Abstract
Thrombocytopenia caused by platelet consumption in thrombi is a major manifestation of hemolytic uremic syndrome (HUS) associated with Shiga toxin (Stx) producing Escherichia coli. Platelets have glycosphingolipid receptors capable of binding Stx, but a direct interaction between the toxin and platelets, leading to platelet activation, has not been reported. In this study, it is shown that Stx1 and its B (binding) subunit (Stx1B), at 10 pg/mL to 10 ng/mL, bound to platelets. Toxin was internalized in platelets within 2 hours. This led to increased platelet aggregation, as demonstrated by confocal microscopy. Preincubation of Stx1B with anti-Stx1 antibody inhibited this reaction. Stx1 induced morphologic changes in platelets seen on scanning electron microscopy. In the presence of platelets and tumor necrosis factor–pretreated human umbilical vein endothelial cells (HUVEC), Stx1 and Stx1B induced the binding of platelets to the endothelial cell membrane and were present at this binding site. Incubation of Stx1 and Stx1B with whole blood increased fibrinogen binding to platelets detected by flow cytometry. Fibrinogen binding was partially inhibited by preincubation with anti-Stx1. Stx1 increased platelet retention measured in a glass bead assay. In addition, plasma from 17 patients with HUS, taken during the acute phase of the disease, increased the retention of normal platelets and normalized after recovery. Taken together, the results of this investigation show that Stx1, Stx1B, and a factor or factors in the plasma of patients with HUS activate platelets. The presence of Stx1 at the binding site of platelets to HUVEC suggests that Stx may be directly involved in the prothrombotic state seen in HUS.
Introduction
Shiga toxin (Stx)-producing Escherichia coli(STEC) have been associated with cases of hemolytic uremic syndrome (HUS). Microangiopathic hemolytic anemia, acute renal failure, and thrombocytopenia characterize this syndrome. Stx has been specifically implicated as a causal factor1 of HUS because cases of the disease have been associated with Stx-producing strains,2 the toxin has been identified in the kidney of a patient with HUS,3 and the toxin has been found to be cytotoxic for renal endothelial and epithelial cells.4-8In addition, animal models have reproduced aspects of HUS using wild-type bacteria that produce the toxin9,10 or purified toxin.11
The toxin is composed of 5 B, or binding, subunits and 1 enzymatically active A subunit.12 The molecular weight of the A subunit is 33 000 kd, and that of the pentameric B subunit is approximately 35 000 kd.13 The B subunit (StxB) binds to a glycosphingolipid receptor on cell membranes, globotriaosyl ceramide (Gb3), containing the terminal disaccharide galactose α(1-4) galactose β.14 A recent study showed that Stx could bind to platelets through Gb3 and a novel platelet glycosphingolipid termed band 0.03.15 Other studies have not been able to reproduce these results.16,17 Binding to the cell mediates endocytosis of the holotoxin. Intracellularly the A subunit is proteolytically nicked, binds to 60S ribosomes, and thereby inhibits protein synthesis leading to cell death.18
Thrombocytopenia is an important feature of HUS. Platelets are consumed in small thrombi, and these thrombi may compromise the blood supply to various organs, particularly the kidney. The mechanism by which these thrombi are formed is unclear, and platelet activation has been suggested. Studies performed on platelets from patients with HUS showed impaired aggregating responses19,20 and reduced β-thromboglobulin (β-TG) content.19 Platelet-derived products such as β-TG, platelet factor 4,21 and soluble P-selectin22 were found to be elevated during acute HUS. Furthermore, changes in platelet ultramorphology were noted.23 Increased platelet-derived microvesicles were found in these patients,24 indicating platelet activation. Plasma from patients with HUS increased aggregation of normal platelets, but the factor causing this was not determined.25
Several previous studies have investigated a direct interaction between Stx and platelets. Culture filtrates from Stx-producing E coli were found to induce platelet-aggregating activity,26 but purified Stx did not increase platelet aggregation16,27,28 or P selectin expression.16 The procoagulative state in HUS has, therefore, been ascribed to endothelial cell damage. Extensive endothelial cell injury has been described, leading to the exposure of subendothelium and the release of platelet-aggregating substances such as von Willebrand factor.29 Endothelial cell activation has been documented by measuring markers of activated endothelium, such as tissue plasminogen activator (t-PA) and plasminogen activator inhibitor 1 (PAI-1), indicating a prothrombotic and hypofibrinolytic state.30
Using methods other than those previously reported in studies of Stx-platelet interactions, we have studied the effect of Stx and plasma from patients with HUS on platelets to address the possibility that Stx interacts directly with platelets and to investigate whether additional factors are involved in platelet activation.
Patients, materials, and methods
Cells
Platelets were obtained from 9 healthy adult volunteers. The volunteers were not using any medications. Venous blood was collected in 5-mL Vacutainer tubes (Becton Dickinson, Plymouth, United Kingdom) containing 0.5 mL 0.129 M sodium citrate. Platelet-rich plasma (PRP) was obtained after centrifugation at 200g for 20 minutes at room temperature. Then the PRP was centrifuged at 2000g for 10 minutes at room temperature, and the pellet was collected. Cells were washed in 0.009 M EDTA, 0.0264 M Na2HPO4 × 2 H2O, 0.14 M NaCl at pH 7.0, centrifuged at 2000g for 10 minutes, and resuspended in endothelial cell serum-free medium (ESFM; Gibco BRL, Paisley, United Kingdom). PRP or washed platelets were used at a final concentration of 2 × 108/mL (PRP was diluted, if necessary, in the donor's platelet poor plasma [PPP]). These cells were identified as platelets by immunofluorescence with anti-CD41 (mouse IgG1 antiplatelet glycoprotein IIb/IIIa) phycoerythrin (PE)-conjugated antibody (Immunotech, Marseilles, France) using flow cytometry or confocal microscopy as described below.
Human umbilical vein endothelial cell (HUVEC) cultures were obtained from pooled umbilical cords taken from newborns and used within 24 hours. HUVECs were isolated according to a previously described method.31 Briefly, the umbilical cords were washed externally in 70% ethanol, flushed with Hanks balanced salt solution (HBSS; Gibco) at room temperature, and incubated with 1.25 g/L collagenase type 1 (Sigma, Stockholm, Sweden) in HBSS for 10 minutes at 37°C. Dissociation solution was flushed away with 20% fetal calf serum (FCS; Gibco) in HBSS and thereafter with cold HBSS to inactivate the collagenase. The solution was centrifuged at 200g for 10 minutes, and the pellet was resuspended in medium M199 (Gibco) with 2 mM L-glutamine (Gibco), 100 U/mL penicillin, 100 μg/mL streptomycin (both from Gibco), 250 ng/mL Fungizone (Gibco), 17% FCS, and 10 mM glucose (Fluka, Switzerland). Cells were plated onto poly-D-lysine (Sigma) –coated chamber slides (Tamro Med Lab, Mölndal, Sweden) or cultured in 0.2% gelatin (Sigma) -coated cell culture flasks (Nunc, Roskilde, Denmark), grown to confluence and passaged after trypsinization onto chamber slides. Cells in chamber slides were incubated at 37°C in an atmosphere of 95% air and 5% CO2 and grown to near confluence.
