were stiffer compared with neutrophils bound to untreated ECs, they still responded to 1 μM fMLP by further increasing their stiffness (Figure2). This increase in stiffness was similar in magnitude whether neutrophils were adhered to untreated or TNF-α–treated ECs. The effect of fMLP was short-lived, and the stiffness of neutrophils returned to pre-fMLP levels after 5 minutes.
fMLP-induced changes in the stiffness of neutrophils adherent to untreated or TNF-α–treated ECs.
Neutrophils were allowed to adhere to untreated ECs (A) or to 24-hour TNF-α–treated ECs (B) for 15 minutes. After measuring the baseline stiffness, 1 μM fMLP or vehicle was added, and neutrophil stiffness was measured 2, 5, 10, and 15 minutes later. Data are expressed as mean ± SEM (n = 4). *P < .05 when compared to the baseline stiffness. ▪, fMLP; ♦, vehicle.
fMLP-induced changes in the stiffness of neutrophils adherent to untreated or TNF-α–treated ECs.
Neutrophils were allowed to adhere to untreated ECs (A) or to 24-hour TNF-α–treated ECs (B) for 15 minutes. After measuring the baseline stiffness, 1 μM fMLP or vehicle was added, and neutrophil stiffness was measured 2, 5, 10, and 15 minutes later. Data are expressed as mean ± SEM (n = 4). *P < .05 when compared to the baseline stiffness. ▪, fMLP; ♦, vehicle.
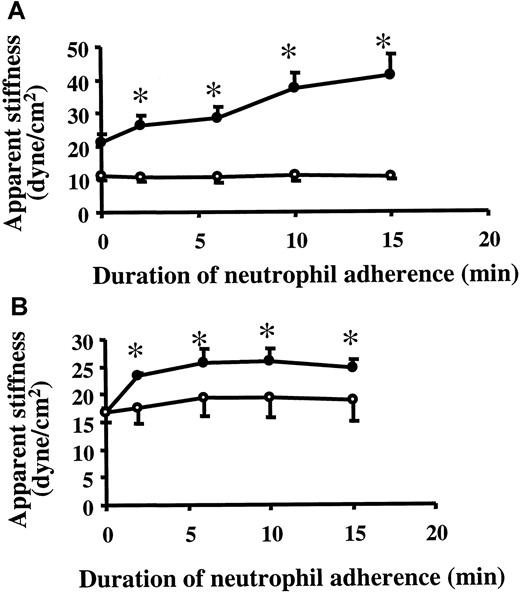
Changes in the biomechanical properties of 24-hour TNF-α–treated endothelial cells on neutrophil adherence
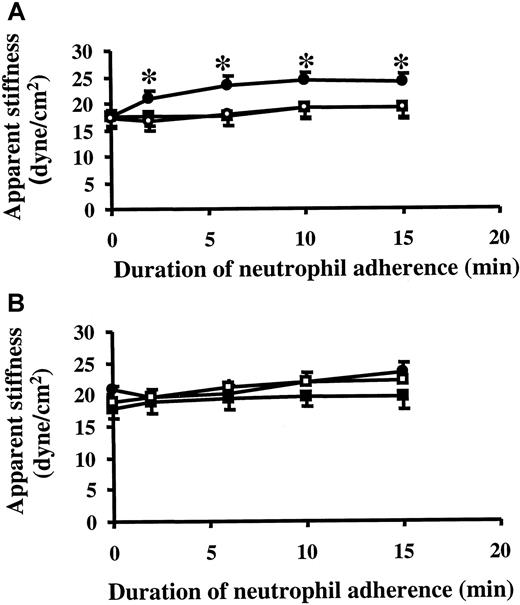
Neutrophil adherence also led to changes in the biomechanical properties of cytokine-activated ECs. ECs were treated with 20 ng/mL TNF-α for 24 hours and were then washed. Neutrophil adherence for 2 minutes induced an increase in the apparent stiffness of TNF-α–treated ECs when measured using ferromagnetic beads bound to β1 integrin on ECs (Figure3A). This change in EC stiffness required TNF-α treatment because neutrophil adherence to untreated ECs did not increase EC stiffness (Figure 3B). This EC stiffening response also depended on the presence of neutrophils because neutrophil supernatants did not increase EC stiffness (Figure 3A). In addition, supernatants from neutrophil-EC incubation did not induce the EC stiffening response (data not shown). Taken together, these data demonstrated that the neutrophil adherence-induced EC stiffening response was not caused by neutrophil-derived mediators during adhesion.
Changes in EC stiffness on neutrophil adherence.
After TNF-α was washed off, the stiffness of 24-hour TNF-α–treated ECs (A) or untreated ECs (B) was measured by magnetic twisting cytometry using ferromagnetic beads bound to β1 integrin as described in “Materials and methods.” After the measurement of baseline stiffness, either purified neutrophil (neutrophil to EC ratio, 1:1), buffer, or neutrophil supernatant was added to the well, and EC stiffness was measured 2, 6, 10, and 15 minutes later. Data are expressed as mean ± SEM (n = 4). *P < .05 when compared to the baseline stiffness. ●, addition of neutrophils; ▪, addition of buffer; ■, addition of neutrophil supernatant.