By light microscopy, more than 90% of the cells had similar morphology. Cells were confirmed as endothelial by positive staining for von Willebrand factor (DAKO, Glostrup, Denmark), endoglin CD105 (DAKO), and CD31 (DAKO) using the alkaline phosphatase antialkaline phosphatase technique. Using the same method, cells stained negatively for cytokeratins MNF116 (DAKO) and CAM 5.2 (Becton Dickinson, San Jose, CA) and negatively for the presence of leukocytes (CD45; DAKO) or fibroblasts (DAKO).
Stx1, Stx1B, and anti-Stx1
Shiga toxin 1 and Shiga toxin 1 B-subunit purified from E coli were kindly provided by James Brunton (Microbiology Department, The Toronto Hospital, Ontario, Canada). The toxin was found to be cytotoxic in the Vero cell assay (VCA)32-34 at 10 pg/mL and above. Stx1B did not have a cytotoxic effect on the VCA. Dilutions of Stx1 and Stx1B were prepared in ESFM. Mouse monoclonal IgG antibody directed to Stx1A and B subunits35 was obtained from Toxin Technology (Sarasota, FL). Preincubation of the antibody with Stx1 for 1 hour at 37°C neutralized cytotoxicity of the toxin in the VCA at antibody concentrations of 1 to 100 μg/mL, and Stx1 concentrations of 10 pg to 1 ng/mL; ie, 1 μg/mL antibody could neutralize the cytotoxic effect of 1 ng/mL toxin, indicating that an antibody-toxin ratio (ng/ng) of 1000:1 was inhibitory in the VCA. This assay does not enable separate neutralization of the A or the B subunit effects, and the exact antibody-toxin ratio required to inhibit toxin binding could therefore not be calculated. An antibody concentration of 200 μg/mL was used in this study.
The effect of Stx1 on platelets was studied using various techniques. Stx1 binding to platelets was studied by flow cytometry and confocal microscopy; internalization and platelet aggregation were examined by confocal microscopy. Stx1-induced changes in platelet morphology were studied by scanning electron microscopy. Toxin induced fibrinogen binding to platelets, and the effect on platelet interactions was studied by flow cytometry and platelet retention, respectively. All experiments were repeated with reproducible results.
Stx binding to platelets detected by flow cytometry
PRP obtained from 6 adult donors was used in these experiments. Two methods of fixation, before and after incubation with Stx1 or Stx1B, were tested with comparable results. PRP (50 μL), was fixed in 0.5% paraformaldehyde (Sigma) for 30 minutes at room temperature and washed twice in phosphate-buffered saline (PBS). Platelets were then resuspended in the donor's PPP (45 μL) and incubated for an additional 45 minutes at 37°C with 5 μL Stx1 or Stx1B, each at a final concentration of 10 pg/mL to 10 ng/mL. Unstimulated platelets were incubated in PPP with 5 μL ESFM. Inhibition experiments were carried out in which Stx1B was preincubated with monoclonal anti-Stx1 antibody at 37°C for 1 hour before addition to platelets. Stx1B, at concentrations of 50 pg/mL to 50 ng/mL, was preincubated with anti-Stx1 (antibody-toxin ratio of 4000:1 to 4 000 000:1 [ng/ng]). The combined Stx1B–anti-Stx1 (5 μL) was added to the platelets and PPP (45 μL) to achieve final Stx1B concentrations of 10 pg/mL to 10 ng/mL. After incubation, platelets were washed twice in PBS (0.14 M NaCl, 0.003 M KCl, 0.01 M phosphate; pH 7.4; Medicago, Uppsala, Sweden).
Platelets were further incubated for 2 hours at 37°C with monoclonal anti-Stx1 or mouse IgG2a negative control (DAKO), both antibodies at 25 μg/mL in 1% bovine serum albumin (BSA; Sigma) in PBS. As a control, certain tubes were incubated in BSA-PBS without the primary antibody. Platelets were then washed twice in PBS and incubated with goat F(ab)2 antimouse immunoglobulin–fluorescein isothiocyanate (FITC) (DAKO) at a concentration of 1:20 in HEPES buffer (137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 5.6 mM glucose, 1 g/L BSA, and 20 mM HEPES, pH 7.4) for 30 minutes on ice and diluted in 500 μL PBS. Stx1/Stx1B binding was identified by flow cytometry using a Facs calibur instrument (Becton Dickinson), which processed data from 10 000 cells per sample. The platelet population was identified by labeling with mouse-antihuman CD61–FITC (1:13 in HEPES buffer; DAKO) and mouse-antihuman CD41–PE (1:10). White blood cells labeled with mouse-antihuman CD14–PE/CD45–FITC (1:6.5; Opticlone, Marseilles, France) could not be visualized in the same gate.
Cell stimulation assays
Washed platelets from 4 healthy adult donors were resuspended in ESFM without additives and incubated with 10 pg/mL to 1 ng/mL Stx1 and 10 pg/mL to 10 ng/mL Stx1B or were left unstimulated at 37°C for 2 hours. In inhibition experiments, 5-fold higher concentrations of Stx1B (50 pg-50 ng/mL) were preincubated with monoclonal anti-Stx1 antibody (antibody-toxin ratio of 4000:1 to 4 000 000:1 [ng/ng]) at 37°C for 1 hour before stimulation of the platelets. At stimulation, the combined Stx1B–anti-Stx1 was diluted in ESFM with platelets to final concentrations of 10 pg/mL to 10 ng/mL Stx1B.
HUVECs at near confluence were washed in PBS, and the culture medium was replaced with ESFM supplemented with 2 mM L-glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, 20 U/mL heparin (Pharmacia), and 1% endothelial cell growth factor (Sigma). All cells were then prestimulated with 40 ng/mL tumor necrosis factor-α (TNF-α; Research Biochemicals International, Natick, MA) for 24 hours to increase the expression of the Stx Gb3 receptor on the surfaces of HUVECs36 At this time 10 pg to 1 ng/mL Stx1 or 10 pg to 10 ng/mL Stx1B and platelets were added to the media and incubated with HUVECs for an additional 2 hours at 37°C. Several wells were not stimulated with Stx1 or platelets; several wells were stimulated with only Stx1, Stx1B, or platelets.
After 2 hours' incubation, platelets incubated in tubes were spun down onto glass slides. Furthermore, the supernatant was removed from HUVECs and spun down onto glass slides (Cytospin; Shandon, Pittsburgh, PA). Glass slides and chamber slides were fixed in 4% paraformaldehyde for 15 minutes, washed twice in PBS, and left overnight in 1% BSA in PBS, after which they were prepared for immunofluorescence.
Platelets incubated with Stx1 (10 pg/mL-1 ng/mL) in tubes were also prepared for scanning electron microscopic examination. Media from the tubes were placed on poly-D-lysine–coated glass slides for 30 minutes at room temperature, after which they were fixed in 2.5% glutaraldehyde (diluted in 0.1 M Sörensen buffer) for 1 hour at room temperature and left overnight in 0.1 M Sörensen buffer at 4°C. Cells were thereafter critical-point dehydrated in a Balzer critical-point apparatus (Balzer, Lichtenstein) and coated with 15 nm gold in a Polarone 5150 sputter coater (Hertfordshire, United Kingdom). Platelets were examined in a Philips 515 scanning electron microscope (Eindhoven, Holland) and digitally recorded with 1024 × 735 pixels per frame.
Immunofluorescence
To detect Stx1 distribution on and in platelets and HUVECs and to detect platelet activation, cells on glass and chamber slides were permeabilized with saponin (Sigma) to allow the entry of FITC- and PE-conjugated antibodies. Cells were blocked in 3% BSA with 0.1% saponin in PBS at room temperature for 1 hour. After they were washed 3 times in 0.1% saponin/PBS, the cells were incubated with 25 μg/mL monoclonal anti-Stx1 or mouse IgG1 negative control (DAKO) in 0.1% saponin/PBS for 2 hours at 37°C. Cells were washed 3 times in 0.1% saponin/PBS, after which goat F(ab)2 antimouse immunoglobulin–FITC (DAKO) was added at a concentration of 1:20 in 0.1% saponin/PBS and incubated with the cells at 37°C for 1 hour. Cells were washed 4 times and counterstained with mouse-antihuman CD41–PE at a concentration of 1:10 in 0.1% saponin/PBS and incubated for 1 hour at 37°C. Cells were washed 4 times, dried, covered with fluorescent mounting medium (DAKO), and examined by confocal microscopy (1024 laser scanning confocal microscope; Bio-Rad, Hemel-Hempstead, United Kingdom) attached to a Nikon (Tokyo, Japan) Diaphot inverted microscope. As a permeabilization control, all steps of the assay of glass slides with platelets were also carried out without saponin.
Fibrinogen binding
Activated platelets bind fibrinogen; this process is followed by aggregation. We examined fibrinogen binding, as determined by flow cytometry, to study Stx1-induced platelet activation. The method used for detection of fibrinogen on platelets by flow cytometry has been previously described.37 Whole blood was obtained separately from 3 healthy adult volunteers in citrated tubes, as described above, and stimulated with 10 pg/mL to 10 ng/mL Stx1 or Stx1B for 1 hour at 37°C or left unstimulated. To assess the specificity of fibrinogen binding to its receptor, 30 μg/mL Reopro (Eli Lilly, Indianapolis, IN) containing abciximab, a Fab fragment of the monoclonal antibody 7E3 against platelet receptor GPIIb/IIIa (which binds fibrinogen),38 was added to some tubes of whole blood and incubated at room temperature for 15 minutes before the addition of 10 pg/mL to 1 ng/mL Stx1. In separate tubes, whole blood was incubated with only 30 μg/mL Reopro.
Inhibition experiments were carried out in which Stx1 or Stx1B were preincubated with monoclonal anti-Stx1 antibody at 37°C for 1 hour before addition to whole blood. Stx1 at concentrations of 500 pg/mL to 50 ng/mL (antibody-toxin ratio of 4000:1 to 400 000:1 ng/ng) and 5 ng/mL to 500 ng/mL Stx1B (antibody-toxin ratio of 400:1 to 40 000:1 ng/ng) were preincubated with anti-Stx1. After this, the combined Stx1–anti-Stx1 or Stx1B–anti-Stx1 was added to whole blood to achieve final Stx1 concentrations of 10 pg/mL to 1 ng/mL or final Stx1B concentrations of 100 pg/mL to 10 ng/mL. In separate tubes, whole blood was incubated with anti- Stx1 alone.
After incubation, whole blood was centrifuged at 140g for 10 minutes to obtain PRP. Then 5 μL PRP was added to polystyrene tubes containing 50 μL HEPES buffer, and 5 μL each of the following antibodies was used to identify the cells as platelets: mouse-antihuman CD41-FITC; mouse-antihuman CD42b-PE (directed to glycoprotein Ib on platelets; both antibodies from DAKO), and as a control antibody mouse IgG1-RD/IgG1-FITC (Coulter, Miami, FL). The presence of leukocytes was ruled out by the lack of labeling with mouse-antihuman CD14-PE/CD45-FITC. Fibrinogen binding was detected by adding 5 μL chicken antihuman fibrinogen-FITC and, as a control, chicken antihuman insulin-FITC (both antibodies from Biopool, Umeå, Sweden). Samples were incubated with the various antibodies for 30 minutes on ice and diluted in 500 μL PBS, and fluorescence intensity was quantified by flow cytometry using an EPICS XL-MCL instrument (Coulter), which processed data from 5000 cells per sample.
Patients
Seventeen children with HUS—as defined by microangiopathic hemolytic anemia, thrombocytopenia, and renal failure—were treated at the Department of Pediatrics, Section for Pediatric Nephrology, University Hospital of Lund, Sweden. All children had a diarrheal prodrome, consisting of watery or bloody diarrhea, before HUS developed. There were 8 boys and 9 girls, aged 1 to 10 years (median, 3 years), all with acute-phase illness. Plasma was collected in sodium citrate tubes on admission (n = 17 samples) and 2 to 24 months (median, 6 months) after recovery (n = 14 samples).
Citrated plasma was collected from 4 children, 1 boy and 3 girls, aged 2 to 15 years seen for bloody diarrhea not associated with HUS. Citrated plasma was also collected from 10 pediatric control subjects, 5 girls and 5 boys, aged 1 to 15 years (median, 7 years). These patients were seen in the outpatient clinic of the Department of Pediatrics for control of diabetes mellitus, IgA nephropathy, nephrotic syndrome, hydronephrosis, pyelonephritis, neurofibromatosis, and anal atresia. Control subjects did not have fever or symptoms of acute illness at the time of blood sampling.