Changes in EC stiffness on neutrophil adherence.
After TNF-α was washed off, the stiffness of 24-hour TNF-α–treated ECs (A) or untreated ECs (B) was measured by magnetic twisting cytometry using ferromagnetic beads bound to β1 integrin as described in “Materials and methods.” After the measurement of baseline stiffness, either purified neutrophil (neutrophil to EC ratio, 1:1), buffer, or neutrophil supernatant was added to the well, and EC stiffness was measured 2, 6, 10, and 15 minutes later. Data are expressed as mean ± SEM (n = 4). *P < .05 when compared to the baseline stiffness. ●, addition of neutrophils; ▪, addition of buffer; ■, addition of neutrophil supernatant.
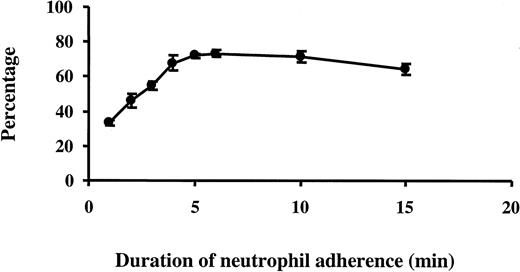
Assays of neutrophil adhesion for up to 15 minutes indicated that neutrophil adherence to 24-hour TNF-α–treated ECs increased over time, and there were more neutrophils adherent to 24-hour TNF-α–activated ECs than to untreated ECs (15.9% vs 7.0% by 15 minutes). The location of the adherent neutrophils with respect to the EC borders was examined using silver staining. Results indicated that there was a time-dependent increase in the percentage of neutrophils found at the EC borders between 1 and 5 minutes of neutrophil adherence, after which the percentage of neutrophils found at the EC borders remained constant at 65% (Figure4). At 1 minute, 33% of adherent neutrophils were at the EC borders. Assuming that neutrophils adhere to ECs randomly and that the radii of these 2 cell types are 3.5 μm and 40 μm, respectively, the chance that neutrophils can be found on the edges of ECs is estimated to be approximately 32% [(π × 402 − π × (40 − 3.5 × 2)2)/ (π × 402) × 100 = 32%]. These data suggest that neutrophils adhere randomly to EC surfaces and then migrate along EC surfaces to the borders within 5 minutes, rather than adhering preferentially to the borders. However, few neutrophils migrate beneath the EC monolayer within 15 minutes, as examined by confocal microscopy. Most neutrophils were found adherent to ECs rather than to the substrate. Measurement of the spaces formed between ECs indicated that only 1.1% ± 0.2% of the well was occupied by the gaps formed between ECs after TNF-α treatment and that neutrophil adherence did not cause widening of the gaps until after 15 minutes. Percentages of the well that represented gaps at 2, 6, 10, and 15 minutes after neutrophil adherence measured 0.9% ± 0.1%, 1.1% ± 0.1%, 1.2% ± 0.1%, and 2.7% ± 0.3%, respectively (n = 3). These data suggested that the increase in percentage neutrophils found at EC edges did not reflect neutrophils adhering to the exposed substrate in the gaps.
Percentages of neutrophils observed along the EC borders.
After 24-hour TNF-α–treated ECs were washed, purified neutrophils (neutrophil to EC ratio, 1:1) were added to the wells and allowed to adhere for 1 to 15 minutes. After two washes, the cells were fixed and stained with silver stain as described in “Materials and methods.” The percentage of neutrophils observed along EC borders was measured. Data are expressed as mean ± SEM from 3 cell wells.
Percentages of neutrophils observed along the EC borders.
After 24-hour TNF-α–treated ECs were washed, purified neutrophils (neutrophil to EC ratio, 1:1) were added to the wells and allowed to adhere for 1 to 15 minutes. After two washes, the cells were fixed and stained with silver stain as described in “Materials and methods.” The percentage of neutrophils observed along EC borders was measured. Data are expressed as mean ± SEM from 3 cell wells.
Neutrophil adherence–induced endothelial cell stiffening response requires changes in the actin cytoskeleton
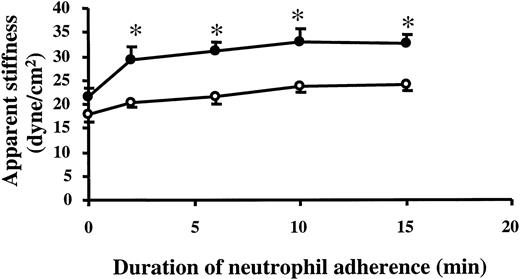
To examine whether this observed increase in stiffness was associated with changes in EC cytoskeleton, ECs were pretreated with cytochalasin D or jasplakinolide. Cytochalasin D decreases the addition rate of G-actin to the barbed ends of F-actin and may have other effects on F-actin dynamics after prolonged exposure.25Therefore, under conditions in which actin is cycling between soluble and polymeric states, cytochalasin D induces net F-actin depolymerization. Jasplakinolide binds to the same sites on F-actin as phalloidin, and it stabilizes F-actin by preventing F-actin depolymerization.26 Pretreatment with 1 μg/mL cytochalasin D for 30 minutes resulted in a 48% decrease in the baseline stiffness of 24-hour TNF-α–treated ECs and completely prevented the stiffening response induced by adherent neutrophils compared with the vehicle-pretreated control cells (Figure5A). In contrast, pretreatment with 5 μM jasplakinolide for 30 minutes did not alter the baseline stiffness of 24-hour TNF-α–treated ECs. However, it did significantly attenuate the EC stiffening response induced by neutrophil adherence (Figure 5B). These results suggest that the EC stiffening response induced by neutrophil adherence depended on changes in the F-actin cytoskeletal network in ECs.