All PPP samples were prepared by centrifugation of whole blood at 2000g for 20 minutes, after which plasma was removed and stored at −80°C until assayed. This study was approved by the ethics committee of the University of Lund.
Bacterial strains isolated from the stools of patients
Fecal samples from the patients with HUS were obtained on admission. Feces were tested for the presence of the EHEC virulence genes eae and stx by polymerase chain reaction (PCR), as previously described.39 If found positive by PCR, the strains were isolated and serotyped. Thirteen of the 17 patients were found to have genes for eae andstx2 in their fecal samples. In 2 patients whose fecal samples revealed negative findings, fecal samples from immediate family members were found to contain the genes for eae andstx2. STEC was isolated from 12 of 17 patients with HUS and family members of the 2 other patients. Of these, 10 were associated with E coli O157, and the others were associated withE coli O145 (n = 3) and O26 (n = 1).
Similarly, fecal samples were collected from the 4 children with bloody diarrhea. Using the same detection methods, these children were found not to have STEC. Two children were infected with Campylobacter jejuni, in the other 2 children, no infectious agent was found.
Platelet retention assay
Platelet retention was measured using a modified Adeplat S test (Semmelweis, Milan, Italy), as previously described.40 41Platelet suspensions were counted by phase microscopy in Bürker chambers, and tubes were coded to avoid bias.
Platelet retention was tested in blood samples from 3 healthy adult volunteers. In 5 experiments, 1 ng/mL Stx1 was added to whole blood from the 3 volunteers and incubated for 1 hour at room temperature, after which platelet retention was measured. In addition, 1 fg/mL to 1 ng/mL Stx1 was added to whole blood (from 2 of these volunteers), and a dose response was registered.
An in vitro exchange assay was carried out in which additional blood samples from the same volunteers were centrifuged at 2000gfor 20 minutes and the PPP was removed and replaced with an equal volume of PPP from the patients with HUS or bloody diarrhea and the control subjects. Plasma was then gently combined with the volunteer's blood cells and incubated for 1 hour at room temperature, after which it was assayed in the platelet retention assay. In vitro separation of plasma and blood cells does not affect the results of the platelet retention assay.40
Statistics
Stx1 and Stx1B binding and the effects of Stx1 and Stx1B on levels of fibrinogen binding and of Stx1 on platelet retention were measured by the Mann-Whitney U test. Similarly measured were differences between patients during acute illness and after recovery and between patients and controls with regard to platelet retention.P ≤ .05 was considered significant.
Results
Stx1 and Stx1B binding to platelets
Platelets were incubated with Stx1 or Stx1B or were left unstimulated. Significant binding in a dose-dependent manner was detected by flow cytometry. Binding was partially neutralized by preincubation with anti-Stx1 antibody. Results are presented in Table 1.
Stx1 and Stx1B uptake in platelets
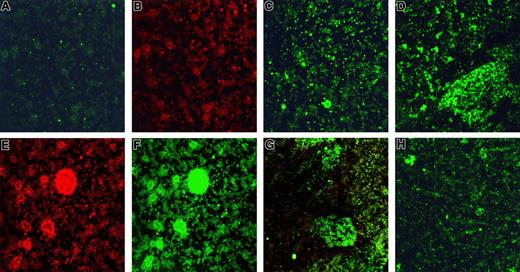
Stx1 and Stx1B were incubated with washed platelets, after which binding and internalization were visualized by fluorescence confocal microscopy. Stx1-FITC staining was not noted in platelets that had not been stimulated with Stx1 or Stx1B (Figure1A). These platelets were identified by the presence of CD41 (Figure 1B). FITC uptake, indicating the internalization of Stx1, was observed in platelets after incubation with Stx1 (Figure 1C) and Stx1B (Figure 1D). This was noted for all concentrations of Stx1 and Stx1B. Preincubation of Stx1B with monoclonal anti-Stx1 inhibited the uptake of Stx1B at all but the highest concentration. No FITC uptake was noted in platelets in which immunofluorescence was carried out without saponin.
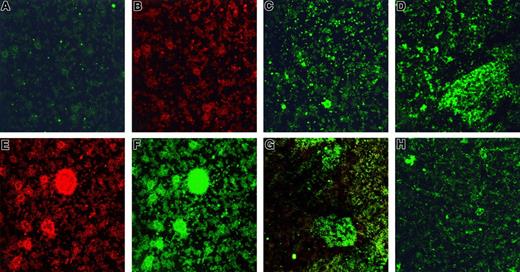
Stx1 uptake and activation of platelets.
(A) Unstimulated human platelets in medium incubated with monoclonal anti-Stx1 and goat-antimouse immunoglobulin FITC. No FITC labeling observed. (B) Same as in panel A, counterstained with mouse-antihuman CD41–PE to identify platelets. (C, D) Platelets incubated for 2 hours with 10 pg/mL Stx1 (C) or 10 ng/mL Stx1B (D). FITC labeling noted in cells. (E) Platelet aggregates forming after incubation with 100 pg/mL Stx1 labeled with mouse-antihuman CD41-PE (to identify platelets). (F) Platelet aggregates containing Stx1-FITC after incubation with 100 pg/mL Stx1 (same as panel E, labeled for Stx1). (G) Platelet aggregates containing Stx1B-FITC after incubation with 10 pg/mL Stx1B (combined labeling for FITC-Stx1 and anti-CD41–PE). (H) Preincubation of Stx1B (final concentration, 10 pg/mL; see “Patients, materials, and methods”) with anti-Stx1 inhibited the uptake of Stx1B and the formation of aggregates (FITC-Stx1 staining). (A-H) Magnification, × 400.
Stx1 uptake and activation of platelets.
(A) Unstimulated human platelets in medium incubated with monoclonal anti-Stx1 and goat-antimouse immunoglobulin FITC. No FITC labeling observed. (B) Same as in panel A, counterstained with mouse-antihuman CD41–PE to identify platelets. (C, D) Platelets incubated for 2 hours with 10 pg/mL Stx1 (C) or 10 ng/mL Stx1B (D). FITC labeling noted in cells. (E) Platelet aggregates forming after incubation with 100 pg/mL Stx1 labeled with mouse-antihuman CD41-PE (to identify platelets). (F) Platelet aggregates containing Stx1-FITC after incubation with 100 pg/mL Stx1 (same as panel E, labeled for Stx1). (G) Platelet aggregates containing Stx1B-FITC after incubation with 10 pg/mL Stx1B (combined labeling for FITC-Stx1 and anti-CD41–PE). (H) Preincubation of Stx1B (final concentration, 10 pg/mL; see “Patients, materials, and methods”) with anti-Stx1 inhibited the uptake of Stx1B and the formation of aggregates (FITC-Stx1 staining). (A-H) Magnification, × 400.