Effect of cytochalasin D and jasplakinolide on EC stiffening response induced by neutrophils.
Twenty-four-hour TNF-α–treated ECs were incubated with 1 μg/mL cytochalasin D or control vehicle (A) or with 5 μM jasplakinolide or control vehicle (B) for 30 minutes along with the anti–β1-integrin–coated beads. Cells were then washed twice, and the stiffness before or 2, 6, 10, and 15 minutes after neutrophil adherence was evaluated using magnetic twisting cytometry as described in “Materials and methods.” Data are expressed as mean ± SEM (n = 4). ●, control vehicle; ○, agonist. *P < .05 when compared to the vehicle-pretreated controls.
Effect of cytochalasin D and jasplakinolide on EC stiffening response induced by neutrophils.
Twenty-four-hour TNF-α–treated ECs were incubated with 1 μg/mL cytochalasin D or control vehicle (A) or with 5 μM jasplakinolide or control vehicle (B) for 30 minutes along with the anti–β1-integrin–coated beads. Cells were then washed twice, and the stiffness before or 2, 6, 10, and 15 minutes after neutrophil adherence was evaluated using magnetic twisting cytometry as described in “Materials and methods.” Data are expressed as mean ± SEM (n = 4). ●, control vehicle; ○, agonist. *P < .05 when compared to the vehicle-pretreated controls.
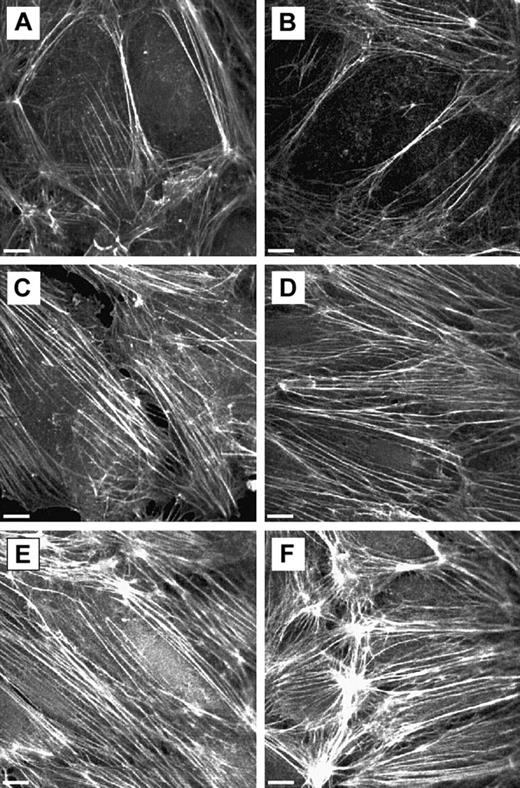
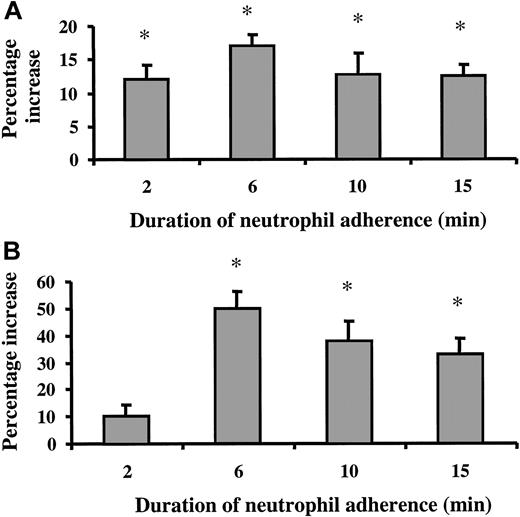
The changes in the EC F-actin cytoskeleton induced by neutrophil adherence were also demonstrated by F-actin staining (Figure6). Neutrophil adherence to untreated ECs had little effect on the F-actin distribution in ECs (Figure 6A-B). Treatment of ECs with 20 ng/mL TNF-α for 24 hours resulted in the disappearance of the dense peripheral bands of F-actin and the formation of stress fibers in the cytoplasm (Figure 6A,C). Neutrophil adherence to 24-hour TNF-α–treated ECs induced more F-actin staining in ECs and the formation of F-actin clusters (Figure 6D-F). The location of these F-actin clusters did not correlate with the sites of neutrophil adherence at the time of observation. F-actin staining in ECs was quantified by measuring the mean pixel intensity of the F-actin fluorescence and the percentage area of the observation field occupied by F-actin (Figure 7A-B). Results indicated that the mean pixel intensity and the percentage area occupied by F-actin increased in response to neutrophil adherence when compared with the ECs treated with buffer for the same duration (Figure7A-B). The increase in the mean pixel intensity of the F-actin staining occurred before the increase in the area occupied by F-actin, and both were maximal at 6 minutes after neutrophil adherence.