Stx1- and Stx1B-induced activation of platelets
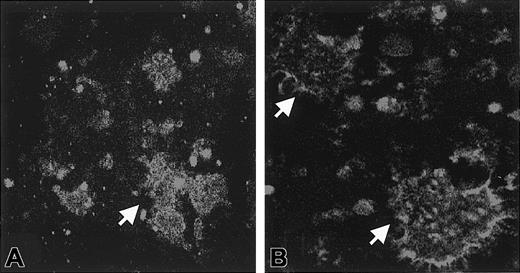
After the incubation of platelets with Stx1, aggregates formed in the media. These aggregates contained platelets as identified by the presence of CD41 (Figure 1E). Marked uptake of Stx1 was demonstrated in these aggregates (Figure 1F). In separate experiments, Stx1B was incubated with platelets, and aggregates with prominent fluorescent Stx1B uptake were noted in the media (Figure 1G). Aggregate formation was noted for all concentrations of Stx1 and Stx1B used. Preincubation of Stx1B with monoclonal anti-Stx1 antibody inhibited the formation of platelet aggregates at all (Figure 1H) but the highest concentration of Stx1B. In immunofluorescence experiments in which the control antibody was used, there was no FITC-conjugated antibody uptake, but platelet aggregates were identified by the presence of CD41. In experiments in which immunofluorescence was assayed without saponin, FITC staining was noted on the periphery of aggregates but not within them (Figure2).
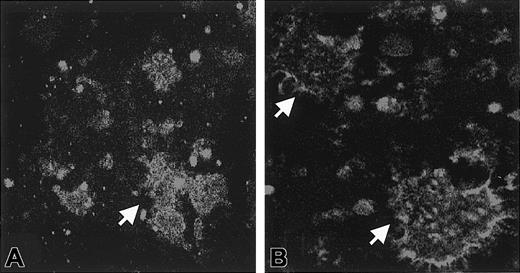
Effect of saponin permeabilization on immunofluorescence.
Washed platelets were incubated with Stx1, and aggregates formed in the medium. In experiments in which immunofluorescence was performed in the presence of saponin, FITC staining was noted inside the aggregates for all concentrations of Stx1 (A, 1 ng/mL Stx1). In experiments in which immunofluorescence was assayed without saponin, FITC staining was noted on the periphery of aggregates. This was demonstrated for all concentrations of Stx1 (B, 1 ng/mL Stx1). Arrows indicate aggregates. Aggregates were identified as platelets by counterstaining with antibody to CD41-PE (not shown). Magnification, × 1000.
Effect of saponin permeabilization on immunofluorescence.
Washed platelets were incubated with Stx1, and aggregates formed in the medium. In experiments in which immunofluorescence was performed in the presence of saponin, FITC staining was noted inside the aggregates for all concentrations of Stx1 (A, 1 ng/mL Stx1). In experiments in which immunofluorescence was assayed without saponin, FITC staining was noted on the periphery of aggregates. This was demonstrated for all concentrations of Stx1 (B, 1 ng/mL Stx1). Arrows indicate aggregates. Aggregates were identified as platelets by counterstaining with antibody to CD41-PE (not shown). Magnification, × 1000.
Stx1 and Stx1B uptake in TNF-α–pretreated HUVECs
FITC uptake was not noted in TNF-α–pretreated HUVECs that were not stimulated with Stx1 or Stx1B (Figure3A). FITC staining was visualized in HUVECs after incubation with Stx1 (Figure 3B) and Stx1B. This was noted for all concentrations of Stx1 and Stx1B. FITC uptake was not observed in HUVECs similarly incubated with Stx1 and visualized with the control and FITC-conjugated antibodies.
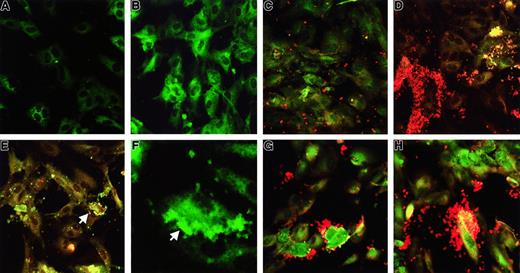
Platelet binding and aggregation on TNF-α–prestimulated HUVECs.
(A) Unstimulated (ie, not incubated with Stx) HUVECs incubated with monoclonal anti-Stx1 and goat-antimouse immunoglobulin FITC. No FITC labeling observed. (B) HUVECs incubated for 2 hours with 100 pg/mL Stx1. Stx1-FITC labeling noted in cells. (C) HUVECs incubated with platelets without Stx1. Scattered platelets noted. (D) HUVECs incubated with 100 pg/mL Stx1 and platelets. Platelet aggregates containing Stx1-FITC and platelet binding to HUVECs noted (panels C and D show combined staining FITC-Stx and anti CD41-PE). (E) HUVECs incubated with 1 ng/mL Stx1 and platelets showing platelet binding to the circumference of HUVEC (see arrow, FITC-Stx and anti CD41-PE). (F) HUVECs incubated with 100 pg/mL Stx1 and platelets. Arrow shows platelet aggregate containing Stx1-FITC binding to HUVEC. (G, H) HUVECs incubated with 100 pg/mL (G) or 10 pg/mL (H) Stx1B and platelets showing uptake of Stx1B in both cell types, aggregation of platelets, and binding to HUVECs (FITC-Stx and anti-CD41–PE). Magnifications: A-E and G-H, × 400; F, × 1300.
Platelet binding and aggregation on TNF-α–prestimulated HUVECs.
(A) Unstimulated (ie, not incubated with Stx) HUVECs incubated with monoclonal anti-Stx1 and goat-antimouse immunoglobulin FITC. No FITC labeling observed. (B) HUVECs incubated for 2 hours with 100 pg/mL Stx1. Stx1-FITC labeling noted in cells. (C) HUVECs incubated with platelets without Stx1. Scattered platelets noted. (D) HUVECs incubated with 100 pg/mL Stx1 and platelets. Platelet aggregates containing Stx1-FITC and platelet binding to HUVECs noted (panels C and D show combined staining FITC-Stx and anti CD41-PE). (E) HUVECs incubated with 1 ng/mL Stx1 and platelets showing platelet binding to the circumference of HUVEC (see arrow, FITC-Stx and anti CD41-PE). (F) HUVECs incubated with 100 pg/mL Stx1 and platelets. Arrow shows platelet aggregate containing Stx1-FITC binding to HUVEC. (G, H) HUVECs incubated with 100 pg/mL (G) or 10 pg/mL (H) Stx1B and platelets showing uptake of Stx1B in both cell types, aggregation of platelets, and binding to HUVECs (FITC-Stx and anti-CD41–PE). Magnifications: A-E and G-H, × 400; F, × 1300.