F-actin distribution in untreated or 24-hour TNF-α–activated ECs on neutrophil adherence.
F-actin was visualized using rhodamine-phalloidin stain as described. (A) Untreated ECs. (B) Untreated ECs with neutrophil adherence for 15 minutes. (C) Twenty-four-hour TNF-α–activated ECs. (D-F) Twenty-four-hour TNF-α–activated ECs with neutrophil adherence for 2, 6, and 10 minutes. Scale bars, 10 μm.
F-actin distribution in untreated or 24-hour TNF-α–activated ECs on neutrophil adherence.
F-actin was visualized using rhodamine-phalloidin stain as described. (A) Untreated ECs. (B) Untreated ECs with neutrophil adherence for 15 minutes. (C) Twenty-four-hour TNF-α–activated ECs. (D-F) Twenty-four-hour TNF-α–activated ECs with neutrophil adherence for 2, 6, and 10 minutes. Scale bars, 10 μm.
Quantification of F-actin staining in 24-hour TNF-α–activated ECs on neutrophil adherence.
F-actin was detected by rhodamine-phalloidin stain as described, and the image was scanned and monitored using a Leica TCS-NT laser scanning confocal microscope. The mean pixel intensity of the F-actin fluorescence per field (A) and the percentage of the field (1000×) occupied by F-actin staining (B) were measured. Data are expressed as percentage changes over the corresponding control (exposing to buffer for the same duration) and presented as mean ± SEM from at least 10 images for each slide. The experiment was repeated 2 times with similar results. *Mean pixel intensity and percentage area in ECs in response to adherent neutrophils were significantly higher compared to addition of buffer for the same duration (P < .05).
Quantification of F-actin staining in 24-hour TNF-α–activated ECs on neutrophil adherence.
F-actin was detected by rhodamine-phalloidin stain as described, and the image was scanned and monitored using a Leica TCS-NT laser scanning confocal microscope. The mean pixel intensity of the F-actin fluorescence per field (A) and the percentage of the field (1000×) occupied by F-actin staining (B) were measured. Data are expressed as percentage changes over the corresponding control (exposing to buffer for the same duration) and presented as mean ± SEM from at least 10 images for each slide. The experiment was repeated 2 times with similar results. *Mean pixel intensity and percentage area in ECs in response to adherent neutrophils were significantly higher compared to addition of buffer for the same duration (P < .05).
Endothelial cell stiffening response involves a phosphoinositide-dependent mechanism
The role of phosphoinositides in EC stiffening response was evaluated using a rhodamine-conjugated, phosphoinositide-binding peptide that is cell permeant. As shown in Figure8, pretreatment of ECs with 10 μM phosphoinositide-binding peptide for 30 minutes significantly attenuated EC stiffening response, whereas pretreatment with the nonconjugated phosphoinositide-binding peptide that was not cell-permeant had no effect. These results suggest that EC stiffening response induced by adherent neutrophils involves a phosphoinositide-dependent mechanism.
Modulation of EC stiffening response by a cell-permeant phosphoinositide-binding peptide.
Twenty-four-hour TNF-α–treated ECs were pretreated with 10 μM phosphoinositide-binding peptide or the control peptide for 30 minutes along with anti–β1-integrin–coated beads. The stiffness before or 2, 6, 10, and 15 minutes after neutrophil adherence was evaluated as described before. Data are expressed as mean ± SEM (n = 3). ●, control peptide; ○, phosphoinositide-binding peptide. *P < .05 when compared to the control peptide–pretreated cells.
Modulation of EC stiffening response by a cell-permeant phosphoinositide-binding peptide.
Twenty-four-hour TNF-α–treated ECs were pretreated with 10 μM phosphoinositide-binding peptide or the control peptide for 30 minutes along with anti–β1-integrin–coated beads. The stiffness before or 2, 6, 10, and 15 minutes after neutrophil adherence was evaluated as described before. Data are expressed as mean ± SEM (n = 3). ●, control peptide; ○, phosphoinositide-binding peptide. *P < .05 when compared to the control peptide–pretreated cells.
Endothelial cell stiffening response induced by adherent neutrophils appears not to be dependent on myosin light-chain–mediated endothelial cell contraction
To examine whether myosin light-chain phosphorylation mediates the EC stiffening response induced by adherent neutrophils, ECs were pretreated with 50 μM BAPTA, an intracellular Ca2+chelator. As shown in Figure 9A, pretreatment with BAPTA did not inhibit EC stiffening response. Moreover, pretreatment for 30 minutes with 1 μM forskolin along with 0.5 mM IBMX, which increases intracellular cyclic adenosine monophosphate (cAMP) levels and attenuates myosin light-chain phosphorylation in ECs,27 28 did not inhibit the EC stiffening response (Figure 9B). Similar results were obtained when ECs were pretreated with 0.1 mM dibutyl-cAMP, a cell-permeant cAMP analogue, for 30 minutes (data not shown). In addition, pretreatment with ML-7, a myosin light-chain kinase (MLCK) inhibitor, did not inhibit the EC stiffening response induced by adherent neutrophils (Figure 9C), whereas ML-7 completely inhibited the EC stiffening response induced by 1 μM endothelin-1 (stiffness of ECs treated with endothelin-1 for 0 and 10 minutes: 21.5 ± 1.5 dyne/cm2and 29.2 ± 2.2 dyne/cm2, respectively;P < .05; n = 4; pretreatment with ML-7 followed by endothelin for 0 or 10 minutes: 20.8 ± 0.9 dyne/cm2 and 20.8 ± 0.6 dyne/cm2, respectively;P > .05; n = 4). Taken together, these data suggest that activation of the calcium-calmodulin–dependent MLCK is not required for neutrophil adherence-induced EC stiffening to occur.
Agents that chelate intracellular Ca2+, increase intracellular cAMP levels, or inhibit MLCK did not inhibit EC stiffening response induced by adherent neutrophils.
Twenty-four-hour TNF-α–treated ECs were pretreated with the agent or its control vehicle for 30 minutes, and the EC stiffness before or 2, 6, 10, and 15 minutes after neutrophil adherence was evaluated as before. (A) Pretreatment with 50 μM BAPTA/am, an intracellular Ca2+ chelator. (B) Pretreatment with 1 μM forskolin along with 0.5 mM IBMX to increase intracellular cAMP levels. (C) Pretreatment with 10 μM ML-7, an MLCK inhibitor. Data are expressed as mean ± SEM (n = 4). ●, control vehicle; ○, agent.
Agents that chelate intracellular Ca2+, increase intracellular cAMP levels, or inhibit MLCK did not inhibit EC stiffening response induced by adherent neutrophils.
Twenty-four-hour TNF-α–treated ECs were pretreated with the agent or its control vehicle for 30 minutes, and the EC stiffness before or 2, 6, 10, and 15 minutes after neutrophil adherence was evaluated as before. (A) Pretreatment with 50 μM BAPTA/am, an intracellular Ca2+ chelator. (B) Pretreatment with 1 μM forskolin along with 0.5 mM IBMX to increase intracellular cAMP levels. (C) Pretreatment with 10 μM ML-7, an MLCK inhibitor. Data are expressed as mean ± SEM (n = 4). ●, control vehicle; ○, agent.
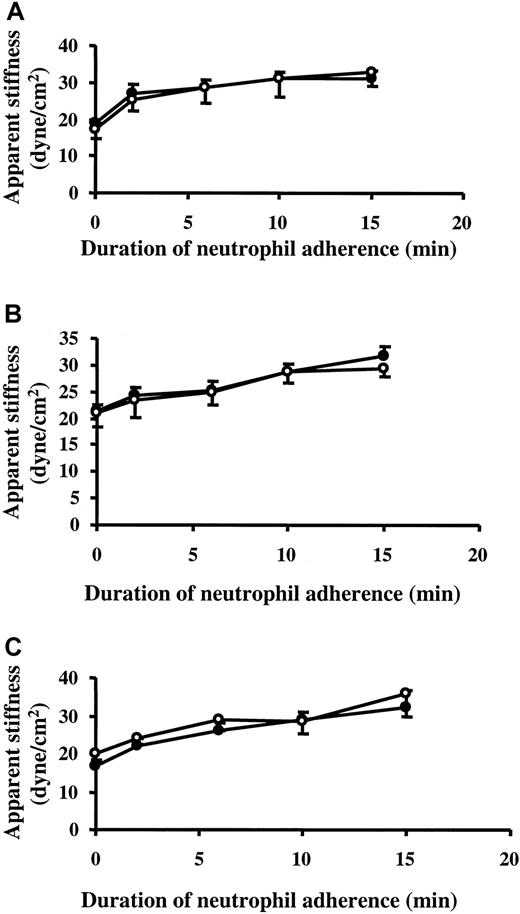
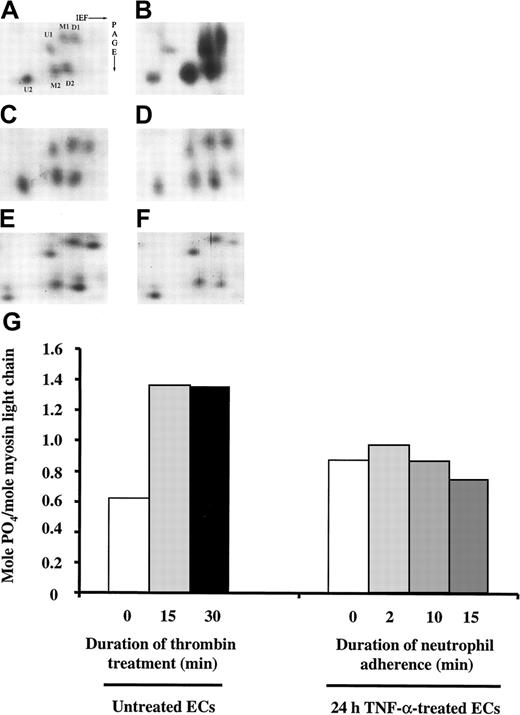
To determine whether myosin light-chain phosphorylation occurred in pulmonary microvascular ECs on neutrophil adherence, 2-D gel electrophoresis was performed to resolve the phosphorylation pattern of the regulatory myosin light-chain isoforms as previously described.23 24 As shown in Figure10, the level of myosin light-chain phosphorylation in untreated ECs was 0.62 mol PO4/mol myosin light chain. As a positive control, the phosphorylation pattern of myosin light chain on thrombin stimulation was examined. Thrombin (1 U/mL) for 15 and 30 minutes increased myosin light-chain phosphorylation to 1.35 and 1.36 mol PO4/mol myosin light chain, respectively (Figure 10A-B,G). Treatment with TNF-α alone for 24 hours induced a small increase in myosin light-chain phosphorylation in ECs, but neutrophil adherence to 24-hour TNF-α–treated ECs for 2, 10, and 15 minutes did not cause any further change (Figure 10C-G). Pretreatment with 10 μM ML-7 had no effect on the phosphorylation pattern of the regulatory myosin light chain before and after neutrophil adherence (data not shown). Thus, the EC stiffening response on neutrophil adherence was not mediated by the mechanisms that involved myosin light-chain phosphorylation.