Stx1 and Stx1B activation of platelets in the presence of HUVECs
The effect of Stx on platelet aggregation was studied in the presence of TNF-α–pretreated HUVEC. In the absence of Stx, scattered platelets were noted around HUVECs (Figure 3C). When Stx1 was incubated with HUVECs, platelet aggregates were noted in the media. These aggregates contained platelets as identified by the presence of CD41, and marked uptake of Stx1 was demonstrated in these aggregates. Stx1 induced platelet aggregation on HUVECs (Figure 3D). Platelets bound to the endothelial cell membrane and distributed in the circumference of the cell (Figure 3E). The toxin was visualized by FITC uptake in platelets and HUVECs at the site of platelet binding to HUVECs (Figure 3F).
Similarly, Stx1B was incubated with TNF-α–pretreated HUVECs and platelets, and induced marked platelet aggregation. Uptake of Stx1B was noted in HUVECs and in platelets. In these experiments, platelets bound to the endothelial cell membrane (Figure 3G-H), and Stx1B was present in platelets and HUVECs at the binding site. Platelet aggregation in the media and on HUVECs was noted for all concentrations of Stx1 and Stx1B. In experiments in which the control antibody was used, there was no FITC-conjugated antibody uptake, but platelet aggregates on HUVECs were identified by the presence of CD41.
Stx1-induced changes in platelet ultramorphology
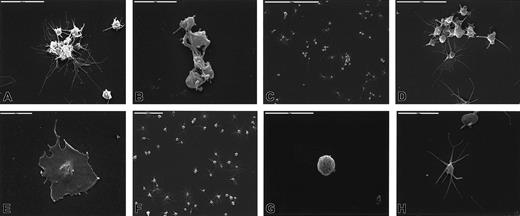
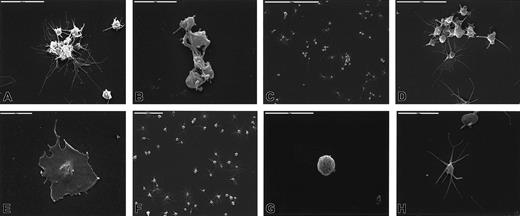
Washed platelets were incubated with Stx1 at 37°C and fixed, and structural changes were studied by scanning electron microscopy. Stx1 induced the formation of aggregates consisting of 8 to 15 platelets (Figure 4A-B). Extended long pseudopodia were noted (Figure 4C-D), and some platelets totally lost their round form and adhered in flattened form to the glass (Figure 4E). Unstimulated platelets did not aggregate (Figure 4F) and exhibited round (Figure 4G), discoid, or elongated form with tactile projections (Figure 4H). These experiments were not carried out for Stx1B.
Stx1-induced changes in platelet ultramorphology.
Platelet aggregates were formed after incubation with 10 pg/mL (A), 100 pg/mL (B), 1 ng/mL (C, D) Stx1. Long pseudopodia were noted (A, B, D), and highly activated platelets flattened and adhered firmly to the glass (E, Stx1 100 pg/mL). Panels F, G, and H show unstimulated platelets that are round, discoid, or elongated and do not aggregate.
Stx1-induced changes in platelet ultramorphology.
Platelet aggregates were formed after incubation with 10 pg/mL (A), 100 pg/mL (B), 1 ng/mL (C, D) Stx1. Long pseudopodia were noted (A, B, D), and highly activated platelets flattened and adhered firmly to the glass (E, Stx1 100 pg/mL). Panels F, G, and H show unstimulated platelets that are round, discoid, or elongated and do not aggregate.
Stx1 and Stx1B induced fibrinogen binding to platelets
Platelet activation has been shown to trigger structural changes leading to the exposure of the glycoprotein (GP) IIb/IIIa receptor, thus enabling the binding of fibrinogen and von Willebrand factor.42 This process leads to platelet aggregation. We examined whether Stx1 and its B subunit increase fibrinogen binding to platelets. Stx1 was incubated with whole blood from healthy volunteers for 1 hour at 37°C, after which flow cytometry showed increased fibrinogen binding to platelets in all experiments (Table2). Preincubation of Stx1 with anti-Stx1 partially inhibited fibrinogen binding. Preincubation of platelets with Reopro (monoclonal antibody to GP IIb/IIIa; Eli Lilly) reduced fibrinogen binding induced by Stx1 (Table 2). The results of one representative experiment are shown in Figure5.
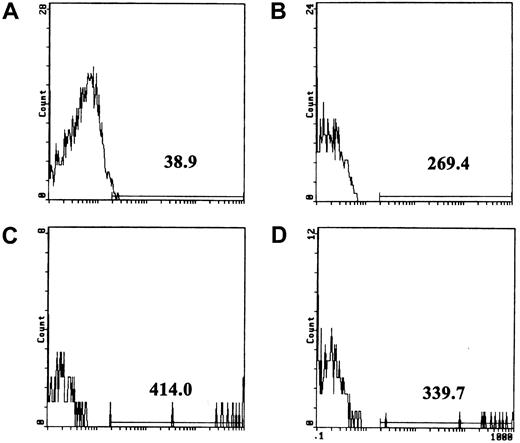
Fibrinogen binding to platelets assayed by flow cytometry.
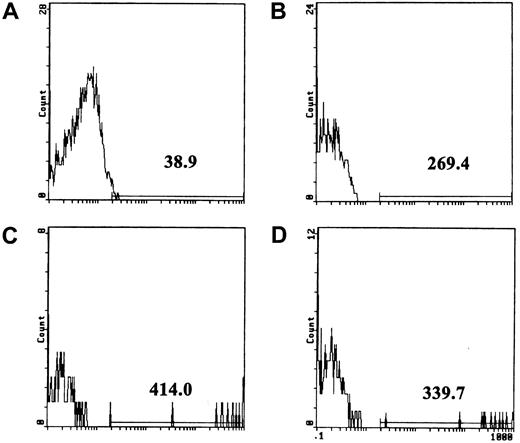
(A) Background fluorescence of unstimulated platelets incubated with the FITC-conjugated anti-insulin control antibody. The line above the x-axis was placed at approximately the point where the fluorescence curve terminated, and the number shows mean fluorescence intensity above the background. (B) Unstimulated platelets incubated with FITC-conjugated antifibrinogen antibody. (C) Fibrinogen binding to platelets stimulated with 100 pg/mL Stx1 (3% of cells were positive in this experiment; range, 3%-10% in 6 experiments). (D) Fibrinogen binding to platelets was reduced by preincubation of 100 pg/mL Stx1 (final concentration; see “Patients, materials, and methods”) with anti-Stx1.
Fibrinogen binding to platelets assayed by flow cytometry.
(A) Background fluorescence of unstimulated platelets incubated with the FITC-conjugated anti-insulin control antibody. The line above the x-axis was placed at approximately the point where the fluorescence curve terminated, and the number shows mean fluorescence intensity above the background. (B) Unstimulated platelets incubated with FITC-conjugated antifibrinogen antibody. (C) Fibrinogen binding to platelets stimulated with 100 pg/mL Stx1 (3% of cells were positive in this experiment; range, 3%-10% in 6 experiments). (D) Fibrinogen binding to platelets was reduced by preincubation of 100 pg/mL Stx1 (final concentration; see “Patients, materials, and methods”) with anti-Stx1.
Stx1B increased fibrinogen binding in a similar manner to the holotoxin (Table 2). Preincubation of Stx1B with monoclonal anti-Stx1 antibody reduced this effect.
Stx1 induced platelet retention
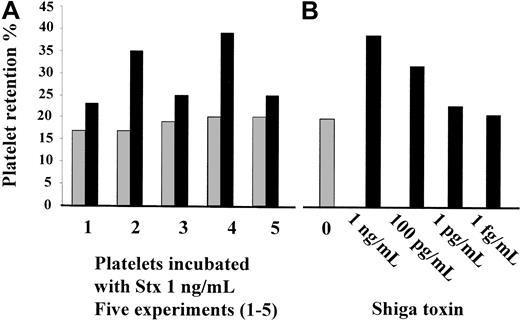
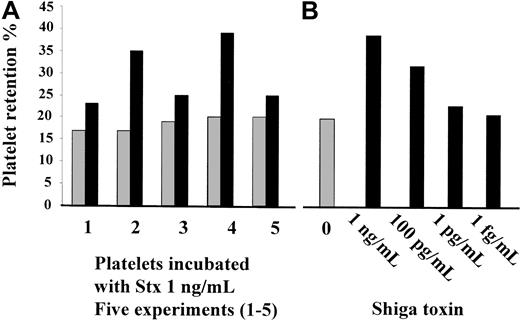
Stx1 was added to whole blood taken from healthy volunteers. The volunteers had baseline platelet retention values of 17%, 17%, 19%, 20%, and 20% in 5 different experiments (median, 19%). In these experiments, 1 ng/mL Stx1 increased platelet retention to 23%, 35%, 25%, 39%, and 25%, respectively (median, 25%;P < .01 compared to controls; Figure6A). A dose response with a range of concentrations between 1 fg/mL and 1 ng/mL was carried out and registered in 2 experiments. Platelet retention values increased in a dose-related manner when 1 pg/mL to 1 ng/mL Stx1 was added to whole blood, but no change was registered for lower concentrations of Stx1. Results from one of these experiments are shown in Figure 6B.
Stx-induced platelet retention.
(A) Stx1 (1 ng/mL) increased platelet retention in whole blood from healthy volunteers; results of 5 experiments are shown (1-5). (B) Dose-related increase in platelet retention after incubation with Stx 1. ░, unstimulated whole blood; ▪, whole blood stimulated with Stx1.
Stx-induced platelet retention.
(A) Stx1 (1 ng/mL) increased platelet retention in whole blood from healthy volunteers; results of 5 experiments are shown (1-5). (B) Dose-related increase in platelet retention after incubation with Stx 1. ░, unstimulated whole blood; ▪, whole blood stimulated with Stx1.
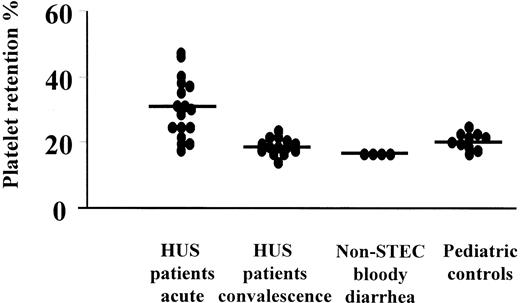
Plasma from patients with HUS activates normal platelets
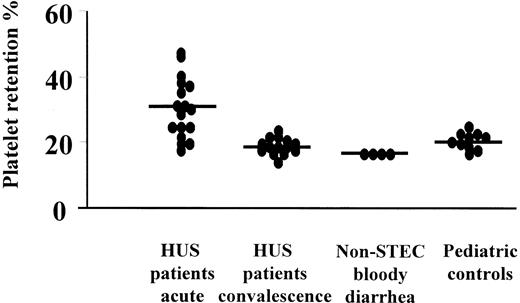
Assay results are presented in Figure7. They indicate that plasma taken during the acute phase of HUS increased retention of normal platelets, whereas plasma taken after recovery, from patients with bloody diarrhea not associated with STEC, and from pediatric controls did not have this effect.
Plasma from patients with HUS increases platelet retention.
Plasma from patients with HUS taken during the acute phase of disease (n = 17; median platelet retention, 30%) showed increased platelet retention compared to plasma from the same patients after recovery (n = 14; median, 18%, P = .0001), patients with non-STEC–associated bloody diarrhea (n = 4; median, 16%;P < .001), and pediatric controls (n = 10; median, 20%; P < .01).
Plasma from patients with HUS increases platelet retention.
Plasma from patients with HUS taken during the acute phase of disease (n = 17; median platelet retention, 30%) showed increased platelet retention compared to plasma from the same patients after recovery (n = 14; median, 18%, P = .0001), patients with non-STEC–associated bloody diarrhea (n = 4; median, 16%;P < .001), and pediatric controls (n = 10; median, 20%; P < .01).
Discussion
In this study, we used various qualitative and quantitative techniques to demonstrate an interaction between Stx and platelets, and we report that the toxin is capable of binding to and activating platelets. In the presence of platelets and endothelial cells, Stx1 induced the binding of platelets to endothelial cells and was present at this binding site. This investigation shows, for the first time, a direct interaction between Stx and platelets leading to activation. In addition to Stx1, a factor or factors in plasma from patients with HUS was also capable of activating platelets, indicating that Stx1 and circulatory factors, possibly released from endothelial cells or platelets, may contribute to the prothrombotic state seen in HUS.