Separation of regulatory myosin light-chain isoforms by 2-D gel electrophoresis.
After ECs treated with buffer or TNF-α for 24 hours were washed twice, neutrophils (neutrophil to EC ratio, 1:1) were added to TNF-α–treated ECs and allowed to adhere for 2, 6, or 15 minutes. After two washes in cold PBS, the cells were lysed and the phosphorylation myosin light-chain isoforms was analyzed using 2-D gel electrophoresis as described in “Materials and methods.” Only the region of the gels containing regulatory myosin light-chain isoforms is shown. (A-F) Myosin light-chain isoforms in untreated ECs (A). ECs treated with 1 U/mL thrombin for 30 minutes (B). Twenty-four-hour TNF-α–treated ECs (C) or 24-hour TNF-α–treated ECs with neutrophils adherent for 2, 10, or 15 minutes (D-F). (G) Quantification of mol PO4/mol myosin light-chain molecules; U, unphosphorylated regulatory myosin light chain; M, monophosphorylated regulatory myosin light chain; D, diphosphorylated myosin light chain.
Separation of regulatory myosin light-chain isoforms by 2-D gel electrophoresis.
After ECs treated with buffer or TNF-α for 24 hours were washed twice, neutrophils (neutrophil to EC ratio, 1:1) were added to TNF-α–treated ECs and allowed to adhere for 2, 6, or 15 minutes. After two washes in cold PBS, the cells were lysed and the phosphorylation myosin light-chain isoforms was analyzed using 2-D gel electrophoresis as described in “Materials and methods.” Only the region of the gels containing regulatory myosin light-chain isoforms is shown. (A-F) Myosin light-chain isoforms in untreated ECs (A). ECs treated with 1 U/mL thrombin for 30 minutes (B). Twenty-four-hour TNF-α–treated ECs (C) or 24-hour TNF-α–treated ECs with neutrophils adherent for 2, 10, or 15 minutes (D-F). (G) Quantification of mol PO4/mol myosin light-chain molecules; U, unphosphorylated regulatory myosin light chain; M, monophosphorylated regulatory myosin light chain; D, diphosphorylated myosin light chain.
Discussion
This study demonstrated that neutrophils adherent to TNF-α–activated ECs were stiffer than neutrophils bound to untreated ECs. This increase in stiffness was associated with an increase in neutrophil adherence and F-actin remodeling. Adhesion of neutrophils to TNF-α–activated ECs also induced an increase in EC stiffness that could not be mimicked by neutrophil supernatants. This EC stiffening response was associated with F-actin reorganization and was inhibited by agents that disrupt or stabilize F-actin. This response was inhibited by a cell-permeant phosphoinositide-binding peptide. However, this stiffening response may not involve myosin light chain–dependent mechanisms because agents that prevent Ca2+-calmodulin–dependent myosin light-chain phosphorylation did not prevent the stiffening response, and the stiffening response was not accompanied by myosin light-chain phosphorylation and paracellular gap formation. These data suggest that neutrophil adherence-induced EC stiffening response may result from phosphoinositide-dependent F-actin remodeling rather than actin-myosin–mediated events.
Our studies indicate that neutrophils adherent to cytokine-activated ECs are stiffer than neutrophils bound to untreated ECs. This measured increase in neutrophil stiffness is likely due to adhesion-induced cytoskeletal rearrangement. Changes in neutrophil shape may also contribute because neutrophils bound to untreated ECs were more spherical and less spread than neutrophils adherent to TNF-α–treated ECs.29 These changes in the biomechanical properties of neutrophils, when they are adherent to TNF-α–activated ECs, may result from the direct engagement of neutrophil adhesion molecules including the β2 integrins. Neutrophil integrins play important roles in mediating adhesion-dependent neutrophil activation.30-32 Cross-linking CD18 in neutrophils using soluble antibodies induces increases in intracellular Ca2+and actin polymerization.2 Adherence to surface-bound anti–β2-integrin antibodies induces neutrophil spreading and production of reactive oxygen species33,34 and actin polymerization,35 which is preceded by the cytoskeletal association of actin-binding proteins including talin, α-actinin, and paxillin and the tyrosine kinases p58c-fgr, p53/56lyn, and p72syk.35 Thus, on adherence to TNF-α–activated ECs, the engagement of neutrophil CD11/CD18 or other adhesion molecules may induce signaling events and subsequent cytoskeletal rearrangements that result in increases in the apparent stiffness.
It is interesting that treatment with fMLP induces a further increase in neutrophil stiffness of 16 dyne/cm2, whether they are bound to untreated ECs or to TNF-α–activated ECs. The fMLP-induced increase in neutrophil stiffness may result directly from signaling through the fMLP receptors, which belong to the 7 transmembrane-spanning G-protein–linked receptor family. These receptors can initiate signaling cascades, leading to cytoskeletal remodeling that can be short-lived and reversible.36Alternatively, the increase in neutrophil stiffness may result from enhanced neutrophil adherence through up-regulation and ligation of the CD11b/CD18 complex and subsequent signaling. The physiological significance of this finding in the process of neutrophil crawling and transmigration remains to be determined, but changes in the biomechanical properties of neutrophils appear to occur through different pathways in response to stimulation through various receptors.
Neutrophil-endothelial adherence also resulted in an increase in EC stiffness. This increase may result from F-actin remodeling, such as F-actin polymerization and enhanced F-actin cross-linking, or from actin-myosin–mediated EC contraction. Our data clearly show that the EC stiffening response induced by adherent neutrophils requires F-actin cytoskeletal remodeling. The stiffening response on neutrophil adherence is accompanied by F-actin reorganization and by increases in total F-actin staining in ECs. Although treatment of ECs with TNF-α for 24 hours caused the disappearance of the dense peripheral bands and the formation of stress fibers, neutrophil adherence resulted in further cytoskeletal changes, as demonstrated by more F-actin staining and F-actin redistribution into aggregates. The F-actin staining intensity increased more rapidly than the percentage area occupied by F-actin (compare Figure 7A,B), suggesting that the actin polymerization and F-actin remodeling in response to neutrophil adherence occurred initially at existing sites of actin filaments. Moreover, pretreatment with cytochalasin D, which disrupts F-actin, or jasplakinolide, which binds to F-actin with higher affinity than phalloidin and stabilizes F-actin, prevents the stiffening response, suggesting that cytoskeletal remodeling was required for the stiffening response.
Our previous studies demonstrated that neutrophil adherence-induced EC stiffening response requires CD18 and depends on ICAM-1–mediated signaling pathways in ECs.29 How these signaling pathways are initiated in ECs on ligation of ICAM-1 during neutrophil adherence and how these signaling events lead to cytoskeletal remodeling are still unclear. In this study, we demonstrated that this stiffening response does not require intracellular Ca2+ but does involve a phosphoinositide-dependent mechanism because it is inhibited by the cell-permeant phosphoinositide-binding peptide. The pathways leading to phosphoinositide production remain to be determined. Clues may be found in the studies of Cui et al,37 who demonstrate that chemokine-induced neutrophil adherence to ECs and subsequent emigration activates phospholipase D in ECs. Phospholipase D activation results in phosphatidic acid generation, which in turn stimulates phosphatidylinositol 4-P 5-kinase (PI 4-P 5-kinase) and results in phosphoinositide 4,5 diphosphate (PIP2) production.38 In addition, the activation of Rho is involved in mediating F-actin changes induced by ICAM-1 cross-linking and by monocyte adhesion,39,40 and the activation of Rho can lead to PIP2 production, possibly through the activation of PI 4-P 5-kinase.41 We hypothesize that phosphoinositides generated during adherence or downstream signaling pathways may act on actin-binding proteins, which in turn modulate F-actin remodeling. Phosphoinositides regulate several actin-binding proteins, resulting in the cumulative effect of inhibiting actin depolymerizing proteins such as gelsolin and profilin and stabilizing filament cross-linking and membrane-linking proteins such as α-actinin and ezrin (reviewed in Flanagan and Janmey42). These biochemical findings are consistent with those of studies showing an increase in cellular F-actin content when PIP2 levels are raised by the overexpression of PI 4-P 5-kinase.43Additional studies investigating the roles of these actin-binding proteins in mediating the EC stiffening response are necessary for understanding the underlying mechanisms.
The data also show that the EC stiffening response appears not to be mediated by Ca2+-calmodulin–dependent MLCK. The stiffening response was not inhibited by an intracellular Ca2+chelator or by MLCK inhibitor ML-7. Moreover, forskolin and dibutyl-cAMP, which are known to attenuate myosin light-chain phosphorylation, also did not inhibit the stiffening response. Because myosin light-chain phosphorylation can occur through Rho-dependent inactivation of myosin phosphatase44,45 and because the activation of Rho is involved in mediating F-actin changes induced by ICAM-1 cross-linking in brain ECs and human umbilical vein endothelial cells (HUVECs),39 40 the phosphorylation pattern of the regulatory light chain of myosin light chain was examined. Although TNF-α treatment for 24 hours induced a small increase in myosin light-chain phosphorylation, neutrophil adherence for 2 to 15 minutes did not result in a further increase. This lack of increase did not result from the saturation of myosin light-chain phosphorylation because marked myosin light-chain phosphorylation was detected on thrombin stimulation. Taken together, these data suggest that myosin light-chain phosphorylation may not mediate the increase in EC stiffness on neutrophil adherence. However, it remains a possibility that Rho-dependent local changes in myosin light-chain phosphorylation that are beyond the detection limit of 2-D gel electrophoresis could occur.