Previous studies have shown that Stx is capable of binding to the Gb3 receptor on many different cell types, such as endothelium, epithelium, erythrocytes, monocytes, and tumor cell lines,4,8,43-45and that receptor expression is a prerequisite for the induction of the microangiopathic process by the toxin.46-48 Cells lacking the toxin receptor were resistant to the toxic effects.49Binding of Stx to Gb3 and band 0.03 on platelets was recently exhibited by high-performance thin-layer chromatography and by flow cytometry.15 Using flow cytometry and confocal microscopy, we obtained similar results demonstrating Stx binding and uptake in intact platelets. Cooling et al15 showed that not all platelets bind Stx and suggested that platelets capable of binding may be older cells. We showed that 5% to 21% of platelets bound Stx1 and Stx1B within 45 minutes. Levels of binding may decrease after internalization occurs. Although the time span required for endocytosis of Stx in platelets is unknown, confocal microscopy demonstrated that most platelets internalized Stx-FITC, but not all platelets aggregated within 2 hours. Binding and uptake were specific and could be partially inhibited by preincubation with monoclonal anti-Stx1 antibody. In agreement with these in vitro results, platelet counts in D+ HUS are usually not extremely low, suggesting that the entire platelet population may not be involved.
Two previous studies could not demonstrate Stx binding to platelets.16 17 We propose that these discrepancies may result from methodological differences related to the length of incubation of platelets with Stx and the temperature at incubation. Specifically, we incubated platelets with Stx at 37°C and believe that this enabled binding to occur.
In vitro and in vivo studies have shown that Stx is capable of inducing cell death by intracellular blocking of protein synthesis18 and by inducing apoptosis.6,7,50,51 Recent studies have also reported that Stx is capable of activating intestinal epithelial cells, monocytes, and monocytic cell lines to produce and secrete cytokines,44,52 53 suggesting that the toxin may have stimulatory effects as well. We show that Stx induced ultramorphologic changes leading to platelet aggregation, increased fibrinogen binding to platelets, and platelet retention. These results indicate that Stx is capable of platelet activation.
Three studies16,27,28 show that Stx does not induce platelet aggregation, as measured by aggregometry. We obtained similar results using a Lumi aggregometer and blood from the same healthy donors mentioned in this study (data not shown). Thus, the lack of aggregation using this method cannot be attributed to donor-specific differences in platelet receptors. For this reason, we used alternative methods for the detection of platelet activation. Platelets aggregates form through sequential stages of adhesion, activation, and aggregation.54 In the presence of high shear stress, adhesion is mediated through the binding of von Willebrand factor (vWF) to glycoprotein Ib (GPIb).42,55,56 After adhesion occurs, shape changes take place in platelets allowing alteration in platelet receptor GPIIb/IIIa, which then binds fibrinogen and vWF, inducing aggregation. We measured platelet activation by retention in a high shear stress flow system41 57 and by detection of fibrinogen binding to platelets secondary to exposure of the GPIIb/IIIa receptor. These techniques rely on physiological aspects of platelet activation and indicate a direct and specific effect of Stx on platelet stimulation.
Endothelial cell damage is a prominent pathologic feature of HUS58 and is considered essential for the procoagulative state seen in this disorder. Injured endothelium allows for the exposure of the subendothelium, which contains vWF and fibrinogen, thus leading to the formation of microthrombi. In vitro experiments have demonstrated that Stx induced a cytotoxic effect on HUVECs4 and that this effect increased when these cells were pretreated with TNF-α because of an increase in expression of Gb3.36 59 We studied the direct interaction between Stx1, TNF-α–pretreated HUVECs, and platelets and found that Stx1 binds to both cells, is internalized, induces binding of platelets to endothelium, and is present at this binding site. The mechanism by which platelets bound to HUVECs in this study is unclear, but Stx1 or its B subunit were observed in both platelets and HUVECs and increased immunofluorescence was noted at the actual binding site. This suggests that Stx, in addition to its direct interaction with platelets, may induce a prothrombotic state by inducing platelet aggregation on endothelium and not only by exposure of the subendothelium.
A factor or factors present in the plasma of patients with acute HUS was also capable of inducing platelet activation. This confirms the observation by others that plasma from patients with HUS contains a platelet-aggregating factor.25 It has been suggested that such a factor may be released from endothelial cells or platelets,25 and increases in vWF, which may enhance platelet aggregation, have been reported in patients with HUS.29 The factor or factors may be released from host cells or may originate from the bacteria. The nature of the circulatory factors capable of increasing platelet activation will have to be addressed in future studies.
The B subunit of Stx is known for its function as a binding subunit.12 A previous study suggested that, at high doses, it might induce apoptosis.50 In the present study, we have shown that the B subunit in pentameric form could induce platelet aggregation in medium and on endothelium. Concentrations of the B subunit were equivalent to those of the holotoxin, though the actual concentration of B subunit per milliliter would be approximately double that for the holotoxin because the latter also consists of the A subunit. Based on the various methods used in this study, we conclude that the optimal concentration of Stx1 and its B subunit capable of causing platelet activation would be 100 pg/mL or higher. We propose that the binding of the B subunit to its receptor on platelets and endothelial cells may transmit an intracellular signal leading to platelet aggregation and binding to endothelium. Thus, although the B subunit may not be cytotoxic, it may induce harmful effects.
It has been suggested that polymorphonuclear leukocytes,17monocytes,44 or erthrocytes60 61 may transport Stx in the circulation from the intestine to the kidney. The results of this investigation suggest that on contact with the intestinal circulation, Stx may bind to platelets and to other blood cells. The toxin may induce platelet consumption in microthrombi already at this stage and may circulate bound to platelets. Because platelets do not contain nuclei and, as such, do not have an active protein synthesis apparatus, they are not as susceptible as other cells to the cytotoxic effects of the toxin and could thus transport the toxin without becoming injured.
The observations reported in this study suggest that Stx can directly interact with platelets leading to aggregation and binding to endothelium. Stx appears to induce this effect through its binding subunit. Circulatory factors possibly released from endothelial cells or platelets may enhance the procoagulant state. Theoretically, our findings would support the recently proposed antiadhesive therapies aimed at blocking the binding of Stx to its glycosphingolipid receptor.62 63
We thank Dr J. Brunton (Microbiology Department, The Toronto Hospital, Canada) for providing purified Stx1 and Stx1B and Dr T. Olofsson (Department of Hematology, Lund University, Sweden) for the use of the flow cytometry instrument. We thank Dr Lars Holmberg (Department of Pediatrics) and Dr Catharina Svanborg (Department of Microbiology, Immunology and Glycobiology), both of Lund University, for help and support, and we thank Gerd Hansson and Lena Jirgård for their technical assistance.
Supported by grants from The Swedish Society of Medicine, The Crafoord Foundation, Alfred Österlunds Foundation, Greta and Johan Kocks Foundations, Anna Lisa and Sven-Eric Lundgren Foundation, The Royal Physiographic Foundation, The Foundation for Fighting Blood Disease, Research funds of the Malmö University Hospital, and Region Skåne.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Diana Karpman, Department of Pediatrics, Lund University, S-22185 Lund, Sweden; e-mail: diana.karpman@skane.se.