Other investigators have shown a role for Ca2+signaling and activation of MLCK in mediating F-actin polymerization, EC contraction, and isometric tension generation in response to chemoattractant-stimulated neutrophils on unstimulated ECs.6,7,23,46 These investigators hypothesized that EC contraction in response to activated neutrophils may facilitate neutrophil transmigration across the EC junctions because agents that inhibit MLCK also reduce neutrophil transmigration across HUVEC monolayers.6,7,23 This apparent discrepancy may be attributed to 3 differences between our studies. First, we examined the changes associated with neutrophil adherence and migration to EC borders only, not with transendothelial cell migration in response to chemoattractant-stimulated neutrophils as described in the previous studies. Most of the adherent neutrophils in our studies were found at the EC borders by 15 minutes, but few migrated beneath the EC monolayer. It is possible that the activation of EC MLCK is associated with the process of transendothelial cell migration but not with adherence or migration toward the EC borders. Second, the studies used 24-hour TNF-α pretreatment and subsequent endogenous presentation of chemokines and adhesion molecules rather than treatment with exogenous chemokines. TNF-α–induced changes in the cytoskeletal structure may also alter subsequent responses to adherent neutrophils. Third, the data may also represent unique characteristics of pulmonary microvascular ECs. In the previous studies investigating the roles of Ca2+ and MLCK in mediating EC cytoskeletal changes in response to neutrophil adherence and transmigration, HUVECs or pulmonary artery ECs were used.6,7,23,46 Compared to cultured ECs from large vessels, microvascular ECs have different cytoskeleton profiles revealed by 2-D gel electrophoresis47and altered contractility in response to vasoactive agonists.48 In addition, the signaling pathways leading to EC shape changes and permeability increases are differentially regulated in microvascular ECs and artery ECs.49 50 Singly or in combination, these differences may account for the observed discrepancy.
The physiological significance of the EC stiffening response induced by adherent neutrophils is not yet clear. We speculate that the EC stiffening response is important in 2 ways. First, changes in EC cytoskeleton may modulate signaling events in ECs in response to neutrophil adherence. Cell activation is often associated with changes in the actin cytoskeletal organization (for example, Berton et al,32,33 Zhou and Brown34), and the activation of certain signaling molecules is modulated by the actin cytoskeleton and actin-binding proteins (for example, Sun et al51,52). Neutrophil adherence-induced EC stiffening response occurs within 2 minutes, and these early changes in EC actin cytoskeleton may mediate downstream signal transduction pathways in ECs. Second, EC stiffening response may modulate neutrophil migration on the EC surfaces to the EC borders. The increase in the percentage of neutrophils found at the EC borders between 1 and 5 minutes after adding neutrophils to EC cultures suggests that neutrophils crawl along the EC surfaces to reach the EC borders, where most neutrophil transmigration occurs during inflammation. Because changes in substrate rigidity alone are sufficient to alter cell adherence and locomotion, as demonstrated in a study by Pelham and Wang53 using cultured fibroblasts, we postulate that this increase in EC stiffness may enhance neutrophil migration to the EC borders and thus modulate neutrophil transmigration across the endothelium. Third, changes in the EC cytoskeleton may be involved in the association of ICAM-1 or other adhesion molecules with the EC actin cytoskeleton and, thus, in the formation of more stable adhesion with neutrophils because ICAM-1 clustering and association with the actin cytoskeleton are required for monocyte adhesion to TNF-activated ECs and monocyte spreading.39
In summary, this study shows that cytoskeleton-dependent stiffening of neutrophils and ECs occurs during neutrophil-EC adhesion. The EC stiffening response involves a phosphoinositide-mediated remodeling of the cytoskeleton but appears not to involve myosin light-chain–mediated EC contraction. These changes in the biomechanical properties of neutrophils and ECs during adhesion may modulate neutrophil emigration across endothelium during inflammatory responses.
Acknowledgment
We thank Dr Jeffrey J. Fredberg for his expertise in magnetic twisting cytometry and for invaluable discussions.
Supported by National Institutes of Health grants HL 48160 and HL 33009, a Clinical Scientist Award in Translational Research from the Burroughs Wellcome Fund (C.M.D.), National Institutes of Health grant HL 56618 (D.S.), and National Heart, Lung, and Blood Institute NRSA fellowship IF32 HL 10177-01.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Claire M. Doerschuk, Rainbow Babies and Children's Hospital, Rm 787, 11100 Euclid Ave, Cleveland, OH 44106; e-mail: cmd22@po.cwru.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal